Abstract
Objective
Mirtax is a generic mirtazapine widely used since 2003. We conducted an open-label, uncontrolled 6-week study to evaluate the efficacy and safety of Mirtax for major depressive disorder (MDD).
Methods
Ninety three MDD patients with the diagnosis of MDD and 17-item Hamilton Depression Rating Scale (HDRS) score ≥14 were recruited. The HDRS, Montgomery-Åsberg Depression Rating Scale (MADRS), and the Clinical Global Impressions-Severity Scale (CGI-S) were administered at baseline, 1, 2, 4 and 6 weeks. Response (≥50% decrease in the HDRS or MADRS score), remission (absolute HDRS score ≤7 or MADRS score ≤10) and CGI-I score ≤2 were also calculated. Adverse event (AE) frequency and severity, weight, blood pressure, and pulse rate were checked to assess safety.
Results
The starting dosage was 11.5±6.4 mg/day, and the maintenance dosage was 23.1±9.4 mg/day. During 6 weeks, HDRS, MADRS and CGI-S scores decreased from 25.1±5.6 to 11.9±8.6 (mean change −13.1±8.3, p<0.001), from 30.2±6.3 to 13.73±10.40 (mean change −16.5±9.8, p<0.001), and from 5.0±0.8 to 2.5±1.3 (mean change −2.5±1.3, p<0.001), respectively. The percentages of responders, remitters by HDRS and patients with a CGI-I score ≤2 were 64.6%, 35.4% and 52.7%, respectively. Significant decreases in HDRS, MADRS and CGI-S scores were confirmed at week 1. The total rate of AEs was 32.3%; the most frequently reported AEs were sedation (4.3%) and constipation (4.3%). Weight was increased from 58.8±10.6 to 60.3±9.3 kg (mean change 0.7±1.7 kg, p=0.004).
Conclusion
This study, as the first clinical trial of generic mirtazapine, demonstrated the efficacy and tolerability of Mirtax for MDD using a single treatment design.
Keywords: Mirtazapine, Generic drugs, Efficacy, Tolerability
INTRODUCTION
Depression is a common and longstanding illness, which contributes to the major global burden of disease. In 2009, the World Health Organization states that approximately 350 million people affected depression, and it is the second leading cause of disability worldwide.1) Antidepressants can be an effective form of the treatment for major depressive disorder (MDD). Among them, mirtazapine is a widely used antidepressant due to its unique pharmacological profile and good psychiatric efficacy. It mainly blocks 5-hydroxytryptamine (5-HT)2, 5-HT3 and α2-auto and heteroreceptors, subsequently enhancing noradrenergic and serotonergic transmission. It also acts as a potent histamine receptor antagonist, which causes a sedative effect.2) Mirtazapine showed good antidepressant efficacy equal to selective serotonin reuptake inhibitor (SSRI)3) or serotonin-norepinephrine reuptake inhibitor (SNRI),4) and advantages especially in the early course of treatment.5)
Since 1996, mirtazapine has been internationally available for the treatment of MDD, including in the United States. During this period, many generic forms of mirtazapine were released following bioequivalence tests. Mirtax (Sandoz Co.) is a widely used generic mirtazapine that was approved by the United States Food and Drug Administration in 2003. In fact, most generics are released following evidence that their pharmacokinetics are similar to those of the original drug,6) and few clinical trials have been conducted. Because of the similar pharmacokinetic profiles, we presume that the effects of generic mirtazapines are similar to those of branded mirtazapine. However, generic drug sometimes did not show the efficacy as same as branded drug.7) About paroxetine, there are potential differences in efficacy and safety between paroxetine mesylate and paroxetine hydrochloride.8) It also is unknown whether generic mirtazapine is effective or not due to a lack of evidence. Therefore, to evaluate the effects of generic mirtazapine, we conducted a phase 4 clinical trial of Mirtax film-coated tablets. This study simply focused on the Mirtax treatment itself without adopting any control group or switching design from branded medication. However, to our knowledge, this is the first clinical trial to evaluate the efficacy and tolerability of generic mirtazapine.
