Abstract
Context
Protease inhibitor (PI)-based therapy is recommended for HIV-infected infants exposed to nevirapine for prevention of mother-to-child HIV transmission. However, there are limitations of continuing PI-based therapy indefinitely and re-use of nevirapine has many advantages.
Objective
To test whether nevirapine-exposed infants who initially achieve viral suppression on PI-based therapy can maintain viral suppression when switched to nevirapine-based therapy.
Design
Randomized equivalence trial conducted between April 2005-May 2009.
Setting
Johannesburg, South Africa.
Patients
195 children who achieved viral suppression <400 copies/ml for ≥ 3 months from a cohort of 323 nevirapine-exposed children who initiated PI-based therapy before 24 months of age.
Interventions
Control group remained on ritonavir-boosted lopinavir, stavudine, and lamivudine (n=99). Switch group substituted nevirapine for ritonavir-boosted lopinavir (n=96).
Main Outcome Measures
Children were followed for 52 weeks post-randomization. Plasma HIV-1 RNA >50 copies/ml was the primary endpoint. Confirmed viremia >1000 copies/ml was used as a criterion to consider regimen change for children in either group (safety endpoint).
Results
Plasma viremia >50 copies/ml occurred less frequently in the switch group (Kaplan-Meier probability, 0.438; 95% confidence interval [CI], 0.334–0.537) than in the control group (0.576; 95% CI, 0.470–0.668) (P=0.02). Confirmed viremia >1000 copies/ml occurred more frequently in the switch group (0.201%; 95% CI, 0.125–0.289) than in the control group (0.022%; 95% CI, 0.004–0.069) (P<0.001). CD4 cell response was better in the switch group (median CD4% at 52 weeks, 34.7) vs the control group (CD4%, 31.3) (P=0.004). Older age (Relative Hazard [RH], 1.71; 95% CI, 1.08-2.72) was associated with viremia >50 copies/ml in the control group. Inadequate adherence (RH, 4.14; 95% CI, 1.18-14.57) and drug resistance (RH, 4.04; 95% CI, 1.40-11.65) pre-treatment were associated with confirmed viremia >1000 copies/ml in the switch group.
Conclusions
Among HIV-infected children previously exposed to nevirapine, switching to nevirapine-based therapy after achieving viral suppression on a ritonavir-boosted lopinavir regimen resulted in lower rates of viremia >50 copies/ml than maintaining the primary ritonavir-boosted lopinavir regimen.
Trial Registration
Introduction
Nevirapine used to prevent mother-to-child HIV transmission selects drug resistant viral mutations among a large proportion of HIV-infected infants1,2 and is associated with reduced viral suppression when non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy is initiated.3 A trial comparing nevirapine-based therapy to protease inhibitor (PI)-based therapy among nevirapine-exposed infants was terminated early when reduced viral suppression was observed in the nevirapine-based therapy arm,4 consistent with an adult study.5 Current guidelines for nevirapine-exposed infants advise that treatment be initiated with ritonavir-boosted lopinavir-based regimens.6
There are many limitations of continuing to use PI-based regimens indefinitely in young children. These include its unpleasant taste that poses adherence challenges for children too young to be prescribed tablets.7,8 Refrigeration is required and dosing has to be modified for co-treatment for tuberculosis.9 Metabolic toxicities are of concern with long-term use during critical periods of child development.10-12 Using this PI in first-line regimens limits second-line options. The high cost of ritonavir-boosted lopinavir is a major disincentive to implementing optimal primary therapy recommendations in several sub-Saharan African countries.
We conducted a clinical trial to test the hypothesis that nevirapine-based therapy would be as effective as ritonavir-boosted lopinavir in maintaining viral suppression among nevirapine-exposed children if only initiated once viral suppression had been achieved on the initial PI-based regimen.
Methods
Study Design
We conducted a randomized, open-label, equivalence trial at one site in Johannesburg, South Africa, among 195 HIV-infected children. The children randomized were accrued from a cohort of 323 nevirapine-exposed children who met clinical and immunologic criteria for treatment when <24 months of age and who initiated a PI-based regimen as their first treatment regimen. Data involving the children in this study population were included in prior publications on immune reconstitution inflammatory syndrome and initial response to protease-inhibitor-based antiretroviral therapy, respectively.13,14 Those who achieved and sustained plasma HIV-1 RNA <400 copies/ml for at least 3 months within the first 12 months of treatment were eligible for randomization to: (1) the switch group, who had nevirapine substituted for ritonavir-boosted lopinavir or to (2) the control group, who remained on the ritonavir-boosted lopinavir-based regimen. Follow-up continued to 52 weeks post-randomization. The study received approval by the Institutional Review Boards of Columbia University and the University of the Witwatersrand. The child's guardian provided signed informed consent. Consent was obtained for screening for eligibility for this study and a specific consent form only for screening was used. If children were found to be eligible for the study, a second consent was obtained for enrollment in the trial. At this point, events prior to randomization, eligibility for randomization, and events post-randomization (conditional on eligibility) were explained.
Study Population
Women with HIV-infected children <24 months of age who reported that nevirapine was used for prevention of mother-to-child transmission were identified and referred from inpatient wards and pediatric HIV clinics to one research site between April 8, 2005 and July 10, 2007. Children were evaluated for eligibility for treatment based on South African guidelines in place at the time.15 Eligibility for treatment included WHO stage III or IV disease, CD4 percentage <25 if younger than 12 months or <20 if older than 12 months, or recurrent (> 2/year) or prolonged (>4 weeks) hospitalization for HIV-related complications. Children needing acute treatment for opportunistic infections (except tuberculosis) or tumors were excluded. These children would have been considered as candidates for initiation of antiretroviral therapy but were not eligible to enroll in the trial. In practice, we did not identify any child during screening for this study who was excluded on these grounds. For most children (n=254) enrolled, treatment was initiated under supervision of the study team. A further 69 children were enrolled after initiating PI-based therapy elsewhere (other local pediatric antiretroviral treatment services) but who otherwise met all study eligibility criteria except that pre-treatment blood samples could not be stored for resistance testing. The 69 children were all started initially on ritonavir-boosted lopinavir, stavudine, and lamivudine, but not by our study team. They all were nevirapine-exposed and met the same criteria for starting antiretroviral therapy as the other children. Informed consent for the trial was obtained at enrollment which for most of the children (n=254) was soon before treatment initiation, but for the 69 children, consent for the study was obtained when they were already on treatment.
