Abstract
Introduced in the 90’s by Prof Moehwald, Lvov and Decher, the layer-by-layer (LbL) assembly of polyelectrolytes has become a popular technique to engineer various types of objects such as films, capsules and free standing membranes, with an unprecedented control at the nanometer and micrometer scales. The LbL technique allows to engineer biofunctional surface coatings, which may be dedicated to biomedical applications in vivo but also to fundamental studies and diagnosis in vitro. Initially mostly developed as 2D coatings and hollow capsules, the range of complex objects created by the LbL technique has greatly expanded in the past 10 years. In this review, our aim was to highlight the recent progress in the field of LbL films for biomedical applications and discuss the various ways to control spatially and temporally the biochemical and mechanical properties of multilayers. In particular, we will discuss three major developments of LbL films: 1) the new methods and templates to engineer LbL films and control cellular processes from adhesion to differentiation, 2) the major ways to achieve temporal control by chemical, biological and physical triggers and, 3) the combinations of LbL technique, cells and scaffolds for repairing 3D tissues, including cardio-vascular devices, bone implants and neuro-prosthetic devices.
INTRODUCTION
The bioactivity of implantable biomaterials is mostly achieved by precisely engineering their spatio-temporally controlled mechanical and biochemical properties. A particularly efficient way of reach this goal consists in functionalizing the surface of the inert materials that are already used in clinics. Bioactive coatings of endoluminal stents [1] perfectly illustrate the recent developments in the field of biofunctional surface coatings of medical devices. Since their introduction in the 1980’s, the use of metallic stents has become common practice during percutaneous coronary intervention. But the concept of coating stents with anti-proliferative agents that decrease the rate of in-stent restenosis has only emerged in the 2000’s [2]. Since then, this apparently simple innovation has led to its widespread application in human and in numerous clinical trials regarding improved drug delivery or biodegradable stents [2]. This story is one successful example of upcoming series of innovation, which will aim at improving the commonly used implantable materials, such as metals and non-degradable polymers, but also at developing fully engineered 3D tissues.
Within the past 20 years, the layer-by-layer (LbL) assembly technique initially developed by the group of Moehwald, Lvov and Decher [3–5] has emerged as a powerful surface coating technique. It has triggered a large number of fundamental studies, in order to understand the mechanisms of film growth or the interactions between cells and multilayers, as well as numerous applications, especially in the biomedical field. In 2006, Kotov and coworkers wrote the first comprehensive review on the use of multilayer films for biomedical applications, highlighting their potentialities as biosensors [6]. In 2010, our group wrote a review on engineering and controlling polyelectrolyte multilayer film properties at the nanometer and micrometer scales with the goal of developing new biomaterial coatings, biomimicking tissue engineered constructs and stem cell “niches” [7]. At that time, LbL was still mostly focused on fundamental material sciences and only few bioactive films had been explored for their potentialities in guiding cell fate (Table 2, [7]) and there was a very few number of in vivo pre-clinical studies in animals (see Table 3, [7]). Besides, the LbL were mostly 2D supported films or 3D capsules, with a limited choice of templates and only relative spatial control. Since 2010, substantial progresses have been made in spatio-temporally controlling the deposition of LbL films, their loading and delivery of drugs and growth factors to study fundamental biological processes, while the uses of LbL techniques for tissue engineering and biomedical applications have significantly expanded and reached the stage of pre-clinical trials in large animals. Several other reviews focused on drug delivery [8], coating of microorganisms [9], stimuli-sensitive films [10], single cell studies, [11,12] and advances in biomedical technologies [13]. Here our aim was precisely to highlight these recent progresses in the field of LbL films for biomedical applications and discuss the various ways to control spatially and temporally the biochemical and mechanical properties of multilayers (Figure 1). In particular, we will discuss the new methods and templates to engineer LbL films and control cellular processes from adhesion to differentiation (part A), the major ways to achieve temporal control by chemical, biological and physical triggers (part B) and, finally, the combinations of LbL techniques, cells and scaffold for engineering 3D assemblies (part C)
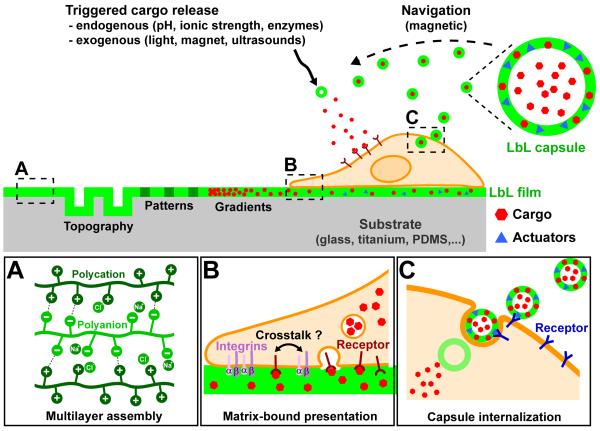
FIGURE 1. A schematic overview of the use of LbL films and microcapsules to influence cell behavior.
The left part illustrates the possibilities of spatially controlling LbL films to present a structured topography, patterns and gradients of rigidity or bioactivity. The right part illustrates the possibilities of loading in LbL films or capsules a cargo of interest (e.g. proteins, drugs, siRNA,…) to be delivered to the cell, as well as actuators (e.g. antibodies, gold NPs, magnetic NPs,…). These actuators can be used for targeting specific cell types or triggering the cargo release. The boxes (A - C) zoom in on areas of interest.
(A) Electrostatic interactions in LbL films. Representation of the electrostatic interactions between polycations and polyanions and the types of charge compensation in a LbL assembly.
(B) Presentation of a cargo by LbL films. This matrix-bound presentation allows spatial confinement of adhesion receptors (e.g. integrins, represented here by their α and β sub-units) and cargo receptors at the ventral side of the cell. Due to the close proximity of cargo receptors and adhesion receptors, a crosstalk between these two types of receptor is possible. The internalization of the cargo by endocytosis is also illustrated.
(C) Presentation of a cargo by LbL capsules. The use of specific actuators (antibodies, glycans, folates, hyaluronic acid) allows the delivery of a loaded cargo to a designated cell type (e.g. tumor cells). The LbL capsules can be internalized by endocytosis in the cell.
A. Spatial Control
In vivo, cells are extremely sensitive to the spatial organization of the biochemical and mechanical cues of their microenvironment [14,15]. These properties play crucial roles in cell adhesion, migration, proliferation, and differentiation, thus impacting physiological and pathological processes, such as morphogenesis, wound healing, cancer metastasis or inflammation (see [16–18] for reviews). A spatial control of the properties of the environment can thus be applied to fundamental studies of cell-cell and cell-environment interactions, as well as to biomedical applications such as tissue engineering, biosensors, and diagnostic devices. This section highlights the techniques recently developed for spatially controlling the biochemical and mechanical properties of LbL films (Figure 2).
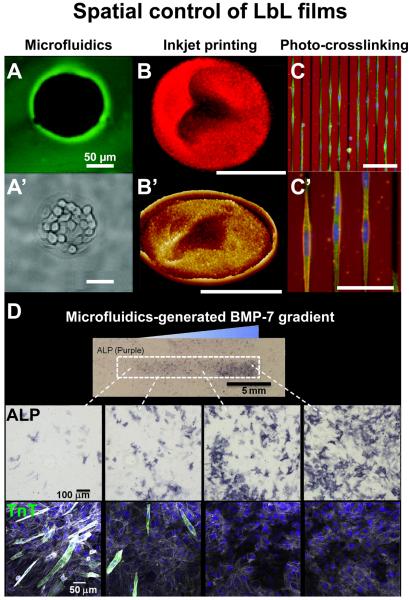
FIGURE 2. Illustrations of recent developments in spatially controlled LbL films.
(A) Fluorescence image and (A’) phase-contrast of the selective deposition by microfluidics of a poly-L-lysine/hyaluronic acid (PLL/HA) film inducing selective growth of L929 cells in the uncoated spherical areas. (B) Fluorescence image and (B’) 3D AFM image of a rhodamine-loaded multilayer disc assembled by inkjet printing. (C) Overview and (C’) magnification of the orientation of C2C12 myoblasts, stained for vinculin (green), actin (red) and nucleus (blue), on linear micropatterns of rigidity engineered by photo-crosslinking of a PLL/HA film. Red background corresponds to the autofluorescence of the chromium mask. (D) Differentiation of C2C12 myoblasts on a PLL/HA film presenting a matrix-bound bone morphogenetic protein-7 (BMP-7) gradient generated by microfluidics. Overview image and representative images of alizarin red (ALP) staining (dark purple) confirms osteogenic differentiation, while immunofluorescent imaging reveals a decrease of troponin T (TnT, green) positive cells with increasing BMP-7 concentration. Actin is stained in gray and nuclei in blue. A and A’ adapted from [20] with permission of The Royal Society of Chemistry, B and B’ from [33] with permission of the American Chemical Society, C, C’ and D from [60] and [66] with permission of Elsevier.
A.1 New methods for spatial control of film deposition
A.1.1 Microfluidics
Microfluidics has been demonstrated to be an interesting tool with multiple applications such as generating spatially arranged surfaces, biosensors, microfabrication, and for the generation of gradients. Microfluidic techniques have been aptly combined with the LbL method yielding interesting arrangements.
For instance, microfluidics allows for the selective deposition of LbL films on surfaces at the microscale, with channel widths or diameters ranging from 50 to 800 μm. Reyes et al. constructed polyethyleneimine (PEI)/poly(styrene sulfonate) (PSS) and poly(allylamine hydrochloride) (PAH)/PSS LbL film lines absorbed on poly(dimethylsiloxane) (PDMS) surfaces by flowing the polyelectrolyte solutions through a microchannel network that was in contact with the PDMS surface. This yielded a patterned substrate in which neuronal cells adhered only on the LbL-deposited lines [19]. Volodkin and co-workers generated films inside a microfluidic chamber containing micropillars (Fig. 2A). This combination resulted in a micropatterned substrate were cells preferentially attach to the areas not covered by the LbL film [20]. Payne et al. coupled electrodeposition with LbL deposition and microfluidics to selectively generate chitosan (CHI)/alginate (ALG) LbL films on an electrode to be used as an electrochemical biosensor [21]. Using microfluidics, Char et al. successfully coated patterned posts with (PAH/PSS) films, a feat previously impossible to accomplish with traditional LbL methods [22]. Their technique opens possibilities in selectively coating materials in the microscale. In a similar manner, Cohen and co-workers were able to successfully coat nanochannels with (PAH/PSS) multilayers by combining microfluidics with LbL deposition [23]. With this method, they can easily coat narrow nanogaps in a microfluidic device with high precision. Microfluidics may also be used to generate microcapsules, as was elegantly proposed by Trau et al. Inspired by the pinball game, droplets are guided with pillars inside a microfluidic chamber, which exposes them alternatively to the polymer solutions [24].
The combination of microfluidics with LbL deposition can also be expanded in the generation of LbL films with gradients of chemical and physical properties to mimic the naturally heterogeneous extracellular surrounding and provoke cell migration towards a more favorable environment. Using a “Christmas tree” microfluidic gradient mixer, Groth and co-workers generated CHI/Heparin (HEP) LbL films with a gradient in pH ranging from pH 5 to pH 9 [25]. They observed that osteoblasts adhere and spread on the basic side of the LbL film and that cells migrated from the low pH to the high pH region of the film.
Lastly, microfluidics can be applied for the high-throughput construction of LbL films. In this regard, Hammond et al. developed a microfluidic device for the high-throughput assembly and screening of LbL films [26]. Their device consists of independent microchannels that permit flow due to capillary action. Multiple microchannels are possible, and microstructures within the channel are also performed via lithographic techniques. They investigated cellular response to a library of LbL films constructed with varying pH, which affected film thickness, and observed that increasing film thickness decreased cell density. They also used their high-throughput method to construct films composed of a green fluorescent protein (GFP) plasmid and varying the polycation. They observed that films constructed with the polycation PEI yielded the highest transfection rates.
A.1.2. Photolithography
Photolithography can be combined with LbL deposition to generate a spatial control with a micrometer scale precision. For instance, Rubner et al. functionalized regions of individual living cells with LbL “backpacks” that deliver a desired payload [27]. By combining photolithography with LbL assembly, they generated a surface containing these backpacks composed of 1) a releasable region, 2) a payload region, and 3) a cell-adhesion region. Living cells seeded onto these surfaces attached to the backpacks, and the backpack was released via the selected release trigger mechanism (pH, temperature, etc.). They attached these backpacks to various types of cells: to T cells for cell sorting using magnetic particles [27], to macrophages where no phagocytosis was observed [28], to B cells that formed cell aggregates of reproducible size [29] and finally to monocytes using a mucin/lectin sacrificial layer [30]. In a different application, Tsukruk et al. engineered micro-bubble constructs from patterned spin-assisted polystyrene (PS)/silk LbL films [31]. Using photolithography a micropattern was created that upon exposure to acetone generated a micro-bubble by PS dissolution. These micro-bubbles can be used for encapsulation of hydrophobic and hydrophilic molecules as well as nanoparticles (NPs), serving as potential platforms for spatially ordered arrays for encapsulation and release systems.
A.1.3. Inkjet printing
Inkjet printing was first combined with LbL assembly by Andres and Kotov, who printed films composed of gold NPs and poly(diallyldimethylammonium chloride) (PDAC) [32]. Tsukruk et al. used it also to selectively deposit LbL films on a large number of substrates [33,34] and to spatially pattern films using both synthetic and natural polymers (Fig. 2B). Moreover, this technique allows for encapsulations of cells: a “nest” of printed LbL films was first deposited, followed by the inkjet deposition of cells, and finally by the deposition of bilayers to complete the encapsulation process [34]. An interesting combination of LbL deposition and inkjet printing was also proposed by Akashi and colleagues to build 3D tissue-micro arrays [35] (see also part C). Cells were printed as an initial layer in droplets of 500 – 750 μm in diameter, followed by gelatin (G)/fibronectin (FN) LbL film deposition on top of the cells. The LbL film permitted the adhesion of a second layer of cells, and the process was repeated to obtain the desired micro-tissue array. They applied this technique to prepare a liver tissue chip to investigate the mechanisms of drug metabolism.
A.3 New 2D templates
One of the reasons that make the LbL technique attractive as a method for surface modification is that it can be applied to any type of substrate. In this manner, substrates that have micro- and nanostructures can be coated to create a surface that provides multiple signals (chemical, physical, etc.) to cells to control intracellular processes.
Nanometer size objects such as nanochannels and viruses can thus be coated with multilayers to engineer new kinds of structures. For instance, a sacrificial nanoporous template was proposed by Roy and colleagues as a substrate for LbL deposition [36]. When removed, this yielded nanotubes of 100 – 500 nm in diameter. Recently, an interesting expansion of the LbL technique was proposed by Yoo and colleagues where nanostructured membranes were created using viruses on a graphene oxide film [37]. They deposited a monolayer of M13 viruses that have a specific affinity to negatively charged graphene nanosheets. These viruses can be aligned via shear force. Thus subsequent M13 layers were deposited via a stacking process, each layer perpendicular to the previous one.
Microstructured surfaces can also be employed as template for controlling spatially LbL deposition. Our team has developed a microstructured PDMS substrate coated with poly(L-lysine) (PLL)/ hyaluronic acid (HA) LbL films to investigate muscle cell alignment and differentiation [38]. Microgrooves of various size as well as (PLL/HA) films of various stiffness were evaluated to obtain an optimal conditions for the formation of parallel-oriented mature myotubes. LbL coatings on microstructured substrates were also developed by Gigli et al. via micro-molding in capillaries [39]. This was performed by coating glass with either (PAH/PSS) or poly(L-arginine) (pARG)/ dextran sulfate (DXS) LbL films, placing a PDMS microgrooved mold on top, filling the mold with polyurethane (PU), curing it, and lifting the PDMS mold. Myoblasts seeded on these substrates elongated along the microgrooves and formed multinucleated myotubes.
