Abstract
Aim
Doubt still remains as to whether peripheral vascular and skeletal muscle dysfunction accompanies the compromised cardiac function associated with heart failure with reduced ejection fraction (HFrEF). The aim of this study was to examine the effect of HFrEF on the haemodynamic and metabolic responses to exercise with both a large (cycle) and a small [knee extensor (KE)] muscle mass in comparison with well-matched healthy controls (Ctrls).
Methods
Utilizing blood sampling and thermodilution blood flow measurements, we studied incremental cycle and KE exercise in 12 patients with HFrEF (ejection fraction: 25 ± 3%) and eight Ctrls.
Results
Incremental cycle exercise in both groups [heart failure with reduced ejection fraction (HFrEF): 23 ± 1 to 116 ± 10; Ctrls: 22 ± 1 to 137 ± 5 W] resulted in a similar rise in blood flow (HFrEF: 1525 ± 132 to 4216 ± 408; Ctrls: 1774 ± 161 to 4713 ± 448 mL min−1), oxygen uptake (HFrEF: 206 ± 24 to 586 ± 34; Ctrls: 252 ± 21 to 747 ± 89 mL min−1) and lactate efflux across the leg (HFrEF: 479 ± 122 to 4929 ± 1255; Ctrls: 537 ± 155 to 5776 ± 1010 mM min−1). Vascular resistance fell similarly in both groups with increasing exercise intensity (HFrEF: 66 ± 10 to 24 ± 3; Ctrls: 69 ± 12 to 24 ± 4 mmHg L−1 min−1). Incremental KE exercise also revealed similar haemodynamic and metabolic responses in both Ctrls and patients.
Conclusion
Although assessed in a relatively small cohort, these data reveal that, when compared with well-matched healthy Ctrls, alterations in peripheral haemodynamics and skeletal muscle metabolism during exercise may not be an obligatory accompaniment to HFrEF.
Keywords: blood flow, cardiac output, exercise pressor reflex, skeletal muscle, vascular resistance
Diminished exercise capacity is a cornerstone symptom of HFrEF, as defined by the American Heart Association (Yancy et al. 2013). This significantly limits physical activity and impairs quality of life (Drexler et al. 1992). Previously, with a focus upon maximal exercise, our group has partitioned the contributors to this reduced exercise capacity in HFrEF and revealed a consistent 25–30% attenuation in both the convective and diffusive components of O2 transport during both cycle and knee extensor (KE) exercise (Esposito et al. 2010b). Interestingly, it was later determined that these deficits could be corrected by exercise training which did not alter peak cardiac output (Esposito et al. 2011). These findings were consistent whether patients with HFrEF were studied during either large muscle mass (cycle) or small muscle mass (KE) exercise. This identified an underlying peripheral O2 transport limitation from blood to skeletal muscle in this pathology which is not simply a consequence of diminished cardiac function, but is likely impacted by inactivity. Although there is already considerable evidence that HFrEF results in altered peripheral responses to even submaximal exercise (Wilson et al. 1984a, Sullivan et al. 1989), some methodological concerns continue to cast doubt on whether these observations are generalizable to all patients with HFrEF and across exercise modalities that have differing cardiac demands.
Attenuated blood flow to working skeletal muscle in patients with HFrEF has been recognized in several studies (Wilson et al. 1984a, Sullivan et al. 1989, Isnard et al. 1996, Magnusson et al. 1997) and is often accompanied by the relatively early increase in blood lactate concentration (Rubin et al. 1980, Wilson et al. 1984a, Sullivan et al. 1989). Indeed, this apparent hypoperfusion and subsequently greater reliance on anaerobic metabolism have been suggested to be a primary cause of exercise intolerance in this population (Weber et al. 1982, Wilson & Ferraro 1983, Sullivan et al. 1989). From these studies, it appears that an increased peripheral vascular resistance, likely mediated by an exaggerated exercise pressor reflex in patients with HFrEF (Garry 2011), may be responsible for these findings. Unfortunately, the interpretation of the exercise pressor reflex and skeletal muscle lactate production for a given absolute work rate in subject groups with fundamental differences in physical fitness is problematic and can yield misleading results. Indeed, without significant effort from the investigators, healthy volunteers are typically far more physically active than patients with HFrEF and this will undoubtedly affect any comparison of haemodynamic and metabolic responses to exercise.
Consequently, using direct intravascular measurements, this study was designed to assess haemodynamics and skeletal muscle metabolism in patients with HFrEF during incremental exercise in comparison with Ctrls who were well matched, both in terms of physical activity and in terms of physical characteristics. Additionally, two modes of incremental exercise, both a small and a large muscle mass, were employed to assess the impact of varying metabolic demand on haemodynamic and metabolic responses. Specifically, we hypothesized that patients with New York Heart Association (NYHA) classes II and III HFrEF, when compared with well-matched Ctrls, will (i) exhibit little or no difference in the blood flow response, oxygen uptake (VO2), skeletal muscle lactate production and vascular resistance in response to incremental cycle exercise and (ii) the similarity between patients and Ctrls in these haemodynamic and metabolic variables will be even more clear during incremental KE exercise.
Materials and methods
Subjects
Twelve male patients with HFrEF and eight well-matched Ctrls volunteered and gave written consent to participate in the study, which had been approved by the University of California, San Diego, Human Subjects Protection Program. The study complies with the 1974 Declaration of Helsinki and with Title 45, US Code of Federal Regulations, Part 46, Protection of Human Subjects, Revised 23 June 2005, effective 23 June 2005.