Procedures
Children older than 6 months received treatment with ritonavir-boosted lopinavir (230 mg/m2), stavudine (1 mg/kg), and lamivudine (4 mg/kg) every 12 hours. Children younger than 6 months or on tuberculosis treatment received ritonavir (400-450 mg/m2), stavudine, and lamivudine every 12 hours. After the children became older than 6 months of age, or after completing tuberculosis treatment, ritonavir was changed to ritonavir-boosted lopinavir. At each visit, drug doses were adjusted according to growth. All medications were administered as syrups.
Caregivers were educated about their child's treatment. Comprehensive adherence counseling was provided, including by peer counselors who conducted home visits if necessary. Participants were encouraged to consult the study team for all clinical problems. Information from in-patient records was abstracted for children who were hospitalized and the clinical circumstances of all deaths were reviewed.
Blood samples were collected pre-treatment and at 4, 12, 24, 36 and 52 weeks post-treatment initiation and were tested for HIV-1 RNA quantity and CD4 cell counts and CD4 percentages were determined. Weight and length were measured and concomitant medications and other clinical conditions were recorded at each visit. Weight-for-age and height-for-age Z-scores were calculated using WHO software.16 Caregivers were requested to return medication bottles which were weighed and the contents reconciled with the expected usage of each medication to determine the extent of adherence. Returning more than 20% of the expected drug volume for any of the three drugs was defined as non-adherence based on expectations that >95% adherence is not necessarily required for suppression.17
Children who achieved viral suppression <400 copies/ml for at least 3 months within the first 12 months of treatment were eligible for randomization. The cut-off of <400 copies/ml was selected as the criterion for randomization for pragmatic reasons because an assay that only quantified to this threshold was in routine use at the time the study was designed. Other criteria for randomization included < grade 2 ALT abnormalities and not receiving tuberculosis treatment. Randomization was done in cohort blocks of variable size between 8 and 12. Allocations were generated by the study statistician and were concealed in opaque envelopes opened on-site at the time of randomization. Once randomization criteria were met, a visit was scheduled to begin the changed regimen for the switch group or to start the post-randomization clock for the controls.
Children randomized to the switch group substituted nevirapine for ritonavir-boosted lopinavir within their treatment regimen. Nevirapine (120mg/m2) was introduced once-per-day for the first 2 weeks and thereafter 200mg/m2 every 12 hours. Children randomized to the control group remained on the ritonavir-boosted lopinavir-based regimen. Both groups received additional adherence counseling, including specific instructions concerning the lead-in schedule and possible adverse effects for the switch group.
Children in both the switch and control groups had blood samples collected for HIV-1 RNA quantification in plasma at 4, 16, 24, 36, and 52 weeks post-randomization and for CD4 cell determination at 16, 24, 36 and 52 weeks. ALT and neutrophil measurements were scheduled at 2, 4, 16, 24, 36 and 52 weeks.
Laboratory Methods
Plasma HIV-1 RNA (Roche version 1.5 Amplicor assay, Roche, Branchburg, NJ) measurements, CD4 cell determinations, blood counts, and liver function tests were conducted by Clinical Laboratory Services in Johannesburg and reported directly to the site for use in clinical management. The Roche standard assay (quantification range, 400-750,000 copies/ml) was used on pre-treatment samples and the ultra-sensitive assay (quantification range, 50-150,000 copies/ml) for post-treatment samples. Available pre-treatment plasma samples were tested in South Africa for mutations in the reverse transcriptase gene using bulk population sequencing at the end of the study using methods previously described.18 Resistance data for children having treatment failure pre-randomization (none of whom were randomized), and for the entire cohort pre-treatment to describe resistance post-nevirapine exposure and compare allele-specific polymerase chain reaction to sequencing have been described.19 Samples from children not achieving viral suppression post-randomization were also tested by population sequencing.20 Resistance was defined using the Stanford Algorithm (http://hivdb.stanford.edu/pages/algs/HIVdb.html).
Study Endpoints
The study protocol defined any viremia >50 copies/ml post-randomization as the primary endpoint following recommendations that full viral suppression is the goal of antiretroviral treatment.21 This was also the lowest threshold discernable with the ultrasensitive assay used during the trial. In addition, as a safety endpoint, all children with two or more HIV-1 RNA measurements >1000 copies/ml were evaluated as candidates for regimen change. If poor adherence was ruled out, children in the switch group who met this safety endpoint were returned to the original ritonavir-boosted lopinavir-based regimen and other potential regimens were considered for children in the control group. Elevated ALT measurements, neutropenia, CD4 cell count response, and growth were compared between the groups as secondary endpoints.
Statistical Analysis
We calculated that 93 children per group were needed to detect a 20% failure rate in the switch group vs 5% in the control group using standard methods for comparing proportions22 and allowing 95% follow-up rate post-randomization and alpha=0.05 and beta=0.2. The slightly larger number randomized (n=195) occurred because randomization was contingent on meeting eligibility after enrollment. We initially planned to enroll 234 children but expanded to 341 children when the proportion meeting eligibility criteria was lower than expected.
