Abstract
Evidence is emerging of the role of membrane progestin receptors (referred to as mPRs herein: members of Progestin and AdipoQ Receptor (Paqr) family) as a novel brain target in mammals, such as rats. In the present study, the role of mPRs in mice was assessed to further elucidate the conservation of this mechanism across species. The brain target investigated was the midbrain ventral tegmental area (VTA) given its described role for rapid actions of progestins for reproduction. Studies tested the hypothesis that if mPRs are required for progestin-facilitated lordosis through actions in the VTA, then knockdown of mPRs in the VTA will attenuate lordosis. Ovariectomized (OVX) mice were subcutaneously injected with estradiol (E2) and progesterone (P4), and infused with antisense oligodeoxynucleotides (AS-ODNs) to mPRαs (Paqr7) and/or mPRβ (Paqr8) or vehicle to the lateral ventricle or VTA. Mice were assessed for reproductive behavior (lordosis and aggression/rejection quotients) in a standard mating task. Results supported our hypothesis. E2 + P4-facilitated lordosis was significantly reduced, and aggression/rejection increased, with infusions of mPRα, mPRβ, or mPRαβ AS-ODNs to the lateral ventricle, compared to vehicle. E2 + P4-facilitated lordosis was significantly decreased, and aggression/rejection increased, with mPRβ or mPRαβ AS-ODNs to the VTA of C57/BL6 mice. Both mPRα and mPRβ AS-ODNs reduced lordosis, and increased aggression/rejection, of wildtype (C57/BL6x129) mice, but not nuclear PR knockout mice. Thus, mPRs may be a novel target of progestins for reproductive behavior of mice.
Keywords: Nongenomic, Progestin, Neurosteroids, Reproduction
1. Introduction
One approach to further understand the mechanisms and brain targets of ovarian hormones for their functional effects is to use a model that includes a behavioral output that is dependent upon these hormones, and manipulate actions of hormones in brain regions of interest. One such well-characterized model utilized to investigate these questions for ovarian steroids is reproductive behavior of female mice. Reproductive behaviors of female rodents depend on ovarian hormones, such as 17β-estradiol (E2) and progesterone (P4) and environmental stimuli. Cyclic increases in E2, followed by P4, are associated with sexual receptivity of mice. Ovariectomy attenuates cyclic increases in these hormones and sexual receptivity of mice [1]. Administration of hormone regimen that produce circulating concentrations, akin to that observed during behavioral estrus, reliably reinstate sexual receptivity commensurate to that which can be observed over the estrous cycle [2–5]. Thus, this model of reproductive behavior in mice is utilized to address questions about the mechanisms and brain targets of ovarian hormones; a focus, in the present study, is the actions and brain targets of progestins, such as P4.
One mechanism of progestins to consider “genomic” signaling involving classical progestin receptors (PRs), which were traditionally considered to be located in the nucleus (and will thus be referred to as nPRs herein), and have actions as transcriptional factors, regulating gene transcription and translation. There is also “non-genomic” signaling of progestins, which can include classical, or nPRs, that are tethered to the membrane and have actions in this location of the cell, and other transmembrane steroid and neurotransmitter targets; reproductive behavior has been one model utilized to understand these different, and potentially complementary, mechanisms of progestins. Among E2-primed rodents, P4 has both genomic and non-genomic effects in the ventral medial hypothalamus (VMH) and midbrain ventral tegmental area (VTA) to mediate mating. As an example of the genomic actions of progestins, in the VMH, P4’s classical actions involving nPRs and induction of gene transcription are important for modulation of reproductive responses [6]. P4’s actions in the VTA, an area of the brain with few non-E2 induced nPRs, influences the intensity and duration of sexual receptivity of rodents exclusively through non-genomic, rapid actions at neuronal membranes, such as via neurotransmitter targets, including GABA and dopamine [7–9]. Another potential target of interest is the membrane progestin receptor, which is a member of the Progestin and AdipoQ Receptor (Paqr) family, identified by Zhu and colleagues [10,11]. These receptors (referred to as mPRs herein) alter progestin binding and rapid non-genomic signaling in various in vitro expression systems, such as Escherichia coli, yeast, and mammalian cell lines. Actions involving mPRs for functional effects, such as those effects for reproduction, have received much less attention to date compared to these in vitro models.
The hypothesis tested in the present series of experiments was that mPRα (Paqr7) and mPRβ (Paqr8), two of the most common variants of mPRs, are targets of progestins for reproductive behavior of mice. A preliminary probe assessed expression of mPRα and mPRβ in peripheral tissues (spleen, heart, lungs, kidney, liver, intestines) and different brain regions (prefrontal cortex, hippocampus, amygdala, hypothalamus, and midbrain) of naturally sexually-receptive mice. Experiments were conducted to assess reproductive responses of OVX, hormone-primed mice following manipulations of mPRα and mPRβ with infusions of AS-ODNs to the lateral ventricle or to the VTA. These comparisons were done to begin to address site specificity of these effects. Moreover, if the AS-ODN treatment was producing other side effects, the notion was that these would be particularly apparent with lateral ventricle infusions. We also examined these effects across different strains of mice. Mice were replete in nPRs (C57/BL6 in Experiments 1 and 2, or PRKO wildtypes on a C57/BL6x129/SvEv background in Experiment 3) or lacking functional nPRs (PRKO mice in Experiment 4) to begin to ascertain if there may be interactions with classical nuclear PRs. We predicted that if mPRs are involved in P4’s non-genomic actions in the VTA for reproduction, knocking down mPRs in the midbrain VTA will selectively reduce lordosis responses of OVX, E2- and P4-primed mice.
2. Experimental
2.1. Experimental overview
A pilot experiment assessed mPR expression in peripheral and central tissues of proestrous C57/BL6 mice (n = 2). For Experiment 1, OVX C57/BL6 mice were administered E2 and P4 and infused with control (n = 15), mPRα (n = 13), mPRβ (n = 13) or mPRαβ (n = 15) AS-ODNs to the lateral ventricle. For Experiment 2, OVX C57/BL6 mice were administered E2 and P4 and infused with control (n = 9), mPRα (n = 9), mPRβ (n = 10) or mPRαβ (n = 14) AS-ODNs to the VTA. For Experiment 3, OVX PRKO wildtypes on a C57/BL6x129 background were administered E2 and P4 and infused with control (n = 11), mPRα (n = 15), mPRβ (n = 13) or mPRαβ (n = 16) AS-ODNs to the VTA. For experiment 4, PRKO mice were administered E2 and P4 and infused with control (n = 13), mPRα (n = 12), mPRβ (n = 11) or mPRαβ (n = 13) AS-ODNs to the VTA. For Experiments 1–4, mice were behaviorally tested and tissues were collected from a subset of animals to verify effects of AS-ODN infusions. A control experiment assessed specificity of effects by determining extent of behavioral responses following mPR manipulations in OVX, C57/BL6 mice administered E2 only and infused with control (n = 12), mPRα anti-sense deoxynucleotides (AS-ODN; n = 4), mPRβ AS-ODN (n = 5) or mPRαβ AS-ODN (n = 5) to the lateral ventricle. These methods utilizing live animals (surgery, drug manipulations, behavioral testing, euthanasia) were approved by the Institutional Animal Care and Use Committee at The University at Albany-SUNY and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85–23).