Particular care was taken to match Ctrl participants based upon physical activity, assessed by both questionnaire [modified Minnesota Leisure Time Physical Activity Questionnaire (Taylor et al. 1978)] and personal interview, in addition to age, sex and quadriceps muscle mass. All patients with HFrEF were clinically stable and had symptoms compatible with NYHA functional classes II–III (n = 5 and 7, respectively), which equates to Weber VO2max Class C/B (Weber et al. 1982). Mean left ventricular ejection fraction in the patients with HFrEF was 25 ± 3%. Other than ß-blockers that were withheld for 48 h prior to the studies, patient medications were not altered.
Catheter placement and experimental protocol
Upon arrival at the laboratory, a radial arterial and common femoral venous line in addition to a thermo-couple in the same common femoral vein was placed, as previously described (Richardson et al. 1995). With catheters in place, both the patients with HFrEF and Ctrls underwent two exercise tests in a balanced design (cycle and KE), separated by at least one and half hours for recovery. In each trial, exercise intensity was incremented progressively every 3 min until exhaustion.
Exercise modalities
Cycle exercise was performed on an electromagnetically braked cycle ergometer (Lode Excalibur Sport; Quinton Instruments, Groningen, the Netherlands). KE exercise was performed with the subject seated on an adjustable chair with the ankle of one leg attached by a rigid bar to a cycle ergometer (Monark, mod. 839E, Vansbro, Sweden), as previously illustrated (Esposito et al. 2010b, Fig. 1).
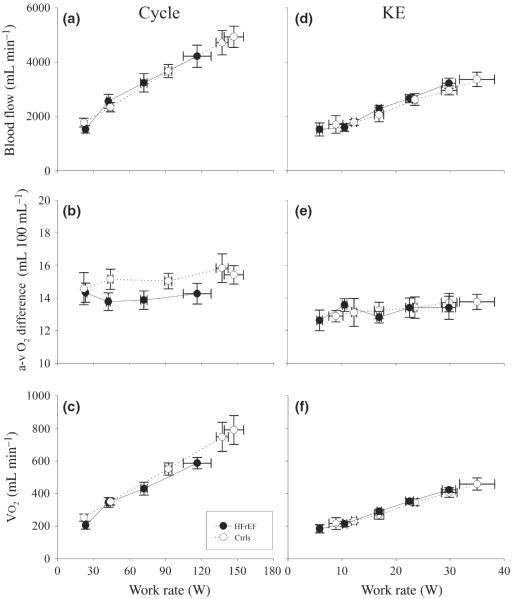
Figure 1.
Blood flow, a-vO2 difference and VO2 during incremental cycle and knee extensor (KE) exercise in patients with heart failure with reduced ejection fraction (HFrEF) and controls (Ctrls).
Measurements and calculations
Mixed expired O2 and CO2, expiratory air flow and ECG were continuously recorded and digitized (Parvo Medics, Salt Lake City, UT, USA). Simultaneous arterial and femoral venous blood samples were collected at rest and during the third minute of each incremental work rate. At each level of work, the following variables were measured: (i) PO2, PCO2, pH (IL model 1302, pH/blood gas analyser; Instrumentation Laboratories, Milan, Italy), oxyhaemoglobin saturation, haemoglobin concentration (Hb) (IL 482 co-oximeter) and lysed whole-blood lactate concentrations (La) (YSI 23L blood lactate analyser; Yellow Springs Instruments, Yellow Springs, OH, USA) from simultaneous arterial and common femoral venous blood samples (blood gas values were corrected to the temperature measured in the common femoral vein) and (ii) common femoral venous blood flow (thermodilution) and arterial and venous vascular pressures (Andersen et al. 1985). Technical aspects of these measurements and subsequent calculations have been previously provided in detail (Knight et al. 1993, Agusti et al. 1994).
Cardiac output
Cardiac output was measured in duplicate at rest and during exercise using an open-circuit acetylene uptake technique, as described previously (Barker et al. 1999).
Catecholamines
Plasma adrenaline and noradrenaline (Ne) were assayed in duplicate by the method of Kennedy and Zeigler (Kennedy & Zeigler 1990), and the rate of Ne spillover was determined, as described previously (Savard et al. 1989), using the following equation:
where Cv and Ca are plasma Ne concentrations in the common femoral vein and radial artery respectively. Ee is the fractional extraction of adrenaline, and PF is the plasma flow, determined from blood flow and haematocrit.
Muscle mass
With the use of thigh length, circumferences and skin-fold measurements, thigh volume was calculated to allow a valid estimate of quadriceps muscle mass, as utilized previously (Jones & Pearson 1969, Andersen et al. 1985, Esposito et al. 2010a). During cycle exercise, the amount of working muscle mass was estimated based on the reported ratio of quadriceps muscle mass to other leg muscles, in a similar fashion as we have previously described (Richardson et al. 1999a).
Muscle biopsy
On a different day, a percutaneous biopsy of vastus lateralis muscle was obtained (Bergstrom needle) from all subjects who wished to take part in this component of the research (nine of the 12 patients with HFrEF and six of the eight Ctrls), as previously described (Bergstrom 1975).
Histochemistry
Eight-millimetre-thick transverse sections of the muscle biopsy samples were cut at −24 °C on a cryostat (Jung-Reichert Cryocut 1800) and kept at −20 °C until histochemical processing, which was performed within a week of sectioning. After 5-min fixation in a Guth and Samaha fixative at room temperature, sections were incubated at 37 °C for 1 h in lead (Pb)–ATPase staining medium to simultaneously stain for skeletal muscle fibre types I and II and capillaries (Rosenblatt et al. 1987).
Tissue preparation for microscopy
The glutaraldehyde-fixed samples were completely cut into thin longitudinal strips and processed for electron microscopy as described previously (Mathieu-Costello 1987). Electron micrographs for morphometry were taken on 70-mm films with a Zeiss 10 electron microscope (Zeiss, Oberkochen, Germany).