A.3 Spatial control of physico-chemical properties
Microcontact printing is one of the most popular techniques to create adhesive islets of proteins. It has already widely been used for stamping micro-features of ECM proteins on glass, tissue culture plastic or hydrogels (see [40] for a detailed review). A variation of this technique named polymer-on-polymer stamping (POPS) was developed by Hammond’s group [41] and used by several other groups to pattern adhesive proteins onto LbL films. This technique has already been described in part 8 of our 2010 review [7] and will not be further discussed here.
A.3.1. Control of film internal porosity
Control of LbL film topography has been recently achieved via modulation of film porosity. To this end, the group of Rubner has initially developed a technique to modulate porosity on LbL films by exposure to acidic solutions [42,43]. This acidic exposure induces a phase separation that changes film porosity. Nanoporous films are formed with apparent pore depths between 60-600 nm. Using this technique, Rajagopalan et al. prepared a variety of LbL surfaces with porosity ranging from the nano- to the micro-scale to investigate the behavior of human corneal epithelial cells [44]. They observed that although the entire range of porosity supported cell adhesion, the nanoscale porosity significantly enhanced cell proliferation and cell migration. A similar approach was used by Murphy et al. in that they created a porous LbL film master via acidic exposure and used soft lithography methods to replicate the film’s porosity onto PDMS surfaces [45]. They cultured both human umbilical vein endothelial cells (HUVECs) and human artery endothelial cells (HAECs) on these porous substrates and observed an increase in cell migration and a decrease in cell proliferation when compared to flat substrates. Another strategy for generating porous LbL films has been proposed by Pauthe and Van Tassel by incorporating NPs as templates during film buildup, which are subsequently removed yielding highly porous films [46,47]. In a study investigating the potential of these films to serve as carriers for bone morphogenetic protein 2 (BMP-2), cells seeded on the BMP-2 loaded porous films demonstrated higher bioactivity than those seeded on non-porous films [46].
A.2.2. Patterning hydrophobicity
One approach exploits hydrophilicity and hydrophobicity properties of LbL films to generate patterned surface. Rubner et al. initially proposed utilizing the in-stability of H-bonded LbL films to create patterned surfaces, by exposing to water un-crosslinked regions of these films causing them to dissolve yielding micro-patterned surfaces [48]. Indeed, Lynn et al. developed superhydrophobic surfaces of PEI/ poly(2-vinyl-4,4-dimethylazlactone) (PVDMA) LbL films by treating them with n-decylamine [49]. Further treatment of these surfaces with d-glucamine converted these surfaces into highly hydrophilic films. Exploiting this behavior they created patterned surfaces with hydrophilic and hydrophobic regions [49] and patterns of model proteins [50].
A.2.3. Chemical gradients obtained by gradual immersion
By post treating PSS/PDAC multilayers with NaCl in a gradual immersion process, the team of Gao et al. has developed multilayers with gradually different swelling ratio while preserving their chemistry [51,52]. They observed that vascular smooth muscle cells (SMCs) migrated in the direction of low hydration, although this effect was only observed on an appropriate cell-seeding density [52]. Interestingly, their technique may be applied to any type of surface, opening the possibility of a multifunctional substrate. The same group generated swelling ratio gradients on LbL films constructed on a PDMS linear grooved substrate [51]. They concluded that the combined effect of topography and swelling gradient drives SMCs migration towards low swelling ratios without diminishing their migration rate.
Barrett and co-workers engineered LbL films with two-dimensional gradients in their physical properties and thickness using a PAH/ poly(acrylic acid) (PAA) system [53,54]. This was obtained by rotating the coated substrate during a gradual immersion process. They obtained films presenting gradients in wettability, thickness, and surface charge. They tested the viability of HEK 293 and embryonic rat spinal commissural neurons, and observed that both cell types preferred an environment of intermediate stiffness composed of moderately charged polyelectrolytes. The authors determined that of all the properties evaluated (wettability, surface charge, and stiffness), the stiffness plays a stronger role in dictating cell survival [54].
A.4. Stiffness patterning
Typical modulation of LbL film stiffness includes chemical crosslinking or photo-crosslinking. Micropatterning of LbL films by means of selective illumination has been first reported by Rubner’s and Zhang’s groups [48,55]. By incorporating photo-cross-linkable polymers such as diazo resins (DAR) or poly(acrylic acid-ran-vinylbenzyl acrylate) (PAArVBA) in LbL films, they investigated the patterning of crosslinked/non-cross-linked microfeatures by exposing the LbL film to ultraviolet (UV) irradiation through a photomask [56,57]. Recently these photopatterned LbL films have been used to guide cell adhesion. Chien et al. thus explored the potentiality of photo-crosslinked synthetic films based on PAA conjugated with 4-azidoaniline to control cell adhesion [58]. They used an alkaline solution to remove the uncrosslinked areas and cells were let to adhere on the underlying bare substrate. In this case, the photo-crosslinked films served as a physical barrier between cells and as a non-adhesive background. The stability of cell patterns could be modulated by modifying the surface chemistry. In addition, these authors showed the ability of these patterned films for co-culturing spatially segregated hepatocytes and fibroblasts.
In collaboration with our group, the Glinel group recently designed a photoreactive HA derivative [59], which can be assembled with PLL to build photo-sensitive multilayer films. These films can be photo-crosslinked through a photomask to create spatial patterns of rigidity (Fig. 2C). We showed that these micropatterns are chemically homogeneous and flat, without any preferential adsorption of adhesive proteins. By varying the pattern geometries, we studied how rigidity patterns impacted myoblast cell adhesion and spreading [60]. A similar strategy was used by Keller et al. to generate stiffness gradients [61] with (PAA/PAH) films, the PAH being grafted with photosensitive benzophenone. Using a gradient density filter that regulates the amount of UV that hits the film, they generated linear gradients with a range of 55 – 140 MPa, having either a shallow or steep slope. Both smooth muscle cells (SMCs) and osteoblast-like cells spread and adhered better on stiffer part of the gradients. Interestingly, SMCs responded differently to the stiffness gradients depending on their slope. They elongated and oriented along the shallow gradient, whilst they migrated up the steep gradient.
For chemical crosslinking, the 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) crosslinking agent has been successfully used by our group and others for the modulation of film stiffness due to its simple chemistry and solubility in aqueous solvents [62]. Gradients of EDC would subsequently generate films containing stiffness gradients. Both microfluidics [63] and gradual immersion [64] have been successfully used to generate stiffness gradients on LbL films. Our team combined a simple microfluidic device with (PLL/HA) films to generate stiffness gradients, using EDC as the crosslinker [63]. We obtained a linear gradient over a range of 200-600 kPa, as confirmed with nanoindentation experiments. We observed that pre-osteoblastic cells responded to the stiffness gradients, where the number of attached cells and their area decreased with decreasing stiffness. On the other hand, Vasilev et al. used a gradual immersion technique with EDC to generate gradients on (PAA/PAH) films [64]. They obtained a gradient with a range of 0.5-100 MPa. They investigated the adhesion, morphology, and proliferation of human dermal fibroblasts on the stiffness gradients. They showed that cells preferentially attached to the stiffer end of the surface, and they exhibit an increase in proliferation rate, cell spreading, and cellular organization.
A.5 Surface gradients of biomolecules
Biochemical and biomechanical cues are presented to cells in the native extracellular environment as gradients. Bioactive molecules as growth factors or cytokines are indeed presented embedded in the ECM and, in the case of inflammatory response, their concentration is gradually increasing towards the wound site. Chemotaxis is a process by which the cells are driven to the site where they are requested, in response to a biochemical gradient. In an attempt of biomimetism, a strong effort is undergoing into the generation of spatially arranged biomaterials that captures the native ECM using the different methods cited above. On that end, LbL films serve as a suitable platform since both their mechanical and biochemical properties can be finely tuned with ease. A number of research groups are exploring the generation of continuous lateral gradients on surfaces coated with LbL films. Techniques available to generate gradients include microfluidics [25,63], gradual immersion [51,52,64], rotational gradual immersion [53,54], and photo-crosslinking [61]. However, these examples mainly focus in generating lateral gradients of physico-chemical properties of LbL films (swelling, stiffness, wettability, charge density, etc.) (see sections A.1.1, A.3.3., and A.4). Presently, our team has developed continuous gradients of a number of biomolecules on LbL films composed of PLL and HA using a microfluidic device. Firstly, LbL films with a gradient of the adhesive peptide RGD were generated by creating a gradient of poly(glutamic acid) (PGA) conjugated with RGD (PGA-RGD) [63]. We observed that myoblast spreading was affected by the gradient, demonstrating a linear decrease of cell area with decreasing concentration of RGD along the microchannel. Secondly, LbL films with a matrix-bound gradient of stromal cell-derived factor 1 (SDF-1) were developed to investigate its effect on cell migration velocities [65]. We observed a graded dependence, were cells had higher velocities at higher SDF-1 concentrations, which decreased with decreasing matrix-bound SDF-1. Lastly, individual or dual matrix-bound gradients of BMP-2 and -7 were generated, either each protein on its own, in parallel, or in an opposite manner [66]. The osteogenic differentiation behavior of myoblastic cells differed between the BMP-2 and BMP-7 gradients. On the BMP-2 gradients, the cells differentiated towards osteogenic cells in a nonlinear manner, while on the BMP-7 gradients the dose dependence towards differentiation was linear (Fig. 2D). Interestingly, a shift in the differentiation behavior of myoblasts cells was observed on both the BMP-2 and BMP-7 gradients, where at a certain BMP concentration myogenic markers commence to appear, while the osteogenic markers are continuously decreasing [66]. However, on the dual parallel gradients no myogenic marker is observed on both BMP-2 and BMP-7 matrix-bound gradients. The versatility of this microfluidic platform in combination with LbL films highlights the potential and importance of biomaterials with spatial properties.
B. Stimuli responsive LbL systems
In the last two decades, stimuli-responsive LbL films have been extensively studied to engineer “smart” coatings and capsules [10,13,67]. Different stimuli were thus reported to trigger a conformational change of these “smart” LbL films, with significant potential in the fields of drug delivery, tissue engineering and biosensors [12]. pH or ionic strength have thus been used to allow a cargo delivery only at specific locations while temperature was utilized to control cell attachment/detachment [10]. Other physical influences, such as magnetic field, ultrasound and light that are widely used in clinics were investigated as promising stimuli for controlling LbL capsules navigation and cargo release [68]. In this section, we will review the recent uses of LbL systems for engineering stimuli-responsive systems.
B.1 Chemically-triggered response
LbL films made of weak polyelectrolytes are strongly sensitive to pH and ionic strength, as these parameters interfere with the electrostatic balance between polycations and polyanions [69,70]. Each mechanism has been used as a triggering signal for pH-responsive drug delivery. A large number of ionizable moieties can confer pH-sensitivity, such as the carboxylic acid, amine, azo, phenylboronic acid, imidazole, pyridine, sulfonamide, and thiol groups [71,72]. Each pH-sensitive polyelectrolyte contains acidic or basic groups that respond to the environmental pH by either protonation or deprotonation. Protonation leads to stronger repulsion, inducing a swelling of the film resulting in increased permeability. Conversely, deprotonation decreases the interaction of polymers, causes shrinking and thus lowers the permeability. Of note, these changes of permeability are reversible. pH-responsive coatings and capsules have thus been extensively studied in the biomedical field because this factor can be easily controlled and targeted, both in vitro and in vivo. Indeed, the pH inside the body is highly regulated by various mechanisms at the tissue scale as well as at the cellular scale. For example, the pH changes along the gastrointestinal tract from the stomach (pH = 1-3) to the intestine (pH = 5-8) whereas tumor tissues or endosomes are slightly acidic (pH = 6.0-6.5). pH-responsive systems could thus be used for releasing drugs only in precisely targeted environments. Kim and co-workers were the first to investigate this possibility by developing a platform for the delivery of hydrophobic drugs conjugated to block copolymer micelles via pH-responsive LbL films [73]. Feng et al. recently demonstrated how powerful this approach could be by coating NPs with pH-responsive LbL films. The potential of these pH-responsive drug nanocarriers was systemically evaluated via multiple in vitro and in vivo studies of the release of the anticancer drug doxorubicin (DOX) [74,75]. They demonstrated that the drug release rate was pH-dependent and increased with the decrease of pH (< 10% at pH 7.4 but > 50% at pH 5.2, after 58h at 37°C). The DOX-loaded nanocarriers also exhibited a sustained intracellular DOX release and a prolonged DOX retention in the nucleus, thus showing a reduced toxicity and prolonged therapeutic efficacy. In vivo assays in healthy rats showed that DOX-loaded nanocarriers had longer systemic circulation time and good tissue compatibility. De Geest and co-workers also reported on the release of fluorescein isothiocyanate (FITC)-dextran from the capsules inside the cells by pH- and enzymatic-mediated degradation of LbL capsules [76]. These results, coupled with the capacity to be functionalized with targeting ligands, make LbL functionalized NPs and LbL capsules promising carriers for efficient and localized delivery of anticancer drugs and other therapeutic agents.
Similarly to pH, ionic strength is also an important parameter for tuning LbL films swelling [77,78], which allows to control loading and release of bioactive molecules [79].
B.2 Biologically-triggered response
B.2.1. Enzyme-responsive LbL systems
Insertion of enzymes into LbL films and capsules have been extensively studied [10,67] as this approach opens an avenue for developing biosensing and drug delivery systems. Enzymes such as organophosphorus hydrolase have thus been used to fabricate sensing coatings for easy optical detection of organophosphorus compounds in solutions [80,81]. But a large majority of the research in this domain has been focused on fabricating multilayers containing oxyreductase enzymes. These thin films can be prepared on electrode surfaces and, when immersed in a solution containing the specific substrates and electrical mediators, the oxyreductase catalyzes the conversion of the substrates from the oxidized to the reduced state and from reduced to the oxidized state. A wide variety of enzymes have been incorporated in LbL films to form biosensors, such as glucose [82–85], lactate [86,87], pyruvate [88] or cholesterol[89,90] oxidases, horseradish peroxidase (HRP) [91,92] or uricase [93]. Due to this versatility, analytes measured by these sensors cover a large range of biological or other related substances such as glucose [94], hydrogen peroxide [92], cholesterol [90], uric acid [93] and pollutants [95]. As applications to diagnose and follow diabetes account for approximately 85% of the world market for biosensors [96], glucoses-sensitive LbL films and capsules have been the most widely studied enzyme-sensitive systems. Qi et al. thus engineered glucose-sensitive LbL capsules by coating glucose oxidase (GOx) and catalase on insulin particles alternately [97]. When these capsules were incubated with external glucose, the release rate of insulin increased markedly. By using LbL films composed of insulin, GOx, and poly(2-(dimethylamino)ethyl methacrylate) poly(DMAEMA), Li’s group demonstrated the possibility of finely tuning insulin release profiles in function of the glucose exposure as well as the potential of this system for controlling glycemic levels in vivo in a diabetic rat model [83,84]. This system paves the way to better controlling glucose levels for diabetic patients without relying on patient compliance and without any adverse inflammatory response.
B.2.2.Targeting
The delivery of loaded cargo to a specific and designated site is the first step in drug delivery. Designing of proper targeting is thus crucial and several approaches have been developed. Antigen–antibody interactions have been the first to be explored, as such interactions are very specific. Caruso et al. thus incorporated anti-immunoglobulin G (IgG) monoclonal antibodies into LbL films before investigating their subsequent interaction with IgG [98]. More recently, they demonstrated the specific binding and uptake of A33 antigen-targeted LbL coated particles and capsules to colorectal cancer cells [99,100].Other receptors have also been widely used as targets, depending on the cells or tissues of interest. Liver parenchymal cells have thus been successfully targeted by glycans-presenting LbL capsules, as these cells express receptors presenting lectins, proteins that recognize sugar complexes [101–103]. Tumor-targeted systems have also been developed, targeting either folate receptors [104] or CD44 glycoproteins [105,106], as these receptors are overexpressed on many cancer cell membranes.