2.2. Housing of animal subjects
Subjects were 8–10 week old, female mice that were C57/BL6, PRKO, or their wildtype counterparts on a C57/BL6x129 background (n = 272). Mice were group-housed (4/5 per cage) in polycarbonate cages (26 × 16 × 12 cm) in a temperature-controlled room (21 ± 1 °C) in the Laboratory Animal Care Facility. Mice were maintained on a 12/12-h reversed light cycle (lights off at 8:00 am) with continuous access to Purina Mice Chow and tap water in their home cages.
2.3. Mouse strain and genotyping
C57/BL6 mice, bred in our colony, were used for Experiments 1 and 2. For Experiments 3 and 4, wildtype (+/+) or homozygous (−/−) PRKO knockout mice were derived from heterozygous (+/−) breeder pairs from a colony that maintained our animal facility. Genotyping was determined by genomic DNA isolated from tails and analyzed by polymerase chain reaction (PCR) modified from Jackson Laboratory protocol and per previous methods to determine the genotype of mice [4,12–15]. PCR was performed by denaturing the DNA at 95 °C for 5 min, followed by 30 cycles of amplification: 94 °C for 1 min, 60 °C for 1 min, 72 °C for 1 min and a final primer extension step at 72 °C for 10 min. The following PR specific primers were used: P1 (5′-TAGACAGTGTCTTAGA CTCGTTGTTG-3′), P2 (5′-GATGGGCACATGGATGAAATC-3′), and a neo gene- specific primer, N2 (5′-GCATGCTCCAGACTGCCTTGG GAAA-3′). Bands of approximately 565 and 500 base pairs were amplified for wild-type and PRKO, respectively.
2.4. Estrous cycle determination
For the expression study, mice were cycled daily. To determine what stage of the estrous cycle each mouse was in, vaginal epithelium of experimental mice was obtained by lavage and examined under a light microscope daily between 0700 and 0900. After two weeks of regular, 4–5 day cycles, tissues were collected of mice when in proestrus or behavioral estrus. Mice were considered in proestrus when their vaginal epithelium had characteristic nucleated cells, 4–5 days following the previous lavage of this type.
2.5. mPR expression
Mice were left intact and cycled and had tissues collected when they were sexually-receptive (in proestrus), associated with high E2 and P4 levels. Whole brains and peripheral tissues were collected at University of Albany. Expression of mPRα and mPRβ were determined at East Carolina State University with reverse transcriptase PCR (RT-PCR) (from tissues from University of Albany) which were frozen immediately following collection and dissection, and shipped on dry ice overnight. Expression of mPRα and mPRβ was determined by RT-PCR for brain, spleen, heart, lungs, kidney, liver, and intestines. In brain, expression was examined in prefrontal cortex, hippocampus, amygdala, hypothalamus, and midbrain. Total RNA was extracted from snap-frozen tissue samples with TRIzol reagent (Invitrogen), homogenized using a sonicator (Sonic Dismembrator Model 100; Fisher Scientific), and purified following the manufacturer’s instructions. Total RNA (1 µg) of each sample was reverse transcribed into cDNA in a 10-µl reaction using Superscript III (Invitrogen). As a negative control to confirm that extracted RNA is free of genomic DNA, samples were also prepared for all tissues using same procedure except without Superscript III (RT minus). The PCR was conducted on the cDNA template for 25 or 30 cycles, respectively, with an annealing temperature of 55 °C, using mPRα- or mPRβ-specific primer pairs (mPRα forward: 5′-ACGCAGCAGACAGCTCCTA-3′ located in exon 1; mPRα reverse: 5′-CACTGCCAAACTGGTACACG-3′ located in exon 2; mPRβ forward: 5′-CTACCTCCCTGCTTGTTTGC-3′, located in exon 2 mPRβ reverse: 5′-GTGGATGTACGGCTCCCTAA-3′, located in exon 3). These mPR PCR primers were specifically designed across intron in two different exons to discriminate any possible amplification of genomic DNA, which would produce large PCR products. The PCR products were run on a 2% agarose gel and imaged using a Fluor Chem 8900 imaging station (Alpha Innotech, Santa Clara, CA).
2.6. Surgical protocol
Adult mice were administered sodium pentobarbital anesthesia (80 mg/kg, IP or to effect) for stereotaxic surgery and ovariectomy. For Experiment 1, and the control experiment to assess effects of priming with E2 only, mice had placement of bilateral guide cannulae aimed at the lateral ventricle (from bregma: AP −0.5, DV −0.5, ML ± 0.5). For experiments 2–4, mice had guide cannulae aimed at the VTA (from bregma: AP = −3.5, DV = −4.5, ML = 0.8), per [16]. Cannulae consisted of 26-gauge stainless steel hypodermic tubing, cut to 4.5 mm, and fitted with 5 mm 33 gauge removable inserts. Cannulae were secured to the skull with dental cement and the surrounding skin was closed with sutures and/or adhesive. Immediately after stereotaxic surgery mice were ovariectomized. Following surgery, mice were neurologically evaluated daily for their ability to right themselves, cage-climb, have proper muscle tone and reflexive responses to their hind limbs being gently extended by an experimenter. Mice were also evaluated for weight gain after surgery. Only mice that passed neurological evaluations and gained weight following surgery were continued in the experiment. Sixteen mice were discontinued in the study because of not passing these post-surgical assessments. Mice were administered post-operative analgesic (liquid ibuprofen dissolved in drinking water to a concentration of 2 mg/ml) in drinking water for 5 days following surgery.
2.7. Hormone and infusion condition
OVX mice were administered E2 (10 µg, 44–48 h before testing) and P4 (4 mg/kg SC, Steraloids, Newport, RI, 6 h before testing, Experiments 1–4) and infused with control, mPRα AS-ODN, mPRβ AS-ODN, or mPRαβ AS-ODN to the lateral ventricle and/or the VTA 0 (immediately), 24, and 44 h before behavioral testing (mPRα AS-ODN: 5′-CGCTCTTCTGGAAGCCGTACA TCTATG-3′; mPRβ AS-ODN: 5-GACTGGAAAG TAAGTAGGTGGCTGGCTGGTCCTC-3′). Infusion volumes were the same for each condition (1 µl in each cannula). The timing of these infusions was done to correspond to hormone-priming of mice and to ensure constant knockdown of mPRs during this time and behavioral testing, given instability of AS-ODNs. Full phosphorothioate HPLC-purified AS-ODNs were synthesized, such that S-oligonucleotides were capped and remaining links were unmodified, and desalted by Invitrogen Life Technologies (Carlsbad, CA). A control experiment assessed the responses to these mPR manipulations ICV in mice that were E2-primed only (10 µg, 44–48 h before testing).