Modified intent-to-treat analyses were conducted excluding those children (n=3) missing virologic outcome data. All virologic and clinical data through 52 weeks post-randomization or up to the time of death or censoring for those lost to follow-up or who died were included. Virologic endpoints, mortality, and loss to follow-up were analyzed using Kaplan-Meier methods and groups are compared using log-rank tests.23 Relative Hazards (RH) were calculated using Cox Proportional Hazards models. The proportional hazards assumption was not violated. Other outcomes were compared across groups using Wilcoxon and t-tests tests for continuous variables (eg, CD4 percentage and anthropometric indicators) and Chi-squared or Fisher exact tests for categorical variables (eg, ALT grades). Analyses were done using SAS version 9.1.3 (Cary, NC). All statistical tests were two-sided and a P value <0.05 was considered statistically significant.
Results
Study population
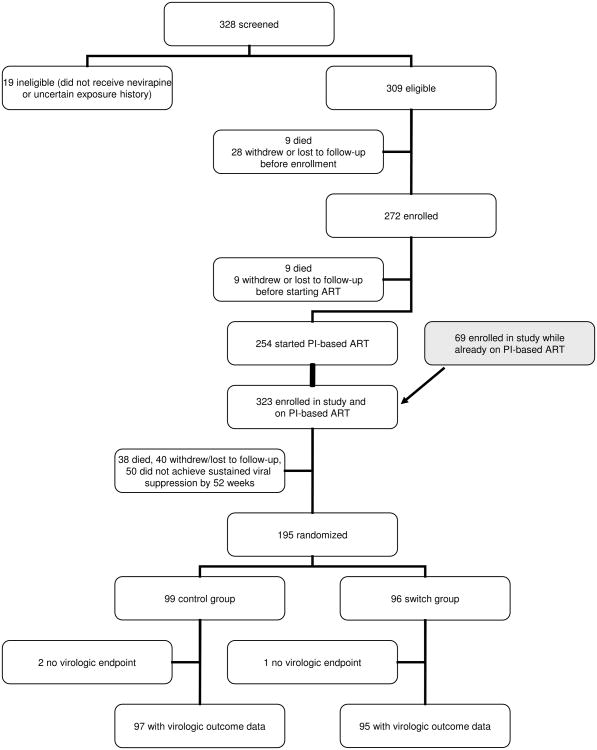
Among 323 HIV-infected children who initiated PI-based therapy during the pre-randomization period, 38 (11.8%) died, 40 (12.4%) did not remain in follow-up, and 50 (15.5%) did not meet criteria for randomization (Figure 1). Children who met criteria for randomization tended to be older at treatment start, had less severe disease, and were more likely to have mothers on treatment (eTable). Children randomized were a median of 10 months of age at treatment start, 55% had >750,000 HIV-1 RNA copies/ml in plasma, and had a median CD4 percentage of 18.5 pre-treatment. A prior report has described pre-randomization characteristics of the study population in detail.14 At randomization, a median of 9 months later, the median CD4 percentage was 29.1. The switch groups and control groups were similar in all pre-randomization characteristics (Table 1).
Figure 1. Disposition of Study Participants in Screening, Enrollment, Randomization, and Follow-up Post-Randomization.
Table 1. Pre-Treatment And Pre-Randomization Characteristics Of 195 Hiv-Infected Children Randomized To Remain On A Ritonavir-Boosted Lopinavir-Based Regimen (Control Group) Or To Switch To A Nevirapine-Based Regimen (Switch Group).
| Control group | Switch group | p-value* | |
|---|---|---|---|
| N | 99 | 96 | |
| Sex: Male | 50 (51%) | 54 (56%) | 0.42 |
| Pre-treatment | |||
| Age at treatment start | |||
| < 6 month | 28 (28%) | 26 (27%) | |
| 6-11 months | 29 (29%) | 40 (42%) | |
| 12-17 months | 25 (25%) | 22 (23%) | |
| 18-24 months | 17 (17%) | 8 (8%) | 0.16 |
| Median (range) in months | 11 (2 – 24) | 9 (2 – 22) | 0.09 |
| HIV-1 RNA quantity | |||
| <100,000 copies/ml | 7 (8%) | 12 (13%) | |
| 100,000-750,000copies/ml | 29 (35%) | 29 (33%) | |
| >750,000 copies/ml | 48 (57%) | 48 (54%) | 0.56 |
| CD4 percentage | |||
| <10 | 16 (17%) | 10 (11%) | |
| 10-14.9 | 16 (17%) | 21 (23%) | |
| > 15 | 61 (66%) | 62 (67%) | 0.36 |
| Median (range) | 19.0 (2.2 – 41.8) | 18.4 (1.5 – 39.3) | 0.68 |
| WHO Stage | |||
| I/II | 16 (21%) | 18 (23%) | |
| III/IV | 61 (79%) | 60 (77%) | 0.73 |
| Weight for age Z-score | |||
| Mean (std) | -2.23 (1.84) | -2.13 (1.48) | 0.71 |
| < -2 STD | 48 (54%) | 43 (49%) | 0.55 |
| Height for age Z-score | |||
| Mean (std) | -3.14 (1.67) | -3.10 (1.70) | 0.88 |
| < -2 STD | 70 (80%) | 62 (73%) | 0.31 |
| Time of randomization | |||
| Age at randomization | |||
| 6-11 months | 6 (6%) | 9 (9%) | |
| 12-17 months | 34 (34%) | 36 (38%) | |
| 18-23 months | 26 (26%) | 29 (30%) | |
| ≥ 24 months | 33 (33%) | 22 (23%) | 0.40 |
| Median (range) in months | 20 (10 – 36) | 19 (9 – 43) | 0.09 |
| CD4 percentage | |||
| < 10 | 0 | 1 (1%) | |
| 10-14.9 | 4 (4%) | 3 (3%) | |
| 15-19.9 | 8 (8%) | 10 (11%) | |
| ≥ 20 | 84 (88%) | 81 (85%) | 0.70 |
| Median (range) | 28.9 (10.9 – 55.7) | 29.5 (7.3 – 52.3) | 0.81 |
| Weight for age Z-score | |||
| Mean (std) | -0.56 (1.21) | -0.59 (1.12) | 0.84 |
| < -2 STD | 9 (9%) | 9 (9%) | 0.95 |
| Height for age Z-score | |||
| Mean (std) | -3.19 (1.49) | -3.07 (1.65) | 0.60 |
| < -2 STD | 75 (76%) | 73 (76%) | 0.96 |
| Median months on therapy before randomization (range) | 9 (4 – 19) | 9 (5 – 21) | 0.88 |
Categorical variables are compared across groups using chi-squared tests; median age, CD4 percentage, and time on therapy are compared using Wilcoxon tests; and height and weight for age are compared using t-tests. Denominators are as shown.
