Abstract
Background:
Substantial evidence from human post-mortem and genetic studies has linked the neurotrophic factor neuregulin 1 (NRG1) to the pathophysiology of schizophrenia. Genetic animal models and in vitro experiments have suggested that altered NRG1 signaling, rather than protein changes, contributes to the symptomatology of schizophrenia. However, little is known about the effect of NRG1 on schizophrenia-relevant behavior and neurotransmission (particularly GABAergic and glutamatergic) in adult animals.
Method:
To address this question, we treated adult mice with the extracellular signaling domain of NRG1 and assessed spontaneous locomotor activity and acoustic startle response, as well as extracellular GABA, glutamate, and glycine levels in the prefrontal cortex and hippocampus via microdialysis. Furthermore, we asked whether the effect of NRG1 would differ under schizophrenia-relevant impairments in mice and therefore co-treated mice with NRG1 and phencyclidine (PCP) (3mg/kg).
Results:
Acute intraventricularly- or systemically-injected NRG1 did not affect spontaneous behavior, but prevented PCP induced hyperlocomotion and deficits of prepulse inhibition. NRG1 retrodialysis (10nM) reduced extracellular glutamate and glycine levels in the prefrontal cortex and hippocampus, and prevented PCP-induced increase in extracellular GABA levels in the hippocampus.
Conclusion:
With these results, we provide the first compelling in vivo evidence for the involvement of NRG1 signaling in schizophrenia-relevant behavior and neurotransmission in the adult nervous system, which highlight its treatment potential. Furthermore, the ability of NRG1 treatment to alter GABA, glutamate, and glycine levels in the presence of PCP also suggests that NRG1 signaling has the potential to alter disrupted neurotransmission in patients with schizophrenia.
Keywords: behavior, microdialysis, neuregulin 1, phencyclidine, schizophrenia
Introduction
Substantial evidence from human and animal studies has linked the neurotrophic factor neuregulin 1 (NRG1) to the pathophysiology of schizophrenia (Stefansson et al., 2002; Petryshen et al., 2005; Buonanno, 2010; Agim et al., 2013). The majority of identified polymorphisms are located in the non-coding region of the NRG1 gene. This suggests a change in NRG1 isoform expression levels, rather than a change in the amino acid sequence of the proteins themselves (Buonanno, 2010). Post-mortem analyses of NRG1 mRNA and protein expression levels in the prefrontal cortex (PFC) and hippocampus have reported increased, decreased, and unchanged levels of NRG1 isoforms and altered isoform type ratios in patients with schizophrenia (Hashimoto et al., 2004; Hahn et al., 2006; Law et al., 2006; Bertram et al., 2007; Chong et al., 2008; Barakat et al., 2010). While differences in NRG1 expression vary between patients, the findings suggest altered NRG1 signaling in the schizophrenia pathophysiology.
Genetic animal models with previously identified or novel mutations in the NRG1 gene have been developed (Lu et al., 2011). These modifications resulted in schizophrenia-relevant behavioral (e.g. hyperlocomotion and sensorimotor gating impairments) and neurochemical (e.g. altered glutamate and γ-aminobutyric acid [GABA] neurotransmission) impairments (Stefansson et al., 2002; Karl et al., 2007; Kato et al., 2010; Wen et al., 2010; Shamir et al., 2012; Luo et al., 2013; Mitchell et al., 2013; Yin et al., 2013). Furthermore, differences in brain NRG1 expression have been identified in several schizophrenia-relevant rodent models (Du Bois et al., 2012; Radonjić et al., 2013; Rhein et al., 2013; Swerdlow et al., 2013). The rodent studies indicate that changes to NRG1 signaling have functional consequences relevant to the schizophrenia pathophysiology.