2.8. Behavioral tasks
In order to ascertain specific changes in behaviors associated with differences in mating, rather than exploration, anxiety, and social behavior [17] mice were tested sequentially in the following tasks. For behavioral testing, assessments were made by experienced experimenters and the Any-Maze video-tracking system (Stoelting, Wood Dale, IL). Mice were first assessed in control tasks (open field, elevated plus maze, social interaction) and then in a standard mating task.
2.9. Reproductive behaviors
Behavioral testing was conducted per previously described methods [4] in a round glass container that is 20.5 cm in diameter and 21.5 in depth. Sexual behavior was assessed by pairing experimental, hormone-primed, female mice with proven stimulus male mice for 10 min or 10 mounts. The percentage of mounts that elicited a lordosis response by a female (lordosis quotient) was scored. Sexual contacts that elicited aggression/rejection of male mounting (kicking, boxing, vocalizing, tail rattling) was calculated, and reported as an aggression quotient.
2.10. Open field
Mice were placed in an open field with an exterior activity monitor (Digiscan Optical Animal Activity Monitor; 39 × 39 × 30 cm; Accuscan Instruments, Columbus, OH, USA). The number of interruptions in the horizontal beams, a measure of spontaneous activity, was mechanically recorded for 5 min [12,18]. The activity monitor has a grid on the floor with 16 total squares of which 12 were considered peripheral. During the 5 min test, the number of entries to peripheral and central squares was observed. The total number of squares entered is another index of spontaneous activity [12,18]. There were no differences in these measures in groups assessed.
2.11. Elevated plus maze
Mice were placed at the juncture of the two open (5 × 40 cm) and two closed arms (5 × 40 × 20 cm) of the elevated plus maze (Columbus Instruments). The number of open arm entries and time spent in the open or closed arms was recorded by an observer for 5 min. The total time spent on the open arms is a measure of antianxiety behavior [12,18–20]. There were no differences in the time spent on the open arms of mice.
2.12. Social interaction
Experimental and conspecific mice are placed in opposite corners of the open field. Time spent by the experimental mouse engaging in social interaction (crawling over and under partner, sniffing of partner, following with contact, anogenital investigation, tumbling, boxing and grooming) with the conspecific is recorded for 5 min [21]. There were no differences in time spent interacting with a conspecific.
2.13. Tissue collection and validation
Immediately after testing, mice were rapidly decapitated. Trunk blood and whole brain were collected to measure hormone levels and examine the expression of mPRα and mPRβ, respectively. Plasma levels of E2 and P4 were determined with radioimmunoassay using previously described methods [22] to validate that physiological levels were achieved with the systemic dosing of E2 and P4 that were utilized. We found that there were proestrous-like levels of E2 in plasma of OVX mice in the experiment, which were all administered E2 (13.6 ± 2.1 pg/ml). As expected, levels of P4 were higher in plasma of mice administered systemic P4 (34.3 ± 3.1 ng/ml) and reached proestrous-like levels, compared to mice that were administered E2 alone in the control experiment (5.6 ± 0.5 ng/ml).
2.14. Infusions placements and site-specificity
Immediately after testing, mice were euthanized by cervical subluxation. Brains were extracted and then frozen on dry ice and stored at −80 until dissection. Brains that were keep frozen on dry ice, were sliced at the level of the midbrain, and cut in 100-µ slices. These slices were inspected visually using a dissecting microscope as previously described. All mice with ICV infusions were found to have infusions to the ventricle. Eighteen of the mice with intended infusions to the VTA had infusions to other sites. The data from these mice that had infusions to sites other than the VTA were excluded from the overall analyses. To determine efficacy of AS-ODNs mPRα and mPRβ expression was examined in micro-punches from the VTA, which were taken from the slices. Tissues were placed in RNAlater (Qiagen) until homogenization and extraction to prevent degradation, and were used for determining mPRα and mPR expression with qPCR.
2.15. Quantitative real-time PCR (qPCR)
Standard qPCR methods were utilized [10]. For extractions, total RNA was isolated from tissue using the Qiagen RNeasy Micro Kit (Valencia, CA) according to the manufacturer’s protocol. Reverse transcription was carried out using Oligo(dT)20 and the Superscript III First-Strand Synthesis System for RT-PCR from Invitrogen (Carlsbad, CA). qPCR was performed using Bio-Rad SYBR Green Supermix (Hercules, CA) and the following gene-specific primers: β-actin forward (5′-GCTCGTCGTCGACAACGGCT-3′), β-actin reverse (5′-CAAACATGATCTGGGTCATCTTCTC-3′), mPRα forward (5′-GCTCTGCTCTGACCACAGTTTTCC-3′ and reverse 5′-CAGCCTCGTTGTGCCGCTGA-3′ and mPRβ forward (5′-TACCAGGGACGCCATGAGAT-3′ and reverse (5′-CCTCAGCCCGTAATACATATTAA-3′). Reactions were run on an Applied Biosystems 7900HT and analyzed using the comparative cycle time (DeltaDeltaCT) method (Applied Biosystems, Foster City, CA). The fold change in comparison to vehicle controls of the delta CT values of mPR versus actin are depicted for the differences in the number of cycles for expression as observed from subjects in each condition [23,24].
2.16. Statistical analyses
One-way analyses of variances (ANOVAs) were used to examine effects of infusion condition (control, mPRα, β, αβ AS-ODN groups) on behavior. When the α level for statistical significance was reached (p ≤ 0.05), Fisher’s Least Significant Difference post hoc tests were used to examine group differences.