METHODS
Study Design
This was an open-label, uncontrolled, prospective 6-week study to evaluate efficacy and tolerability of Mirtax in MDD patients. It was conducted at 9 centres in Korea from June 2012 to December 2013. Recruitment was accomplished by recommendation from clinicians at the outpatient clinic without any advertisement process. Enrolment criteria were: (1) adult patients over 20 years of age who were diagnosed with MDD according to Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria, (2) a 17-item Hamilton Depression Rating Scale (HDRS) score of ≥14 at screening, (3) lack of sufficient treatment for the present episode or necessary medication changes due to tolerability problems. Sufficient pharmacological treatment was defined as using appropriate dosage of antidepressant for at least 6 weeks. Appropriate dosages were regarded as 20–40 mg/day of fluoxetine, 20–40 mg/day of paroxetine, 20–40 mg/day of citalopram, 10–20 mg/day of escitalopram, 50–200 mg/day of sertaline, 75–225 mg/day of venlafaxine and 150–300 mg/day of bupropion. Patients treated with mirtazapine at the point of screening that were also at risk for suicidality or other psychiatric diagnoses were excluded. The screening period was within 2 weeks before baseline measurement; however, when patients had no specific reason for disqualification, screening was permitted following baseline measurement on the same day. Previous antidepressants were stopped after screening. Exclusion criteria were pregnancy or lactation, a medical condition that could interfere with everyday life activities, and psychotic symptoms or previous diagnosis of bioplar disorder or any psychotic disorder. Current primary diagnosis other than MDD, lack of responses during current or a past episode of depression to two or more antidepressant at clinically appropriate doses for a minimum of 6 weeks, and severe suicidal risk were also under the exclusion criteria.
The visit schedule included: screening, baseline, and 1, 2, 4, and 6 weeks. During that period, dosages were started and maintained flexibly at the discretion of the investigator. Any other antidepressants, antipsychotics, mood stabilizers, psychostimulants and buspirone were prohibited; benzodiazepine (≤3 mg/day of lorazepam-equivalent dosage) and hypnotics were allowed.
The study was conducted according to the Declaration of Helsinki and Good Clinical Practice. Informed consent was obtained from all participants following an extensive explanation of the nature and procedures of the study. The study protocol was approved by the Institutional Review or Ethics Committee of each study site.
Efficacy and Tolerability Assessments
Efficacy was evaluated using the HDRS,9) Montgomery-Åsberg Depression Rating Scale (MADRS),10) the Clinical Global Impressions-Severity Scale (CGI-S) and the Clinical Global Impressions-Improvement Scale (CGI-I).11) The primary efficacy was measured as the mean HDRS change from baseline to week 6. Additional efficacy measures included the mean changes in MADRS, CGI-S scores, and response, remission, and CGI-I ≤2 rates at week 6. A response was defined as a ≥50% decrease in the HDRS or MADRS score, and remission was defined as an absolute HDRS score ≤7 or MADRS score ≤10.12)
Tolerability and safety were evaluated with the frequency and severity of AEs, and mean changes in weight, blood pressure, and pulse rate from baseline to week 6. AEs were filled up in the constructed response questionnaire in the Novartis Adverse Event Report Form.
Statistical Analyses
Efficacy and safety were analysed in an intent-to-treat (ITT) group, and the last-observation-carried-forward (LOCF) method was applied for endpoint analysis. All patients who received at least one dosage of the study medication were included in the safety analysis.
Data are presented as means±standard deviation for quantitative variables and frequencies (percentage) for categorical variables. Quantitative data were analysed by Student’s t-tests and categorical data were analysed by chi-squared tests. Repeated measures analysis of variance (ANOVA) was used to determine the changes in group with adjusting for the time and repeated measures analysis of covariance (ANCOVA) with baseline score as covariate also used to check the different decreases between mild to moderate and severe subgroups. Response and remission rates of each subgroup were also compared. Analyses were performed using SAS software (ver. 9.2; SAS Institute Inc., Cary, NC, USA). Statistical significance was defined as p<0.05.
