Abstract
Objective
Prior reports suggest that autism spectrum disorder (ASD) is associated with atypically excessive early brain growth. Recent cross-sectional studies suggest that later cortical development during adolescence/adulthood might also be aberrant, though longitudinal designs are required to evaluate atypical growth trajectories. The present study sought to examine longitudinal changes in cortical thickness and surface area among adolescents and young adults with ASD.
Method
Two (70 total) high-resolution anatomic magnetic resonance imaging scans approximately two years apart were acquired from 17 adolescents with ASD and 18 typically developing (TD) adolescents, matched on age (range=14–24 years old), IQ, sex ratio, and handedness. The FreeSurfer image analysis suite was utilized to quantify longitudinal changes in cortical thickness and surface area.
Results
Accelerated cortical thinning for the ASD group as compared to the TD group was found in two areas in the left hemisphere, the posterior portion of ventral temporal cortex and superior parietal cortex (cluster corrected p<.01). For ventral temporal cortex, cortical thinning was associated with everyday executive function impairments, and thinner cortex at time 2 was correlated with ASD social symptoms. Differences in surface area changes were not detected.
Conclusion
The present longitudinal study extends prior cross-sectional research by demonstrating increased cortical thinning (in portions of temporal and parietal cortex) but comparable surface area growth rates in ASD compared to TD controls during adolescence and into young adulthood. These findings provide further evidence for atypical cortical development beyond the early years in ASD marked by increased cortical thinning in late adolescence/young adulthood.
Keywords: autism, longitudinal, cortical thickness, surface area, executive function
Introduction
Autism spectrum disorder (ASD) is characterized by impairments in social and communicative functioning and the presence of restricted interests/repetitive behaviors1. Although findings are mixed, some previous studies using brain volume from magnetic resonance imaging (MRI) suggest that ASD is associated with an age-dependent increase in brain size, which likely peaks sometime during the early postnatal years2 (though findings reliant upon head circumference data have been recently called into question3). Furthermore, when found, brain volume increases, particularly in gray matter, may be regionally specific4.
Relatively recent methodological advances have allowed a focus on the lower-order components of gray matter volume. Gray matter volume represents the product of cortical thickness (CT) and surface area (SA). Each of these components is relatively highly heritable, but with generally non-overlapping genetic influences5 and distinct developmental trajectories6. While both CT and SA largely follow an inverted U-shaped trajectory of development with an increase early in development followed by loss in adolescence, the pattern is more protracted for SA than for CT6. This non-linear growth pattern, along with high individual variability7, complicates comparisons and integration of findings across cross-sectional studies of ASD.
Indeed, only a few studies to date have examined separately the age-related changes in CT and/or SA in ASD via either correlations with age in cross-sectional samples or using the most stringent test, longitudinal designs. Three of these longitudinal studies have been conducted to date covering early childhood, the early school years, and childhood to adulthood, respectively, though none have explicitly examined differences in longitudinal SA changes. Hazlett et al.8, in the context of a longitudinal study of young children with ASD (ages 2–5 years) versus a control group comprised of similarly aged typically developing (TD) and developmentally delayed children, found comparable increases in lobar-level CT during this period of development though disproportionately elevated gray matter volumes in ASD, suggesting that SA (though it wasn’t quantified separately) underlies this volumetric increase. There is increasing evidence that cortical growth trajectories do not simply normalize after this early developmental peak in brain size is reached in ASD9,10. Hardan et al.9 also examined lobar-level longitudinal changes in CT in 18 children with ASD versus 16 controls, though their sample included school-age (predominantly preadolescent: ages 8–12 years at first scan) children. Using lobar-level means, they found greater cortical thinning in the ASD group, particularly in temporal and occipital regions. Finally, in the most recent study incorporating a mixed longitudinal/cross-sectional design, Zielinski et al.10 focused on CT alone and found aberrant trajectories in ASD compared to controls in the 3- to 39-year age range. Cross-sectional examinations of associations between age and cortical structure in ASD have focused primarily upon the school-age years and adulthood. For example, Mak-Fan et al.11 and Wallace et al.12 showed greater age-related decreases in CT in children, adolescents, and young adults with ASD when compared to TD control groups. Similarly, in studies including mainly adolescents and adults, Scheel et al.13 and Raznahan et al.14 demonstrated different age–CT relationships in ASD groups as compared to TD controls. In contrast, the two studies of age associations with SA14,15 demonstrate no significant differences between individuals with ASD and TD controls. These longitudinal and cross-sectional studies converge to implicate a continued aberrant developmental course of cortical growth, particularly CT in ASD, beyond the preschool years. Nevertheless, no study has examined longitudinal changes in both of these components of cortical development (i.e., CT and SA) during adolescence and young adulthood among individuals with ASD.