3. Results
3.1. mPRα and mPRβ are expressed in the midbrain VTA of proestrous mice
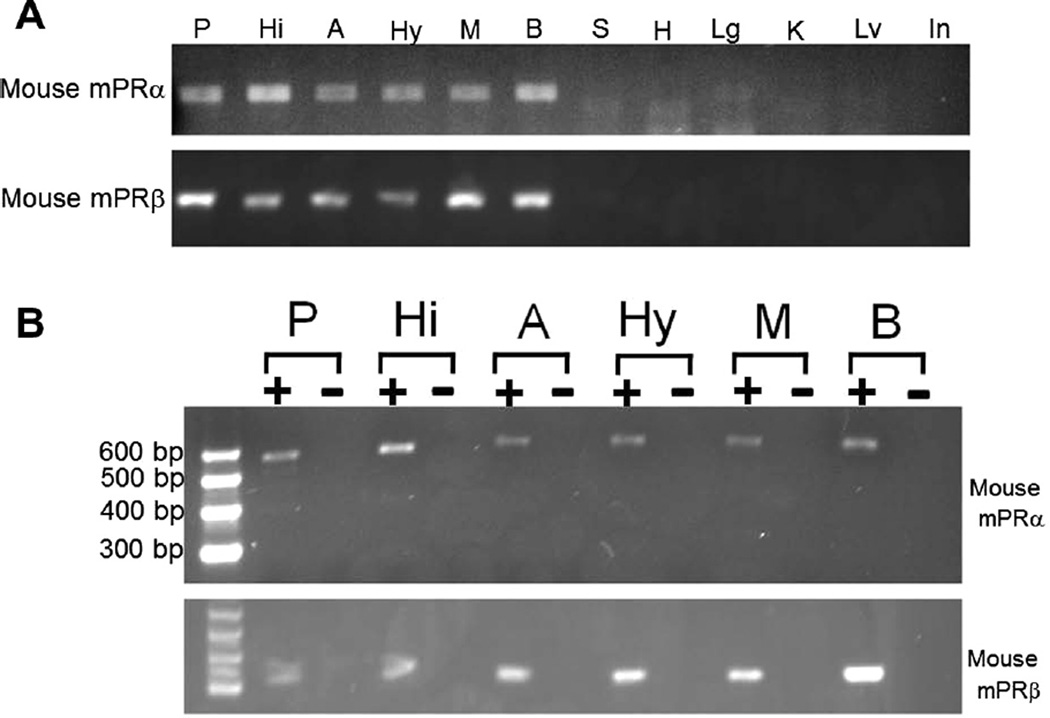
The first set of results in this study demonstrated that mPRs were expressed in midbrain. Analyses of samples from proestrous mice demonstrated that mPRβ was expressed highly in the brain, but was undetectable in the peripheral tissues (spleen, heart, lung, kidney, liver, intestines) with limited number of PCR cycles (30 cycles, Fig. 1). Further analyses of expression of mPRs in the different parts of brain tissues (prefrontal cortex, hippocampus, amygdala, hypothalamus, midbrain) was also analyzed using RT-PCR. Expression of mPRα and mPRβ were high in the midbrain (Fig. 1). As a negative control, samples were also prepared for these tissues using the same procedure except without Superscript III (RT−) to demonstrate that extracted DNA was free of genomic DNA. There was no evidence of genomic DNA in samples. Thus, mPRs are expressed in brain regions of interest.
Fig. 1.
Expression of membrane progestin receptor α (mPRα, Paqr7) and mPRβ (Paqr8) transcripts in proestrus C57/BL6 mouse peripheral tissues and brain regions, as analyzed by RT-PCR. P: prefrontal cortex; Hi; hippocampus; A: amygdala; Hy: hypothalamus; M: midbrain; B: brain; B S: spleen; H: heart; Lg: lungs; K: kidney; Lv: liver; and In: intestines. Samples were prepared for all tissues samples using same procedure with (+, indicated in Panel B) or without Superscript III (−, indicated in Panel B). Forward and reverse PCR primers were specifically designed to be located in two different exons. Specific PCR products amplified from the transcripts with expected size were observed (525 bp for mPRα, and 328 bp for mPRβ). There was no evidence of genomic DNA contamination.
3.2. Experiment 1: among C57/BL6 mice, mPR knockdown via ICV infusions reduced lordosis and increased aggression
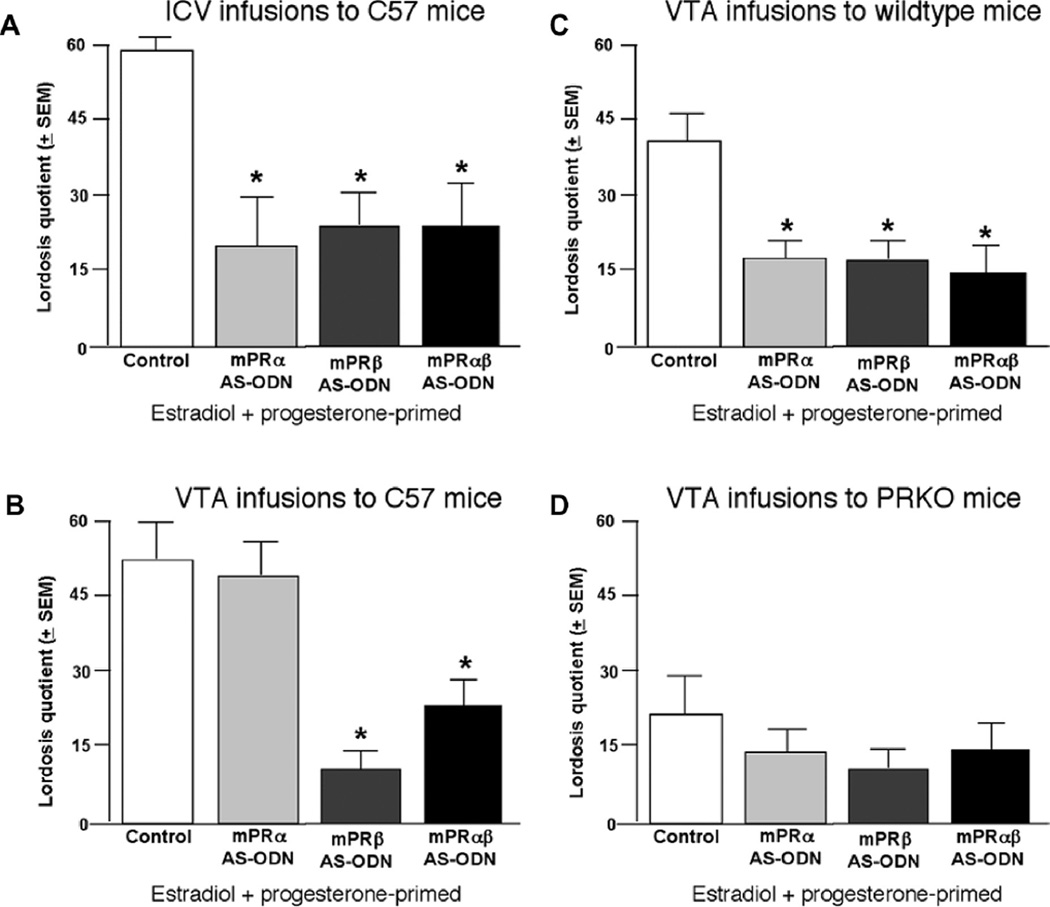
Lateral ventricle infusions of AS-ODNs were utilized in this experiment to assess more widespread knockdown of mPRs, particularly in the hypothalamus. Infusions of ODNs ICV had significant effects on reproductive behaviors. There was a main effect of infusion condition for lordosis quotients (F3,52 = 7.237, p < 0.01) and aggression quotients (F3,52 = 2.858, p < 0.05). Post hoc tests revealed that infusions of mPRα, mPRβ, and mPRαβ AS-ODNs significantly reduced incidence of lordosis (Fig. 2, Panel A) and increased incidence of aggressive responses (Table 1), compared to control infusions. Differences between knockdown of mPRα or mPRβ were not noted with lateral ventricle infusions. Thus, mPR knockdown reduced lordosis and increased aggression towards males during mating task.
Fig. 2.