Morphometry
The relative cross-sectional area and number of type I and type II fibres were estimated under a light microscope (250×) on histochemical sections by point-counting using an eyepiece square grid test A100 (Weibel 1979). Capillary density (i.e. capillary number per fibre cross-sectional area), capillary-to-fibre ratio (i.e. capillary number per fibre number), capillary number around a fibre and fibre cross-sectional area were measured by point-counting on 1-μm-thick sections examined at a magnification of 400× with a light microscope. The volume density of mitochondria per volume of muscle fibre was estimated by point-counting at a final magnification of 49 000× on ultrathin transverse sections.
Statistical analysis
Data were analysed using parametric statistics, following mathematical confirmation of normal distribution using Shapiro–Wilk tests. Between-group subject characteristics were assessed using independent-sample t-tests. Comparisons of patients with HFrEF and control data collected during both cycle and KE exercise were performed with a two-way [health status (two levels)] and exercise intensity (four and five levels, cycle and KE exercise, respectively) anova. Following a significant main effect and/or interaction, paired-sample t-tests were employed to make post hoc comparisons at each level of the within-subject factor. Statistical significance was set at α < 0.05. Data are expressed as mean ± standard error (SE).
Results
Assessment of normal distribution and statistical power
The Shapiro–Wilk tests revealed P-values of >0.05; thus, the null hypothesis that the data were normally distributed was not rejected, and parametric statistics were employed. Post hoc power analyses of the major statistical comparisons revealed a power of >0.8.
Participants characteristics and medications
There were no statistically significant differences in the level of physical activity or age, sex, body mass and quadriceps muscle mass between the patients with HFrEF and Ctrls (Table 1). During the incremental cycle exercise, the Ctrls achieved a greater maximal work rate and a greater VO2max, when expressed in absolute terms or relative to body mass (Table 1). Additionally, B-type natriuretic peptide (BPN), an indicator of increased ventricular wall stress related to reduced ejection fraction, was also greater in the patients with HFrEF compared with the Ctrls. The majority of the morphometric assessments from the muscle biopsies were not statistically different between groups. The exception to this similarity was mitochondrial density, which was significantly lower in the patients compared with Ctrls. The medication regimen of the patients with HFrEF was unaltered for the study except for ß-blockers that were withheld for 48 h prior to testing. None of the Ctrls were currently taking any medication (Table 2).
Table 1.
Characteristics of controls (Ctrls) and patients with HFrEF
| Ctrls | HFrEF | |
|---|---|---|
| NYHA class | – | II–III |
| HFrEF aetiology (ischaemic/non-ischaemic) | – | 11/1 |
| Sinus rhythm | 8/8 | 12/12 |
| Activity level (range 0–10) | 3 ± 1 | 2 ± 1 |
| Age (years) | 52 ± 2 | 53 ± 1 |
| Stature (cm) | 177 ± 2 | 179 ± 2 |
| Body mass (kg) | 88 ± 5 | 98 ± 6 |
| VO2max (mL kg−1 min−1) | 21.2 ± 1.7 | 15.2 ± 1.1* |
| BNP (pg mL−1) | 36 ± 5 | 1010 ± 126* |
| Quadriceps muscle-specific data | ||
| Quadriceps (one leg) muscle mass (kg) | 2.2 ± 0.1 | 2.2 ± 0.1 |
| KE exercise VO2max (mL min−1 100 g−1) | 21.1 ± 2.1 | 15.3 ± 1.3* |
| Fibre cross-sectional area (μm2) | 3853 ± 606 | 3092 ± 237 |
| % area of type I fibres | 41 ± 4 | 37 ± 4 |
| % area of type II fibres | 59 ± 4 | 63 ± 4 |
| Capillary density (capillaries mm−2) | 415 ± 46 | 442 ± 21 |
| Capillary-to-fibre ratio | 1.52 ± 0.10 | 1.36 ± 0.12 |
| Number of capillaries around a fibre | 3.8 ± 0.1 | 3.3 ± 0.3 |
| Mitochondrial volume density (%) | 4.4 ± 0.4 | 3.3 ± 0.3* |
| Lipid droplets volume density (%) | 0.30 ± 0.06 | 0.36 ± 0.07 |
NYHA, New York Heart Association; VO2max, maximal oxygen uptake during cycle exercise; BNP, brain-type natriuretic peptide; KE, knee extensor; HFrEF, heart failure with reduced ejection fraction Data are expressed as mean ± SE.
P < 0.05 (HFrEF vs Ctrls).
Table 2.
Medication use by controls (Ctrls) and patients with heart failure with reduced ejection fraction (HFrEF)
| Ctrls | HFrEF | |
|---|---|---|
| Digoxin | 0/8 | 12/12 |
| Diuretics | 0/8 | 12/12 |
| Long-acting nitrates | 0/8 | 7/12 |
| Statins | 0/8 | 6/12 |
| Aspirin | 0/8 | 7/12 |
| ß-Blockers | 0/8 | 10/12* |
| Warfarin | 0/8 | 4/12 |
| ACE inhibitors | 0/8 | 9/12 |
| Ca2+ channel blockers | 0/8 | 3/12 |
Data are expressed as mean ± SE.
Withheld for 48 h prior to the study.
Blood flow, a-vO2 difference and VO2 during cycle and KE exercise
During cycle exercise, blood flow increased in a similar linear fashion with increasing work rate in both patients with HFrEF and Ctrls. Ultimately, the Ctrls achieved a higher maximal work rate and a significantly greater cycle exercise blood flow than the patients (Fig. 1a). The a-vO2 difference during cycle exercise revealed very little increase, if any, across increasing work rates in patients with HFrEF and Ctrls and was similar between groups (Fig. 1b). VO2 during cycle exercise increased in a similar linear fashion with increasing work rate in both patients with HFrEF and Ctrls. With the attainment of a higher work rate, the Ctrls achieved a greater VO2max (Fig. 1c). In essence, the responses to KE exercise, in terms of blood flow (Fig. 1d), a-vO2 difference (Fig. 1e) and VO2 (Fig. 1f), were comparable to the cycle exercise with both groups responding in a very similar fashion across the scope of work. Again, ultimately, the Ctrls achieved a greater maximal work rate in this exercise modality than the patients with HFrEF and subsequently a greater blood flow and VO2max (Fig. 1d,f).