Using data from a group of higher functioning adolescents and young adults with ASD and a sample of TD controls matched on age, IQ, sex ratio, handedness, and duration between scans, we examined longitudinal CT and SA changes. Consistent with prior cross-sectional12 and mixed cross-sectional/longitudinal10 studies, we expect to find greater cortical thinning in the ASD compared to the TD control group within this developmental window. Furthermore, consistent with prior cross-sectional findings14,15, we predict comparable changes in SA for participants with ASD and TD participants within this age range.
Method
Participants
17 adolescents and young adults with higher-functioning ASD and 18 TD adolescents and young adults matched on age, IQ, sex ratio, handedness, and time between scans (see Table 1) underwent MRI scanning. Each participant provided two scans each for a total of 70 structural MRI brain images (note that all of the participants described here were part of a much larger sample from which cross-sectional results based on a single MRI scan per individual have been previously reported12,15). All 17 participants with ASD met DSM-IV diagnostic criteria as assessed by an experienced clinician (12 Asperger’s syndrome, 4 high-functioning autism, and 1 pervasive developmental disorder-not otherwise specified). All participants with ASD also received the Autism Diagnostic Interview (ADI or ADI-R16,17) and the Autism Diagnostic Observation Schedule (ADOS18) administered by a trained, research-reliable clinician. Participants with ASD received either module 3 (n=4) or 4 (n=13) of the ADOS. All scores of participants with ASD met the cut-off for the category designated as “Broad ASD” according to criteria established by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/National Institute on Deafness and Other Communication Disorders (NIDCD) Collaborative Programs for Excellence in Autism (CPEA19). Exclusion criteria for the group with ASD included an IQ<70 or any known comorbid medical conditions, such as Fragile X syndrome or other genetic disorders, and brain trauma/injury. In the group with ASD, 13 individuals were taking one or more psychotropic medications at one or more scan sessions: 8 were taking stimulants, 8 were taking selective serotonin reuptake inhibitors (SSRIs), 3 were taking atypical antipsychotics, 1 was taking an anxiolytic, 2 were taking an anticonvulsant, and 1 was taking a tricyclic.
Table 1.
Demographic Characteristics
| ASD (n=17) Mean (SD) |
TD (n=18) Mean (SD) |
|
|---|---|---|
| Age at scan 1, y | 17.37 (2.41) | 17.46 (1.45) |
| Age at scan 2, y | 19.12 (2.51) | 19.60 (1.61) |
| Time between scans, y | 1.72 (0.83) | 2.10 (0.95) |
| Full scale IQa | 116.59 (13.05) | 116.17 (9.54) |
| Verbal IQa | 116.35 (15.07) | 114.11 (11.66) |
| Performance IQa | 113.47 (12.22) | 114.17 (6.35) |
| Sex ratio (Male:Female) | 15:2 | 17:1 |
| Handedness (Right:NonRight) | 15:2 | 16:2 |
| ADI Social | 19.12 (5.60) | |
| ADI Verbal Communication | 14.76 (3.58) | |
| ADI Restricted and Repetitive Behavior | 5.94 (2.08) | |
| ADOS Social | 9.71 (3.14) | |
| ADOS Communication | 4.12 (1.80) | |
| ADOS Stereotyped Behavior | 1.24 (1.60) |
Note: ADI = Autism Diagnostic Interview; ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; TD = typically developing.