RESULTS
Patients and Medications
After screening, a total of 93 patients (70 females, 75.3%) entered the study (Table 1). Most patients (61, 65.6%) were in their 40s. Seventy-two patients (77.4%) were free from antidepressant treatment at baseline, and 65 patients (69.9%) had a prevalence period of less than 1 year. Baseline HDRS, MADRS and CGI-S scores were 25.0±5.6, 30.3±6.3 and 5.0±0.8, respectively. Mean starting dosage at baseline was 11.5±6.4 mg/day and mean maintenance dosage at week 4 was 23.1±9.4 mg/day.
Table 1.
Baseline demographic and clinical characteristics
| Variable | Data |
|---|---|
| Age (yr) | 50.87±11.06 |
| Sex (female) | 70 (75.3) |
| Married | 78 (83.9) |
| Prevalence period of depression (mon) | 18.40±47.35 |
| Treatment period of depression (mon) | 16.45±49.06 |
| Previous history of antidepressant use | 21 (22.6) |
| Comorbid medical illness | |
| Hypertension | 24 (39.3) |
| Dyslipidaemia | 11 (18.0) |
| Diabetes | 9 (14.8) |
| Renal disorder | 2 (3.3) |
| Liver disorder | 1 (1.6) |
| Urinary incontinence | 1 (1.6) |
| Baseline concomitant medications | |
| Anxiolytics | 59 (74.7) |
| Hypnotics | 27 (34.2) |
| β-blocker | 9 (11.4) |
| Baseline score of scales | |
| HDRS | 25.08±5.61 |
| MADRS | 30.23±6.34 |
| CGI-S | 5.0±0.8 |
Values are presented as number (%) or mean±standard deviation.
HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; CGI-S, Clinical Global Impressions-Severity.
Fourteen patients (15.1%) cancelled their consents due to simple change of mind during the first week and were regarded as baseline dropouts. Other dropouts were due to loss to follow-up (17, 18.3%), adverse events (7, 7.5%), protocol violations (4, 4.3%), low compliance (1, 1.1%), and one quit at the discretion of the investigator (1.1%). At the end of the trial, 49 patients (52.7%) finished the study. ITT analyses included a initially total 93 patients.
Efficacy
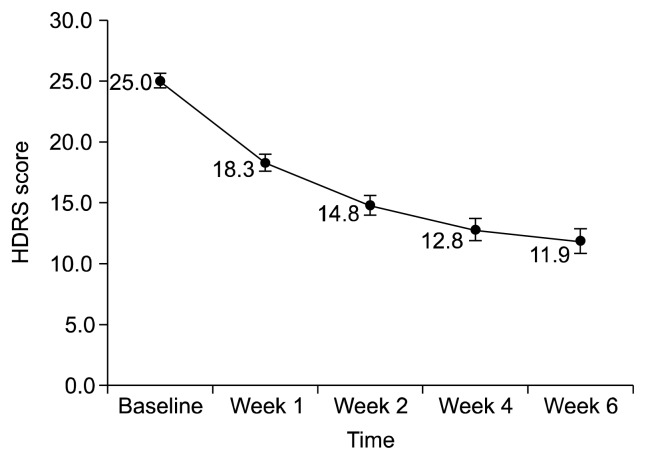
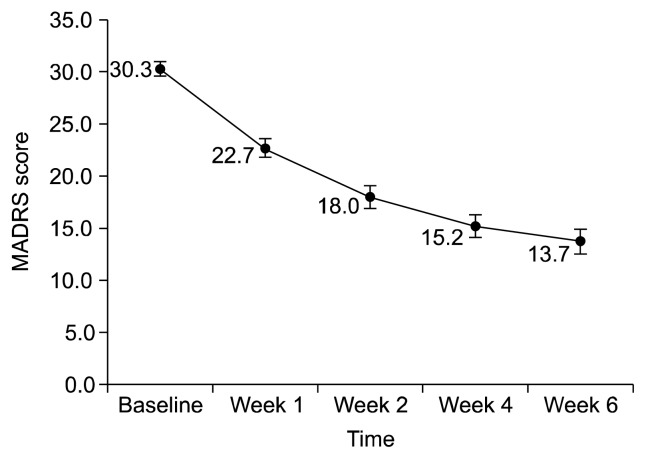
At week 6, the HDRS and MADRS scores were decreased to 11.9±8.6 (mean change −13.1±8.3, p<0.001; Fig. 1) and 13.73±10.40 (mean change −16.5±9.8, p<0.001; Fig. 2). The percentages of responders were 64.6% (HDRS) and 60.8% (MADRS), and those of patients in remission were 35.4% (HDRS) and 43.0% (MADRS) (Table 2). CGI-S was decreased to 2.5±1.3 (mean change −2.5±1.3, p<0.001) and there were 49 patients (52.7%) with a CGI-I score ≤2. Significant decreases in HDRS, MADRS and CGI-S scores were confirmed at week 1. The response and remission rate also showed great increase during the first week (Table 2).