B.3. Physically-triggered response
B.3.1. Temperature-responsive LbL systems
Poly(N-isopropylacrylamide) (PNIPAAm) and elastin-like recombinamers (ELRs) are the most used thermo-responsive polymers for conferring thermo-responsiveness to LbL films. Upon heating above 32°C, PNIPAAm sharply changes from a hydrophilic to a hydrophobic state [107]. Serpe et al. were the first to apply the LbL technique to produce temperature responsive PNIPAAm-co-acrylic acid/PAH coatings [108]. ELRs are genetically engineered peptide-based materials that mimic the structure of natural elastin [109]. Below a certain transition temperature, the free polymer chains are disordered, consisting of fully hydrated random coils. When the temperature is increased above the transition temperature, the structure loses the water molecules from hydrophobic hydration and the chains fold and assemble into a β-spiral conformation [110,111]. Multilayer films with temperature-responsive properties due to PNIPAAm or ERLs have been fabricated to control the cell attachment/detachment to a surface for cell sheet technology, drug delivery and to fabricate surfaces with acute wettability transitions [112–114].
B.3.2. Light-responsive LbL systems
Photosensitive LbL systems were first investigated by Sukhorukov’s, Möhwald’s and Caruso’s groups, who embedded organic (e.g. an azo dye) or inorganic (e.g. gold or silver NPs) chromophores in non-adsorbing LbL films [115–117]. Upon accumulation of light energy, the chromophore heats locally, thus increasing the permeability of the LbL films. This property has mostly been used in PEM capsules for triggering drug release [118] as well as encapsulation [119]. Of note, if local temperature is only slightly superior to the glass transition temperature of the LbL film, the drug is released without damaging the capsules, as the LbL network can self-seals upon switching off the irradiation [120]. Interestingly, as gold nanorods can have one or two absorption peaks depending on the aspect ratio, Skirtach et al. demonstrated the possibility of selective release from capsules containing different payloads [121].
Several groups thus investigated the effects of light-triggered drug release from LbL capsules [122] on cancer cells [123,124] and fibroblasts [125], highlighting the possibility of using LbL capsules for local drug delivery in vivo and biomedical application. Thus, by embedding both gold and magnetite NPs in the shell of LbL capsules, Gorin et al. paved the way to remotely addressing and locally activating drug-loaded microcapsules [126].
B.3.3. Electrically-responsive LbL systems
Electrical control of multilayer buildup [127,128], disassembly [129–132], morphology [133] or loading/release [134,135] have been widely studied as such electrically-controlled systems have strong potential for biomedical applications. Indeed, the release of a drug can be induced by switching on or off an electric current, which is an attractive option for actively and remotely controlling the release of a therapeutic from an implantable device (Fig. 3A). Hammond’s group started to explore this possibility with first a model system for delivering DXS [136] before designing multilayers made of gentamicin sulfate (GS), an antibiotic, and NPs of Prussian Blue (PB). As PB NPs are negatively charged in the absence of an applied potential but neutral at an anodic potential, the film can be destabilized by application of a small (< 1V) electric potential, triggering the release of the GS [134]. The control over the drug dosage is thus possible by tuning the film thickness as well as the magnitude of the applied voltage, whereas the drug release kinetics ranging from triggered burst release to on/off, or pulsatile release, is achieved by applying different electric potential profiles. The in vitro efficacy of the released GS was confirmed against Staphylococcus aureus bacteria, suggesting a strong potential for controlling release autonomously or remotely in implantable or transdermal controlled drug release devices.
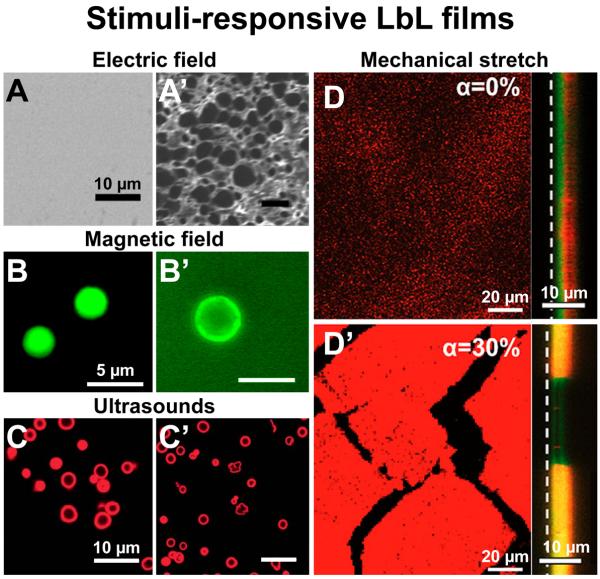
FIGURE 3. Illustrations of recent developments in stimuli-responsive LbL films.
(A) SEM images (top-view) of LPEI/PAA LbL films before and (A’) after 1h exposure to an electric field, inducing a strong change of the film permeability. (B) Fluorescence images of hybrid capsules formed with PSS/PAH, Fe3O4 nanoparticles and a DDAC bilayer membrane loaded with calcein before and (B’) after alternating magnetic field irradiation at 360 kHz and 234 Oe for 30 min, inducing the immediate release of the calcein. (C) Confocal images of BSARhodamine-loaded microcapsules of PSS/PAH before and (C’) after ultrasonic irradiation at 3.19 W for 5 min, inducing some partial and full ruptures of the microcapsules. (D) Confocal images of a (PLL/HA)/(PAH/PSS) incubated with trypsinRhodamine solution in a non-stretched and (D’) a 30% stretch state, inducing trypsinRhodamine diffusion across the PAH/PSS barrier and within the PLL/HA reservoir. Left images were acquired in (x,y) plane whereas right images were acquired in (x,z) plane at the reservoir–barrier interface. The white dashed line indicates the film-silicone substrate interface.
A, A’, B, B’, D and D’ adapted from [133], [142] and [155] with permission of the American Chemical Society. C and C’ adapted from [149] with permission of The Royal Society of Chemistry.
Recently, electrically active LbL films (biosensors) emerged with considerable interest for biomedical applications. Electroactive thin films have thus been developed for sensing glucose or hydrogen peroxide, by using GOx [21,137–139] or HRP [139,140].
B.3.4. Magneto-responsive LbL systems
By incorporating magnetic NPs within LbL films, several groups have recently demonstrated the possibility of targeting and triggering the drug delivery from LbL films or capsules. External magnetic fields have thus been used to change the permeability of microcapsules, either by embedding ferromagnetic cobalt NPs coated with gold shells into LbL capsule walls [141] or by using a more complex system made of LbL films, lipid bilayers, and Fe3O4 [142] NPs. Therefore, external magnetic fields may be used to “switch on” the unloading of these microcapsules (Fig. 3B). Similar techniques have been developed to induce a controlled rupture of magnetic NPs-loaded LbL microcapsules, leading to a slow and precise drug delivery [143], or even provoke the burst of these microcapsules, producing a local but massive release of the drug [144]. Gorin et al. reported the engineering of nanocomposite PEM microcapsules with both gold and magnetite nanoparticles in the shell. Iron oxide nanoparticles thus allowed for control over capsules positioning by external magnetic fields whereas laser irradiation leads to the opening of the capsules. These multifunctional microcapsules are of great interest for medical and biological applications [126].
B.3.5.Ultrasound-responsive LbL systems
Ultrasound-responsive systems for controlled drug delivery are a very attractive, non-invasive approach and have been successfully used for medical treatment and diagnosis. Several publications demonstrated the possibility of using high power ultrasound (100–500W, 20 kHz) for destroying LbL capsules or decreasing the permeability of their shell [145–148]. Nevertheless, it remains challenging to engineer LbL capsules sensitive to ultrasound at frequencies and power close to those appropriate in medical use (1–3W, 850 kHz). Even if the presence of NPs embedded in the shell of microcapsules strongly enhanced the ultrasonically-triggered release of encapsulated compounds, only a few publications demonstrated the possibility of releasing a cargo from microcapsules composed of polymers and 20 nm gold NPs upon exposition to ultrasounds close to these characteristics [149], thus opening up the possibilities for in vitro and in vivo use of ultrasound-responsive polyelectrolyte capsules (Fig. 3C).
B.3.6. Mechanically-responsive LbL systems
Over the last decade, Schaaf’s group investigated the use of mechanical stimuli to trigger a response from LbL films [150,151]. Recently, they engineered LbL films with strata playing the role of reservoir capped by strata blocking the diffusion of molecules outside of the reservoir. The reservoir strata are thick, hydrated gel-like structures allowing the loading and easy diffusion of active molecules [152] whereas the barrier strata are thinner, denser structures preventing diffusion of active molecules, polyelectrolytes and even of small ions [3]. Upon stretching, these multicompartment LbL films release their payload, opening an elegant avenue to applications as drug-releasing patches or biomechanically responsive implant coatings. The group thus demonstrated the possibility of modulating cellular adhesion [153], activating enzymatic catalysis [154] or to deliver an anticancer drug initially loaded within the architecture (Fig. 3D) [155]. Similarly, LbL microcapsule rupture and release of a cargo can be achieved by disrupting the shell membrane by mechanical deformation. This idea produced a new method of studying release and mechanical strength upon mechanical deformation that could provide information for optimizing microcapsules for intracellular delivery in particular and delivery in general (capsules that resist release due to mechanical stress during uptake, for instance) [156].
C. 3D assemblies using LbL
As suggested in the previous sections, LbL coatings have a tremendous potential for biomedical applications. In this section, we will review recent and innovative uses of multilayers for creating 3D devices. We will first discuss the encapsulation of biological elements such as viruses or cells, which pave the way to the isolation of injected elements from the host immune system while allowing proper nutrient and waste transport [157]. The possibility of producing multilayered membranes, especially as wound healing dressings, will then be addressed. Finally we will describe the latest developments in LbL-based tissue engineering and implant coatings. Of note, the use of multilayer-based capsules for drug delivery is mostly detailed in part B and will thus not be described here.
C.1. New 3D templates with nano- and micrometer size
Initially, researchers used mostly inert objects such as polystyrene (PS) beads or calcium carbonate cores to prepare polyelectrolyte microcapsules. In a recent past, new types of templates have been proposed, which enable to engineer more biomimetic objects (Figure 4) and to broaden the perspectives of applications of these nano- or micrometer size storage or delivery systems.
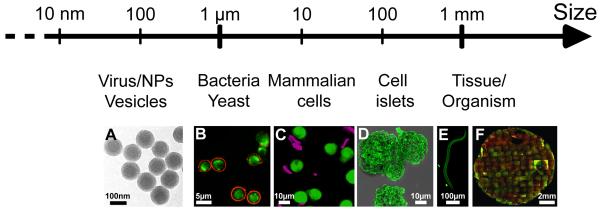
FIGURE 4. LbL coatings of 3D objects over a wide range of sizes.
Recent uses of the LbL technique to coat 3D objects from nanometric objects to millimer size tissues or organisms: A). polystyrene latex NPs, B) yeast, e.g. yEGFP-expressing S. cerevisiae, C) mammalian cells e.g. monocytes, D) cell islets e.g. pancreatic islets, E) organisms, e.g. nematodes C. elegans and F) millimetric engineered tissues, e.g. 3D tissue made of human dermal fibroblasts and HUVECs. A, C, D and E adapted from [106], [30], [178] and [245] with permission of the American Chemical Society. B adapted from [246] with permission of The Royal Society of Chemistry. F adapted from [247] with permission of Elsevier.
Coating of ~100 nm diameter lipid vesicles (or liposomes) by small interfering RNA (siRNA) and polyarginine (pARG) can be used to protect them from circulating enzymes, to enhance their half-life in the serum and to deliver siRNA to cells (Fig. 4A) [106]. These liposomes can be loaded with an anti-cancer drug, such as DOX, in order to kill cancer cells. In this case, the lipid vesicle does not need to be removed.
In contrary, removal of the template is needed to fabricate hollow microcapsules. In this field, new types of templates are currently being used. Living organisms such as bacteria may serve as template once dissolved after encapsulation. In 2001, Neu et al. already showed the dissolution of Escherichia coli (E. Coli) with NaOCl after sequential deposition of (PAH/PSS) layers [158]. As a result, by using different cell types (bacteria versus red blood cells), these authors obtained hollow capsules of varying size and shape. In 2012, Lederer et al. used a genetically modified and elongated E. coli as biotemplate [159]. The bacterial coating by (PAH/PSS)6 films and its subsequent dissolution led to polyelectrolyte tubes of 0.7 μm in diameter and 5-50 μm in length. These tubes were then functionalized by metallization and could be used as metal nanowires for electrical devices.
New shapes have been proposed with different aspect ratios. The aspect ratio of an object has been found to be of crucial importance in its internalization [160]. Discoidal or ellipsoidal templates, which enable to mimic one of the simplest but crucial cell of our body, the red blood cell (RBC) have been recently engineered. RBCs are characterized by their discoidal shape and ability to deform into narrow vessels called capillaries. Recently, using biconcave discoidal particles as template, Gao’s team succeeded in fabricating multilayer microcapsules mimicking the shape, size, and functionalities of RBCs [161]. They studied the capsule deformation and recovery processes through narrow capillaries and showed that these capsules can deliver oxygen when hemoglobin is assembled on their surface.
In the family of colloidal particles used as templates for LbL assemblies, the calcium carbonate cores are described to be attractive [162]. The group of H. Möhwald recently reported a variety of morphologies obtained by varying some parameters of the particle synthesis like the stirring time, pH and Na2CO3/CaCl2 concentration and ratio [163]. Spheroid-like, ellipsoid-like and cubic-like particles were used as template for LbL build-up and subsequently dissolved in EDTA solution. As a result, these hollow anisotropic microcapsules present high flexibility and loading capacity.
Using a different approach, Kharlampieva et al. fabricated capsules from cross-linked poly(methacrylic acid) (PMAA) multilayers using sacrificial discoid silicon templates [164]. These capsules exhibited pH-induced transitions from discoids to oblate ellipsoids. The degree of capsule shape transition was controlled by the pH-tuned volume change (from 7.4 to 4.0), which in turn was regulated by the capsule wall composition, PMAA only or PMAA/poly(N-vinylpyrrolidone) (PVPON) multilayer. These authors showed that the (PMAA/PVPON)5 discoidal capsules interacted differently with J774A.1 macrophages, HMVEC endothelial cells, and 4T1 breast cancer cells. The percentage of internalization of the capsules depended on their shape, the discoidal capsules exhibiting a 60% lower internalization as compared to the spherical ones. Using silica particles with various aspect ratio as templates, Caruso and co-workers reported the fabrication of PMAA capsules exhibiting different aspect ratios and studied the influence of the aspect ratio on internalization dynamics and intracellular fate [165]. They found that the internalization efficiency increased when the aspect ratio increased but that the capsules were always located in lysosomes.
These examples show that the LbL microcapsules of controlled shapes have a great potential as novel carrier for cellular uptake.
C.2 Encapsulated/LbL coated cells
Layer-by-layer encapsulation of living biological cells and other microorganisms is a promising tool for engineering cells with enhanced properties and artificial microorganisms (Figure 4). LbL bioencapsulation via sequential adsorption of oppositely charged functional nanoscale components may have several goals and applications: (1) encapsulation of viruses, (2) functionalization of isolated microorganisms, (3) functionalization of isolated mammalian cells and (4) encapsulation of cell aggregates, or formation of thick tissues [9]. However, it is important to keep in mind that the use of polymeric assemblies has to be carefully studied because of cytotoxicity of some cationic molecules that is time and concentration dependent [166,167].