Primary Endpoints Post-Randomization
Loss to follow-up and mortality was low in both groups. Four children died post-randomization. Two children in the control group died: one of bacterial pneumonia and the other of unknown causes; two children in the switch group died: one of fulminant sepsis and one due to pre-existing renal pathology. None of the deaths was considered drug-related.
There was better virologic suppression in the switch group based on the primary endpoint of viremia of >50 copies/ml. In the control group, the Kaplan-Meier probability of having at least one measurement >50 copies/ml was 0.576 (95% CI: 0.470 – 0.668), which was higher than in the switch group for whom the probability was 0.438 (95% CI: 0.334 – 0.537) (Table 2, P=0.02).
Table 2. Primary Endpoints Through 52 Weeks Post-Randomization Among 99 Children Retained on a Ritonavir-Boosted Lopinavir-Based Regimen (Control Group) and 96 Children Switched to a Nevirapine-Based Regimen (Switch Group).
| Control group | Switch group | p-value | |
|---|---|---|---|
| N | 99 | 96 | |
| Probability of lost to follow-up by 52 weeks | 0.031 | 0.053 | 0.46 |
| (95% CI) | (0.008 – 0.080) | (0.020 – 0.110) | |
| N lost to follow-up before 52 weeks | 3 | 5 | |
| Probability of mortality by 52 weeks | 0.020 | 0.021 | 0.97 |
| (95% CI) | (0.004 – 0.065) | (0.004 – 0.068) | |
| N died | 2 | 2 | |
| Probability of HIV-1 RNA > 50 copies/ml* | 0.576 | 0.438 | 0.02 |
| (95% CI) | (0.470 – 0.668) | (0.334 – 0.537) | |
| N with > one measurement >50 copies/ml | 55 | 40 |
Three children (2 in the control and 1 in the switch group) were missing virologic data and were excluded. Virologic data through 52 weeks post-randomization or up to the time of death or censoring were included. Probabilities are calculated using Kaplan-Meier methods and are compared across group using log-rank tests.
Secondary Endpoints
Confirmed viremia of >1000 copies/ml, a safety endpoint, was more common among children in the switch group than the control group. In the control group, the probability of confirmed viremia >1000 copies/ml was 0.022 (95% CI, 0.004 – 0.069), whereas in the switch group, the probability was 0.201 (95% CI, 0.125 – 0.289) (Table 3, P<0.001). Of 18 children in the switch group with confirmed viremia >1000 copies/ml, 3 achieved viral suppression again without regimen change, and 9 achieved it after being switched back to ritonavir-boosted lopinavir. Three children discontinued study participation soon after viral elevations were noted and three were returned to ritonavir-boosted lopinavir but discontinued study participation before the next measurement. In all six cases severe household disruption affecting adherence, including changes in caregivers, maternal illness and relocation, were known to have occurred.
Table 3. Secondary Endpoints Through 52 Weeks Post-Randomization Among 99 Children Retained on a Ritonavir-Boosted Lopinavir-Based Regimen (Control Group) and 96 Children Switched to a Nevirapine-Based Regimen (Switch Group).
| Control group | Switch group | p-value* | |
|---|---|---|---|
| N (Probability) of confirmed viremia > 1000 copies/ml* | 2 (0.022) | 18 (0.201) | <0.001 |
| (95% CI) | (0.004-0.069) | (0.125 – 0.289) | |
| N (%) highest ALT measurement† | |||
| Grade 1 | 20 (20%) | 35 (36%) | |
| Grade 2 | 2 (2%) | 11 (11%) | |
| Grade 3/4 | 4 (4%) | 7 (7%) | 0.0003 |
| N (%) lowest neutropenia† | |||
| Grade 1 | 8 (8%) | 15 (16%) | |
| Grade 2 | 5 (5%) | 8 (8%) | |
| Grade 3/4 | 3 (3%) | 5 (5%) | 0.18 |
| Median CD4% (25th - 75th percentiles)§ | |||
| Week 16 | 30.3 (26.2 – 35.3) | 34.6 (27.6 – 39.5) | 0.005 |
| Week 24 | 30.0 (24.3 – 34.2) | 33.9 (28.9 – 41.1) | <0.0001 |
| Week 36 | 31.8 (26.4 – 35.9) | 33.2 (27.3 – 39.6) | 0.08 |
| Week 52 | 31.3 (26.5 – 37.4) | 34.7 (29.6 – 40.8) | 0.004 |
| N (%) CD4% declined by 10% between randomization and 52 weeks | 14 (15%) | 3 (3%) | 0.005 |
| [95% CI]† | [8.87 - 24.56] | [0.84 - 9.81] | |
| Mean (std) weight-for-age Z-score§ | |||
| Week 16 | -0.39 (1.10) | -0.30 (1.04) | 0.59 |
| Week 24 | -0.40 (1.15) | -0.30 (1.08) | 0.56 |
| Week 36 | -0.43 (1.23) | -0.35 (1.07) | 0.63 |
| Week 52 | -0.38 (1.19) | -0.44 (1.09) | 0.69 |
| N (%) Weight-for age Z score declined by 1 Z-score between randomization and 52 weeks | 13 (13%) | 4 (4%) | 0.03 |
| [95% CI]† | [7.45 - 21.76] | [1.36 - 11.04] | |
Three children (2 in the control and 1 in the switch group) were missing virologic data and were excluded. Virologic data through 52 weeks post-randomization or up to the time of death or censoring were included. Probabilities are calculated using Kaplan-Meier methods and are compared across group using log-rank tests.