Fig. 1.
Change in Hamilton Depression Rating Scale (HDRS) score over 6 weeks.
Fig. 2.
Change in Montgomery-Åsberg Depression Rating Scale (MADRS) score over 6 weeks.
Table 2.
Percentage of patients classified as responders and remitters by HDRS and MADRS score
| Time | HDRS | MADRS | ||
|---|---|---|---|---|
|
|
|
|||
| Response rate | Remission rate | Response rate | Remission rate | |
| Week 1 | 13.9 | 6.3 | 11.4 | 6.3 |
| Week 2 | 44.3 | 16.5 | 41.8 | 16.5 |
| Week 4 | 50.6 | 29.1 | 54.4 | 31.6 |
| Week 6 | 64.6 | 35.4 | 60.8 | 43.0 |
HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale.
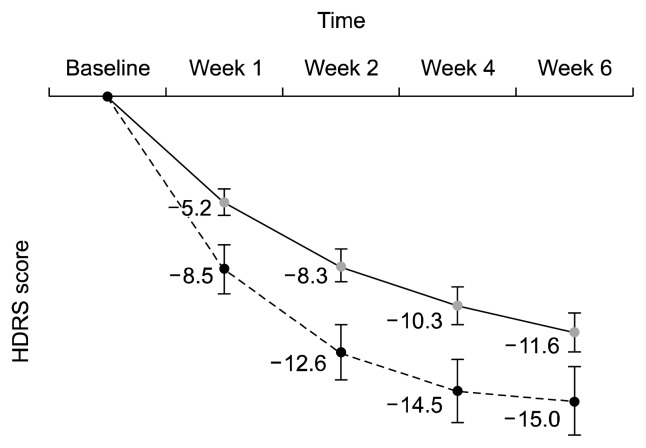
When participants were divided into a mild-to-moderate subgroup (baseline HDRS ≤24, n=48) and a severe subgroup (baseline HDRS >24, n=45), their decreases of HDRS scores from baseline were significantly different between two subgroups (p=0.006, Fig. 3). That is, the HDRS scores were more decreased in the severe subgroup than in the mild-to-moderate subgroup. It was much the same for the MADRS scores (p=0.012). However, final response rates at week 6 were not different as 68.2% (mild-to-moderate subgroup) and 60.0% (severe subgroup, p= 0.125), while the remission rates were significantly different as 47.7% (mild-to-moderate subgroup) and 20.0% (severe subgroup, p<0.001).
Fig. 3.
Difference in decreases of Hamilton Depression Rating Scale (HDRS) score between mild to moderate-to moderate patients and severe patients.
A solid line: patients whose baseline HDRS ≤24 (n=48); a dotted line: patients whose baseline HDRS >24 (n=45).
Adverse Events (AEs) and Safety
There were a total of 45 AEs in 30 patients, and the total AE rate was 32.3%. The most frequently reported adverse events were sedation (4.3%) and constipation (4.3%), followed by dyspepsia (3.2%) and thirst (3.2%) (Table 3). Among them, about two-thirds (30 cases) were mild in severity, and nine cases of adverse events in seven patients (7.5%) were related to the drug. Three cases of severe adverse events occurred in three patients (3.2%), including suicide, unintentional drug intoxication and acute tonsillitis, of which causalities with drug were low.