Mean (±SEM) lordosis quotients (LQs) of mice administered vehicle (control), mPRα AS-ODNs, mPRβ AS-ODNs, or both mPRα/β AS-ODNs. Panel A: E2 + P4-primed C57/BL6 (“C57”) mice that were administered AS-ODNs intracerebroventicularly (ICV). Panel B: E2 + P4-primed C57/BL6 mice that were administered AS-ODNs to the ventral tegmental area (VTA). Panel C: E2 + P4-primed C57/BL6x129 (“wildtype”) mice that were administered AS-ODNs to the VTA. Panel D: E2 + P4-primed progestin receptor knockout (PRKO) mice that were administered AS-ODNs to the VTA. *indicates significant difference from vehicle control group p < 0.05.
Table 1.
Effects on aggression/rejection behavior of female mice during mating task (mean ± sem).
| Condition | Brain infusion condition | |||
|---|---|---|---|---|
| Veh | mPRα AS-ODN | mPRβ AS-ODN | mPRαβ AS-ODN | |
| Experiment 1: effects of AS-ODNs to the lateral ventricle of ovx, E2 + P4-primed C57/BL6 mice | ||||
| Aggression Quotient | 57 ± 7 | 80* ± 9 | 98* ± 2 | 87* ± 7 |
| Experiment 2: effects of AS-ODNs to the VTA of ovx, E2 + P4-primed C57/BL6 mice | ||||
| Aggression Quotient | 57 ± 7 | 80* ± 9 | 98* ± 2 | 87* ± 7 |
| Experiment 3: effects of AS-ODNs to the VTA of ovx, E2 + P4-primed C57/BL6X129 mice | ||||
| Aggression Quotient | 72 ± 7 | 79 ± 8 | 94 ± 4 | 89 ± 6 |
| Experiment 4: effects of AS-ODNs to the VTA of ovx, E2 + P4-primed PRKO mice | ||||
| Aggression Quotient | 78 ± 7 | 89 ± 5 | 92 ± 4 | 78 ± 8 |
indicates significant effect of AS-ODN to alter behavior compared to vehicle control (p < 0.05).
3.3. Experiment 2: among C57/BL6 mice, knocking down mPRβ, more so than mPRα, in the VTA attenuated lordosis and increased aggression
To directly assess effects of mPR knockdown in the midbrain, infusions of AS-ODNs were directed at the midbrain of C57/BL6 mice. There was a significant effect of VTA infusions on lordosis quotients (F3,38 = 11.283, p < 0.01) and aggression quotients (F3,38 = 5.252, p < 0.01). Post hoc tests revealed that mice infused with mPRβ or mPRαβ AS-ODNs had significantly lower lordosis quotients, compared to the control infusion condition (Fig. 2, Panel B). All AS-ODN infusions to the VTA increased aggression quotients compared to vehicle control (Table 1). Thus, knocking down mPRβ, more so than mPRα, attenuated lordosis.
3.4. Experiment 3: among wildtype (C57/BL6x129) mice, knocking down mPRs in the VTA attenuates lordosis
Cross-strain comparisons were completed to begin to address the notion that this is a basic mechanism of progestin action. There was a significant effect of VTA infusions on lordosis quotients (F3,51 = 2.707, p ≤ 0.05). Mice infused with mPRα, mPRβ or mPRαβ had significantly lower lordosis quotients compared to the control infusion condition (Fig. 2, Panel C), but no differences were observed for aggression (Table 1). Thus, these data suggest some differences in sexual receptivity and aggression towards males in this task between these two C57/BL6 strains, as well as sensitivity to mPR knockdown.
3.5. Experiment 4: among PRKO mice, mPR knockdown in the VTA did not attenuate lordosis
A question is the potential role of nuclear PRs. There were no significant effects of VTA infusions of AS-ODNs on reproductive measures, such as lordosis quotients or aggression quotients, among PRKO mice (Fig. 2 Panel D, Table 1). As expected, PRKO mice had attenuated reproductive responding; this was not further reduced by manipulations of mPRs.
3.6. Control conditions, tasks and validation measures – effects via mPRs may be specific to progestins, not E2, for reproductive responding
To assess whether effects of mPR manipulations were specific to P4, this experiment assessed effects of AS-ODNs in mice primed with E2 alone. Among mice that were E2-primed alone, lordosis quotients or aggression quotients were not altered by ICV infusions of mPRα (lordosis 32 ± 2%; aggression 80 ± 9%), mPRβ (lordosis 22 ± 7%; aggression 80 ± 21%), mPRαβ (lordosis 15 ± 10%; aggression 80 ± 17%) AS-ODNs, compared to control vehicle (lordosis 30 ± 3%; aggression 90 ± 5%). As for non-reproductive, control tasks assessed, there were no differences in open field, elevated plus maze, or social interaction tasks between groups following infusions ICV or to the VTA (data not shown). qPCR results supported knock down of mPRs in midbrain, particularly with infusions to the VTA (compared to ICV infusions; Table 2). Thus, effects via mPRs may be specific to progestins, not E2, for reproductive responding.
Table 2.
Effects on mPR expression from experiments (mean ± sem). Minus (−) indicates average fold decrease compared to the control.
| Condition | Brain infusion condition | |||
|---|---|---|---|---|
| Veh | mPRα AS-ODN | mPRβ AS-ODN | mPRαβ AS-ODN | |
| Experiment 1: effects of AS-ODNs to the lateral ventricle on ovx, E2 and P4-primed C57/BL6 mice | ||||
| n | 6 | 4 | 3 | 2 |
| MPRα fold change in midbrain | 0.4 ± 0.1 | −0.2 ± 0.6 | 0.3 ± 0.1 | |
| MPRβ fold change in midbrain | −1.4 ± 0.7 | −0.8 ± 0.6 | 0.5 ± 0.1 | |
| Experiment 2: effects of AS-ODNs to the VTA on ovx, E2 and P4-primed C57/BL6 mice | ||||
| n | 6 | 4 | 3 | 6 |
| MPRα fold change in midbrain | −1.1 ± 0.2 | −0.1 ± 0.5 | −5.2 ± 4.6 | |
| MPRβ fold change in midbrain | −0.7 ± 0.7 | −1.9 ± 1.4 | 1.0 ± 0.6 | |
| Experiment 3: effects of AS-ODNs to the VTA on ovx, E2 and P4-primed C57/BL6X129 mice | ||||
| n | 5 | 4 | 3 | 6 |
| MPRα fold change in midbrain | 0.1 ± 0.1 | 0.8 ± 0.1 | −0.3 ± 0.5 | |
| MPRβ fold change in midbrain | −1.0 ± 1.2 | −1.5 ± 0.6 | −2.3 ± 0.5 | |
| Experiment 4: effects of AS-ODNs to the VTA on ovx, E2 and P4-primed PRKO mice | ||||
| n | 6 | 4 | 3 | 2 |
| MPRα fold change in midbrain | 0.4 ± 0.1 | −0.2 ± 0.6 | 0.3 ± 0.1 | |
| MPRβ fold change in midbrain | −1.4 ± 0.7 | −0.8 ± 0.6 | 0.5 ± 0.1 | |
4. Discussion
The hypothesis that mPRs are involved in P4’s actions in the VTA, relevant for reproductive behavior of E2-primed mice, was supported. mPRα and mPRβ were expressed in the midbrain VTA of proestrous mice. AS-ODN infusions of mPRβ AS-ODNs to the lateral ventricle or VTA of C57/BL6 or wildtype (C57/BL6x129) mice reduced lordosis quotients and increased aggression quotients. PRKO mice had reduced reproductive responding than did wildtypes, and did not demonstrate significant decrements in reproductive behavior with mPR AS-ODN infusions. These effects were hormone and strain-specific. Infusions to E2-primed mice were less effective than that of E2- and P4-primed mice suggesting progestin-specificity for these effects of mPR manipulations. Strain sensitivity is seen by differences in pattern of response with C57/BL6 and C57/BL6x129 mice for mPRα, but not mPRβ, manipulations. Interestingly, the failure to see effects in PRKO versus PRKO wildtype mice suggests that some actions of mPRs may require nPRs, as well as other targets. Together, these findings suggest that mPRs may be one novel target in VTA for P4-facilitated lordosis of mice.