Proportions were compared across groups using chi-squared tests. No children were missing ALT data, one child in the switch group was missing data on neutropenia, 7 children in the control and 3 in the switch group were missing data on CD4 percentage and one child in the switch group was missing data on weight.
Groups were compared using t-tests.
Viral RNA from 15/18 children in switch group and 2/2 children in the control group with confirmed viremia >1000 copies/ml could be sequenced to determine drug resistance genotype. Major NNRTI resistance could be detected among 13/15 (86.7%) children in the switch group and in neither of the two children in the control group. Y181C was the most common mutation occurring among 10/15 (66.7%) of children. Five of six children in the switch group with wild type virus pre-treatment had NNRTI resistance at time of failure (2 had Y181C, 2 had V106A, and 1 had K101E and G190A. NRTI mutations could be detected among 12/15 (80.0%) children in the switch group and 1/2 children in the control group. All were M184V/I except for one D67N.
Elevated ALT measurements were common in the switch group but tended to be of low grades. Neutropenia was rare in either group. CD4 percentage increased at a significantly slower pace in the control group than in the switch group. Weight-for-age Z-scores were similar on average but more children in the control group than in the switch group experienced a decline in weight-for-age post-randomization (Table 3).
Predictors of Viremia
Children who were older at the time of randomization were more likely to have viremia >50 copies/ml (RH, 1.71; 95% CI, 1.08-2.72) than younger children but this age trend was only significant in the control group (Table 4). Adherence ascertained by pharmacy reconciliation was not associated with viremia >50 copies/ml in either group (Table 4). However, inadequate adherence was associated with viremia >1000 copies/ml in the switch group (RH, 4.14; 95% CI, 1.18-14.57).
Table 4. Predictors of the Primary Endpoint (Viremia >50 copies/ml) and the Safety Endpoint (Confirmed Viremia >1000 copies/ml) in the Control Group and Switch Group Separately.
| Primary endpoint: viremia >50 copies/ml | ||||||
|---|---|---|---|---|---|---|
| Control | N (probability) Viremic [95% CI] | p-value | Switch | N (probability) Viremic [95% CI] | p-value | |
| N* | 99 | 55 | 96 | 40 | ||
| Age at randomization | ||||||
| 6-11 months | 6 | 2 (0.333) [0.046 – 0.676] | 9 | 3 (0.375) [0.087 – 0.674] | ||
| 12-17 months | 34 | 13 (0.408) [0.239 – 0.570] | 36 | 15 (0.457) [0.280 – 0.618] | ||
| 18-23 months | 26 | 15 (0.605) [0.386 – 0.767] | 29 | 9 (0.323) [0.161 – 0.496] | ||
| ≥ 24 months | 33 | 25 (0.764) [0.577 – 0.877] | 0.01 | 22 | 13 (0.591) [0.361 – 0.762] | 0.12 |
| Drug resistance results | ||||||
| Major NNRTI mutations | 11 | 7 (0.659) [0.298 – 0.866] | 20 | 11 (0.605) [0.342 – 0.791] | ||
| No major NNRTI mutations | 61 | 33 (0.570) [0.433 – 0.686] | 0.60 | 51 | 20 (0.406) [0.269 – 0.539] | 0.16 |
| Adherent | 89 | 52 (0.595) [0.483 – 0.689] | 79 | 33 (0.436) [0.322- 0.544] | ||
| Inadequate adherence* | 3 | 4 (0.667) [0.009 – 0.774] | 0.45 | 6 | 3 (0.600) [0.126 – 0.882)] | 0.41 |
| Safety endpoint: confirmed viremia >1000 copies/ml | ||||||
| Control | N (probability) Viremic [95% CI] | p-value | Switch | N (probability) Viremic [95% CI] | p-value | |
| N | 99 | 97 | 96 | 78 | ||
| Age at treatment start | ||||||
| < 6 month | 28 | 0 | 26 | 2 (0.087) [0.015 – 0.243] | ||
| 6-11 months | 29 | 2 (0.074) [0.013 – 0.211] | 40 | 10 (0.271) [0.140 – 0.419] | ||
| 12-17 months | 25 | 0 | 22 | 6 (0.273) [0.111 – 0.464] | ||
| 18-24 months | 17 | 0 | 0.18 | 8 | 0 | 0.13 |
| Drug resistance results | ||||||
| Major NNRTI mutations | 11 | 0 | 20 | 8 (0.447) [0.214 – 0.657] | ||
| No major NNRTI mutations | 61 | 2 (0.036) [0.007 – 0.109] | 0.55 | 51 | 6 (0.120) [0.049 – 0.226] | 0.005 |
| Adherent | 82 | 2 (0.026) [0.005 – 0.082] | 79 | 13 (0.172) [0.097 – 0.265] | ||
| Inadequate adherence* | 8 | 0 | 0.66 | 6 | 3 (0.600) [0.126 – 0.882] | 0.02 |
Three children (2 in the control and 1 in the switch group) were missing virologic data and were excluded. Virologic data through 52 weeks post-randomization or up to the time of death or censoring were included. Probabilities are calculated using Kaplan-Meier methods and are compared across group using log-rank tests. The results presented are from univariate models.