Table 3.
Adverse events during the study period (≥2%)
| Adverse event | Total (n) |
|---|---|
| Sedation | 4 |
| Constipation | 4 |
| Dyspepsia | 3 |
| Thirst | 3 |
| Headache | 2 |
| Dry mouth | 2 |
| Nausea | 2 |
| Palpitation | 2 |
Twenty-nine patients received no AE treatment, and 23 patients remained in the trial, while 7 patients were withdrawn due to AEs. The AEs related with dropout were sedation (2), headache (2), thirst (1), dyspepsia (1) and suicide (1 patient). At the end of the trial, the AEs of 17 patients had improved, while 6 patients were still experiencing them.
Weight increased from 58.8±10.6 to 60.3±9.3 kg (mean change 0.7±1.7 kg, p=0.004) over 6 weeks. Systolic blood pressure did not change (from 119.5±5 to 118.8±12.0 mmHg, mean change −1.27±7.7 mmHg, p=0.222), but diastolic blood pressure was decreased from 77.1±9.4 to 75.9±8.9, mean change −2.4±8.1 mmHg, p=0.031). Pulse rate did not change (from 74.8±9.3 to 73.9±5.9 beats/min, mean change 0.5±6.4 beats/min, p=0.584). These findings were not clinically significant.
DISCUSSION
Although this study employed an open-label and non-comparative design, the results demonstrated the efficacy and tolerability of Mirtax for the treatment of MDD. Significant decreases in the HDRS, MADRS, and CGI-S scores over 6 weeks were confirmed. Rates of CGI-I score ≤2, responders and remitters at week 6 also further support the efficacy of Mirtax. Decreases of HDRS scores during 6 weeks were found out more in severe subgroup, while the remission rate at 6 week was above two times more in mild-to-moderate patients group. The incidence of AEs was very low, and most AEs were tolerable and not severe. Drug causality was low, including the severe AEs.
How does it compare with branded mirtazapine? The results from between-groups design are hard to compare with single treatment of this study uniformly. Nevertheless, studies including ours and previous ones revealed generally similar character in efficacy at week 6. Wheatley et al.13) reported a 63.3% response rate and a 23.3% remission rate in a comparison study with fluoxetine. Benkert et al.14) reported a 58.3% response rate and a 40.9% remission rate in a comparison study with paroxetine. Asian people also showed similar efficacy in a comparison study with fluoxetine (58% response rate and 35% remission rate).15) In two meta-analyses, the pooled response rate for mirtazapine was 67.1%,16) which is in agreement with our findings (64.6%), but the remission rate for mirtazapine was 43.4%,17) which is somewhat higher than that reported here (35.4%, standard: HDRS score).
The most distinguished result about branded mirtazapine might have a faster onset of action. Many studies of branded mirtazapine showed efficacy prior to week 2 as compared with SSRIs, which was confirmed by a meta-analysis.18) Another meta-analysis indicated 13% response rate and 3.4% remission rate for mirtazapine in week 1.19) These findings coincided relatively well with our study which demonstrated significant improvements in both HDRS and MADRS, and 13.9% of response rate and 6.3% of remission rate at week 1. In addition, response rate and remission rate markedly rose to 44.3% and 16.5%, respectively, at week 2. Considering above findings, Mirtax may have a earlier efficacy similar to branded mirtazapine potentially.
In this study, one characteristic feature was revealed. The degree of decreasing HDRS and MADRS scores was different between severe and mild to moderate patients as having more decrease in severe patients, while the remission rate of a mild-to-moderate subgroup (47.7%) was above two times more than that of a severe subgroup (20.0%). It suggested that mild-to-moderate subgroup seems to have a better therapeutic effect from Mirtax than severe subgroup, even though decreases of HDRS scores from baseline were more in severe subgroup.