Standard score (population mean = 100, SD = 15).
Parents of TD children and the TD adults underwent telephone screenings. TD individuals were excluded from participation if they had ever received mental health treatment for anxiety, depression, or any other psychiatric condition, taken psychiatric medications, required special services in school, been diagnosed with a genetic disorder or neurological disorder, or had brain trauma/injury that could potentially affect cognitive functioning and/or brain development. IQ scores were obtained from all participants. All Full Scale IQ (FSIQ) scores were 85 or above, as measured by the Wechsler Abbreviated Scale of Intelligence (ASD: n=15, TD: n=18), Wechsler Adult Intelligence Scale-III (ASD: n=1), or Wechsler Intelligence Scale for Children-IV (ASD: n=1). Parent ratings of everyday executive functioning (Behavior Rating Inventory of Executive Function [BRIEF]), which serve as a complement to diagnostic measures, were also acquired, and in addition to ASD symptom severity measures (ADI and ADOS), were utilized as behavioral correlates of neuroimaging findings. Informed assent and consent were obtained from all participants and/or their parent/guardian when appropriate in accordance with a National Institutes of Health Institutional Review Board-approved protocol.
Neuroimaging
Two high-resolution (1.07 x 1.07 x 1.2 mm) T1-weighted structural images were obtained axially from each participant with a magnetization-prepared rapid gradient echo (MPRAGE) sequence (124 slices, 1.2 mm slice thickness, 224 x 224 acquisition matrix, flip angle=6°, field of view=24 cm) on the same 3T General Electric Signa scanner (Milwaukee, Wisconsin) using an 8-channel head coil. The FreeSurfer image analysis methods have been thoroughly described previously20,21,22. Briefly, FreeSurfer (version 5.1) was used to generate a cortical surface model providing a measure of CT and SA at each vertex. The resulting surface models generated were reviewed for accuracy and manually edited for all participants in both groups because of over-inclusion of white matter around the optic nerve. CT was quantified at each surface location or vertex as the distance (in mm) from the gray/white boundary to the pial surface. This method of CT measurement has been validated against postmortem brains/histological analysis23 and hand tracings24,25 and has shown good reliability across sites and platforms26. Pial SA was quantified as the sum of all tessellations. Vertex-level CT and SA values were obtained and mapped onto a normalized cortical surface. These CT and SA maps were smoothed with a 15mm full width at half maximum (FWHM) kernel. FreeSurfer’s longitudinal analysis stream, wherein an unbiased within-subject template space and average image27 are created using robust, inverse consistent registration28, allows for accurate within-subject registration in order to assess change in cortical morphology. Information from this participant template is used to initialize the longitudinal image processing in several locations to increase repeatability and statistical power. Because of the now well-established effect of head motion on neuroimaging results, a subjective rating of head motion (on a 1–5 scale with higher scores indicative of more motion) from the raw T1-weighted structural images was acquired for all participants, and an objective quantification of head motion29 during a resting state scan was obtained for most participants (31 of 35; 2 missing from each group) at time 2.
Analysis
Group differences in the slopes of change in CT and SA from time 1 to time 2 were examined on the surface maps vertex by vertex using a least squares general linear model. To control for multiple comparisons, cluster correction was completed using Monte Carlo simulation with 10,000 iterations (vertex-wise threshold of p<.01). This group comparison was run again entering full-scale IQ as a covariate to ensure that IQ variance did not contribute to any group differences. Furthermore, if significant longitudinal group differences were found, CT and/or SA from clusters surviving multiple comparison correction were examined for cross-sectional group differences at times 1 and 2 separately. Finally, the mean longitudinal changes (e.g., CT loss) and cross-sectional group differences at times 1 and/or 2 (i.e., in CT or SA) in cortical structure for clusters surviving multiple comparison correction were correlated (using Pearson’s r) with behavioral functioning.