Inadequate adherence was defined as returning more than 20% of the expected drug volume for any of the three drugs based on pharmacy reconciliation at the time of the outcome
Pre-treatment NNRTI resistance mutations were detected among 31/143 (21.7%) children tested (25 had Y181C, 4 had K103N, 1 had Y188C, and 1 had G190A). As expected, there was no association between pre-treatment NNRTI resistance mutations and viremia in the control group. In the switch group, there was a non-significant trend toward an association using the endpoint of >50 copies/ml. Pre-treatment NNRTI mutations were strongly related to confirmed viremia >1000 copies/ml in the switch group (RH, 4.04; 95% CI, 1.40-11.65). The probability of confirmed viremia >1000 copies/ml was 0.447 (95% CI, 0.214 – 0.657) among those who had NNRTI mutations pre-treatment compared to 0.120 (95% CI, 0.049 – 0.226) among those who did not (P=0.005). Pre-treatment NNRTI mutations were more common among children ≤12 months of age at the time of initiating therapy (29.0%) than among older children (8.0%) but this difference did not lead to worse outcomes in children who were younger when starting therapy. Younger age, whether categorized at the time of starting treatment or at the time of randomization, was not associated with viremia >1000 copies/ml in the switch group (Table 4).
Comment
A recent trial demonstrated that nevirapine-exposed, HIV-infected children should initiate therapy with a PI-based regimen,4 but whether this regimen needs to be continued indefinitely is unclear. Our data indicate that children who switch to nevirapine-based therapy once they have achieved viral suppression after an average of 9 months of ritonavir-boosted lopinavir-based therapy are more likely to achieve viremia <50 copies/ml than children who remain on their original regimen. However, a sizable minority (20%) experienced breakthrough viremia >1000 copies/ml that required consideration for therapy change. This outcome was strongly related to pre-treatment NNRTI mutations and was rare (2%) among children maintained on their original regimen. These seemingly inconsistent results highlight the promise and pitfalls of switching nevirapine-exposed infants. Switching allows treatment options to be expanded and the desirable benefits of a nevirapine-based regimen to be accrued. However, for about 20% of exposed children the regimen is suboptimal. Therefore, switching can only be considered in situations in which adequate virologic monitoring can be conducted, both to identify who is eligible to switch, and to identify, as early as possible, children who should be returned to the ritonavir-boosted lopinavir-based regimen.
Switching following a suppressive regimen has not, to our knowledge, been previously investigated as a strategy to overcome pre-existing drug resistance in either adults or children. Several trials in adults have evaluated switching from PI-based therapy to NNRTI-based therapy for reasons of toxicity, adherence, and quality of life.24-31 The overall conclusions are that switching can be accomplished safely, while maintaining virologic suppression, improving adherence, and reducing some toxicities.24-31 PI-associated toxicities may be less common in children, particularly prior to puberty, but are of concern as therapy is being given during developmentally-critical periods and long-term consequences may be serious.10-12 There are two non-randomized reports of children switched from PI-based regimens to NNRTI-based regimens.,32,33 Both reported sustained viral suppression and improved lipid profiles.32,33
NNRTI resistance mutations detected by standard population sequencing pre-treatment were strongly related to confirmed viremia >1000 copies/ml in the switch group. Among those without pre-treatment resistance, 88% did not reach this safety endpoint post-switch (Table 4). These data suggest that screening for mutations pre-treatment would be clinically-useful if the switching strategy were to be employed. However, in practice, drug resistance testing is likely to be difficult to accomplish in low-resource settings. Nevertheless, strategies to utilize targeted drug resistance testing should be considered as the cost of resistance testing could be justified based on the potential for cost-savings from the less expensive regimen.
It was unexpected that younger age at treatment initiation was not associated with virologic response in the switch group. Among nevirapine-exposed women, the proportion who have detectable mutations declines with time after exposure and response to first-line NNRTI-based therapy is generally better with longer time after exposure.3,34-36 Child response to exposure may be different than adult response to exposure, or the ritonavir-boosted lopinavir-based induction regimen may have modified the relationship.
Older age was strongly related to intermittent and/or low level viremia in the control group. Low-level viremia occurred among more than half (55%) of all children and more than three-quarters (76.4%) of children older than 24 months at randomization in the control group. Few prior studies have included large numbers of ritonavir-boosted lopinavir-treated children of this age or described viral response in such detail. Ritonavir-boosted lopinavir syrup poses substantial adherence challenges among young children, given its unpleasant taste.7,8 As children become old enough and strong enough to resist their caregivers, the taste of this drug may play a larger role in adherence. Potency could be compromised if children do not consume adequate volumes. More palatable formulations in pediatric doses are urgently needed. A limitation of our study is that blinding was not possible. Thus we cannot distinguish pharmacologic and virologic differences between the regimens from behavioral changes that may result from poor palatability. We used standard recommended doses of ritonavir-boosted lopinavir based on body surface area although some have questioned whether these doses are sufficient.37 Since higher doses are thought to be necessary for younger children, inadequate dosing would not explain the observed age gradients.
CD4 cell response was weaker in the control group. Although CD4 percentages were mostly in the normal range and increased over time in both groups, the increase was larger in the switch group. Mean weight-for-age Z-scores were similar between the groups, but a larger proportion of children in the control group dropped a Z-score at some point post-randomization. One possible explanation may be appetite suppression related to the poor palatability of ritonavir-boosted lopinavir. Similar differences have been noted in some other studies.4
Guidelines now recommend starting treatment among all HIV-infected infants as soon as possible after diagnosis following a trial demonstrating better outcomes if treatment is initiated immediately rather than waiting until standard prognostic indicators are reached.38 Thus, large numbers of HIV-infected infants should be initiating ritonavir-boosted lopinavir-based treatment, but the high cost of this regimen poses a barrier in many low-resource settings. Our results suggest that a majority of nevirapine-exposed children who are successfully treated with initial ritonavir-boosted lopinavir-based regimens and achieve viral suppression could benefit from the switch strategy which would allow costs of pediatric treatment programs to be reduced. However, switching should only be undertaken with adequate virologic monitoring. Although the value of virologic monitoring in HIV treatment is strongly emphasized in well-resourced settings,21,39 most programs in low-resource settings do not include it as part of routine services because of cost. Simple algorithms could be developed for targeted virologic testing to safely implement the switch strategy.
