Abstract
Although vaccine supply chains in many countries require additional stationary storage and transport capacity to meet current and future needs, international donors tend to donate stationary storage devices far more often than transport equipment. To investigate the impact of only adding stationary storage equipment on the capacity requirements of transport devices and vehicles, we used HERMES (Highly Extensible Resource for Modeling Supply Chains) to construct a discrete event simulation model of the Niger vaccine supply chain. We measured the transport capacity requirement for each mode of transport used in the Niger vaccine cold chain, both before and after adding cold rooms and refrigerators to relieve all stationary storage constraints in the system. With the addition of necessary stationary storage, the average transport capacity requirement increased from 88% to 144% for cold trucks, from 101% to 197% for pickup trucks, and from 366% to 420% for vaccine carriers. Therefore, adding stationary storage alone may worsen or create new transport bottlenecks as more vaccines flow through the system, preventing many vaccines from reaching their target populations. Dynamic modeling can reveal such relationships between stationary storage capacity and transport constraints.
Keywords: immunization, storage, transport, vaccine supply chains
Vaccine supply chains in many countries currently experience bottlenecks due to limited storage or transport capacity, and these are expected to worsen with the introduction of 12 new vaccines by 2019.1 In efforts to improve vaccine supply chains, international donors have donated stationary storage devices far more often than transport equipment.2–4 The HERMES (Highly Extensible Resource for Modeling Supply Chains) modeling team at the University of Pittsburgh used a dynamic simulation model of the Niger vaccine supply chain to investigate the impact of adding stationary storage equipment on the capacity requirements of transport devices and vehicles.
Methods
We used HERMES to construct a discrete-event simulation model of the Niger vaccine supply chain. Previous publications provide detailed descriptions of this model.5–7 The model simulated the population demand and Expanded Program on Immunization vaccine schedule for 2015, as projected in the Niger Comprehensive Multiyear Plan.8 The transport capacity requirement is the amount of space needed to carry an order of vaccines, expressed as a percentage of the space available in a vehicle or transport device. It is calculated as follows:
Transport capacity requirement = Transport capacity required ÷ Transport capacity available
A transport capacity requirement of more than 100% indicates a transport bottleneck. We measured the transport capacity requirement for each of the 3 types of vehicles and transport devices used in the Niger vaccine cold chain (cold trucks, pickup trucks, and vaccine carriers) under the current system, without additional storage or transport equipment. We then added cold rooms and refrigerators to relieve all stationary storage constraints and measured the transport capacity requirement under this altered system.
Results
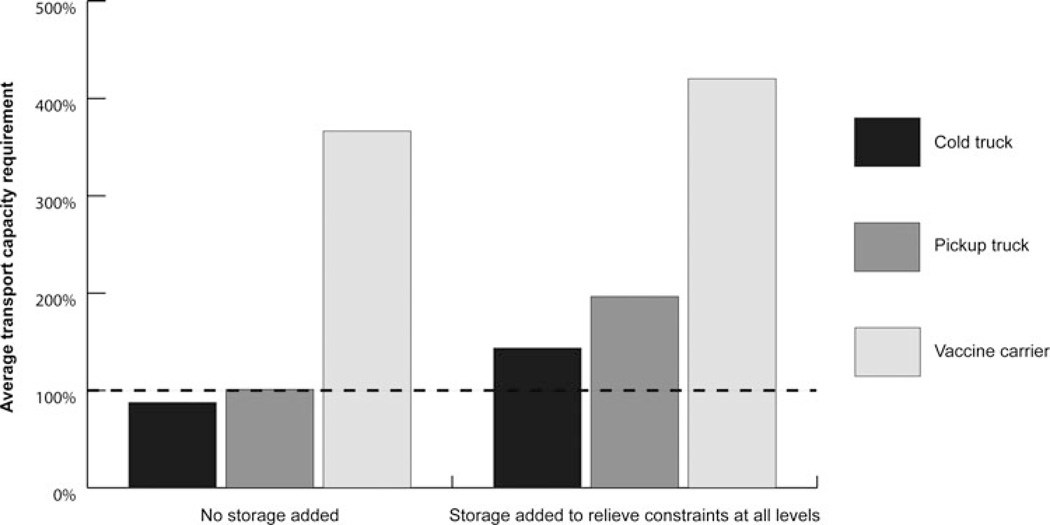
The Figure displays the average transport capacity requirement for each type of transport equipment, before and after the addition of stationary storage to relieve all storage constraints. Relieving storage constraints exacerbated existing transport bottlenecks and created new bottlenecks for routes that did not experience constraints before storage was added. With the addition of stationary storage, the average transport capacity requirement increased from 88% to 144% for cold trucks, from 101% to 197% for pickup trucks, and from 366% to 420% for vaccine carriers.