Results
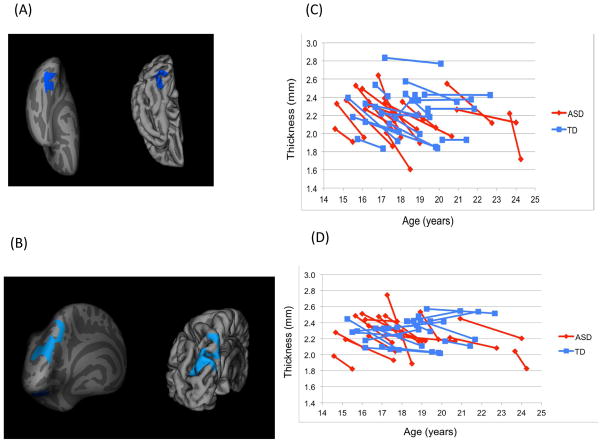
There was accelerated cortical thinning for the group with ASD as compared to the TD group with two areas in the left hemisphere, the posterior region of the ventral occipitotemporal cortex (ASD: M=−0.20mm/year, SD=0.18; TD: M=−0.02mm/year, SD=0.08) and superior parietal cortex (ASD: M=−0.14mm/year, SD=0.10; TD: M=−0.01mm/year, SD=0.06) surviving cluster correction (p<.01; see Figure 1, Table 2 [results are not meaningfully altered when a corrected threshold of p<.05 is used instead]). Moreover, the rate of cortical thinning differed significantly from zero in both the ventral occipitotemporal cortex (t=−4.62, p<.001) and superior parietal cortex (t=−5.68, p<.001) for the ASD group, while these rates in the TD group were not significantly different from zero (ps>.20). There was no evidence for significantly increased cortical thickening at any brain location during this age range in ASD. Cross-sectional comparisons at times 1 and 2 in these regions showed that while there were no group differences in CT at time 1, the group with ASD had thinner cortex in both clusters at time 2: left ventral occipitotemporal (t=2.70, p=.01) and left superior parietal (t=2.83, p=.008) cortices.
Figure 1.
Inflated and pial surface maps (dark gray=sulci; light gray=gyri) of (A) inferior and (B) posterior views of the left hemisphere showing greater rates of cortical thinning in the group with autism spectrum disorders (ASD) as compared to the group of typically developing (TD) controls (areas in blue are cluster corrected p<.01; clusters described in Table 2). Note: Line graphs of cortical thickness values are shown for each participant in the (C) left ventral occipitotemporal cluster (shown in Figure 1A) and (D) left superior parietal cluster (shown in Figure 1B) at time points 1 and 2.
Table 2.
Regions Differing Significantly in Longitudinal Rate of Cortical Thinning Between the Autism Spectrum Disorders Group and the Group of Matched Typically Developing Controls
| Region | Size (mm2) | X | Y | Z | Clusterwise p value | Number of vertices |
|---|---|---|---|---|---|---|
| Left Superior Parietal | 2,022 | −7.9 | −60.4 | 51.1 | .0001 | 3,424 |
| Left Ventral Occipitotemporal | 886 | −19.9 | −81.4 | −2.9 | .0009 | 1,081 |
Note: Cluster corrected p < .01.
As opposed to the differences in CT changes documented above, longitudinal changes in SA did not differ between groups after cluster correction for multiple comparisons.
Given sex differences in brain structure in both typical development30 and in ASD31,32,33, we re-ran analyses excluding females, resulting in a reduced sample of participants with ASD (n=15) and TD participants (n=17). Restricting the sample to include only males did not change results so that greater cortical thinning was found (in the same left hemisphere regions as indicated above) in males with ASD compared to TD males (cluster corrected p<.01). These longitudinal results were again driven by group differences found only at time 2 in left ventral occipitotemporal (t=3.23, p=.003) and left superior parietal (t=2.84, p=.008) cortices.
Adding IQ as a covariate and rerunning the original longitudinal analyses described above did not alter the pattern of results, consistent with the groups being well matched on IQ.
Groups did not differ in amount of head motion using either metric of head motion (ts<0.80, ps>.42), there was no significant association between these metrics and rate of change in cortical thickness in either cluster surviving correction (rs<.21, ps>.22), and including either of these head motion metrics as covariates in group comparisons of rate of CT change in either cluster surviving correction did not alter the above-reported findings. Taken together, these results suggest that findings could not be attributed to differences in head motion.