Acknowledgments
Funding/Support: The study was supported by grants from the National Institutes of Child Health and Human Development (NICHD) HD 47177 and Secure the Future Foundation.
Role of the Sponsors: The funders had no role in the design and conduct of the study, the collection, management, analysis and interpretation of the data, or the preparation, review or approval of the manuscript.
Footnotes
Author Contributions: Drs. Coovadia, Abrams, Tsai and Kuhn had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The study was designed by LK, EJA AC, TM, GS. Clinical aspects of the protocol and patient recruitment and follow-up were the responsibility of AC, RS, TM, LM, GS. Drug resistance testing was done by GH and LM. Data management and statistical analyses were done by LK, CH, WYT. All authors have reviewed the data and provided input into the interpretation of the results and have reviewed the manuscript for critical content.
Financial Disclosures: None of the authors have any conflict of interest to declare.
Previous Presentation: These data were presented in part at the 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 20, 2009; Cape Town, South Africa. Abstract MOAB103.
Additional Contributions: We would like to thank the members of the Data Safety and Monitoring Board: Edmund Caparelli, MD (University of California, San Diego, California); Mark Cotton, MD (University of Stellenbosch, South Africa); Victor DeGruttola, PhD (Harvard University, Boston, Massachusetts); Brian Eley, MD (University of Cape Town, South Africa); Mary-Glenn Fowler, MD (Johns Hopkins University, Baltimore, Maryland); Paul Palumbo, MD (Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire); and Andrea Ruff, MD (Johns Hopkins University, Baltimore, Maryland). We would also like to thank the following people for assistance with scientific aspects of the study: Lynne Mofenson, MD (National Institute of Child Health and Human Development (NICHD], Bethesda, Maryland); Kevin Ryan, PhD (NICHD, Bethesda, Maryland); and Deborah Persaud, MD (Johns Hopkins University, Baltimore, Maryland). None of the individuals named here received compensation from the study. We appreciate the dedication of the participants and their care-givers and the clinical and administrative study team.
References
- 1.Arrive E, Newell ML, Ekouevi DK, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 2.Martinson NA, Morris L, Gray G, et al. Selection and persistence of viral resistance in HIV-infected children after exposure to single-dose nevirapine. J Acquir Immune Defic Syndr. 2007;44:148–153. doi: 10.1097/QAI.0b013e31802b920e. [DOI] [PubMed] [Google Scholar]
- 3.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 4.Palumbo P, Violari A, Lindsey J, et al. Nevirapine (NVP) vs lopinavir-ritonavir (LPV/r)-based antiretroviral therapy (ART) in single dose nevirapine (sdNVP)-exposed HIV-infected infants: preliminary results from the IMPAACT P1060 trial. 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. 2009; 19-22 July 2009; Cape Town, South Africa. [Google Scholar]
- 5.Lockman S A5208/OCTANE Study Team. Lopinavir/ritonavir+Tenofovir/Emtricitabine is superior to Nevirapine+Tenofovir/Emtricitabine for women with prior exposure to single-dose nevirapine. 16th Conference on Retroviruses and Opportunistic Infections. 2009; Feb 8-11; Montreal, Canada. [Google Scholar]
- 6.World Health Organization. Geneva-Switzerland: 2010. [accessed August 3 2010]. Antiretroviral therapy for HIV infection in infants and children: Towards universal access. http://www.who.int/hiv/pub/paediatric/paed-prelim-summary.pdf. [PubMed] [Google Scholar]
- 7.Davies MA, Boulle A, Fakir T, Nuttall J, Eley B. Adherence to antiretroviral therapy in young children in Cape Town, South Africa, measured by medication return and caregiver self-report: a prospective cohort study. BMC Pediatrics. 2008;8:34. doi: 10.1186/1471-2431-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simoni JM, Montgomery A, Martin E, New M, Demas PA, Rana S. Adherence to antiretroviral therapy for pediatric HIV infection: A qualitative systematic review with recommendations for research and clinical management. Pediatrics. 2007;119:e1371–e1383. doi: 10.1542/peds.2006-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren Y, Nuttall JJ, Egbers C, et al. Effect of rifampicin on lopinavir pharmacokinetics in HIV-infected children with tuberculosis. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2008;47:566–569. doi: 10.1097/QAI.0b013e3181642257. [DOI] [PubMed] [Google Scholar]
- 10.McComsey GA, Leonard E. Metabolic complications of HIV therapy in children. AIDS. 2004;18:1753–1768. doi: 10.1097/00002030-200409030-00004. [DOI] [PubMed] [Google Scholar]
- 11.Bitnun A, Sochett E, Babyn P, et al. Serum lipids, glucose homeostasis and abdominal adipose tissue distribution in protease inhibitor-treated and naive HIV-infected children. AIDS. 2003;17:1319–1327. doi: 10.1097/00002030-200306130-00006. [DOI] [PubMed] [Google Scholar]
- 12.Farley J, Gona P, Crain M, et al. Prevalence of elevated cholesterol and associated risk factors among perinatally HIV-infected children (4-19 years old) in Pediatric AIDS Clinical Trials Group 219C. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2005;38:480–487. doi: 10.1097/01.qai.0000139397.30612.96. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Kuhn L, Coovadia A, et al. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reitz C, Coovadia A, Ko S, et al. Initial response to protease-inhibitor-based antiretroviral therapy among children less than 2 years of age in South Africa: effect of cotreatment for tuberculosis. J Infect Dis. 2010;201:1121–1131. doi: 10.1086/651454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Department of Health. Guidelines for the management of HIV-infected children. 2005 Pretoria: http://www.doh.gov.za/docs/policy-f.html.