Mean arterial pressure, Ne spillover and vascular resistance during cycle and KE exercise
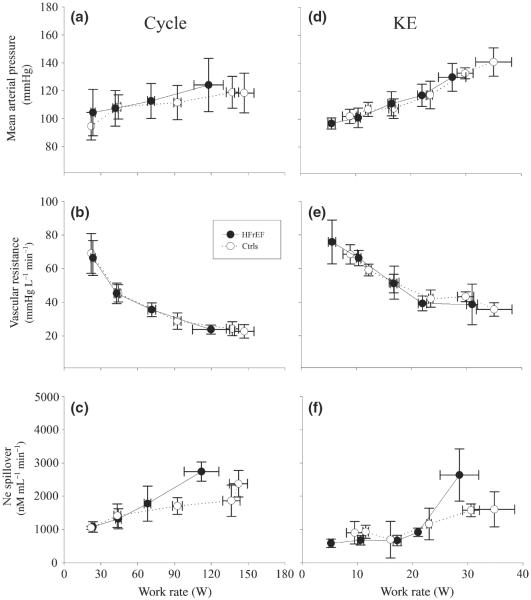
During cycle exercise, mean arterial pressure (MAP) increased with increasing work rate in both patients with HFrEF and Ctrls. Although the Ctrls achieved a greater maximal work rate, MAP was not higher than that of the patients with HFrEF due to the moderate slope of the work rate to MAP relationship (Fig. 2a). Despite steadily increasing Ne spillover that tended to be greater in the patients with HFrEF (Fig. 2c), leg vascular resistance fell with increasing work rate during cycle exercise in a very similar fashion in both the patients with HFrEF and the Ctrls (Fig. 2b). Due to the hyperbolic relationship between vascular resistance and work rate during cycle exercise, vascular resistance was not ultimately lower in the Ctrls, despite achieving a higher power output (Fig. 2b). In essence, the response to KE exercise in terms of MAP (Fig. 2d), Ne spillover (Fig. 2f) and vascular resistance (Fig. 2e) were comparable to cycle exercise, with both groups responding in a very similar fashion across all work rates. Again, although the Ctrls achieved a greater maximal work rate than the patients with HFrEF, there was not a statistically significant difference in end exercise MAP, Ne spillover or vascular resistance (Fig. 2d,f,e). Only Ne spillover tended to be greater at the higher work rate, but, due to large variability, this did not achieve statistical significance (Fig. 2f).
Figure 2.
Mean arterial pressure, vascular resistance and noradrenaline spillover during incremental cycle and knee extensor (KE) exercise in patients with HFrEF and controls (Ctrls).
Skeletal muscle lactate efflux during cycle and KE exercise
During both cycle and KE exercise, skeletal muscle lactate efflux increased with increasing work rate in a very similar fashion in both patients with HFrEF and Ctrls. Ultimately, the Ctrls achieved a higher maximal work rate in both exercise modalities and a significantly greater lactate efflux than the patients (Fig. 3a,b).
Figure 3.
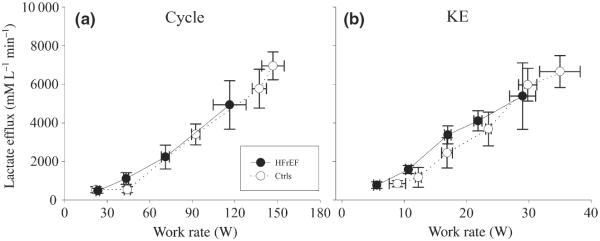
Lactate efflux during incremental cycle and knee extensor (KE) exercise in patients with HFrEF and controls (Ctrls).
Heart rate and cardiac output during cycle and KE exercise
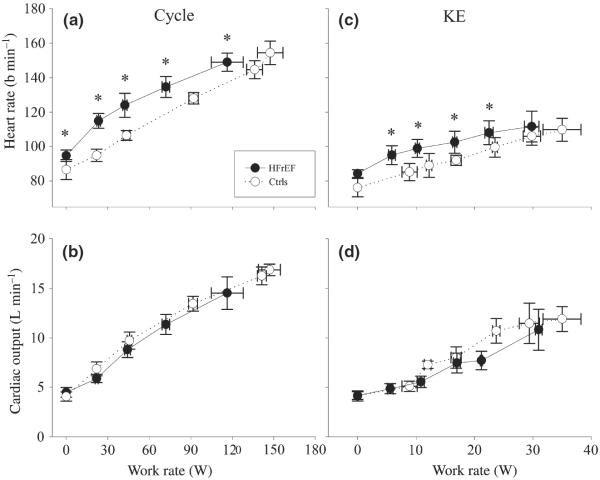
Heart rate (HR) increased with increasing work rate in both patients with HFrEF and Ctrls during cycle and KE exercise, but was significantly elevated in the patients compared with Ctrls throughout exercise in both modalities (Fig. 4a,c). Cardiac output increased essentially linearly, with no difference between patients and Ctrls during both cycle and KE exercise (Fig. 4b,d). It should be noted that as a consequence of withholding the patient's ß-blocker therapy, there was a clear increase in the HR achieved during the study-related cycle exercise testing compared with previous clinical exercise testing of these patients with HFrEF in the past (from approx. 130 b min−1 to 145 b min−1).