There were also significant correlations between parent behavior ratings and CT loss in the ASD group in brain regions showing greater thinning in ASD such that the rate of thinning in the ventral occipitotemporal cortex was correlated with executive function problems as indexed by the Global Executive Composite from the BRIEF (r=-.55, p=.03). Finally, correlations between behavior and brain were also noted at time 2: in the ventral occipitotemporal cortex, increased ASD social symptom severity from the ADI was associated with thinner cortex (r=-.56, p=.02). However, neither of these correlations survives application of a Bonferroni multiple comparison correction procedure.
Discussion
The present longitudinal study complements and extends prior cross-sectional research by demonstrating extended cortical thinning in ASD during adolescence and into young adulthood. Specifically, in portions of the temporal and parietal lobes, thinning of the cortex appears to have slowed in TD individuals, while this process continues to occur in these regions in individuals with ASD during this developmental window. In contrast, SA, another component of cortical volume, appears to exhibit comparable growth rates for TD individuals and individuals with ASD during this period. These findings provide further evidence for continued atypical cortical development beyond the early years in ASD marked by increased cortical thinning in late adolescence/young adulthood. Finally, linking these structural abnormalities with behavior, we found that higher parent ratings of executive functioning problems were associated with greater degrees of cortical thinning in the group with ASD, and that thinner cortex at time 2 was associated with increased ASD social symptom severity.
Consistent with our hypotheses, we found greater cortical thinning in participants with ASD than TD participants but comparable changes in SA during late adolescence and into young adulthood. As in the present study, thinner temporal and parietal cortices, particularly in the left hemisphere, were also documented by others13,34 in cross-sectional studies of higher functioning adults on the autism spectrum. Moreover, atypical associations between age and CT14 or atypical longitudinal cortical thinning9,10 in both temporal and parietal regions have also been noted in prior research. Our finding of no group differences in the longitudinal development of SA is consistent with at least one previous cross-sectional report from another lab, which found no age by group interaction effects on SA in ASD as compared to TD individuals14.
The normal process of cortical thinning appears to be extended, and perhaps more protracted, in ASD. While cortical thinning is slowing in TD individuals, there is continued thinning in temporal and parietal regions in ASD during adolescence and young adulthood. Coupled with MRI findings of thicker cortex in early development35, the increased cortical thinning during adolescence and young adulthood in ASD could reflect a leftward shift and alteration of the expected inverted U-shaped pattern of typical CT development6, perhaps related to synaptogenesis/neurogenesis gone awry. Speculatively, it could be that in ASD, early cortical overgrowth is followed by an overcorrection via either earlier synaptic pruning or over-pruning during adolescence, a period characterized by selective synaptic elimination and arborization of dendrites and axons36. Longer-term longitudinal studies incorporating early childhood and later development will be needed to address such postulations, though recent research appears to support this contention10. Moreover, future studies that include more than two time points of MRI data, as has proven informative in recent studies of typical6 and ASD10 development, would be particularly helpful to begin to construct and compare trajectories of brain development in ASD. More to the point, depending upon the developmental window examined, curvilinear patterns (and differences in these patterns) might emerge for ASD. The present study provides evidence for increased cortical thinning in ASD during late adolescence and young adulthood; however, this CT loss could be mitigated relative to TD controls during later portions of young adulthood and into middle adulthood, as findings from one recent study suggest10. Only longer-term studies with more data collection time points can adequately address such possibilities.
We have also linked the degree of cortical thinning in the left ventral occipitotemporal region with parent ratings of executive functioning problems in ASD. Everyday executive functioning problems, which are indexed by multifactorial behavioral measures, have been consistently documented in ASD37,38. Additionally, we found that greater ASD social symptom severity, as measured by the ADI, was associated with thinner cortex in left ventral occipitotemporal cortex at time 2 in the group with ASD. Taken together, these findings suggest that this atypical pattern of cortical thinning is associated with behavioral atypicalities characteristic of ASD, although findings do not survive Bonferroni correction procedures.