FIGURE.
Transport Capacity Requirement Before and After the Addition of Stationary Storagea
aA transport capacity requirement of more than 100% indicates a transport bottleneck.
Conclusion
Transport constraints cannot be ignored when augmenting vaccine supply chains. Adding stationary storage equipment alone may actually worsen or create new transport bottlenecks, due to a greater number of vaccines flowing through the system. These bottlenecks ultimately prevent vaccines from arriving where they are needed. Dynamic modeling helped reveal this relationship between stationary storage capacity and transport constraints. HERMES can generate a detailed model of any vaccine supply chain anywhere in the world. For example, in the United States, HERMES could be used to evaluate supply chains to distribute routine immunizations, influenza vaccines, and vaccines in response to epidemics.
Acknowledgments
This work was supported by the Bill and Melinda Gates Foundation via the Vaccine Modeling Initiative and the National Institute of General Medical Sciences Models of Infectious Disease Agent Study (MIDAS). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data and preparation, review, or approval of the manuscript.
Footnotes
The HERMES Project team consists of (in alphabetical order): Tina-Marie Assi, PhD, Shawn T. Brown, PhD (Technical Lead), Brigid E. Cakouros, MPH, Sheng-I Chen, PhD, Diana L. Connor, MPH (co-coordinator), Erin G. Claypool, PhD, Leila A. Haidari, MPH, Veena Karir, PharmD, MS, Bruce Y. Lee, MD, MBA (scientific lead), Jim Leonard, Leslie E. Mueller, MPH, Bryan A. Norman, PhD, Proma Paul, MHS, Jayant Rajgopal, PhD, Michelle M. Schmitz, BA, Rachel B. Slayton, PhD, Angela R. Wateska, MPH (co-coordinator), Joel S. Welling, PhD, and Yu-Ting Weng, MS. For further questions regarding HERMES, contact Dr Lee (BYL1@pitt.edu) or Dr Brown (stbrown@psc.edu).
No other financial disclosures were reported by the authors of this article.
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Kaufmann JR, Miller R, Cheyne J. Vaccine supply chains need to be better funded and strengthened, or lives will be at risk. Health Aff (Millwood) 2011;30(6):1113–1121. doi: 10.1377/hlthaff.2011.0368. [DOI] [PubMed] [Google Scholar]
- 2.Maternal and Child Health Integrated Program. Impact of New Vaccine Introduction on Developing Country Immunization Programs: A Review of the Grey Literature. Washington, DC: USAID Bureau for Global Health; 2011. [Google Scholar]
- 3.Rinzin YC. 16 kerosene coolers for “power” less BHUs. [Accessed May 25, 2012];Kuensel Online. http://www.kuenselonline.com/2011/?p=27760. Published 2012.
- 4.Isselmou BO. UNICEF and Government of Japan boost “cold chain” for immunization in Mauritania. UNICEF; 2011. [Accessed May 25, 2012]. http://www.unicef.org/mdg/Mauritania_59245.html. [Google Scholar]
- 5.Assi TM, Brown ST, Djibo A, et al. Impact of changing the measles vaccine vial size on Niger’s vaccine supply chain: a computational model. BMC Public Health. 2011;11:425. doi: 10.1186/1471-2458-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee BY, Assi TM, Rajgopal J, et al. Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger. Am J Public Health. 2012;102(2):269–276. doi: 10.2105/AJPH.2011.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BY, Cakouros BE, Assi TM, et al. The impact of making vaccines thermostable in Niger’s vaccine supply chain. Vaccine. 2012;30:5637–5643. doi: 10.1016/j.vaccine.2012.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. [Accessed May 25, 2012];Niger Comprehensive Multiyear Plan 2011–2015. http://www.who.int/immunization_financing/countries/cmyp/niger/en/index.html. Published 2011.