About tolerability and safety, only 30 patients (32.3%) reported spontaneous AEs, which is about half of previous reports (68.1%14) and 66.4%20)). There was a 47.3% dropout rate, which was higher than previous studies (40%).21,22) We collected AE reports from 84.9% of participants, with the exception of 14 patients who withdrew their consent by simple change of mind before the first week. Considering these points, this study demonstrated a very low incidence of AEs.
Among AEs, sedation was the most frequent, but its incidence (4.3%) was considerably lower than that of the branded drug, which has a sedative rate of up to 54%.23) Also dizziness which had been reported frequently in previous studies (19.7%)15) was not reported. These differences might be caused by the relatively low dosage used in this study. This study used a flexible dosage setting based on investigator judgment. As a result, the starting dosage was only 11.5±6.4 mg/day, and the maintenance dosage was 23.1±9.4 mg/day, which were relatively low compared with previous studies. The low incidence of AEs may support this notion.
In general, a higher starting dosage appears to increase sedation, although it decreases over time after repeat dosing.24) A higher dosage might have a less marked sedative effect when antihistamine activity is offset after increased noradrenergic transmission.25) The low incidence of sedation and other AEs might be due to the low starting and maintenance dosages. These results may be representative of real-world practice because many clinicians carefully prescribe mirtazapine at less than 15 mg/day from the beginning.
Finally, we confirmed that generic mirtazapine also causes weight gain; however, the degree of weight gain was not clinically significant. Weight gain of 0.7±1.7 kg during 6 weeks might occur naturally and regarded safe in the general clinical situation. Considering that two-thirds of AEs were mild and only 7.5% of AEs were related with drug, Mirtax was found to be well tolerated in real clinical situation.
Our study had a number of limitations including single treatment design and small number of participants. The process also was not as strict as phase 3 clinical trial. There was no formal inter-rater reliability assessment before the study. The average in demographic data was influenced by skewed data and hard to evaluate age and sex effects due to small sample size.
However, this study, as the first clinical trial of generic mirtazapine, demonstrated its efficacy and tolerability over 6 weeks. Additionally, it suggested that Mirtax had fast onset of action similar to branded mirtazapine, and the possibility of differences in side effect profiles.
Even though our initial study might not provide high level of evidence, it showed the importance of investigating the generic antidepressants. To address variability between branded and generic drugs, studies into switches from the branded to the generic drug would be more suitable. Further investigation and clinical experience are required to gather those evidences.
Acknowledgments
This study was supported by Sandoz. All data were collected and analyzed by DreamCIS, the CRO (Contract Research Organization) company.
REFERENCES
- 1.Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, et al. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/S1121189X00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szegedi A, Schwertfeger N. Mirtazapine: a review of its clinical efficacy and tolerability. Expert Opin Pharmacother. 2005;6:631–641. doi: 10.1517/14656566.6.4.631. [DOI] [PubMed] [Google Scholar]
- 3.Thompson C. Mirtazapine versus selective serotonin reuptake inhibitors. J Clin Psychiatry. 1999;60( Suppl 17):18–22. discussion 46–48. [PubMed] [Google Scholar]
- 4.Benkert O, Szegedi A, Philipp M, Kohnen R, Heinrich C, Heukels A, et al. Mirtazapine orally disintegrating tablets versus venlafaxine extended release: a double-blind, randomized multicenter trial comparing the onset of antidepressant response in patients with major depressive disorder. J Clin Psychopharmacol. 2006;26:75–78. doi: 10.1097/01.jcp.0000194622.99986.d6. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin S, Doraiswamy PM. Review of the use of mirtazapine in the treatment of depression. Expert Opin Pharmacother. 2011;12:1623–1632. doi: 10.1517/14656566.2011.585459. [DOI] [PubMed] [Google Scholar]