By using a longitudinal design in the present study, we provide the strongest support to date for the notion of aberrant cortical growth during adolescence and young adulthood in ASD. However, what genetic and/or environmental forces underpin the accelerated cortical thinning in ASD remains unknown. As previously mentioned, twin studies demonstrate relatively high heritability for CT during childhood, adolescence, and adulthood5, which is regionally specific and developmentally dynamic39. Future research should explore links between genetic/environmental factors and longitudinal changes in CT in ASD.
It is important to acknowledge and consider limitations. The current study included only higher-functioning individuals with ASD; therefore, whether this pattern of results would also be found within a sample of lower-functioning individuals with ASD remains unclear. More work on cortical development among lower-functioning individuals, particularly adolescents and adults with ASD with a co-occurring intellectual disability, is needed as very little is known outside of the early childhood years. However, a focus on higher-functioning individuals with ASD allowed an isolation of ASD-specific effects on CT and SA, unclouded by the presence of intellectual disability, particularly given that prior studies suggest that intellectual disability is associated with aberrant cortical development10. The sample size in the present study was relatively small, though the within-subjects design allowed detection of group differences of large magnitude. Nevertheless, replication of this study with larger samples to detect possible subtler group differences is needed, particularly given the increasing focus on and interest in development beyond childhood in ASD. Although there were not enough individuals with ASD free from psychotropic medication usage to analyze their data separately, we are reassured that psychostimulants have been shown to mitigate atypical cortical thinning associated with attention-deficit/hyperactivity disorder (ADHD)40; thus, we document cortical thinning in spite of the fact that 47% of the individuals with ASD were taking psychostimulants. It remains unclear what impact other psychotropic medication (e.g., SSRIs and atypical antipsychotics) might have on cortical development in ASD; thus, future research should examine their effects as well.
In summary, the present study, by examining cortical growth trajectories in ASD during adolescence and young adulthood, implicates a specific aspect of brain structure, CT (as opposed to SA), as developmentally aberrant. These findings provide further credence to the possible utility of not only CT, but also trajectories of cortical structure as informative developmental endophenotypes for future genetic studies of ASD and potential targets for the effects of intervention in ASD.
Acknowledgments
This work was supported by the Intramural Research Program at NIMH, National Institutes of Health (NIH) under grant number 1-ZIA-MH002920-05. Ethics approval for this study was granted by the NIH Combined Neuroscience Institutional Review Board under protocol number 10-M-0027.
The authors would like to express their gratitude to the individuals and families who volunteered their time to contribute to this research.
Footnotes
Disclosure: Dr. Kenworthy has received financial compensation as an author of the Behavior Rating Inventory of Executive Function, which is used in this study. Drs. Wallace, Giedd, Martin, Mr. Eisenberg, Ms. Robustelli, and Mr. Dankner report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Gregory L. Wallace, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD. George Washington University, Washington, DC.
Mssr. Ian W. Eisenberg, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD.
Ms. Briana Robustelli, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD.
Mssr. Nathan Dankner, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD.
Dr. Lauren Kenworthy, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD.
Dr. Jay N. Giedd, Child Psychiatry Branch, NIMH.