The present data confirm and extend the literature on the role of non-genomic effects of progestins in the VTA for lordosis. Until now, the studies of mPRs have almost exclusively focused the functions and signaling of mPRα. The unique functions of other mPR isoform, particular mPRβ have not been clearly demonstrated. Our results are first evidence showing that mPRβ is a plausible mediator for progestin-facilitated lordosis in mice. We have previously reported that traditional signaling via nPRs in this region is not required for P4’s actions to facilitate lordosis. There are very few nPRs in the VTA of adult rodents and those that are localized to the VTA are not E2 induced [7,8]. Notably, mPR knockdown in the VTA attenuated lordosis of E2 and/or P4 facilitated lordosis among C57/BL6 and PRKO wildtype, but not PRKO, mice. These findings suggest that actions at mPRs in the VTA do not mediate the expression of lordosis that can be seen among these mice with E2 and/or P4 priming. Progesterone enhances lordosis when applied to the VTA of mutant mice lacking PRs, or of rats that have nPRs knocked down with AS-ODNs [4,5,25]. Thus, actions at mPRs may be influenced by E2 and/or P4 and/or nPR expression. A question of interest is to what extent the other targets in the midbrain, such as GABA, oxytocin, and dopamine, play a role for lordosis and if progestins may be having synergistic effects through these multiple non-genomic targets [7–9,26,27].
The present data provide additional information about expression and functional effects of mPRs among mice. It is important to note these cross-species effects regarding the mPRs. The mPRs were first characterized in amphibian and fish. These results, and others, have shown expression and functional effects in rodents, such as rats [28–31]. For example, expression of mPRβ, as measured by in situ hybridization and histochemistry, was greater than mPRα in the hippocampus, lateral and medial septum, thalamus, and regions of the midbrain of E2-primed rats [29–31]. Moreover, among proestrous rats with high circulating E2 and/or P4, mPRα and mPRβ expression are increased during proestrus, compared to diestrus, of rats in the mediobasal hypothalamus [30], and we have noted high levels in the midbrain of proestrous rats [28]. In the present study, similar reproductive effects were observed among mice as we have recently reported in rats [28]. Expression of mPRα and mPRβ was observed in brain areas including hypothalamus and midbrain of proestrous mice. Among C57/BL6 mice, E2 and P4-facilitated lordosis was significantly reduced with administration of AS-ODNs for mPRα, mPRβ, or mPRαβ to the lateral ventricle, compared to vehicle, reductions in lordosis when AS-ODNs were administered to the VTA of this strain were not observed with mPRα AS-ODNs. Interestingly, there was evidence that another control strain, the C57/BL6x129 wildtypes of PRKO mice, had reduced lordosis with mPRα and/or mPRβ. Moreover, a goal was to begin to ascertain potential interactions between nuclear PRs and mPRs, by using PRKO mice. However, PRKO mice showed such an attenuated response to hormone-priming for reproductive behaviors, we were not able to elucidate this question. These cross-species similarities (rats and mice) provide some evidence of conservation of this target and functional relevance; yet, strain differences noted here also suggest epigenetic and/or other factors to consider beyond the scope of the present study.
Differences in behavioral phenotypes have been noted for other steroid receptor subtypes, such as estrogen receptors [32,33] and, even more recently, thyroid hormone receptors α and β [34], for reproductive and non-reproductive behaviors. A consideration for future investigations is divergence in downstream effectors of mPR isoforms for steroids’ functional effects. For instance, one notion is that knockdown of thyroid hormone receptor α alters GABA signaling [35]. A question is the role of non-genomic effects of E2, as has been described for other complex, socially-relevant mouse behaviors (e.g. learning) [36]. To begin to ascertain this, mice were primed with E2 alone. E2-facilitated lordosis was not significantly reduced with administration of mPR AS-ODNs, but there was a pattern of reduction with infusions mPRβ or co-administration of mPRα and mPRβ AS-ODNs, compared to vehicle. Studies focused on further understanding rapid estrogen receptor signaling suggest that altering membrane targets (e.g. GPR30), down-regulated expression of variant of ERα (ERα36) breast cancer SK-BR-3 cells [37]. Interactions between membrane ERs, intracellular ERs, and other membrane targets for complex neural functions have also been described (e.g. in the case of feeding regulation and reproductive behaviors) [38–42]. As such, an important question, given the present results, is that there may be divergent downstream effectors of mPRs to consider further.
Limitations of the present study are as follows. First, our experiment focused on actions of mPRs in the midbrain VTA. In addition to the midbrain VTA, the hypothalamus is an important brain region in mediating progestin-facilitated lordosis. The hypothalamus has high expression of classic targets of progestins, nPRs, which are involved in reproductive behaviors of female rodents. A question not addressed in the present study was the role of mPRs as a membrane target for progestins’ actions for lordosis in the hypothalamus or other regions. Perhaps hormone-primed rats had reduced lordosis responding following lateral ventricle infusions of mPRα and/or mPRβ AS-ODNs coincident with reduction in mPRs in the hypothalamus. The selective expression of mPRβ in the brain, but not in the body, implies it may be possible in the future to target these receptors without the liability of trophic effects in peripheral reproductive tissues. As such, future studies manipulating mPRs in other regions, are of considerable interest. Second, in these experiments, we effectively tried to verify our knock down of mPR following lateral ventricle and midbrain VTA infusions of AS-ODNS in experimental mice with qPCR. However, the approach that we utilized for taking tissue punches (which were 3–5 mg of tissue) of these regions and extracting RNA for RT-PCR precluded examination of mPR proteins in these samples. Additionally, tissues were run using a qPCR technique to ascertain the extent of mPR knockdown from using AS-ODNs. Effects using these techniques were likely not as robust as if we were able to determine effects on protein levels of mPRs. AS-ODNs are considered to be effective in reducing RNA or protein expression by about 40%. Given that information, and that we saw a modest decrease in RNA expression with AS-ODNs, our results that mPR AS-ODNs can significantly impair lordosis is notable. We hope to address the short-comings of these approaches in our future work.