- 16.WHO Child Growth Standards (2005) and the WHO Anthro 2005 software and macros. 2005 http://www.who.int/childgrowth/software/en/
- 17.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43:939–941. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 18.Pillay V, Ledwaba J, Hunt G, et al. Antiretroviral drug resistance surveillance among drug-naive HIV-1 infected individuals in Gauteng Province, South Africa in 2002 and 2004. Antiviral Therapy. 2008;13(Suppl 2):101–107. [PubMed] [Google Scholar]
- 19.Hunt G, Taylor B, Coovadia A, et al. Development of drug resistance among a cohort of HIV-infected infants exposed to nevirapine for prevention of mother-to-child transmission initiating PI-based ART in South Africa. 16th Conference on Retroviruses and Opportunistic Infections; Montreal, Canada: CROI. 2009. Abstract 957. [Google Scholar]
- 20.Moorthy A, Kuhn L, Coovadia A, et al. Plasma frequencies of nevirapine resistance influence virologic responses to nevirapine maintenance therapy in single-dose exposed HIV-infected children initially treated with lopinavir HAART. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco, CA: CROI. 2010. Abstract 159. [Google Scholar]
- 21.Hammer SM, Eron JJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the international AIDS society - USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 22.Fleiss JL. Statistical Methods for Rates and Proportions. Second. New York: Wiley; 1981. [Google Scholar]
- 23.Collett D. Modelling Survival Data in Medical Research. New York: Chapman-Hall; 1994. [Google Scholar]
- 24.Bucher HC, Kofler A, Nuesch R, Young J, Battegay M, Opravil M. Meta-analysis of randomized controlled trials of simplified versus continued protease inhibitor-based antiretroviral therapy in HIV-1-infected patients. AIDS. 2003;17:2451–2459. doi: 10.1097/00002030-200311210-00007. [DOI] [PubMed] [Google Scholar]
- 25.Murphy RL, Smith WJ. Switch studies: a review. HIV Medicine. 2002;3:146–155. doi: 10.1046/j.1468-1293.2002.00102.x. [DOI] [PubMed] [Google Scholar]
- 26.Negredo E, Bonjoch A, Clotet B. Benefits and concerns of simplification strategies in HIV-infected patients. Journal of Antimicrobial Chemotherapy. 2006;58:235–242. doi: 10.1093/jac/dkl191. [DOI] [PubMed] [Google Scholar]
- 27.Ena J, Leach A, Nguyen P. Switching from suppressive protease inhibitor-based regimens to nevirapine-based regimens: a meta-analysis of randomized controlled trials. HIV Medicine. 2008;9:747–756. doi: 10.1111/j.1468-1293.2008.00627.x. [DOI] [PubMed] [Google Scholar]
- 28.Martinez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349:1036–1046. doi: 10.1056/NEJMoa021589. [DOI] [PubMed] [Google Scholar]
- 29.Raffi F, Bonnet B, Ferre V, et al. Substitution of a nonnucleoside reverse transcriptase inhibitor for a protease inhibitor in the treatment of patients with undetectable plasma human immunodeficiency virus type 1 RNA. Clin Infect Dis. 2000;31:1274–1278. doi: 10.1086/317424. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz L, Negredo E, Domingo P, et al. Antiretroviral treatment simplification with nevirapine in protease inhibitor-experienced patients with HIV-associated lipodystrophy: 1-year prospective follow-up of a multicenter, randomized, controlled study. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2001;27:229–236. doi: 10.1097/00126334-200107010-00003. [DOI] [PubMed] [Google Scholar]
- 31.Negredo E, Cruz L, Paredes R, et al. Virological, immunological, and clinical impact of switching from protease inhibitors to nevirapine or to efavirenz in patients with human immunodeficiency virus infection and long-lasting viral suppression. Clin Infect Dis. 2002;34:504–510. doi: 10.1086/324629. [DOI] [PubMed] [Google Scholar]
- 32.McComsey G, Bhumbra N, Ma JF, Rathore M, Alvarez A, First Pediatric SS. Impact of protease inhibitor substitution with efavirenz in HIV-infected children: results of the First Pediatric Switch Study. Pediatrics. 2003;111:e275–e281. doi: 10.1542/peds.111.3.e275. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Tome MI, Amador JT, Pena MJ, Gomez ML, Conejo PR, Fontelos PM. Outcome of protease inhibitor substitution with nevirapine in HIV-1 infected children. BMC Infectious Diseases. 2008;8:144. doi: 10.1186/1471-2334-8-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chi BH, Sinkala M, Stringer EM, et al. Early clinical and immune response to NNRTI-based antiretroviral therapy among women with prior exposure to single-dose nevirapine. AIDS. 2007;21:957–964. doi: 10.1097/QAD.0b013e32810996b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose NVP and virologic response to non-nucleoside reverse transcriptase inhibitor-based therapy. Clin Infect Dis. 2009;48:462–472. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhn L, Semrau K, Ramachandran S, et al. Mortality and virologic outcomes after access to antiretroviral therapy among a cohort of HIV-infected women who received single-dose nevirapine in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2009;52:132–136. doi: 10.1097/QAI.0b013e3181ab6d5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verweel G, Burger DM, Sheehan NL, et al. Plasma concentrations of the HIV-protease inhibitor lopinavir are suoptimal in children aged 2 years and below. Antiviral Therapy. 2007;12:453–458. [PubMed] [Google Scholar]
- 38.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Working Group on Antiretroviral Therapy and Medical Management of HIV-infected Children. Guidelines for the use of antiretroviral agents in pediatric HIV infection. [Accessed Nov 2009];2009 Feb;23:1–139. http://aidsinfo.nih.gov/contentFiles/PediatricGuidelines.pdf. 2009. [Google Scholar]