Dr. Alex Martin, Laboratory of Brain and Cognition, National Institute of Mental Health (NIMH), Bethesda, MD.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Raznahan A, Wallace GL, Antezana L, et al. Compared to what? Early brain overgrowth in autism and the perils of population norms. Biol Psychiatry. 2013;15:563–575. doi: 10.1016/j.biopsych.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmen SJ, Hulshoff Pol HE, Kemner C, et al. Increased gray-matter volume in medication-naïve high-functioning children with autism spectrum disorder. Psychol Med. 2005;35:561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- 5.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange N, Giedd JN, Castellanos FX, Vaituzis AC, Rapoport JL. Variability of human brain structure size: ages 4–20 years. Psychiatry Res. 1997;74:1–12. doi: 10.1016/s0925-4927(96)03054-5. [DOI] [PubMed] [Google Scholar]
- 8.Hazlett HC, Poe MD, Gerig G, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardan AY, Libove RA, Keshavan MS, Melhem NM, Minshew NJ. A preliminary longitudinal magnetic resonance imaging study of brain volume and cortical thickness in autism. Biol Psychiatry. 2009;66:320–326. doi: 10.1016/j.biopsych.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielinski BA, Prigge MB, Nielsen JA, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–1812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak-Fan KM, Taylor MJ, Roberts W, Lerch JP. Measures of cortical grey matter structure and development in children with autism spectrum disorder. J Autism Dev Disord. 2012;42:419–427. doi: 10.1007/s10803-011-1261-6. [DOI] [PubMed] [Google Scholar]
- 12.Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–3754. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheel C, Rotarska-Jagiela A, Schilbach L, et al. Imaging derived cortical thickness reduction in high-functioning autism: key regions and temporal slope. NeuroImage. 2011;58:391–400. doi: 10.1016/j.neuroimage.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 14.Raznahan A, Toro R, Daly E, et al. Cortical anatomy in autism spectrum disorder: an in vivo mri study on the effect of age. Cereb Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 15.Wallace GL, Robustelli B, Dankner N, Kenworthy L, Giedd JN, Martin A. Increased gyrification, but comparable surface area in adolescents with autism spectrum disorders. Brain. 2013;136:1956–1967. doi: 10.1093/brain/awt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Couteur A, Rutter M, Lord C, et al. Autism Diagnostic Interview - a standardized investigator-based instrument. J Autism Dev Disord. 1989;19:363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- 17.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised - a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 18.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 19.Lainhart JE, Bigler ED, Bocian M, et al. Head circumference and height in autism: A study by the Collaborative Program of Excellence in Autism. Am J Med Genet A. 2006;140A:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis - I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis - II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–1105. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 24.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 25.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 26.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 27.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. NeuroImage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. NeuroImage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gotts SJ, Saad ZS, Jo HJ, et al. The perils of global signal regression for group comparisons: a case study of autism spectrum disorders. Front Hum Neurosci. 2013;7:356. doi: 10.3389/fnhum.2013.00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloss CS, Courchesne E. MRI neuroanatomy in young girls with autism: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2007;46:515–523. doi: 10.1097/chi.0b013e318030e28b. [DOI] [PubMed] [Google Scholar]
- 32.Craig MC, Zaman SH, Daly EM, et al. Women with autistic-spectrum disorder: magnetic resonance imaging study of brain anatomy. Br J Psychiatry. 2007;191:224–228. doi: 10.1192/bjp.bp.106.034603. [DOI] [PubMed] [Google Scholar]
- 33.Schumann CM, Bloss CS, Barnes CC, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–4427. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Anatomical differences in the mirror neuron system and social cognition network in autism. Hum Brain Mapp. 2006;28:441–449. doi: 10.1093/cercor/bhj069. [DOI] [PubMed] [Google Scholar]
- 35.Raznahan A, Lenroot R, Thurm A, et al. Mapping cortical anatomy in preschool aged children with autism using surface-based morphometry. NeuroImage Clin. 2012;2:111–119. doi: 10.1016/j.nicl.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Rosenthal M, Wallace GL, Lawson R, et al. Impairments in real-world executive function increase from childhood to adolescence in autism spectrum disorders. Neuropsychology. 2013;27:13–18. doi: 10.1037/a0031299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Granader Y, Wallace GL, Hardy KK, et al. Characterizing the factor structure of parent reported executive function in autism spectrum disorders: the role of cognitive inflexibility. J Autism Dev Disord. 2014;44:3056–3062. doi: 10.1007/s10803-014-2169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenroot RK, Schmitt JE, Ordaz SJ, et al. Developmental changes in genetic and environmental influences on the human cerebral cortex during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw P, Sharp WS, Morrison M, et al. Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 2009;166:58–63. doi: 10.1176/appi.ajp.2008.08050781. [DOI] [PMC free article] [PubMed] [Google Scholar]