Despite these limitations, the results of the present research informs us about two putative non-genomic mechanisms underlying progestin-facilitated lordosis. First, nuclear steroid receptors, which are activated by binding to a hormone, are able to interact with other transcription factors without direct binding to DNA. There is evidence for nPRs, despite having well-defined transcriptional activity [43], acting through separate non-genomic signaling function [44] in breast cancer signaling and Xenopus ooctye maturation [45–47]. Non-genomic actions of steroids cause rapid introduction of second messenger signal transduction cascades, including the rapid increase in intracellular calcium concentrations, which has been implicated in neuroprotective effects of progestins [48]. As well, there is rapid activation of protein kinase, a protein kinase C and MAPK, which we have shown are involved in progestin-facilitated lordosis in the midbrain VTA [49]. However, the precise mechanisms of the classical nPR at the membrane remains to be understood. The failure of significant, albeit apparent, reductions in lordosis among PRKO mice with mPR AS-ODNs infusions and the level of expression imply that PRKO mice do not over-express mPR proteins, and may rely on these actions for progestin-facilitated signaling in the VTA. What we do not know is whether this pattern of effects could be related to the classical PR being targeted to the membrane to mediate these effects. Second, mPRs seem to be targets for progestins’ actions in the VTA to facilitate lordosis. mPRs are included in the PAQR family, which contains 4 adiponectin receptor like (class I receptors), 5 unique mPR members (mPRα, mPRβ, mPRγ, mPRδ, and mPRε; class II receptors), and 2 hemolysin-like receptors [50–52]. The mPR family of proteins has seven integral transmembrane domains and mediates rapid progestin signaling in various model systems, including fish, amphibians, and mammals [28–31,53–66]. The mPRα and mPRβ are the most well-studied of the mPRs; albeit, effects for reproductive behavior of mammals are just beginning to be understood and there are many factors to consider in progestin signaling at the membrane [67]. For example, data from a series of studies has demonstrated differential regulation of mPRs for induction of oocyte maturation in goldfish [68–70]. The expression/localization and the functional effects of mPRγ, mPRδ, and mPRε are still relatively unknown. Here, a role for mPRβ in the VTA mediating progestin-facilitated lordosis among C57/BL6 mice was supported, a finding which is similar to effects we have observed in Long-Evans rats [28]. These findings extend what has previously been reported in the literature regarding mPR functions, and suggest a functional role of mPRβ for a hard-wired behavior in a brain region (the midbrain) that is conserved across species. Of continued interest are the relative roles of nPRs and mPRs for their involvement in rapid functional effects of progestins.
Acknowledgments
This research was supported by an EAGER grant from the National Science Foundation (IOS-0957148). Assistance provided by Carolyn Koonce, Danielle Llaneza, Danielle Osborne, Dr. Jason Paris, Anthony Santarelli, and Jennifer Torgersen is appreciated.
References
- 1.Edwards DA. Induction of estrus in female mice: estrogen progesterone interactions. Horm Behav. 1970;1:299–304. [Google Scholar]
- 2.Guttenberg I. Plasma levels of “free” progestin during the estrous cycle in the mouse. Endocrinology. 1961;68:1006–1009. doi: 10.1210/endo-68-6-1006. [DOI] [PubMed] [Google Scholar]
- 3.Corpechot C, Collins BE, Carey MP, Tsouros A, Robel P, Fry JP. Brain neurosteroids during the mouse oestrous cycle. Brain Res. 1997;766:276–280. doi: 10.1016/s0006-8993(97)00749-x. [DOI] [PubMed] [Google Scholar]
- 4.Frye CA, Vongher JM. Progesterone has rapid and membrane effects in the facilitation of female mouse sexual behavior. Brain Res. 1999;815:259–269. doi: 10.1016/s0006-8993(98)01132-9. [DOI] [PubMed] [Google Scholar]
- 5.Frye CA, Vongher JM. Progesterone and 3α,5α-THP enhance sexual receptivity in mice. Behav Neurosci. 2001;115:1118–1128. [PubMed] [Google Scholar]
- 6.Blaustein JD. Progestin receptors: neuronal integrators of hormonal and environmental stimulation. Ann N Y Acad Sci. 2003;1007:238–250. doi: 10.1196/annals.1286.023. [DOI] [PubMed] [Google Scholar]
- 7.Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav. 2001;40:226–233. doi: 10.1006/hbeh.2001.1674. [DOI] [PubMed] [Google Scholar]
- 8.Frye CA. The role of neurosteroids and non-genomic effects of progestins and androgens in mediating sexual receptivity of rodents. Brain Res Brain Res Rev. 2001;37:201–222. doi: 10.1016/s0165-0173(01)00119-9. [DOI] [PubMed] [Google Scholar]
- 9.Frye CA. Neurosteroids’ effects and mechanisms for social, cognitive, emotional, and physical functions. Psychoneuroendocrinology. 2009;34:S143–S161. doi: 10.1016/j.psyneuen.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O’Malley BW, et al. Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 2006;186:312–322. doi: 10.1007/s00213-006-0309-3. [DOI] [PubMed] [Google Scholar]
- 13.Frye CA, Sumida K, Lydon JP, O’Malley BW, Pfaff DW. Mid-aged and aged wildtype and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3alpha,5alpha-THP-facilitated lordosis. Psychopharmacology (Berl) 2006;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA, Walf AA. Progesterone enhances learning and memory of aged wildtype and progestin receptor knockout mice. Neurosci Lett. 2010;472:38–42. doi: 10.1016/j.neulet.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frye CA. Progesterone attenuates depressive behavior of younger and older adult C57/BL6, wildtype, and progesterone receptor knockout mice. Pharmacol Biochem Behav. 2011;99:525–531. doi: 10.1016/j.pbb.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Academic Press; 1997. [Google Scholar]
- 17.Crawley JN. What’s wrong with my mouse: behavioral phenotyping of transgenic and knockout mice. Hoboken, NJ: John Wiley & Sons; 2007. [Google Scholar]
- 18.Frye CA, Walf AA, Rhodes ME, Harney JP. Progesterone enhances motor, anxiolytic, analgesic, and antidepressive behavior of wild-type mice, but not those deficient in type 1 5α-reductase. Brain Res. 2004;1004:116–124. doi: 10.1016/j.brainres.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Wahlsten D, Metten P, Crabbe JC. A rating scale for wildness and ease of handling laboratory mice: results for 21 inbred strains tested in two laboratories. Genes Brain Behav. 2003;2:71–79. doi: 10.1034/j.1601-183x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 20.Reddy DS, O’Malley BW, Rogawski MA. Anxiolytic activity of progesterone in progesterone receptor knockout mice. Neuropharmacology. 2005;48:14–24. doi: 10.1016/j.neuropharm.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye CA, Paris JJ, Rhodes ME. Increasing 3α,5α-THP following inhibition of neurosteroid biosynthesis in the ventral tegmental area reinstates anti-anxiety, social, and sexual behavior of naturally receptive rats. Reproduction. 2009;137:119–128. doi: 10.1530/REP-08-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 25.Frye CA, Murphy RE, Platek SM. Anti-sense oligonucleotides, for progestin receptors in the VMH and glutamic acid decarboxylase in the VTA, attenuate progesterone-induced lordosis in hamsters and rats. Behav Brain Res. 2000;115:55–64. doi: 10.1016/s0166-4328(00)00242-4. [DOI] [PubMed] [Google Scholar]