Figure 4.
Heart rate, stroke volume and cardiac output during incremental cycle and knee extensor (KE) exercise in patients with HFrEF and controls (Ctrls). *P < 0.05 HFrEF vs Ctrls.
Discussion
Heart failure with reduced ejection fraction is associated with not only compromised cardiac function, but also peripheral vascular and skeletal muscle dysfunction; however, whether one should generalize to all patients and all forms of exercise from these latter observations is still in doubt. Therefore, this study sought to examine the effect of HFrEF on the haemodynamic and metabolic responses to incremental exercise with both a large (cycle) and a small (KE) muscle mass and compare the findings with well-matched Ctrls. Despite having a significantly attenuated maximal work capacity, the major haemodynamic (cardiac output, blood flow and vascular resistance) and metabolic (VO2 and lactate efflux) variables assessed in patients with HFrEF were remarkably similar to the Ctrls throughout both incremental cycle and KE exercise. Although assessed in a relatively small cohort, these data reveal that, when compared with well-matched healthy Ctrls, alterations in peripheral haemodynamics and skeletal muscle metabolism during exercise may not be an obligatory accompaniment to HFrEF.
Peripheral haemodynamics in HFrEF
Although a cursory review of the literature can certainly lead to the conclusion that there are intrinsic abnormalities in skeletal muscle associated with HFrEF that lead to alterations in vascular resistance and subsequently skeletal muscle blood flow (Wilson et al. 1984a, Sullivan et al. 1989, Isnard et al. 1996, Magnusson et al. 1997), this is not supported by all investigations. Indeed, there are several studies that have reported a normal increase in blood flow to skeletal muscle, upon exertion, in patients with HFrEF (Wiener et al. 1986, Massie et al. 1988, Arnold et al. 1990, Wilson et al. 1993, Barlow et al. 1998, Shoemaker et al. 1999). This apparently normal response appears to be in the face of a variety of alterations specific to the vascular/skeletal muscle interface (greater sympathetic vasoconstrictor tone, decreased capillarity and smaller capillary diameter (LeJemtel et al. 1986, Sullivan et al. 1989, Duscha et al. 1999) which have all been associated with HFrEF. Therefore, currently, the contribution of these skeletal muscle changes to peripheral haemodynamics during exercise in HFrEF is not well understood.
It is likely that additional support in the literature for the concept of skeletal muscle-specific haemodynamic abnormalities in HFrEF may be a consequence of the regular use of whole body exercise, such as cycling, with the goal of evaluating muscle function (Maltais et al. 1996, 1998, Sala et al. 1999). Ideally, to study the muscle function itself in HFrEF, the amount of muscle recruited should be small enough that the patient can achieve maximal muscular work before the influence of central cardiac limitations. Indeed, the original impetus to perform the current multi-modality study was that a large muscle mass exercise paradigm in patients with HFrEF may shroud peripheral muscle limitations by the attainment of a patient's reduced cardiac ceiling, before truly taxing the locomotor muscles. The current comparison between patients with HFrEF and carefully selected activity and age-matched healthy Ctrls reveals remarkably similar blood flow and vascular resistance responses throughout incremental exercise during both cycle (Figs 1a and 2b) and KE exercise (Figs 1d and 2e). This is of significant importance because these two exercise paradigms have very different cardiac demands (Fig. 4), one which challenges maximum cardiac output in patients with HFrEF (cycle) and one that does not (KE).
The single-leg KE exercise model (Andersen et al. 1985) allows the measurement of O2 supply and utilization to a known mass of active muscle (Richardson et al. 1998a) under conditions of limited cardiac demand and thus is an ideal exercise paradigm with which to study the skeletal muscle of patients with HFrEF (Richardson et al. 1999b). Therefore, these findings, of similar haemodynamic responses in both patients with HFrEF and healthy Ctrls during both cycle and KE exercise, are of particular interest, as these paradigms, although having very different central haemodynamic demands, lead to essentially the same conclusion, normal peripheral haemodynamics in patients with NYHA classes II and III HFrEF. This conclusion is in agreement with the work of Magnusson et al. (1997) who studied habitually active patients with HFrEF and healthy individuals solely during one-leg KE and reported no difference in blood flow at submaximal workloads. However, in addition to only assessing KE and not cycle exercise in the same individuals, the NE spillover and lactate efflux data were minimally discussed by the authors. In fact, NE spillover was only presented in relation to absolute work rate and not relative intensity, as such an effort-dependent variable should be when two groups differ in terms of maximal exercise capacity, and thus, the mechanistic insight from that study was limited.
Lastly, it should be noted that previous studies have revealed that the peripheral consequences of HFrEF are related to the severity of the disease (Wilson et al. 1984b, Copp et al. 2010); thus, the current data may have been affected by a lack of NYHA class IV patients. However, in this modern era, a scarcity of NYHA class IV patients is not uncommon due to the practice of implanting left ventricular assist devices and successful heart transplantation in such severe patients.
Skeletal muscle metabolism in HFrEF
There are many reports of intrinsic abnormalities in skeletal muscle associated with HFrEF (Massie et al. 1987, Mancini et al. 1988). Indeed, a variety of alterations specific to skeletal muscle, such as muscle atrophy, fibre-type changes, reduced mitochondrial enzymes and decreased mitochondrial volume density (Drexler et al. 1992, Mancini et al. 1992, Massie et al. 1996, Harrington et al. 1997)(Table 1), have all been recognized in combination with the already described reduction in the vascular/skeletal muscle interface. In human-based studies of HFrEF, the most common responses to exercise attributed to metabolic dysfunction have been an attenuated VO2 for a given absolute work rate and a concomitant increase in lactate efflux from the muscle bed (Wilson et al. 1984a, Sullivan et al. 1989). It is noteworthy that although the former could be interpreted as an increase in economy, others have warned that the lower VO2 and elevated lactate production are more likely evidence of an accumulating and accelerated O2 deficit leading to premature fatigue (Poole et al. 2011).