C.2.1. Encapsulation of viruses
Numerous drug delivery systems are currently being developed. In this context, hollow LbL capsules made after core dissolution are one of the possibilities to release molecules at a specific target [67,68]. But natural templates may be used advantageously. For instance, viruses are already equipped with the delivery machinery and using them as a vehicle would be promising. Besides spherical viruses, the bacteriophage T7 was encapsulated in a polyelectrolyte/silica shell for further use in drug delivery [9]. The group of Aoyagi succeeded in using an LbL assembly for the decoration of hemagglutinating virus of japan envelope with HA and glycol CHI [168]. By optimizing the buildup conditions, the viruses maintained their fusion abilities and resisted to the low pH of endosomes. Virus degradation being an issue for vaccine administration, the coating of viruses with polymers may preserve them from premature degradation [169].
C.2.2. Encapsulation of bacteria/yeast
The diagnosis of bacteria as mycoplasma is limited by the poor success rate at culturing the bacteria from clinical samples. There is a need for innovative materials that could detect mycoplasma with high sensitivity. LbL films loaded with silver NPs were shown to act as a platform for the detection of Mycoplasma pneumoniae strains using surface enhanced Raman scattering (SERS) [170]. However, all bacteria are not deleterious. For instance, probiotics are “friendly” bacteria that protect us against harmful bacteria by maintaining an acidic environment. Thus, during therapeutic ingestion, these bacteria need to be protected from harsh environmental condition (Fig. 4B), like the gastric or intestinal pH or enzymes. LbL encapsulation of Lactobacillus acidophilus by CHI and carboxymethyl cellulose layers was shown to enhance survival of cells due to the impermeability of the coating to proteolytic enzymes [171]. Saccharomyces boulardii was trapped in CHI/DXS capsules as well [172]. Another example of living cell encapsulation was given by Flemke et al., who reported living E. coli encapsulation in microspheres made of (PSS/PAH) and proteins [173]. In this method, the cells were kept alive and a capsule was built around a 5 μm diameter porous calcium carbonate core containing the cells. After core dissolution by ethylenediaminetetraacetic acid (EDTA), the cells were trapped in the polyelectrolyte capsule. The technology may be useful for biomolecules or drug screening but needs to be improved as the cell viability only reached 40%.
C2.3. Encapsulation of mammalian cells for protection against the immune system
Using cells in regenerative medicine implies that their injection in the body, and in the case of allografts, lead to an immune response. Graft rejection being the major issue in cell therapy, there are now strategies to overcome the detection of implanted cells by the immune system. Cell encapsulation before injection is one solution to hide the cells from the immune system and prevent the risk of rejection. For instance, in the case of type I diabetes, the immune system seeks to destroy the insulin-producing islet cells that are transplanted into the patient. Isolating these islets by doing “immune-isolation” may facilitate the transplantation of pancreatic islets to restore glucose sensing and insulin secretion functions. Coating the cells would mask their surface, thereby eliminating the need for immunosuppressive therapy during transplantation. The first reported study of pancreatic islet encapsulation was published in 1980 by Lim and Sun [174]. The authors obtained prolonged survival by encapsulating living cells in cross-linked alginate microcapsules. Since then, several polymers have been developed to control the permeability, the thickness and the stability of the coating. The LbL technique was shown to be efficient for this application, as it relies on non-covalent interactions and avoids covalent conjugation to the islet surface. Indeed, covalent bounds are a process that is not suitable for long-term stability due to the fast degradation of the conjugation bounds and the damaging of membrane proteins [175]. The sequential deposition of cytocompatible PLL-graft-poly(ethylene glycol) (PLL-PEG) copolymers and ALG on pancreatic islets was an effective thin coating, maintaining viability and function upon transplantation [176,177]. Many efforts are made to decrease the thickness of the coating as a thick coating may impair the cell response and increase the size of the implant. To this purpose, Stabler et al. developed a hyper-branched ALG and poly(amidoamine) dendrimers that are deposited LbL on the pancreatic islets [178]. As a result, six bioorthogonal layers of polymers generated a stable ultrathin coating with unimpaired cell viability (Fig. 4D).
Besides, coatings able to modulate immune system by themselves are emerging. For instance, encapsulation of co-cultures of pancreatic cells and mesenchymal stem cells (MSCs) may help the pancreatic islets to escape cytokine-induced apoptosis [179]. Also, the use of a natural antioxidant as film building block leads to a cytoprotective coating by suppression of cytokine synthesis in vivo [180].
C2.4 Mimetic ECM coatings: from single cell to engineered tissues
Besides isolation from the immune system, cell encapsulation may be used to assemble cells into thicker and 3D tissues or to protect the cells from physical stresses, such as centrifugation. One of the challenges of 3D tissue engineering in vitro is that cells behave differently compared to in vivo. In particular, contact inhibition leads to an inhibition of cell proliferation. Cell coating would isolate the cells from each other and maintain them in a growing state. The group of Akashi developed a strategy to create 3D tissues by coating the cells with LbL films made of ECM proteins. In their initial studies, the surface of a fibroblast layer was coated by a LbL film made of fibronectin (FN)/gelatin (G) before depositing an additional cellular layer, in order to form a cellular multilayer [181]. Then, the process was changed to a single cell LbL coating of FN/G prior to cell deposition, a technique which they called the cell-accumulation technique. These tissues can even be vascularized by addition of HUVECs [182]. In 2013, mouse fibroblasts were embedded in the FN/G coating and cultured for 4 weeks [183]. The cells were able to proliferate and, as a consequence, the thickness of the tissue obtained after 4 weeks of culture increased from around 10 μm to 50 μm (i.e. ~ 5 times). Recently, the same group studied the mechanisms of in vitro vascularization using 3D tissues constructs by the cell accumulation technique. They succeeded in introducing blood and lymph capillaries into MSCs tissues (Fig. 4F) [184].
Also, they showed that the encapsulation of hepatocyte carcinoma cells in their ECM-based LbL films increased the cell viability when cells were submitted up to 18 cycles of centrifugation [185]. The protective effect of the films depended on their thickness, a minimum thickness of 5 nm being required. The protective effect of the coating was attributed to the inhibition of the leakage of cytoplasmic molecules during centrifugation. The cell accumulation technique offers interesting perspectives in the engineering of thick tissues.
C.3 Substrate-releasable assemblies
C.3.1. Free standing membranes
A LbL film may be a scaffold by itself, an independent biomaterial with its own shape and mechanical properties. A thick LbL film removed from its underlying substrate is called a free-standing (FS) film. FS membranes can be obtained by three major methods (Figure 5): i) using a sacrificial layer or ii) a supporting layer or iii) detaching the film by spontaneous release. The internal architecture of such FS membranes can be controlled during the film buildup and they are easily handled [13]. The application of FS films in biology and medicine is growing as they can serve as a platform for targeted cell culture with the possibility to be loaded with drugs for localized control of drug release. Thus the development of FS films may improve culture conditions by selecting surface chemistry or mechanical properties adapted to a cell line or a specific tissue [186]. They may also be employed as patches for regenerative medicine.
FIGURE 5. Methods for the production of LbL freestanding membranes made of polyelectrolytes or living cells.
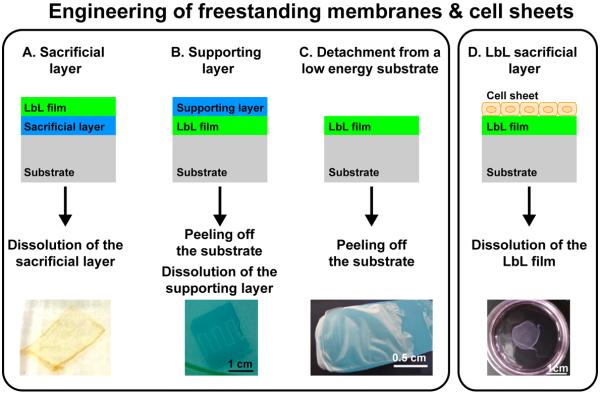
(A) The LbL films can be released from the substrate by dissolution of a sacrificial layer, (B) by peeling off the substrate using a dissolvable supporting layer or (C) by direct in case of low interactions between the LbL film and the underlying substrate. (D) A freestanding cell sheet can be produced by dissolution of a sacrificial LbL film, on top of which cells are forming a sheet. These freestanding membranes can be used for tissue engineering and repair. A, B and C adapted from [191], [194] and [197] with permission of the American Chemical Society. D adapted from [200] with permission of Elsevier.
Use of a sacrificial or supporting layer
The detachment of the films from a surface can be made by dissolution of a sacrificial layer between the film and the underlying substrate (Fig. 5A). Typically, organic solvents to dissolve or etch the substrate are used. However, these strategies using “harsh” chemicals are not compatible with the production of biomimetic scaffolds, which require mild conditions for their production. Nowadays, the sacrificial layer can be made of weak polyelectrolytes [187–189], or polysaccharides [190]. The dissolution of the sacrificial layer may be triggered by pH or light. For instance, H-bonded films made at low pH can be dissolved at neutral pH [191]. Similarly, visible light can be used to convert an anionic to a neutral polymer, thereby releasing the electrostatic interactions [192].
Alternatively, a supporting layer can be deposited on top of the LbL film (Fig. 5B). The film and the supporting layer are then peeled off from the substrate. After the dissolution of the supporting layer, a FS membrane is thus formed. By this method, 35 nm thick CHI/ALG nanosheets supported by a poly(vinylalcohol) (PVA) film were prepared and used to wound a perforated lesion [186]. Also, thin conductive FS films (<100 nm) were obtained after dissolution of a supporting layer of PVA [193]. These may be patterned to form electrodes on tissues or smart conductive substrates for guiding cell adhesion and differentiation [194].
Detachment from the substrate
The presence of a sacrificial layer and its subsequent dissolution may create mechanical or chemical defects in the LbL film. Therefore, another strategy was proposed. It consists in building the LbL film on a low energy surface such as Teflon or polypropylene (PP) [195]. This strategy appears particularly suitable for polysaccharide or polypeptides, which are more sensitive than synthetic polyelectrolytes. It was applied for the first time to polysaccharide-based LbL films (Fig. 5C) [196]. In addition, our group recently showed that polysaccharide-based FS membranes can be made by depositing CHI/ALG films onto a PPsubstrate and subsequently removing it after drying of the films, without any additional post-processing step [197]. The pH and concentration of the polyelectrolytes were tuned so that the thickness of the FS membranes could reach up to 35 μm. The FS membranes were permeable to model drugs (dextran FITC), which opens possibilities for their future use in drug delivery.
C.3.2. Cell sheets
A multilayer biomaterial may be composed itself of living cells. However, classical culture dishes are optimized for having strong cell/matrix adhesions. Thus, a confluent cell sheet usually cannot be detached and used as a biomaterial per se. The Okano group has developed cell sheets that are released from a thermo-responsive polymer after incubation at 20°C [198,199]. These cell sheets may be directly implanted in vivo and applied for heart or bone tissue engineering. Cell sheets may also be obtained using a PEM film as an underlying detachable substrate (Fig. 5D). For instance, PLL/HA films may be detached by ferrocyanide, which is not cytotoxic [200]. Synthetic PAH/PSS films onto which human gingival fibroblasts are cultured, may be detached by degradation of a sacrificial layer containing disulfide-containing polycation and polyanion [201]. This method provides chemically detachable platforms for tissue engineering and tissue repair.
For the fabrication of artificial biofilms, the LbL films offer the possibility to serve as a reservoir for living cells, such as yeast or bacteria. Artificial biofilms composed of cells embedded into the LbL film were constructed on a thin layer of calcium carbonate microparticles [202]. Then the particles are dissolved for the release of the “symbiotic” multicellular biofilms.
C.4 Localized delivery from naturalor synthetic scaffolds
The “bulk” of the multilayer film enables the incorporation of many bioactive molecules. In view of their versatility, LbL films are widely explored as drug delivery materials [7,12,13]. In vitro studies have largely proven that the release of bioactive components, such as growth factors, siRNA, or anti-bacterial agents from LbL films is possible in vitro [7]. Moving a step further in the control of the cell environment and regenerative medicine led the scientific community to the coating of 3D implants. As the role of implantable devices is to restore function by replacing damaged tissues, it is interesting to improve their integration by delivering appropriate signals from their surfaces. In regenerative medicine, there are many fields of applications of scaffolds coated with LbL films containing drugs. The main applications concern bone regeneration, vascular devices and neural electrodes (Figure 6), but others are also being developed such as insulin delivery [203] and ophthalmology [204,205]. In these areas, researchers have already reached the steps of pre-clinical development, i.e. in vivo experiments in small and large animals. Here, we limited this review to three major medical applications for neural prosthetic devices, vascular scaffolds and bone implants.
FIGURE 6. LbL coating for 3D scaffolds in vivo.
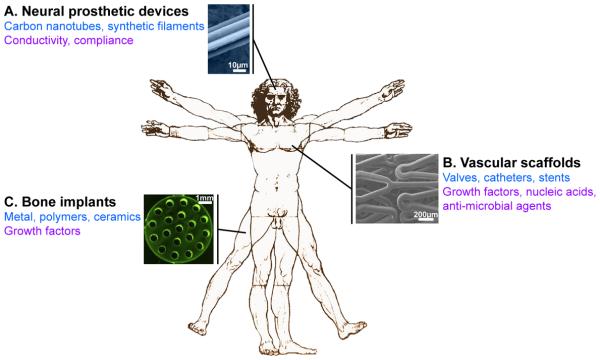
Three main medical applications are concerned by LbL functional coatings. (A) On neural prosthetic devices, the LbL coating provides conductivity and compliance to the carbon nanotubes or synthetic filaments (illustration of a neural electrode). (B) Vascular replacement or repair is approached by coating decellularized matrices, catheters or stents that deliver growth factors (VEGF), nucleic acids (SiRNA or plasmid DNA) or antimicrobial agents (β-peptide, Nitric oxide, cateslytin…) (illustration of a LbL-coated stent). (C) Bone regeneration is addressed by the delivery of BMP-2 from LbL-coated metal, polymer or ceramic implants (illustration of a titanium bone implant). In black: medical field; in blue: scaffolds used; in purple: biochemical, physical or mechanical signals provided by the LbL coating. A and C adapted from [207] and [242] with permission of Elsevier. B adapted from [216] with the permission of the American Chemical Society.
C.4.1. Neural prosthetic devices
Implantation of devices in our body is often a double-edged sword. Even though the beneficial effects have to be prevalent, the side effects of implantation like bleeding and scar tissue formation may affect the long term functionality of the implant. This is why a major interest is to mimic as close as possible the mechanical properties of the natural tissue to favor the integration. It is particularly true in the case of the brain tissue where the mechanical properties of the soft tissue (~ 1 kPa) are extremely different from those used in the prosthetic devices by ~8 orders of magnitude (e.g. ~100 GPa for metal nanowires). An optimal neural electrode would then be a compliant device with adapted electrical properties. The group of Kotov is particularly active in this area. They developed tissue-compliant carbon nanotubes (CNTs) with electrochemical performance particularly suitable for neuroprosthetic devices [206]. The flexible neural electrodes were made of by LbL-assembled nanotubes coated with PSS/PVA. The advantages of LbL-assembled CNTs were improved adhesion, strength, flexibility, conductivity, and impedance of the conductive films [206]. For further functionalization of neural implants and gene silencing, SiRNA may be introduced as nanoparticles in a LbL film and be delivered over several days during degradation of the LbL film coating (Fig. 6A) [207].
Such coatings provide a direct bioactive action on the neighboring cells and are highly innovative for the treatment of spinal cord injuries. To date, more studies are needed as the biocompatibility and the absence of inflammatory response in vivo should be further assessed.