- 26.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 27.Young KA, Liu Y, Wang Z. The neurobiology of social attachment: a comparative approach to behavioral, neuroanatomical, and neurochemical studies. Comp Biochem Physiol C Toxicol Pharmacol. 2008;148:401–410. doi: 10.1016/j.cbpc.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frye CA, Walf AA, Kohtz AS, Zhu Y. Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats. Horm Behav. 2013;64:539–545. doi: 10.1016/j.yhbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Intlekofer KA, Petersen SL. Distribution of mRNAs encoding classical progestin receptor, progesterone membrane components 1 and 2, serpine mRNA binding protein 1, and progestin and ADIPOQ receptor family members 7 and 8 in rat forebrain. Neuroscience. 2011;172:55–65. doi: 10.1016/j.neuroscience.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Arbogast LA. Gene expression profiles of intracellular and membrane progesterone receptor isoforms in the mediobasal hypothalamus during prooestrus. J Neuroendocrinol. 2009;21:993–1000. doi: 10.1111/j.1365-2826.2009.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuloaga DG, Yahn SL, Pang Y, Quihuis AM, Oyola MG, Reyna A, et al. Distribution and estrogen regulation of membrane progesterone receptor-β in the female rat brain. Endocrinology. 2012;153:4432–4443. doi: 10.1210/en.2012-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill RA, Boon WC. Estrogens, brain, and behavior: lessons from knockout mouse models. Semin Reprod Med. 2009;27:218–228. doi: 10.1055/s-0029-1216275. [DOI] [PubMed] [Google Scholar]
- 33.Jensen EV, Jacobson HI, Walf AA, Frye CA. Estrogen action: a historic perspective on the implications of considering alternative approaches. Physiol Behav. 2010;99:151–162. doi: 10.1016/j.physbeh.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasudevan N, Morgan M, Pfaff D, Ogawa S. Distinct behavioral phenotypes in male mice lacking the thyroid hormone receptor α1 or β isoforms. Horm Behav. 2013;63:742–751. doi: 10.1016/j.yhbeh.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guadaño-Ferraz A, Benavides-Piccione R, Venero C, Lancha C, Vennström B, Sandi C, DeFelipe J, Bernal J. Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits. Mol Psychiatry. 2003;8:30–38. doi: 10.1038/sj.mp.4001196. [DOI] [PubMed] [Google Scholar]
- 36.Frick KM. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 2012;126:29–53. doi: 10.1037/a0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, et al. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21:263–270. doi: 10.1111/j.1365-2826.2009.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roepke TA, Ronnekleiv OK, Kelly MJ. Physiological consequences of membrane-initiated estrogen signaling in the brain. Front Biosci. 2011;16:1560–1573. doi: 10.2741/3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305:E632–E640. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 43.Tian J, Kim S, Heilig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz RJ, Chang C, Schrader WT, O’Malley BW. Effect of progesterone receptors on transcription. Ann N Y Acad Sci. 1977;286:147–160. doi: 10.1111/j.1749-6632.1977.tb29413.x. [DOI] [PubMed] [Google Scholar]
- 45.Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 46.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, et al. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 47.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Luoma JI, Stern CM, Mermelstein PG. Progesterone inhibition of neuronal calcium signaling underlies aspects of progesterone-mediated neuroprotection. J Steroid Biochem Mol Biol. 2012;131:30–36. doi: 10.1016/j.jsbmb.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frye CA, Walf AA. Membrane actions of progestins at dopamine type 1-like and GABAA receptors involve downstream signal transduction pathways. Steroids. 2008;73:906–913. doi: 10.1016/j.steroids.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 51.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, et al. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 52.Smith JL, Kupchak BR, Garitaonandia I, Hoang LK, Maina AS, Regalla LM, et al. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas P, Tubbs C, Detweiler C, Das S, Ford L, Breckenridge-Miller D. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids. 2005;70:427–433. doi: 10.1016/j.steroids.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Hanna R, Pang Y, Thomas P, Zhu Y. Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors alpha and beta in transfected cells. J Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 55.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 56.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–116. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, et al. Expression of membrane progesterone receptors on human T lymphocytes and Jurkat cells and activation of G-proteins by progesterone. J Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 58.Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, et al. Membrane progesterone receptors localization in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Lemale J, Bloch-Faure M, Grimont A, El Abida B, Imbert-Teboul M, Crambert G. Membrane progestin receptors alpha and gamma in renal epithelium. Biochim Biophys Acta. 2008;1783:2234–2240. doi: 10.1016/j.bbamcr.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 60.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on GnRH release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tubbs C, Thomas P. Progestin signaling through an olfactory G protein and membrane progestin receptor-alpha in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology. 2009;150:473–484. doi: 10.1210/en.2008-0512. [DOI] [PubMed] [Google Scholar]
- 62.Cai Z, Stocco C. Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology. 2005;146:5522–5532. doi: 10.1210/en.2005-0759. [DOI] [PubMed] [Google Scholar]
- 63.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 64.Nutu M, Weijdegård B, Thomas P, Thurin-Kjellberg A, Billig H, Larsson DG. Distribution and hormonal regulation of membrane progesterone receptors beta and gamma in ciliated epithelial cells of mouse and human fallopian tubes. Reprod Biol Endocrinol. 2009;7:89. doi: 10.1186/1477-7827-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nutu M, Weijdegård B, Thomas P, Bergh C, Thurin-Kjellberg A, Pang Y, et al. Membrane progesterone receptor gamma: tissue distribution and expression in ciliated cells in the fallopian tube. Mol Reprod Dev. 2007;74:843–850. doi: 10.1002/mrd.20685. [DOI] [PubMed] [Google Scholar]
- 66.Meffre D, Labombarda F, Delespierre B, Chastre A, De Nicola AF, Stein DG, et al. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. doi: 10.1016/j.neuroscience.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 67.Petersen SL, Intlekofer KA, Moura-Conlon PJ, Brewer DN, Del Pino Sans J, Lopez JA. Novel progesterone receptors: neural localization and possible functions. Front Neurosci. 2013;7:164. doi: 10.3389/fnins.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokumoto T. Identification of membrane progestin receptors (mPR) in goldfish oocytes as a key mediator of steroid non-genomic action. Steroids. 2012;77:1013–1016. doi: 10.1016/j.steroids.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Tokumoto T, Tokumoto M, Oshima T, Shimizuguchi K, Fukuda T, Sugita E, et al. Characterization of multiple membrane progestin receptor (mPR) subtypes from the goldfish ovary and their roles in the induction of oocyte maturation. Gen Comp Endocrinol. 2012;177:168–176. doi: 10.1016/j.ygcen.2012.03.005. [DOI] [PubMed] [Google Scholar]