Much as with the haemodynamic assessments, the current comparisons between patients with HFrEF and carefully selected activity and age-matched healthy Ctrls reveal a remarkably similar VO2 and skeletal muscle lactate efflux throughout incremental exercise and this was the case during both cycle (Figs 1c and 3a) and KE exercise (Figs 1f and 2c). Although not typical of the literature, these observations are internally consistent with previous interpretations of the finding that, in HFrEF, if VO2 is reduced, then skeletal muscle efflux is reciprocally increased; in our study, there was no difference in either VO2 or lactate efflux between the current patients with HFrEF and Ctrls.
An explanation for the discrepancies between the current data and prior work (Wilson et al. 1984a, Sullivan et al. 1989) hinges on two important issues. The first is that the current study paired patients with HFrEF with equally inactive Ctrls, resulting in more similar exercise capacities in both groups (Table 1). Intimately related to the first issue is the second; specifically, previous investigations have failed to adequately recognize that it is not appropriate to compare skeletal muscle lactate production in two groups with vastly different exercise capacities at the same absolute work rates. Specifically, any submaximal effort will always be a far greater relative effort for the group with the lower maximum work rate and this will stimulate a greater catecholamine release which will promote glycolysis (predominantly adrenaline via cAMP) and the subsequent production of lactate [lactate production is not solely reflective of oxygen availability with increasing work rate (Richardson et al. 1998b, 2001)]. Indeed, there is a strong positive correlation between adrenaline concentration (and blood lactate concentration) and exercise intensity (Hughson et al. 1995, Richardson et al. 1998b). Both altitude exposure and following extended bed rest, where maximum work rate is diminished, are examples of scenarios that lead to elevated adrenaline levels and subsequently higher lactate concentrations at any given submaximal absolute work rate (Saltin et al. 1968, Brooks et al. 1991). However, this, as in HFrEF, should not be considered as evidence of skeletal muscle dysfunction or an intrinsic abnormality. Rather, what happens in each of these scenarios is that the relative intensity of exercise is increased and this leads to a catecholamine-mediated increase in lactate concentration for any given submaximal absolute work rate. In the current study, likely due to the comparison with the well-matched Ctrls with not too dissimilar aerobic capacities [Table 1, with this relatively small disparity probably a consequence of the diminished maximal convective and diffusive components of O2 transport associated with HFrEF (Esposito et al. 2010b)], there was no apparent difference in skeletal muscle lactate efflux, despite being compared at the same absolute work rate (Fig. 3a,b). Hence, as assessed in this study, there was no evidence of altered peripheral metabolic dysfunction in these patients with other significant symptoms of HFrEF.
Skeletal muscle dysfunction vs disuse
Although there is considerable evidence of altered skeletal muscle structure and often function in HFrEF, an issue that has, somewhat unavoidably, clouded conclusions regarding such skeletal muscle research is the difference between skeletal muscle dysfunction (abnormal or impaired function) and disuse. Certainly, patients with HFrEF experience locomotor muscle disuse as a consequence of their condition, but, especially in the case of ischaemic cardiomyopathy, inactivity is one likely cause of the pathology itself. In the realm of human-based research, where, commonly, activity levels of the most accessible healthy middle-aged to older volunteers are far from typical of patients with HFrEF, there is a tendency for studies to magnify the differences between patients with HFrEF by comparing younger or relatively more physically active age-matched Ctrls (Sullivan et al. 1989, Isnard et al. 1996), although it must be acknowledged that this was not always the case (Mettauer et al. 2001, Duscha et al. 2002, Rehn et al. 2012). Thus, the selection of appropriately inactive Ctrls becomes an essential component of the experimental design of research focused on the assessment of skeletal muscle function and HFrEF. These findings are in agreement with other reports in the literature that have examined role of deconditioning in HFrEF-related myopathy (REFs Mattauer et al. and Rehn et al.) and concluded that inactivity likely plays a major role in the peripheral changes observed with this pathology.
In the current study, great lengths were taken to exclude any healthy volunteers who, based upon interview and physical activity questionnaire results, were deemed to be more physically active than members of HFrEF patient cohort. It should be noted that it was far more difficult to find Ctrls that fit this criteria and were willing to participate in this complex study than it was to find patients with HFrEF. The attainment of this goal is supported by the similar quantitative assessment of physical activity in both patients and Ctrls and the relatively minimal difference in maximal aerobic capacity (Table 1). This, although certainly not insignificant, when compared with other work that documented at least 100% difference or greater in aerobic capacity between patients and Ctrls (Sullivan et al. 1989, Isnard et al. 1996), should be considered relatively minimal. Despite exhaustive efforts to well match the patients and Ctrls, it should be noted that the morphometric analyses of muscle structure still revealed tendencies (capillarity) and significant evidence (mitochondrial volume density) in the patients with HFrEF of exaggerated characteristics associated with greater inactivity compared with controls (Table 1). However, we still contend that this approach, of activity matching the patients and Ctrls, contributed significantly to the current finding of extremely similar peripheral haemodynamic and metabolic responses in the patients with HFrEF and the well-matched Ctrls.
Experimental considerations
It must be acknowledged that, in terms of this study's failure to observe any evidence of altered vascular and metabolic responses to submaximal exercise in patients with HFrEF, this work refutes a relatively large body of prior research. As this study was limited to the analysis of only 12 male patients with HFrEF and eight well-matched Ctrls, it is recommended that further studies, preferably, with greater numbers of subjects, be carried out to confirm or deny these findings. Additionally, the performance of this type of invasive study, in a sick cohort, with strict exclusion and subject matching criteria, and the requirement that medications (ß-blockers) to be withheld in the eligible patients, was not without difficulty. As a consequence, this study occurred over a relatively long period of time, spanning the years 2002–2009. Recognizing the ever-changing standard of care for patients with HFrEF (e.g. medications), this could also be considered a limitation of the current investigation.