C.4.2. Vascular scaffolds
For vascular applications, the implants aim at restoring vascular physiology and integrity. It is expected that innovative vascular scaffolds will potentially overcome the limitations of traditional implants, such as the risk of late thrombosis, local inflammation or infection caused by the presence of the foreign body. Several strategies have been developed during the past years taking advantage of the possibility to incorporate bioactive molecules and to spatially control their delivery.
Decellularized tissue for valve replacement
Regarding heart valve tissue engineering, two major problems are faced post-implantation: the important inflammation and fibrosis, and the risk of blood coagulation. The use of bioprosthesis for heart valve replacement began around 10 years ago and decellularized valves are commercially available since 2004 [208]. However, the bare scaffolds have important drawbacks such as inflammation and thrombogenicity. In order to enhance the functionality of decellularized aortic heart valve, they may be coated with LbL film loaded with growth factors for a controlled release. De Cock et al., observed a 4 days sustained release of basic fibroblast growth factor (bFGF) and heparin (HEP) from a LbL coated aortic heart valve leaflets [209]. Similarly, the coating by a CHI/HEP multilayer significantly reduced leukocytes adhesion, erythrocyte hemolysis, and whole blood clotting time [210]. In an another study, a film made of HEP and vascular endothelial growth factor (VEGF) was found to improve the hemocompatibility of the decellularized valve with a sustained release of VEGF over 5 days [211].
Stents
The first stents were made from bare stainless steel and were perceived as foreign objects by the body that reacts by inflammatory response, ultimately causing hyperplasia, where the proliferation of vascular smooth muscle cells (SMCs) causes a re-narrowing of the vessel (in-stent restenosis) [212]. Subsequent thrombosis (late-stent thrombosis), due to the lack of endothelialization, remains a significant complication following the implantation of coronary stents [213]. One solution is to cover the stents with a coating delivering or presenting biomolecules minimizing the proliferation of SMCs and with an antithrombotic action, i.e. avoiding the adhesion of the circulating platelets. Furthermore, the coating has to be protective for the surrounding coronary environment but inert or biocompatible enough to overcome the risk of immune response. Drug-eluting stents were designed to solve these issues using mostly thick coatings (> 200 μm) of synthetic polymers as drug carriers [2]. The use of LbL films as a stent coating is taking a considerable step towards a better device integration (Figure 6). The use of HEP as an antithrombotic polysaccharide rendered them thromboresistant by providing anticoagulant properties [214], the incorporation of Sirolimus enhanced hemocompatibility by controlling the proliferation of endothelial cells [215,216] (Fig. 6B) and the loading of VEGF promoted re-endothelialization of the stent [217]. As an antithrombotic agent, the incorporation of nitric oxide donors has proven to reduce platelet adhesion [218]. As an alternative, the incorporation of antibodies into the LbL films is a strategy to reduce failures that may occur after stent implantation. The presentation of a monoclonal antibody directed against a platelet glycoprotein receptor reduced platelet adhesion and aggregation [219] and the display of anti-CD34 antibody promoted attachment of vascular cells and rapid endothelialization [214]. SiRNA and plasmid DNA delivery from LbL films was developed for the design of nonviral, gene-based approaches for prevention of post implantation complications or treatment of cardiovascular disease, and were called gene-eluting stent. For example, a gene of interest may be delivered via siRNA nanocomplexes incorporated in a LbL film [220] or be released from a LbL film made of a hydrolytically degradable poly(β-amino ester) [221] like the plasmid DNA encoding for protein kinase C to reduce hyperplasia [222]. Moreover, competitiveness of endothelial cells over SMCs was enhanced by delivery of plasmid DNA coding for hepatocyte growth factor (HGF) [223].
Catheters: antifungal coatings
Bacterial infection is one of the most common problems that lead to complications of blood-contacting biomedical devices such as catheters [224]. Drug delivery systems are required to functionalize medical tubing most usually made of PDMS, polyurethane (PU) or polyethylene (PE). In this section, we describe how the LbL method demonstrated its effectiveness in catheter coating for antimicrobial activity. To note, infection may not be limited to vascular devices but also to other implantable devices, such as bone implants.
Hospital acquired infections are often associated with the insertion of medical devices, catheters in particular. They represent the point of entry of the pathogens in the patient and increase dramatically the mortality rate [225]. Functionalization of the catheter tubes with an antifungal agent loaded in a LbL film made of PLL/PGA led to a decrease in the biofilm formed by the pathogen Candida albicans [226,227]. PLL/PGA films loaded with an antifungal β-peptide can provide an antifungal activity to any PE or PDMS tubing, after deposition of a pre-coating layer of branched PEI to prevent film detachment from the polymeric surface. LbL films can thus serve as drug reservoir and are able to overcome the non-adhesive properties of the polymeric tubing and well known fouling properties of PDMS.
Likewise, the antimicrobial agent cateslytin was incorporated in a CHI/HA films by modification of the HA [228]. The peptide was released during the degradation of the LbL film by the hyaluronidase secreted by the pathogens (S. aureus and C. albicans). This method could be applied as an antibacterial and antifungal coating of medical device. Besides antimicrobial peptides, superoxide ions also exhibit antibacterial activity. The group of Meyerhoff showed, using organoselenium containing films, that it is possible to generate superoxide ions and to kill a broad spectrum of bacteria [218].
C.4.3. Bone regeneration
Since 2010 and our first review on the topic [7], the main evolution in the studies of bone tissue engineering appears to be the translation to in vivo studies. As in the vascular devices field, a particularity is that all kinds of materials, i.e. metals, polymers (synthetic or natural) and ceramics are used as implantable materials in the repair of bone defects, fractures and consolidation. As LbL films can be deposited on any kind of substrate, this renders them particularly attractive for modifying the surface of these implantable materials, especially for the incorporation of bioactive molecules. Molecules that can trigger bone growth are called osteoinductive. Growth factors from the BMP family are known for their osteoinductive properties and are already used in the clinics since 2003, in difficult situations where auto- or allografts are not possible. However, to date, the only approved use is a solution of BMP-2 in association with a collagen sponge, which has a very low retention capacity for the BMPs [229]. As a consequence, BMP-2 is rapidly released from the collagen sponge and there is no spatial control of its delivery. Besides, the dosage is supra-physiological and side effects such as ectopic bone growth, edema and pain have been reported [230]. To address this issue, several methods LbL using have been developed to deliver BMP-2 locally to cells [7]. Moreover, BMP-2 incorporation into LbL films may mimic the natural presentation of growth factors by the ECM. The two major strategies that were used are: i) BMP-2 loading as a regular layer in the film [231,232], depending on the properties of the polyelectrolytes, the films may be hydrolytically degradable as is the case for the poly(β)amino esters; ii) BMP-2 post-loading in crosslinked LbL film, which plays the role of a reservoir for the proteins [233].
Lastly, several proofs of concept were made in preclinical studies using small animals and relevant implantable biomaterials, especially those used already in clinical practice or currently in development. Dendri-graft of PLL may be used to enhance the number of interaction sites with BMP-2 and to act as “nanoreservoirs” [234]. Such films were deposited on poly(ε-caprolactone) scaffolds. The systems for BMP-2 delivery are then being built on either solid or porous scaffolds like polycaprolactone or collagen [235,236].
Hydrolytically degradable films are interesting in that they enable the trapping and subsequent release of different types of growth factors. Hammond and co-workers used a tetralayer system made of a first base of hydroxyapatite (HAP), CHI and PAA and a second cushion of BMP-2 in association with a poly(β)aminoester [237]. This coating was deposited on titanium and poly(etheretherketone) (PEEK) screw and implanted in the femur of rats. These authors showed that bone formation around the implant was increased in the presence of the BMP-2 containing film. More recently, these authors incorporated an additional growth factor in the film, the platelet-derived growth factor (PDGF) and deposited the coating on a poly(lactic-co-glycolic acid) (PLGA) supporting membrane [238]. They showed that both growth factors induced bone repair in a critical-size rat calvaria model as early as 2 weeks after implantation. This system would also enable the delivery of a growth factor and antibiotic. Data obtained in vitro have proven its feasibility [239] but further tests need to be done to assess the effectiveness in vivo.
Our group has developed a different strategy, which consists in loading BMP proteins in a polypeptide/polysaccharide film that is chemically crosslinked. Chemical crosslinking enhanced film stability and mechanical properties [62,240] but also enabled to tune the amount of proteins loaded and released from the films. Ceramic granules made of tricalcium phosphate (TCP) and HAP were used as bone fillers [241]. BMP-2 adsorbed onto LbL-coated granules could be stored and remained bioactive over at least 3 weeks. In vivo, both uncoated and LbL-coated TCP/HAP granules loaded with BMP-2 exhibited both osteoconductive and osteoinductive properties. Recently, we showed that BMP-2 containing films coated on porous titanium scaffolds are osteoinductive in vivo (Fig. 6C). In addition, the films were stable after drying and sterilization by exposure to gamma-irradiation [242]. Interestingly, they were still osteoinductive in vivo after drying and sterilization at 50 kGy.
Apart from the natural polymers often used (PLL, ALG, HA…) for BMP-2 delivery, the group of Kim developed a titanium coating made of graphene oxide layers as a carrier for the controlled delivery of BMP-2 where the hydrophobic domains of graphene oxide interacted with the proteins by hydrophobic π–π stacking [243]. Although not tested in vivo, this innovative model may pave the way for the development of orthopedic or dental implants coated with inorganic LbL films.
Instead of using BMP-2 as osteoinductive agent, another strategy consists in incorporating the BMP-2 gene in multilayers in order to induced BMP-2 production by cells. To this end, cationic lipids/BMP-2 plasmid DNA complexes and HA have been used [244]. Although being an interesting strategy, the result is not yet fully convincing as it only slightly accelerates bone formation.
CONCLUSIONS
Since the introduction of the LbL assembly technique in the 1990s, its versatility has led to a multitude of devices for fundamental studies, biomedical applications and up to clinical trials. By combining microfabrication techniques and bioactive molecules, the ability of spatially modulating the biochemical and mechanical properties of multilayers has thus been exploited in fundamental studies of cell-cell and cell-environment interactions, as well as biosensors and diagnostic devices (see part A). The possibility of precisely controlling the permeability of LbL films and capsules has also been extensively investigated, mostly to design stimuli-sensitive coatings and capsules, with significant potential in the field of drug delivery (see part B). Last but not least, the coating potentials offered by multilayers have been intensively used for protecting cells from the host immune system in the context of cell therapy as well as for functionalizing “traditional” implantable materials in the context of vascular surgery and orthopedics (see part C).
The use of LbL films for high throughput analysis is today only at its beginning but their ability to be precisely controlled and tuned should most probably lead to further developments in a near future. LbL-based substrates could indeed serve as well-defined, multi-functional models that complement the conventional cell cultures on plastic for the in vitro study of biological processes. By using natural polymers, LbL substrates also open an interesting avenue for complementing animal models; in particular as animal testing for cosmetics and chemicals are prohibited since 2013 under the 7th Amendment to the Cosmetics Directive (Council Directive 76/768/EEC) in the European Union. The ability to create ECM-like environments that mimic the structural and functional properties of the ECM in vivo would indeed enable design of accurate in vitro models that could be used for high throughput studies of fundamental biology as well as to examine the functional effects of patient-specific mutations and develop cell-based therapies.
The possibility of loading and delivering growth factors and biomolecules, including VEGF, BMP, antibiotics, siRNA, which have been obtained by a large number of groups all over the word, are also particularly encouraging. A question that will also have to be solved in the future is the availability of these biomolecules and of the film components as medical grade products, as any medical application is possible if the products are not qualified. Besides, the film components themselves will have to be approved by regulatory agencies and researchers will need to show that there is no specific negative effects due to the LbL films used as carrier.
Although still mostly in vitro proofs of concepts, even though some are already in clinical trials, this adaptable bioactivity provided by LbL systems paves the way to in vitro diagnosis and, on a longer term, to clinical translation. Nevertheless, the translation of biomedical devices from the bench to the clinics takes time (10-20 years) and can be strongly impacted by the regulatory issues decided by the national regulatory agencies. Moreover, the development of LbL-based devices requires very diverse fields of expertise, from chemistry to biology, material sciences and medicine. Large, transdisciplinary collaborations as well as the involvement of funding agencies, biotechnology companies and companies that produced implants is thus now crucial for fully exploiting the biomedical potential of LbL systems, either 3D engineered tissues, bioactive LbL coated implants or encapsulated cells for cell-based therapies.
Acknowledgments
This work was supported by the European Commission (under FP7) via an ERC starting grant (BIOMIM, GA 259370) and by the Association Française contre les Myopathies (AFM telethon, project n°316530). CM is indebted to AFM telethon for providing a post-doctoral fellowship (n°16673).