- 6.Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578–1592. doi: 10.1016/S0149-2918(03)80157-1. [DOI] [PubMed] [Google Scholar]
- 7.Margolese HC, Wolf Y, Desmarais JE, Beauclair L. Loss of response after switching from brand name to generic formulations: three cases and a discussion of key clinical considerations when switching. Int Clin Psychopharmacol. 2010;25:180–182. doi: 10.1097/YIC.0b013e328337910b. [DOI] [PubMed] [Google Scholar]
- 8.Pae CU, Misra A, Ham BJ, Han C, Patkar AA, Masand PS. Paroxetine mesylate: comparable to paroxetine hydrochloride? Expert Opin Pharmacother. 2010;11:185–193. doi: 10.1517/14656560903451708. [DOI] [PubMed] [Google Scholar]
- 9.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742– 747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 10.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Asberg Depression Rating Scale (SIGMA) Br J Psychiatry. 2008;192:52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 11.Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 12.Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 13.Wheatley DP, van Moffaert M, Timmerman L, Kremer CM. Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. Mirtazapine-Fluoxetine Study Group. J Clin Psychiatry. 1998;59:306–312. doi: 10.4088/JCP.v59n0606. [DOI] [PubMed] [Google Scholar]
- 14.Benkert O, Szegedi A, Kohnen R. Mirtazapine compared with paroxetine in major depression. J Clin Psychiatry. 2000;61:656–663. doi: 10.4088/JCP.v61n0911. [DOI] [PubMed] [Google Scholar]
- 15.Hong CJ, Hu WH, Chen CC, Hsiao CC, Tsai SJ, Ruwe FJ. A double-blind, randomized, group-comparative study of the tolerability and efficacy of 6 weeks’ treatment with mirtazapine or fluoxetine in depressed Chinese patients. J Clin Psychiatry. 2003;64:921–926. doi: 10.4088/JCP.v64n0810. [DOI] [PubMed] [Google Scholar]
- 16.Papakostas GI, Homberger CH, Fava M. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J Psychopharmacol. 2008;22:843–848. doi: 10.1177/0269881107083808. [DOI] [PubMed] [Google Scholar]
- 17.Thase ME, Nierenberg AA, Vrijland P, van Oers HJ, Schutte AJ, Simmons JH. Remission with mirtazapine and selective serotonin reuptake inhibitors: a meta-analysis of individual patient data from 15 controlled trials of acute phase treatment of major depression. Int Clin Psychopharmacol. 2010;25:189–198. doi: 10.1097/YIC.0b013e328330adb2. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe N, Omori IM, Nakagawa A, Cipriani A, Barbui C, Churchill R, et al. Mirtazapine versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2011;(12):CD006528. doi: 10.1002/14651858.CD006528.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quitkin FM, Taylor BP, Kremer C. Does mirtazapine have a more rapid onset than SSRIs? J Clin Psychiatry. 2001;62:358–361. doi: 10.4088/JCP.v62n0509. [DOI] [PubMed] [Google Scholar]
- 20.Leinonen E, Skarstein J, Behnke K, Agren H, Helsdingen JT. Efficacy and tolerability of mirtazapine versus citalopram: a double-blind, randomized study in patients with major depressive disorder. Nordic Antidepressant Study Group. Int Clin Psychopharmacol. 1999;14:329–337. doi: 10.1097/00004850-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Claghorn JL, Lesem MD. A double-blind placebo-controlled study of Org 3770 in depressed outpatients. J Affect Disord. 1995;34:165–171. doi: 10.1016/0165-0327(95)00014-E. [DOI] [PubMed] [Google Scholar]
- 22.Vartiainen H, Leinonen E. Double-blind study of mirtazapine and placebo in hospitalized patients with major depression. Eur Neuropsychopharmacol. 1994;4:145–150. doi: 10.1016/0924-977X(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 23.Dewan MJ, Anand VS. Evaluating the tolerability of the newer antidepressants. J Nerv Ment Dis. 1999;187:96–101. doi: 10.1097/00005053-199902000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kasper S, Praschak-Rieder N, Tauscher J, Wolf R. A risk-benefit assessment of mirtazapine in the treatment of depression. Drug Saf. 1997;17:251–264. doi: 10.2165/00002018-199717040-00005. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto K, Kawano N, Sasada K, Kohmura K, Yamamoto M, Ebe K, et al. Effects of low-dose mirtazapine on driving performance in healthy volunteers. Hum Psychopharmacol. 2013;28:523–528. doi: 10.1002/hup.2327. [DOI] [PubMed] [Google Scholar]