Conclusions
Despite having a significantly attenuated maximal work capacity, the major haemodynamic (cardiac output, blood flow and vascular resistance) and metabolic (VO2 and lactate efflux) variables assessed in this study were remarkably similar in both patients with HFrEF and Ctrls throughout incremental exercise requiring the use of either a large (cycle) or a small muscle mass (KE). Although assessed in a relatively small cohort, these data reveal that, when compared with well-matched healthy Ctrls, alterations in peripheral haemodynamics and skeletal metabolism during exercise may not be an obligatory accompaniment to HFrEF and question the importance of the proposed peripheral dysfunction in this population.
Acknowledgments
The authors wish to thank all the subjects who took part in this study, for their committed participation and sacrifice to be a part of this involved research project. This work was supported by grants from National Heart, Lung, and Blood Institute (HL 091830); and the Veterans Administration (Merit Grant E6910R).
Footnotes
Author contributions F. Esposito takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. P. D. Wagner takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. R. S. Richardson takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Conflict of interest No conflict of interests to declare.
References
- Agusti AGN, Roca J, Barbera JA, Casademont J, Rodriguezroisin R, Wagner PD. Effect of sampling site on femoral venous blood gas values. J Appl Physiol. 1994;77:2018–2022. doi: 10.1152/jappl.1994.77.4.2018. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjøgaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–1653. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Ribeiro JP, Colucci WS. Muscle blood flow during forearm exercise in patients with severe heart failure. Circulation. 1990;82:465–472. doi: 10.1161/01.cir.82.2.465. [DOI] [PubMed] [Google Scholar]
- Barker RC, Hopkins SR, Kellogg N, Olfert IM, Brutsaert TD, Gavin TP, Entin PL, Rice AJ, Wagner PD. Measurement of cardiac output during exercise by open-circuit acetylene uptake. J Appl Physiol. 1999;87:1506–1512. doi: 10.1152/jappl.1999.87.4.1506. [DOI] [PubMed] [Google Scholar]
- Barlow CW, Davey PP, Qayyum MS, Conway J, Paterson DJ, Robbins PA. Leg blood flow and increased potassium release during exercise in chronic heart failure: effect of physical training. J Card Fail. 1998;4:105–114. doi: 10.1016/s1071-9164(98)90250-0. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- Brooks GA, Butterfield GE, Wolfe RR, Groves BM, Mazzeo RS, Sutton JR, Wolfel EE, Reeves JT. Decreased reliance on lactate during exercise after acclimatization to 4,300 m. J Appl Physiol. 1991;71:333–341. doi: 10.1152/jappl.1991.71.1.333. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Ferreira LF, Poole DC, Musch TI. Progressive chronic heart failure slows the recovery of microvascular O2 pressures after contractions in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 2010;299:H1755–H1761. doi: 10.1152/ajpheart.00590.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler H, Riede U, Munzel T, Konig H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33:1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Entin PL, Wagner PD, Richardson RS. The skeletal muscle VEGF mRNA response to acute exercise in patients with chronic heart failure. Growth Factors. 2010a;28:139–147. doi: 10.3109/08977190903512602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010b;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD, Richardson RS. Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol. 2011;58:1353–1362. doi: 10.1016/j.jacc.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry MG. Abnormalities of the exercise pressor reflex in heart failure. Exerc Sport Sci Rev. 2011;39:167–176. doi: 10.1097/JES.0b013e31822a5621. [DOI] [PubMed] [Google Scholar]
- Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, Coats AJ. Skeletal muscle function and its relation to exercise tolerance in chronic heart failure. J Am Coll Cardiol. 1997;30:1758–1764. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Green HJ, Sharratt MT. Gas exchange, blood lactate, and plasma catecholamines during incremental exercise in hypoxia and normoxia. J Appl Physiol. 1995;79:1134–1141. doi: 10.1152/jappl.1995.79.4.1134. [DOI] [PubMed] [Google Scholar]
- Isnard R, Lechat P, Kalotka H, Chikr H, Fitoussi S, Salloum J, Golmard JL, Thomas D, Komajda M. Muscular blood flow response to submaximal leg exercise in normal subjects and in patients with heart failure. J Appl Physiol. 1996;81:2571–2579. doi: 10.1152/jappl.1996.81.6.2571. [DOI] [PubMed] [Google Scholar]
- Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone volumes in young male and female adults. J Physiol. 1969;204:63P–66P. [PubMed] [Google Scholar]
- Kennedy B, Zeigler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2154. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Hyperoxia increases leg maximal oxygen uptake. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- LeJemtel TH, Maskin CS, Lucido D, Chadwick BJ. Failure to augment maximal limb blood flow in response to one-leg versus two-leg exercise in patients with severe heart failure. Circulation. 1986;74:245–251. doi: 10.1161/01.cir.74.2.245. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylvéen C, Karlberg KE, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovasc Res. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Maltais F, Simmard A, Simard C, Jobin J, Desagnes P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288–293. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- Maltais F, Jobin J, Sullivan MJ, Bernard S, Whittom F, Killian KJ, Desmeules M, Belanger M, LeBlanc P. Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol. 1998;84:1573–1580. doi: 10.1152/jappl.1998.84.5.1573. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Ferraro N, Tuchler M, Chance B, Wilson JR. Detection of abnormal calf muscle metabolism in patients with heart failure using phosphorus-31 nuclear magnetic resonance. Am J Cardiol. 1988;62:1234–1240. doi: 10.1016/0002-9149(88)90266-4. [DOI] [PubMed] [Google Scholar]
- Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- Massie BM, Conway M, Yonge R, Frostick S, Sleight P, Ledingham J, Radda G, Rajagopalan B. 31P nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol. 1987;60:309–315. doi: 10.1016/0002-9149(87)90233-5. [DOI] [PubMed] [Google Scholar]
- Massie BM, Conway M, Rajagopalan B, Yonge R, Frostick S, Ledingham J, Sleight P, Radda G. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure. Evidence for abnormalities unrelated to blood flow. Circulation. 1988;78:320–326. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- Massie BM, Simonini A, Sahgal P, Wells L, Dudley GA. Relation of systemic and local muscle exercise capacity to skeletal muscle characteristics in men with congestive heart failure. J Am Coll Cardiol. 1996;27:140–145. doi: 10.1016/0735-1097(95)00416-5. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O. Capillary tortuosity and degree of contraction or extension of skeletal muscles. Microvasc Res. 1987;33:98–117. doi: 10.1016/0026-2862(87)90010-0. [DOI] [PubMed] [Google Scholar]
- Mettauer B, Zoll J, Sanchez H, Lampert E, Ribera F, Veksler V, Bigard X, Mateo P, Epailly E, Lonsdorfer J, Ventura-Clapier R. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–954. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- Poole DC, Hirai DM, Copp SW, Musch TI. Muscle oxygen transport and utilization in heart failure: implications for exercise (in)tolerance. Am J Physiol Heart Circ Physiol. 2011;302:H1050–H1063. doi: 10.1152/ajpheart.00943.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehn TA, Munkvik M, Lunde PK, Sjaastad I, Sejersted OM. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease-specific myopathy or a result of deconditioning? Heart Fail Rev. 2012;17:421–436. doi: 10.1007/s10741-011-9289-4. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Knight DR, Poole DC, Kurdak SS, Hogan MC, Grassi B, Wagner PD. Determinants of maximal exercise VO2 during single leg knee-extensor exercise in humans. Am J Physiol. 1995;268:H1453–H1461. doi: 10.1152/ajpheart.1995.268.4.H1453. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Frank RL, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med. 1998a;19:182–187. doi: 10.1055/s-2007-971901. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Leigh JS, Wagner PD. Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol. 1998b;85:627–634. doi: 10.1152/jappl.1998.85.2.627. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999a;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL, Wagner PD. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999b;159:881–885. doi: 10.1164/ajrccm.159.3.9803049. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Newcomer SC, Noyszewski EA. Skeletal muscle intracellular PO(2) assessed by myoglobin desaturation: response to graded exercise. J Appl Physiol. 2001;91:2679–2685. doi: 10.1152/jappl.2001.91.6.2679. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JD, Kuzon WM, Jr, Plyley MJ, Pynn BR, McKee NH. A histochemical method for the simultaneous demonstration of capillaries and fiber type in skeletal muscle. Stain Technol. 1987;62:85–92. doi: 10.3109/10520298709107973. [DOI] [PubMed] [Google Scholar]
- Rubin SA, Chatterjee K, Parmley WW. Metabolic assessment of exercise in chronic heart failure patients treated with short-term vasodilators. Circulation. 1980;61:543–548. doi: 10.1161/01.cir.61.3.543. [DOI] [PubMed] [Google Scholar]
- Sala E, Roca J, Marrades R, Alonso J, Gonzalez de Suso J, Moreno A, Barbera J, Nadal J, de Jover L, Rodriguez-Roisin R, Wagner P. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1726–1734. doi: 10.1164/ajrccm.159.6.9804136. [DOI] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:VII1–VII78. [PubMed] [Google Scholar]
- Savard GK, Richter EA, Strange S, Kiens B, Christensen NJ, Saltin B. Norepinephrine spillover from skeletal muscle during exercise: role of muscle mass. Am J Physiol. 1989;257:H1812–H1818. doi: 10.1152/ajpheart.1989.257.6.H1812. [DOI] [PubMed] [Google Scholar]
- Shoemaker JK, Naylor HL, Hogeman CS, Sinoway LI. Blood flow dynamics in heart failure. Circulation. 1999;99:3002–3008. doi: 10.1161/01.cir.99.23.3002. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- Weber KT, Kinasewitz GT, Janicki JS, Fishman AP. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation. 1982;65:1213–1223. doi: 10.1161/01.cir.65.6.1213. [DOI] [PubMed] [Google Scholar]
- Weibel E. Practical Methods for Biological Morphometry. Academic Press; London, New York, NY, Toronto, ON: 1979. [Google Scholar]
- Wiener DH, Fink LI, Maris J, Jones RA, Chance B, Wilson JR. Abnormal skeletal muscle bioenergetics during exercise in patients with heart failure: role of reduced muscle blood flow. Circulation. 1986;73:1127–1136. doi: 10.1161/01.cir.73.6.1127. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Ferraro N. Exercise intolerance in patients with chronic left heart failure: relation to oxygen transport and ventilatory abnormalities. Am J Cardiol. 1983;51:1358–1363. doi: 10.1016/0002-9149(83)90312-0. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Martin JL, Ferraro N. Impaired skeletal muscle nutritive flow during exercise in patients with congestive heart failure: role of cardiac pump dysfunction as determined by the effect of dobutamine. Am J Cardiol. 1984a;53:1308–1315. doi: 10.1016/0002-9149(84)90085-7. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Martin JL, Schwartz D, Ferraro N. Exercise intolerance in patients with chronic heart failure: role of impaired nutritive flow to skeletal muscle. Circulation. 1984b;69:1079–1087. doi: 10.1161/01.cir.69.6.1079. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]