References
- [1].Takahashi H, Letourneur D, Grainger DW. Biomacromolecules. 2007;8:3281. doi: 10.1021/bm700540p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Farhatnia Y, Tan A, Motiwala A, Cousins BG, Seifalian AM. Biotechnol. Adv. 2013;31:524. doi: 10.1016/j.biotechadv.2012.12.010. [DOI] [PubMed] [Google Scholar]
- [3].Decher G. Science. 1997;277:1232. (80-. ) [Google Scholar]
- [4].Moya S, Sukhorukov G, Auch M, Donath E, Möhwald H. J. Colloid Interface Sci. 1999;216:297. doi: 10.1006/jcis.1999.6286. [DOI] [PubMed] [Google Scholar]
- [5].Lvov Y, Onda M, Ariga K, Kunitake T. J. Biomater. Sci. Polym. Ed. 1998;9:345. doi: 10.1080/09205063.1998.9753060. [DOI] [PubMed] [Google Scholar]
- [6].Tang Z, Wang Y, Podsiadlo P, Kotov NA. Adv. Mater. 2006;18:3203. [Google Scholar]
- [7].Boudou T, Crouzier T, Ren K, Blin G, Picart C. Adv. Mater. 2010;22:441. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- [8].Zelikin AN. ACS Nano. 2010;4:2494. doi: 10.1021/nn100634r. [DOI] [PubMed] [Google Scholar]
- [9].Fakhrullin RF, Lvov YM. ACS Nano. 2012;6:4557. doi: 10.1021/nn301776y. [DOI] [PubMed] [Google Scholar]
- [10].Deshmukh PK, Ramani KP, Singh SS, Tekade AR, Chatap VK, Patil GB, Bari SB. J. Control. Release. 2013;166:294. doi: 10.1016/j.jconrel.2012.12.033. [DOI] [PubMed] [Google Scholar]
- [11].Volodkin D, von Klitzing R, Moehwald H. Polymers (Basel) 2014;6:1502. [Google Scholar]
- [12].Shukla A, Almeida B. Nanomedicine and Nanobiotechnology. 2014;6:411. doi: 10.1002/wnan.1269. [DOI] [PubMed] [Google Scholar]
- [13].Costa RR, Mano JF. Chem. Soc. Rev. 2014;43:3453. doi: 10.1039/c3cs60393h. [DOI] [PubMed] [Google Scholar]
- [14].Eyckmans J, Boudou T, Yu X, Chen CS. Dev. Biol. 2011;21:35. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Elbert DL, Herbert CB, Hubbell JA. Langmuir. 1999;15:5355. [Google Scholar]
- [16].Charras G, Sahai E. Nat. Rev. Mol. Cell Biol. 2014 doi: 10.1038/nrm3897. DOI 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- [17].Iskratsch T, Wolfenson H, Sheetz MP. Nat. Rev. Mol. Cell Biol. 2014;15:1. doi: 10.1038/nrm3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Watt FM, Huck WTS. Nat. Rev. Mol. Cell Biol. 2013;14:467. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- [19].Reyes DR, Perruccio EM, Becerra SP, Locascio LE, Gaitan M. Langmuir. 2004;20:8805. doi: 10.1021/la049249a. [DOI] [PubMed] [Google Scholar]
- [20].Madaboosi N, Uhlig K, Schmidt S, Jäger MS, Möhwald H, Duschl C, Volodkin DV. Lab Chip. 2012;12:1434. doi: 10.1039/c2lc40058h. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Liu Y, Cheng Y, Kim E, Rubloff GW, Bentley WE, Payne GF. Adv. Mater. 2011;23:5817. doi: 10.1002/adma.201103726. [DOI] [PubMed] [Google Scholar]
- [22].Lee M, Park W, Chung C, Lim J, Kwon S, Ahn KH, Lee SJ, Char K. Lab Chip. 2010;10:1160. doi: 10.1039/b919753b. [DOI] [PubMed] [Google Scholar]
- [23].DeRocher JP, Mao P, Han J, Rubner MF, Cohen RE. Macromolecules. 2010;43:2430. [Google Scholar]
- [24].Kantak C, Beyer S, Yobas L, Bansal T, Trau D. Lab Chip. 2011;11:1030. doi: 10.1039/c0lc00381f. [DOI] [PubMed] [Google Scholar]
- [25].Kirchhof K, Andar A, Yin HB, Gadegaard N, Riehle MO, Groth T. Lab Chip. 2011;11:3326. doi: 10.1039/c1lc20408d. [DOI] [PubMed] [Google Scholar]
- [26].Castleberry SA, Li W, Deng D, Mayner S, Hammond PT. ACS Nano. 2014;8:6580. doi: 10.1021/nn501963q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Nano Lett. 2008;8:4446. doi: 10.1021/nl802404h. [DOI] [PubMed] [Google Scholar]
- [28].Doshi N, Swiston AJ, Gilbert JB, Alcaraz ML, Cohen RE, Rubner MF, Mitragotri S. Adv. Mater. 2011;23:H105. doi: 10.1002/adma.201004074. [DOI] [PubMed] [Google Scholar]
- [29].Swiston AJ, Gilbert JB, Irvine DJ, Cohen RE, Rubner MF. Biomacromolecules. 2010;11:1826. doi: 10.1021/bm100305h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Polak R, Crouzier T, Lim RM, Ribbeck K, Beppu MM, Pitombo RNM, Cohen RE, Rubner MF. Biomacromolecules. 2014;15:3093. doi: 10.1021/bm5006905. [DOI] [PubMed] [Google Scholar]
- [31].Ye C, Kulkarni DD, Dai H, Tsukruk VV. Adv. Funct. Mater. 2014;24:4364. [Google Scholar]
- [32].Andres CM, Kotov NA. J. Am. Chem. Soc. 2010;132:14496. doi: 10.1021/ja104735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Suntivich R, Shchepelina O, Choi I, Tsukruk VV. ACS Appl. Mater. Interfaces. 2012;4:3102. doi: 10.1021/am3004544. [DOI] [PubMed] [Google Scholar]
- [34].Suntivich R, Drachuk I, Calabrese R, Kaplan DL, Tsukruk VV. Biomacromolecules. 2014;15:1428. doi: 10.1021/bm500027c. [DOI] [PubMed] [Google Scholar]
- [35].Matsusaki M, Sakaue K, Kadowaki K, Akashi M. Adv. Healthc. Mater. 2013;2:533. doi: 10.1002/adhm.201200299. [DOI] [PubMed] [Google Scholar]
- [36].Roy CJ, Dupont-Gillain C, Demoustier-Champagne S, Jonas AM, Landoulsi J. Langmuir. 2010;26:3350. doi: 10.1021/la903121e. [DOI] [PubMed] [Google Scholar]
- [37].Lee YM, Jung B, Kim YH, Park a R., Han S, Choe W-S, Yoo PJ. Adv. Mater. 2014;26:3899. doi: 10.1002/adma.201305862. [DOI] [PubMed] [Google Scholar]
- [38].Monge C, Ren K, Berton K, Guillot R, Peyrade D, Picart C. Tissue Eng. Part A. 2012;18:1664. doi: 10.1089/ten.tea.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Palamà IE, D’Amone S, Coluccia AML, Gigli G. Biotechnol. Bioeng. 2013;110:586. doi: 10.1002/bit.24626. [DOI] [PubMed] [Google Scholar]
- [40].Alom Ruiz S, Chen CS. Soft Matter. 2007;3:168. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- [41].Jiang X, Zheng H, Gourdin S, Hammond PT. Langmuir. 2002;18:2607. [Google Scholar]
- [42].Hiller J, Mendelsohn JD, Rubner MF. Nat. Mater. 2002;1:59. doi: 10.1038/nmat719. [DOI] [PubMed] [Google Scholar]
- [43].Mendelsohn JD, Barrett CJ, Chan VV, Pal AJ, Mayes AM, Rubner MF. Langmuir. 2000;16:5017. [Google Scholar]
- [44].Hajicharalambous CS, Lichter J, Hix WT, Swierczewska M, Rubner MF, Rajagopalan P. Biomaterials. 2009;30:4029. doi: 10.1016/j.biomaterials.2009.03.020. [DOI] [PubMed] [Google Scholar]
- [45].McKee CT, Wood J. a, Ly I, Russell P, Murphy CJ. Biophys. J. 2012;102:1224. doi: 10.1016/j.bpj.2012.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Gand A, Hindié M, Chacon D, van Tassel PR, Pauthe E. Biomatter. 2014;4:e28823. doi: 10.4161/biom.28823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wu C, Aslan S, Gand A, Wolenski JS, Pauthe E, Van Tassel PR. Adv. Funct. Mater. 2013;23:66. [Google Scholar]
- [48].Yang SY, Rubner MF. J. Am. Chem. Soc. 2002;124:2100. doi: 10.1021/ja017681y. [DOI] [PubMed] [Google Scholar]
- [49].Manna U, Broderick AH, Lynn DM. Adv. Mater. 2012;24:4291. doi: 10.1002/adma.201200903. [DOI] [PubMed] [Google Scholar]
- [50].Manna U, Lynn DM. ACS Appl. Mater. Interfaces. 2013;5:7731. doi: 10.1021/am4026467. [DOI] [PubMed] [Google Scholar]
- [51].Han L, Mao Z, Wu J, Guo Y, Ren T, Gao C. Colloids Surf. B. Biointerfaces. 2013;111:1. doi: 10.1016/j.colsurfb.2013.05.011. [DOI] [PubMed] [Google Scholar]
- [52].Han L, Mao Z, Wu J, Guo Y, Ren T, Gao C. Biomaterials. 2013;34:975. doi: 10.1016/j.biomaterials.2012.10.041. [DOI] [PubMed] [Google Scholar]
- [53].Sailer M, Barrett CJ. Macromolecules. 2012;45:5704. [Google Scholar]
- [54].Sailer M, Lai Wing Sun K, Mermut O, Kennedy TE, Barrett CJ. Biomaterials. 2012;33:5841. doi: 10.1016/j.biomaterials.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [55].Shi F, Dong B, Qiu D, Sun J, Wu T, Zhang X. Adv. Mater. 2002;14:805. [Google Scholar]
- [56].Shi F, Wang Z, Zhao N, Zhang X. Langmuir. 2005;21:1599. doi: 10.1021/la0478393. [DOI] [PubMed] [Google Scholar]
- [57].Olugebefola SC, Ryu SW, Nolte AJ, Rubner MF, Mayes AM. Langmuir. 2006;22:5958. doi: 10.1021/la060315d. [DOI] [PubMed] [Google Scholar]
- [58].Chien H-WW, Chang T-YY, Tsai W-BB. Biomaterials. 2009;30:2209. doi: 10.1016/j.biomaterials.2008.12.060. [DOI] [PubMed] [Google Scholar]
- [59].Pózos-Vásquez C, Boudou T, Dulong V, Nicolas C, Picart C, Glinel K. Langmuir. 2009;25:3556. doi: 10.1021/la803577t. [DOI] [PubMed] [Google Scholar]
- [60].Monge C, Saha N, Boudou T, Pózos-Vásquez C, Dulong V, Glinel K, Picart C. Adv. Funct. Mater. 2013;23:3432. doi: 10.1002/adfm.201203580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Martinez JS, Lehaf AM, Schlenoff JB, Keller TCS, Keller TC., 3rd Biomacromolecules. 2013;14:1311. doi: 10.1021/bm301863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Richert L, Boulmedais F, Lavalle P, Mutterer J, Ferreux E, Decher G, Schaaf P, Voegel JC, Picart C. Biomacromolecules. 2004;5:284. doi: 10.1021/bm0342281. [DOI] [PubMed] [Google Scholar]
- [63].Almodóvar J, Crouzier T, Selimović Š, Boudou T, Khademhosseini A, Picart C. Lab Chip. 2013;13:1562. doi: 10.1039/c3lc41407h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hopp I, Michelmore A, Smith LE, Robinson DE, Bachhuka A, Mierczynska A, Vasilev K. Biomaterials. 2013;34:5070. doi: 10.1016/j.biomaterials.2013.03.075. [DOI] [PubMed] [Google Scholar]
- [65].Dalonneau F, Liu XQ, Sadir R, Almodovar J, Mertani HC, Bruckert F, Albiges-Rizo C, Weidenhaupt M, Lortat-Jacob H, Picart C. Biomaterials. 2014;35:4525. doi: 10.1016/j.biomaterials.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Almodovar J, Guillot R, Monge C, Vollaire J, Selimovic S, Coll JL, Khademhosseini A, Picart C. Biomaterials. 2014;35:3975. doi: 10.1016/j.biomaterials.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Delcea M, Mohwald H, Skirtach AG. Adv. Drug Deliv. Rev. 2011;63:730. doi: 10.1016/j.addr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- [68].Antipina MN, Sukhorukov GB. Adv. Drug Deliv. Rev. 2011;63:716. doi: 10.1016/j.addr.2011.03.012. [DOI] [PubMed] [Google Scholar]
- [69].Shiratori SS, Rubner MF. Macromolecules. 2000;33:4213. [Google Scholar]
- [70].Linford MR, Auch M, Möhwald H. J. Am. Chem. Soc. 1998;120:178. [Google Scholar]
- [71].Cabane E, Zhang X, Langowska K, Palivan CG, Meier W. Biointerphases. 2012;7:9. doi: 10.1007/s13758-011-0009-3. [DOI] [PubMed] [Google Scholar]
- [72].Kim S, Kim J-H, Jeon O, Kwon IC, Park K. Eur. J. Pharm. Biopharm. 2009;71:420. doi: 10.1016/j.ejpb.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kim B-S, Lee H-I, Min Y, Poon Z, Hammond PT. Chem. Commun. 2009:4194. doi: 10.1039/b908688a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Feng W, Zhou X, He C, Qiu K, Nie W, Chen L, Wang H, Mo X, Zhang Y. J. Mater. Chem. B. 2013;1:5886. doi: 10.1039/c3tb21193b. [DOI] [PubMed] [Google Scholar]
- [75].Feng W, Nie W, He C, Zhou X, Chen L, Qiu K, Wang W, Yin Z. ACS Appl. Mater. Interfaces. 2014;6:8447. doi: 10.1021/am501337s. [DOI] [PubMed] [Google Scholar]
- [76].De Geest BG, Vandenbroucke RE, Guenther a. M., Sukhorukov GB, Hennink WE, Sanders NN, Demeester J, De Smedt SC. Adv. Mater. 2006;18:1005. [Google Scholar]
- [77].Mjahed H, Voegel J-C, Senger B, Chassepot A, Rameau A, Ball V, Schaaf P, Boulmedais F. Soft Matter. 2009;5:2269. [Google Scholar]
- [78].Fery A, Schöler B, Cassagneau T, Caruso F. Langmuir. 2001;17:3779. [Google Scholar]
- [79].Wang X, Zhang L, Wang L, Sun J, Shen J. Langmuir. 2010;26:8187. doi: 10.1021/la904558h. [DOI] [PubMed] [Google Scholar]
- [80].Constantine CA, Gattás-Asfura KM, Mello SV, Crespo G, Rastogi V, Cheng T-C, DeFrank JJ, Leblanc RM. J. Phys. Chem. B. 2003;107:13762. [Google Scholar]
- [81].Constantine C. a, Mello SV, Dupont A, Cao X, Santos D, Oliveira ON, Strixino FT, Pereira EC, Cheng T-C, Defrank JJ, Leblanc RM. J. Am. Chem. Soc. 2003;125:1805. doi: 10.1021/ja028691h. [DOI] [PubMed] [Google Scholar]
- [82].Ding Z, Guan Y, Zhang Y, Zhu XX. Polymer (Guildf) 2009;50:4205. [Google Scholar]
- [83].Luo J, Cao S, Chen X, Liu S, Tan H, Wu W, Li J. Biomaterials. 2012;33:8733. doi: 10.1016/j.biomaterials.2012.08.041. [DOI] [PubMed] [Google Scholar]
- [84].Chen X, Luo J, Wu W, Tan H, Xu F, Li J. Acta Biomater. 2012;8:4380. doi: 10.1016/j.actbio.2012.08.014. [DOI] [PubMed] [Google Scholar]
- [85].Hodak J, Etchenique R, Calvo EJ, Singhal K, Bartlett PN. Langmuir. 1997;13:2708. [Google Scholar]
- [86].Sirkar K, Revzin A, Pishko MV. Anal. Chem. 2000;72:2930. doi: 10.1021/ac991041k. [DOI] [PubMed] [Google Scholar]
- [87].Xu J-J, Zhao W, Luo X-L, Chen H-Y. Chem. Commun. 2005:792. doi: 10.1039/b416548a. [DOI] [PubMed] [Google Scholar]
- [88].Simonian AL, Revzin A, Wild JR, Elkind J, Pishko MV. Anal. Chim. Acta. 2002;466:201. [Google Scholar]
- [89].Yang M, Yang Y, Yang H, Shen G, Yu R. Biomaterials. 2006;27:246. doi: 10.1016/j.biomaterials.2005.05.077. [DOI] [PubMed] [Google Scholar]
- [90].Ram MK, Bertoncello P, Ding H, Paddeu S, Nicolini C. Biosens. Bioelectron. 2001;16:849. doi: 10.1016/s0956-5663(01)00208-1. [DOI] [PubMed] [Google Scholar]
- [91].Sun C, Li W, Sun Y, Zhang X, Shen J. Electrochim. Acta. 1999;44:3401. [Google Scholar]
- [92].Rosca V, Catalin Popescu I. Electrochem. commun. 2002;4:904. [Google Scholar]
- [93].Hoshi T, Saiki H, Anzai J-I. Talanta. 2003;61:363. doi: 10.1016/S0039-9140(03)00303-5. [DOI] [PubMed] [Google Scholar]
- [94].Liu A, Anzai J-I. Anal. Bioanal. Chem. 2004;380:98. doi: 10.1007/s00216-004-2728-5. [DOI] [PubMed] [Google Scholar]
- [95].Sultana N, Schenkman JB, Rusling JF. J. Am. Chem. Soc. 2005;127:13460. doi: 10.1021/ja0538334. [DOI] [PubMed] [Google Scholar]
- [96].Turner APF. Chem. Soc. Rev. 2013;42:3184. doi: 10.1039/c3cs35528d. [DOI] [PubMed] [Google Scholar]
- [97].Qi W, Yan X, Fei J, Wang A, Cui Y, Li J. Biomaterials. 2009;30:2799. doi: 10.1016/j.biomaterials.2009.01.027. [DOI] [PubMed] [Google Scholar]
- [98].Caruso F, Niikura K, Furlong DN, Okahata Y. Langmuir. 1997;13:3427. doi: 10.1021/ac961220r. [DOI] [PubMed] [Google Scholar]
- [99].Cortez C, Tomaskovic-Crook E, Johnston APR, Radt B, Cody SH, Scott AM, Nice EC, Heath JK, Caruso F. Adv. Mater. 2006;18:1998. [Google Scholar]
- [100].Cortez C, Tomaskovic-Crook E, Johnston APR, Scott AM, Nice EC, Heath JK, Caruso F. ACS Nano. 2007;1:93. doi: 10.1021/nn700060m. [DOI] [PubMed] [Google Scholar]
- [101].Qi W, Wang A, Yang Y, Du M, Bouchu MN, Boullanger P, Li J. J. Mater. Chem. 2010;20:2121. [Google Scholar]
- [102].Zhang F, Wu Q, Chen Z-C, Zhang M, Lin X-F. J. Colloid Interface Sci. 2008;317:477. doi: 10.1016/j.jcis.2007.09.065. [DOI] [PubMed] [Google Scholar]
- [103].Yu X, Pishko MV. Biomacromolecules. 2011;12:3205. doi: 10.1021/bm200681m. [DOI] [PubMed] [Google Scholar]
- [104].Zhou J, Romero G, Rojas E, Ma L, Moya S, Gao C. J. Colloid Interface Sci. 2010;345:241. doi: 10.1016/j.jcis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- [105].Boudou T, Kharkar P, Jing J, Guillot R, Pignot-Paintrand I, Auzely-Velty R, Picart C. ASME Summer Bioeng. Conf. Fajardo, Puerto Rico: 2012. [Google Scholar]
- [106].Deng ZJ, Morton SW, Ben-Akiva E, Dreaden EC, Shopsowitz KE, Hammond PT. ACS Nano. 2013;7:9571. doi: 10.1021/nn4047925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schild H. Prog. Polym. Sci. 1992;17:163. [Google Scholar]
- [108].Serpe MJ, Jones CD, Lyon LA. Langmuir. 2003;19:8759. [Google Scholar]
- [109].Rodríguez-Cabello JC, Martín L, Alonso M, Arias FJ, Testera AM. Polymer (Guildf) 2009;50:5159. [Google Scholar]
- [110].Urry D, Shaw R, Prasad K. Biochem. Biophys. Res. 1985;130:50. doi: 10.1016/0006-291x(85)90380-8. [DOI] [PubMed] [Google Scholar]
- [111].Rodríguez-Cabello J. Chem. Phys. Lett. 2004;388:127. [Google Scholar]
- [112].Serpe MJ, Yarmey KA, Nolan CM, Lyon LA. Biomacromolecules. 2005;6:408. doi: 10.1021/bm049455x. [DOI] [PubMed] [Google Scholar]
- [113].Costa RR, Custodio CA, Arias FJ, Rodriguez-Cabello JC, Mano JF. Small. 2011;7:2640. doi: 10.1002/smll.201100875. [DOI] [PubMed] [Google Scholar]
- [114].Martins GV, Mano JF, Alves NM. Langmuir. 2011;27:8415. doi: 10.1021/la200832n. [DOI] [PubMed] [Google Scholar]
- [115].Tao X, Li J, Mohwald H. Chemistry (Easton) 2004;10:3397. doi: 10.1002/chem.200400024. [DOI] [PubMed] [Google Scholar]
- [116].Radt B, Smith TA, Caruso F. Adv. Mater. 2004;16:2184. [Google Scholar]
- [117].Skirtach AG, Antipov AA, Shchukin DG, Sukhorukov GB. Langmuir. 2004;20:6988. doi: 10.1021/la048873k. [DOI] [PubMed] [Google Scholar]
- [118].Angelatos AS, Radt B, Caruso F. J. Phys. Chem. B. 2005;109:3071. doi: 10.1021/jp045070x. [DOI] [PubMed] [Google Scholar]
- [119].Bédard M, Skirtach AG, Sukhorukov GB. Macromol. Rapid Commun. 2007;28:1517. [Google Scholar]
- [120].Skirtach AG, Karageorgiev P, Bédard MF, Sukhorukov GB, Möhwald H. J. Am. Chem. Soc. 2008;130:11572. doi: 10.1021/ja8027636. [DOI] [PubMed] [Google Scholar]
- [121].Skirtach AG, Karageorgiev P, De Geest BG, Pazos-Perez N, Braun D, Sukhorukov GB. Adv. Mater. 2008;20:506. [Google Scholar]
- [122].Borges J, Rodrigues LC, Reis RL, Mano JF. Adv. Funct. Mater. 2014;24:5624. [Google Scholar]
- [123].Skirtach AG, Munoz Javier A, Kreft O, Kohler K, Piera Alberola A, Mohwald H, Parak WJ, Sukhorukov GB. Angew. Chemie. 2006;45:4612. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]
- [124].Munoz Javier A, del Pino P, Bedard MF, Ho D, Skirtach AG, Sukhorukov GB, Plank C, Parak WJ. Langmuir. 2008;24:12517. doi: 10.1021/la802448z. [DOI] [PubMed] [Google Scholar]
- [125].Palankar R, Skirtach AG, Kreft O, Bedard M, Garstka M, Gould K, Mohwald H, Sukhorukov GB, Winterhalter M, Springer S. Small. 2009;5:2168. doi: 10.1002/smll.200900809. [DOI] [PubMed] [Google Scholar]
- [126].Gorin DA, Portnov SA, Inozemtseva OA, Luklinska Z, Yashchenok AM, Pavlov AM, Skirtach AG, Mohwald H, Sukhorukov GB. Phys. Chem. Chem. Phys. 2008;10:6899. doi: 10.1039/b809696a. [DOI] [PubMed] [Google Scholar]
- [127].Rydzek G, Thomann JS, Ben Ameur N, Jierry L, Mesini P, Ponche A, Contal C, El Haitami AE, Voegel JC, Senger B, Schaaf P, Frisch B, Boulmedais F. Langmuir. 2010;26:2816. doi: 10.1021/la902874k. [DOI] [PubMed] [Google Scholar]
- [128].Van Tassel PR. Curr. Opin. Colloid Interface Sci. 2012;17:106. [Google Scholar]
- [129].Boulmedais F, Tang CS, Keller B, Vörös J. Adv. Funct. Mater. 2006;16:63. [Google Scholar]
- [130].Sato K, Kodama D, Naka Y, Anzai J. Biomacromolecules. 2006;7:3302. doi: 10.1021/bm060819q. [DOI] [PubMed] [Google Scholar]
- [131].Wang F, Li D, Li G, Liu X, Dong S. Biomacromolecules. 2008;9:2645. doi: 10.1021/bm800766t. [DOI] [PubMed] [Google Scholar]
- [132].Diéguez L, Darwish N, Graf N, Vörös J, Zambelli T. Soft Matter. 2009;5:2415. [Google Scholar]
- [133].Cho C, Jeon JW, Lutkenhaus J, Zacharia NS. ACS Appl. Mater. Interfaces. 2013;5:4930. doi: 10.1021/am400667y. [DOI] [PubMed] [Google Scholar]
- [134].Schmidt DJ, Moskowitz JS, Hammond PT. Chem. Mater. 2010;22:6416. doi: 10.1021/cm102578j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Zahn R, Vörös J, Zambelli T. Curr. Opin. Colloid Interface Sci. 2010;15:427. [Google Scholar]
- [136].Wood KC, Zacharia NS, Schmidt DJ, Wrightman SN, Andaya BJ, Hammond PT. Proc. Natl. Acad. Sci. USA. 2008;105:2280. doi: 10.1073/pnas.0706994105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zhang S, Yang W, Niu Y, Sun C. Sensors Actuators B Chem. 2004;101:387. [Google Scholar]
- [138].Liu G, Lin Y. Electrochem. commun. 2006;8:251. [Google Scholar]
- [139].Merchant SA, Tran TO, Meredith MT, Cline TC, Glatzhofer DT, Schmidtke DW. Langmuir. 2009;25:7736. doi: 10.1021/la9004938. [DOI] [PubMed] [Google Scholar]
- [140].Li W, Xian M, Wang Z, Sun C, Zhao M. Thin Solid Films. 2001;386:121. [Google Scholar]
- [141].Lu Z, Prouty MD, Guo Z, Golub VO, Kumar CSSR, Lvov YM. Langmuir. 2005;21:2042. doi: 10.1021/la047629q. [DOI] [PubMed] [Google Scholar]
- [142].Katagiri K, Nakamura M, Koumoto K. ACS Appl. Mater. Interfaces. 2010;2:768. doi: 10.1021/am900784a. [DOI] [PubMed] [Google Scholar]
- [143].Hu S-H, Tsai C-H, Liao C-F, Liu D-M, Chen S-Y. Langmuir. 2008;24:11811. doi: 10.1021/la801138e. [DOI] [PubMed] [Google Scholar]
- [144].Wang W, Liu L, Ju X-J, Zerrouki D, Xie R, Yang L, Chu L-Y. Chemphyschem. 2009;10:2405. doi: 10.1002/cphc.200900450. [DOI] [PubMed] [Google Scholar]
- [145].Shchukin DG, Gorin DA, Möhwald H. Langmuir. 2006;22:7400. doi: 10.1021/la061047m. [DOI] [PubMed] [Google Scholar]
- [146].De Geest BG, Skirtach AG, Mamedov AA, Antipov AA, Kotov NA, De Smedt SC, Sukhorukov GB. Small. 2007;3:804. doi: 10.1002/smll.200600441. [DOI] [PubMed] [Google Scholar]
- [147].Skirtach AG, De Geest BG, Mamedov A, Antipov AA, Kotov NA, Sukhorukov GB. J. Mater. Chem. 2007;17:1050. [Google Scholar]
- [148].Kolesnikova TA, Gorin DA, Fernandes P, Kessel S, Khomutov GB, Fery A, Shchukin DG, Möhwald H. Adv. Funct. Mater. 2010;20:1189. [Google Scholar]
- [149].Pavlov AM, Saez V, Cobley A, Graves J, Sukhorukov GB, Mason TJ. Soft Matter. 2011;7:4341. [Google Scholar]
- [150].Hemmerle J, Roucoules V, Fleith G, Nardin M, Ball V, Lavalle P, Marie P, Voegel JC, Schaaf P. Langmuir. 2005;21:10328. doi: 10.1021/la052157g. [DOI] [PubMed] [Google Scholar]
- [151].Mertz D, Hemmerle J, Mutterer J, Ollivier S, Voegel JC, Schaaf P, Lavalle P. Nano Lett. 2007;7:657. doi: 10.1021/nl062657+. [DOI] [PubMed] [Google Scholar]
- [152].Vodouhe C, Le Guen E, Garza JM, Francius G, Dejugnat C, Ogier J, Schaaf P, Voegel JC, Lavalle P. Biomaterials. 2006;27:4149. doi: 10.1016/j.biomaterials.2006.03.024. [DOI] [PubMed] [Google Scholar]
- [153].Davila J, Chassepot A, Longo J, Boulmedais F, Reisch A, Frisch B, Meyer F, Voegel J-CC, Mesini PJ, Senger B, Metz-Boutigue M-HH, Hemmerle J, Lavalle P, Schaaf P, Jierry L, Mésini PJ, Hemmerlé J. J. Am. Chem. Soc. 2012;134:83. doi: 10.1021/ja208970b. [DOI] [PubMed] [Google Scholar]
- [154].Mertz D, Vogt C, Hemmerle J, Mutterer J, Ball V, Voegel JC, Schaaf P, Lavalle P. Nat. Mater. 2009;8:731. doi: 10.1038/nmat2504. [DOI] [PubMed] [Google Scholar]
- [155].Barthes J, Mertz D, Bach C, Metz-Boutigue MH, Senger B, Voegel JC, Schaaf P, Lavalle P. Langmuir. 2012;28:13550. doi: 10.1021/la302550q. [DOI] [PubMed] [Google Scholar]
- [156].Fernandes PAL, Delcea M, Skirtach AG, Möhwald H, Fery A. Soft Matter. 2010;6:1879. [Google Scholar]
- [157].de Villiers MM, Otto DP, Strydom SJ, Lvov YM. Adv. Drug Deliv. Rev. 2011;63:701. doi: 10.1016/j.addr.2011.05.011. [DOI] [PubMed] [Google Scholar]
- [158].Neu B, Voigt A, Mitlöhner R, Leporatti S, Gao CY, Donath E, Kiesewetter H, Möhwald H, Meiselman HJ, Bäumler H. J. Microencapsul. 2001;18:385. doi: 10.1080/02652040010000398. [DOI] [PubMed] [Google Scholar]
- [159].Lederer FL, Günther TJ, Weinert U, Raff J, Pollmann K. Microb. Cell Fact. 2012;11:163. doi: 10.1186/1475-2859-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ross AM, Jiang Z, Bastmeyer M, Lahann J. Small. 2012;8:336. doi: 10.1002/smll.201100934. [DOI] [PubMed] [Google Scholar]
- [161].She S, Li Q, Shan B, Tong W, Gao C. Adv. Mater. 2013;25:5814. doi: 10.1002/adma.201302875. [DOI] [PubMed] [Google Scholar]
- [162].Parakhonskiy BV, Yashchenok AM, Konrad M, Skirtach AG. Adv. Colloid Interface Sci. 2014;207:253. doi: 10.1016/j.cis.2014.01.022. [DOI] [PubMed] [Google Scholar]
- [163].Yashchenok A, Parakhonskiy B, Donatan S, Kohler D, Skirtach A, Möhwald H. J. Mater. Chem. B. 2013;1:1223. doi: 10.1039/c2tb00416j. [DOI] [PubMed] [Google Scholar]
- [164].Kozlovskaya V, Alexander JF, Wang Y, Kuncewicz T, Liu X, Godin B, Kharlampieva E. ACS Nano. 2014;8:5725. doi: 10.1021/nn500512x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Shimoni O, Yan Y, Wang Y, Caruso F. ACS Nano. 2013;7:522. doi: 10.1021/nn3046117. [DOI] [PubMed] [Google Scholar]
- [166].Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. Biomaterials. 2003;24:1121. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- [167].Brunot C, Ponsonnet L, Lagneau C, Farge P, Picart C, Grosgogeat B. Biomaterials. 2007;28:632. doi: 10.1016/j.biomaterials.2006.09.026. [DOI] [PubMed] [Google Scholar]
- [168].Okada T, Uto K, Sasai M, Lee CM, Ebara M, Aoyagi T. Langmuir. 2013;29:7384. doi: 10.1021/la304572s. [DOI] [PubMed] [Google Scholar]
- [169].Ulanova LS, Isapour G, Maleki A, Fanaian S, Zhu K, Hoenen A, Xu C, Evensen Ø, Griffiths G, Nyström B. J. Appl. Polym. Sci. 2014;131 n/a. [Google Scholar]
- [170].Rivera-Betancourt OE, Sheppard ES, Krause DC, Dluhy R. a. Analyst. 2014;139:4287. doi: 10.1039/c4an00596a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Priya AJ, Vijayalakshmi SP, Raichur AM. J. Agric. Food Chem. 2011;59:11838. doi: 10.1021/jf203378s. [DOI] [PubMed] [Google Scholar]
- [172].Ben Thomas M, Vaidyanathan M, Radhakrishnan K, Raichur AM. J. Food Eng. 2014;136:1. [Google Scholar]
- [173].Flemke J, Maywald M, Sieber V. Biomacromolecules. 2013;14:207. doi: 10.1021/bm3016362. [DOI] [PubMed] [Google Scholar]
- [174].Lim F, Sun A. Science. 1980;210:908. doi: 10.1126/science.6776628. (80-. ) [DOI] [PubMed] [Google Scholar]
- [175].Teramura Y, Kaneda Y, Iwata H. Biomaterials. 2007;28:4818. doi: 10.1016/j.biomaterials.2007.07.050. [DOI] [PubMed] [Google Scholar]
- [176].Wilson JT, Cui W, Kozlovskaya V, Kharlampieva E, Pan D, Qu Z, Krishnamurthy VR, Mets J, Kumar V, Wen J, Song Y, Tsukruk VV, Chaikof EL. J. Am. Chem. Soc. 2011;133:7054. doi: 10.1021/ja110926s. [DOI] [PubMed] [Google Scholar]
- [177].Mets JM, Wilson JT, Cui W, Chaikof EL. Adv. Healthc. Mater. 2013;2:266. doi: 10.1002/adhm.201200148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Gattas-Asfura KM, Stabler CL, Gattás-Asfura KM. ACS Appl. Mater. Interfaces. 2013;5:9964. doi: 10.1021/am401981g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Bhaiji T, Zhi Z-L, Pickup JC. J. Biomed. Mater. Res. A. 2012;100:1628. doi: 10.1002/jbm.a.34111. [DOI] [PubMed] [Google Scholar]
- [180].Kozlovskaya V, Zavgorodnya O, Chen Y, Ellis K, Tse HM, Cui W, Thompson JA, Kharlampieva E. Adv. Funct. Mater. 2012;22:3389. doi: 10.1002/adfm.201200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Matsusaki M, Kadowaki K, Nakahara Y, Akashi M. Angew. Chemie. 2007;46:4689. doi: 10.1002/anie.200701089. [DOI] [PubMed] [Google Scholar]
- [182].Nishiguchi A, Yoshida H, Matsusaki M, Akashi M. Adv. Mater. 2011;23:3506. doi: 10.1002/adma.201101787. [DOI] [PubMed] [Google Scholar]
- [183].Chetprayoon P, Kadowaki K, Matsusaki M, Akashi M. Acta Biomater. 2013;9:4698. doi: 10.1016/j.actbio.2012.08.019. [DOI] [PubMed] [Google Scholar]
- [184].Nishiguchi A, Matsusaki M, Asano Y, Shimoda H, Akashi M. Biomaterials. 2014;35:4739. doi: 10.1016/j.biomaterials.2014.01.079. [DOI] [PubMed] [Google Scholar]
- [185].Matsuzawa A, Matsusaki M, Akashi M. Langmuir. 2013;29:7362. doi: 10.1021/la303459v. [DOI] [PubMed] [Google Scholar]
- [186].Fujie T, Kinoshita M, Shono S, Saito A, Okamura Y, Saitoh D, Takeoka S. Surgery. 2010;148:48. doi: 10.1016/j.surg.2009.12.009. [DOI] [PubMed] [Google Scholar]
- [187].Dubas ST, Schlenoff JB. Langmuir. 2001;17:7725. [Google Scholar]
- [188].Ono SS, Decher G. Nano Lett. 2006;6:592. doi: 10.1021/nl0515504. [DOI] [PubMed] [Google Scholar]
- [189].Gui Z, Qian J, Du B, Yin M, An Q. J. Colloid Interface Sci. 2009;340:35. doi: 10.1016/j.jcis.2009.08.008. [DOI] [PubMed] [Google Scholar]
- [190].Mamedov AA, Kotov NA. Langmuir. 2000;16:5530. [Google Scholar]
- [191].Lee H, Sample C, Cohen RE, Rubner MF. ACS Macro Lett. 2013;2:924. doi: 10.1021/mz400398s. [DOI] [PubMed] [Google Scholar]
- [192].Pennakalathil J, Hong J. ACS Nano. 2011;5:9232. doi: 10.1021/nn203490q. [DOI] [PubMed] [Google Scholar]
- [193].Greco F, Zucca A, Taccola S, Menciassi A, Fujie T, Haniuda H, Takeoka S, Dario P, Mattoli V. Soft Matter. 2011;7:10642. [Google Scholar]
- [194].Greco F, Zucca A, Taccola S, Mazzolai B, Mattoli V. ACS Appl. Mater. Interfaces. 2013;5:9461. doi: 10.1021/am402142c. [DOI] [PubMed] [Google Scholar]
- [195].Lutkenhaus JL, Hrabak KD, McEnnis K, Hammond PT. J. Am. Chem. Soc. 2005;127:17228. doi: 10.1021/ja053472s. [DOI] [PubMed] [Google Scholar]
- [196].Larkin AL, Davis RM, Rajagopalan P. Biomacromolecules. 2010;11:2788. doi: 10.1021/bm100867h. [DOI] [PubMed] [Google Scholar]
- [197].Caridade SG, Monge C, Gilde F, Boudou T, Mano JF, Picart C. Biomacromolecules. 2013;14:1653. doi: 10.1021/bm400314s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Haraguchi Y, Shimizu T, Sasagawa T, Sekine H, Sakaguchi K, Kikuchi T, Sekine W, Sekiya S, Yamato M, Umezu M, Okano T. Nat. Protoc. 2012;7:850. doi: 10.1038/nprot.2012.027. [DOI] [PubMed] [Google Scholar]
- [199].Pirraco RP, Iwata T, Yoshida T, Marques AP, Yamato M, Reis RL, Okano T. Lab. Investig. 2014;94:663. doi: 10.1038/labinvest.2014.55. [DOI] [PubMed] [Google Scholar]
- [200].Zahn R, Thomasson E, Guillaume-Gentil O, Vörös J, Zambelli T, Voros J. Biomaterials. 2012;33:3421. doi: 10.1016/j.biomaterials.2012.01.019. [DOI] [PubMed] [Google Scholar]
- [201].Chassepot A, Gao L, Nguyen I, Dochter A, Fioretti F, Menu P, Kerdjoudj H, Baehr C, Schaaf P, Voegel J-C, Boulmedais F, Frisch B, Ogier J. Chem. Mater. 2012;24:930. [Google Scholar]
- [202].Konnova S. a, Kahraman M, Zamaleeva AI, Culha M, Paunov VN, Fakhrullin RF. Colloids Surf. B. Biointerfaces. 2011;88:656. doi: 10.1016/j.colsurfb.2011.07.056. [DOI] [PubMed] [Google Scholar]
- [203].Yoshida K, Hasebe Y, Takahashi S, Sato K, Anzai J-I. Mater. Sci. Eng. C. Mater. Biol. Appl. 2014;34:384. doi: 10.1016/j.msec.2013.09.045. [DOI] [PubMed] [Google Scholar]
- [204].Hu X, Hao L, Wang H, Yang X, Zhang G, Wang G, Zhang X. Int. J. Polym. Sci. 2011;2011:1. [Google Scholar]
- [205].Hu XH, Tan HP, Li D, Gu MY. Mater. Technol. 2014;29:8. [Google Scholar]
- [206].Zhang H, Patel PR, Xie Z, Swanson SD, Wang X, Kotov N. a. ACS Nano. 2013;7:7619. doi: 10.1021/nn402074y. [DOI] [PubMed] [Google Scholar]
- [207].Hartmann H, Hossfeld S, Schlosshauer B, Mittnacht U, Pego AP, Dauner M, Doser M, Stoll D, Krastev R. J. Control. Release. 2013;168:289. doi: 10.1016/j.jconrel.2013.03.026. [DOI] [PubMed] [Google Scholar]
- [208].Dohmen PM, Konertz W. Ann. Thorac. Cardiovasc. Surg. 2009;15:362. [PubMed] [Google Scholar]
- [209].De Cock LJ, De Koker S, De Vos F, Vervaet C, Remon J-P, De Geest BG. Biomacromolecules. 2010;11:1002. doi: 10.1021/bm9014649. [DOI] [PubMed] [Google Scholar]
- [210].Ye X, Hu X, Wang H, Liu J, Zhao Q. Acta Biomater. 2012;8:1057. doi: 10.1016/j.actbio.2011.11.011. [DOI] [PubMed] [Google Scholar]
- [211].Ye X, Wang H, Zhou J, Li H, Liu J, Wang Z, Chen A, Zhao Q. PLoS One. 2013;8:e54622. doi: 10.1371/journal.pone.0054622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Mani G, Feldman MD, Patel D, Agrawal CM. Biomaterials. 2007;28:1689. doi: 10.1016/j.biomaterials.2006.11.042. [DOI] [PubMed] [Google Scholar]
- [213].Tan A, Farhatnia Y, de Mel A, Rajadas J, Alavijeh MS, Seifalian AM. J. Biotechnol. 2013;164:151. doi: 10.1016/j.jbiotec.2013.01.020. [DOI] [PubMed] [Google Scholar]
- [214].Lin Q, Ding X, Qiu F, Song X, Fu G, Ji J. Biomaterials. 2010;31:4017. doi: 10.1016/j.biomaterials.2010.01.092. [DOI] [PubMed] [Google Scholar]
- [215].Shen L, Wu Y, Zhang F, Wu L, Dong C, Gao Y, Sun A, Zou Y, Qian J, Sun J, Zhong W, Ge J. Clin. Res. Cardiol. 2012;101:917. doi: 10.1007/s00392-012-0476-7. [DOI] [PubMed] [Google Scholar]
- [216].Su L-C, Chen Y-H, Chen M-C. ACS Appl. Mater. Interfaces. 2013;5:12944. doi: 10.1021/am403615q. [DOI] [PubMed] [Google Scholar]
- [217].Wang HG, Yin TY, Ge SP, Zhang Q, Dong QL, Lei DX, Sun DM, Wang GX. J. Biomed. Mater. Res. Part A. 2013;101:413. doi: 10.1002/jbm.a.34339. [DOI] [PubMed] [Google Scholar]
- [218].Cai W, Wu J, Xi C, Ashe AJ, Meyerhoff ME. Biomaterials. 2011;32:7774. doi: 10.1016/j.biomaterials.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Luo LL, Wang GX, Li YL, Yin TY, Jiang T, Ruan CG. J. Biomed. Mater. Res. A. 2011;97:423. doi: 10.1002/jbm.a.33066. [DOI] [PubMed] [Google Scholar]
- [220].Hossfeld S, Nolte A, Hartmann H, Recke M, Schaller M, Walker T, Kjems J, Schlosshauer B, Stoll D, Wendel HP, Krastev R. Acta Biomater. 2013;9:6741. doi: 10.1016/j.actbio.2013.01.013. [DOI] [PubMed] [Google Scholar]
- [221].Saurer EM, Jewell CM, Roenneburg DA, Bechler SL, Torrealba JR, Hacker TA, Lynn DM. Biomacromolecules. 2013;14:1696. doi: 10.1021/bm4005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Bechler SL, Si Y, Yu Y, Ren J, Liu B, Lynn DM. Biomaterials. 2013;34:226. doi: 10.1016/j.biomaterials.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Chang H, Ren K-F, Zhang H, Wang J-L, Wang B-L, Ji J. J. Biomed. Mater. Res. B. Appl. Biomater. 2014:1. doi: 10.1002/jbm.b.33224. [DOI] [PubMed] [Google Scholar]
- [224].Dwyer A. Semin. Dial. 2008;21:542. doi: 10.1111/j.1525-139X.2008.00499.x. [DOI] [PubMed] [Google Scholar]
- [225].Kojic EM, Darouiche RO. Clin. Microbiol. Rev. 2004;17:255. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Karlsson AJ, Flessner RM, Gellman SH, Lynn DM, Palecek SP. Biomacromolecules. 11:2321. doi: 10.1021/bm100424s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Raman N, Lee M-R, Palecek SP, Lynn DM. J. Control. Release. 2014;191:54. doi: 10.1016/j.jconrel.2014.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Cado G, Aslam R, Séon L, Garnier T, Fabre R, Parat A, Chassepot A, Voegel J-C, Senger B, Schneider F, Frère Y, Jierry L, Schaaf P, Kerdjoudj H, Metz-Boutigue M-H, Boulmedais F. Adv. Funct. Mater. 2013 n/a. [Google Scholar]
- [229].Geiger M. Adv. Drug Deliv. Rev. 2003;55:1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- [230].Carragee EJ, Hurwitz EL, Weiner BK. Spine J. 2011;11:471. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- [231].Leguen E, Chassepot A, Decher G, Schaaf P, Voegel JC, Jessel N. Biomol. Eng. 2007;24:33. doi: 10.1016/j.bioeng.2006.05.023. [DOI] [PubMed] [Google Scholar]
- [232].Macdonald ML, Samuel RE, Shah NJ, Padera RF, Beben YM, Hammond PT. Biomaterials. 2011;32:1446. doi: 10.1016/j.biomaterials.2010.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Crouzier T, Ren K, Nicolas C, Roy C, Picart C. Small. 2009;5:598. doi: 10.1002/smll.200800804. [DOI] [PubMed] [Google Scholar]
- [234].Mendoza-Palomares C, Ferrand A, Facca S, Fioretti F, Ladam G, Kuchler-Bopp S, Regnier T, Mainard D, Benkirane-Jessel N. ACS Nano. 2012;6:483. doi: 10.1021/nn203817t. [DOI] [PubMed] [Google Scholar]
- [235].Ferrand A, Eap S, Richert L, Lemoine S, Kalaskar D, Demoustier-Champagne S, Atmani H, Mély Y, Fioretti F, Schlatter G, Kuhn L, Ladam G, Benkirane-Jessel N. Macromol. Biosci. 2014;14:45. doi: 10.1002/mabi.201300283. [DOI] [PubMed] [Google Scholar]
- [236].Eap S, Ferrand A, Schiavi J, Keller L, Kokten T, Fioretti F, Mainard D, Ladam G, Benkirane-Jessel N. Nanomedicine. 2013;9:1253. doi: 10.2217/nnm.13.122. [DOI] [PubMed] [Google Scholar]
- [237].Shah NJ, Hyder MN, Moskowitz JS, Quadir M. a, Morton SW, Seeherman HJ, Padera RF, Spector M, Hammond PT. Sci. Transl. Med. 2013;5:191ra83. doi: 10.1126/scitranslmed.3005576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Shah NJ, Hyder MN, Quadir MA, Dorval Courchesne N-M, Seeherman HJ, Nevins M, Spector M, Hammond PT. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12847. doi: 10.1073/pnas.1408035111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Min J, Braatz RD, Hammond PT. Biomaterials. 2014;35:2507. doi: 10.1016/j.biomaterials.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Richert L, Engler AJ, Discher DE, Picart C. Biomacromolecules. 2004;5:1908. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- [241].Crouzier T, Sailhan F, Becquart P, Guillot R, Logeart-Avramoglou D, Picart C. Biomaterials. 2011;32:7543. doi: 10.1016/j.biomaterials.2011.06.062. [DOI] [PubMed] [Google Scholar]
- [242].Guillot R, Gilde F, Becquart P, Sailhan F, Lapeyrere A, Logeart-Avramoglou D, Picart C. Biomaterials. 2013;34:5737. doi: 10.1016/j.biomaterials.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].La W-G, Park S, Yoon H-H, Jeong G-J, Lee T-J, Bhang SH, Han JY, Char K, Kim B-S. Small. 2013;9:4051. doi: 10.1002/smll.201300571. [DOI] [PubMed] [Google Scholar]
- [244].Jiang Q-H, Liu L, Peel S, Yang G-L, Zhao S-F, He F-M. Clin. Oral Implants Res. 2013;24:853. doi: 10.1111/j.1600-0501.2011.02383.x. [DOI] [PubMed] [Google Scholar]
- [245].Minullina RT, Osin YN, Ishmuchametova DG, Fakhrullin RF. Langmuir. 2011;27:7708. doi: 10.1021/la2006869. [DOI] [PubMed] [Google Scholar]
- [246].Kozlovskaya V, Harbaugh S, Drachuk I, Shchepelina O, Kelley-Loughnane N, Stone M, Tsukruk VV. Soft Matter. 2011;7:2364. [Google Scholar]
- [247].Matsusaki M, Ajiro H, Kida T, Serizawa T, Akashi M. Adv. Mater. 2012;24:454. doi: 10.1002/adma.201103698. [DOI] [PubMed] [Google Scholar]