The neurotrophic factor NRG1 is expressed throughout the nervous system, with expression levels strongly influenced by neuronal activity (Liu et al., 2011). Recent research has identified an involvement of NRG1 signaling in neurotransmission of the adult brain, such as GABA release (Woo et al., 2007; Wen et al., 2010), GABA receptor currents (Woo et al., 2007; Chen et al., 2010), and receptor expression levels (Okada and Corfas, 2004; Allison et al., 2011; Mitchell et al., 2013). Furthermore, glutamate release (Gu et al., 2005; Pitcher et al., 2011; Yin et al., 2013) and N-Methyl-D-aspartic acid (NMDA) receptor functions are also reportedly influenced by NRG1 signaling (Gu et al., 2005; Bjarnadottir et al., 2007; Bennett, 2009; Pitcher et al., 2011). The neurotransmission effects of NRG1 appear to be primarily mediated through the epidermal growth factor receptor tyrosine kinase ERBB4, but might also involve other epidermal growth factor receptor tyrosine kinase (ERBB) receptor isoforms (Iwakura and Nawa, 2013; Mei and Nave, 2014). ERBB4 is expressed on GABAergic interneurons and glia in the PFC and hippocampus (Gerecke et al., 2001; Longart et al., 2007; Calvo et al., 2010; Fazzari et al., 2010; Neddens et al., 2011), with other ERBB isoforms expressed throughout the central and peripheral nervous system (Iwakura and Nawa, 2013). The in vitro studies suggest that NRG1 signaling is involved in neurotransmission relevant to schizophrenia pathophysiology, potentially contributing to differences observed in the GABAergic and glutamatergic neurotransmitter systems in patients (Moghaddam and Javitt, 2012; Inan et al., 2013). However, the consequence of altered NRG1 signaling on schizophrenia-relevant neurotransmission differences has not yet been explored.
Genetic animal models and in vitro studies indicate a role for NRG1 signaling in schizophrenia, but how NRG1 affects schizophrenia-relevant behavior and neurotransmission (particularly GABAergic and glutamatergic) in adult animals is unknown. To address this question, we treated adult mice with the extracellular signaling domain of NRG1 and assessed spontaneous behavior (locomotor activity and acoustic startle response), as well as extracellular neurotransmitter levels (GABA, glutamate, and glycine). Furthermore, we asked whether the effect of NRG1 would differ under schizophrenia-relevant impairments in mice, since differences in NRG1 expression levels have been found in patients with schizophrenia. We therefore co-treated mice with NRG1 and phencyclidine (PCP), a commonly used substance for modeling schizophrenia-like behavioral and neurotransmission impairments in rodents (Adell et al., 2012; Javitt et al., 2012; Moghaddam and Krystal, 2012; Pratt et al., 2012). With our experiments we show that NRG1 treatment prevented PCP-induced impairments, which gives rise to the idea of NRG1 having antipsychotic-like properties.
Methods
Animals
Ten-week-old male C57BL6j mice were purchased from Animal Resource Centre for locomotor activity and microdialysis experiments and Australian BioResources for sensorimotor gating experiments. Animals were group-housed in open-lid cages with minimal enrichment (M1 polypropylene cages; Able Scientific) and maintained on a 12h light/dark cycle (light on at 0600 hours) with food and water available ad libitum in the animal facilities of the University of Wollongong (open field and microdialysis experiments) and Neuroscience Research Australia (sensorimotor gating experiments). All research and animal care procedures were approved by either the Animal Ethics Committee of the University of Wollongong (AE10/07 and AE11/18) or University of New South Wales (4149794) and were in agreement with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Mice were acclimatized for at least one week prior to experiments. For behavioral studies, testing order was pseudo-randomized to account for diurnal variations. For microdialysis studies, testing order was constant to allow the comparison of molecular responses.
Drugs
All compounds were obtained from Sigma except where specified otherwise. Drugs were freshly prepared on each treatment day. Recombinant human NRG1 (NRG1-β1/HRG1-β1 EGF domain CF; R&D Systems) and PCP were dissolved in either artificial cerebrospinal fluid (ACSF, 119mM NaCl; 2.5mM CaCl2; 2.5mM KCl; 1.3mM MgSO4; 1mM NaH2PO4; 26.2mM NaHCO3; 11mM glucose, 7.4 pH, 290 mOsm/kg) for i.c.v. injections and retrodialysis or in 0.9% NaCl (vehicle) for i.p. injections. For behavioral testing, each mouse received no more than three treatments with an inter-test interval of one week.
Open Field Behavior
The receptors for NRG1 are expressed throughout the nervous system and have also been found on muscle tissue (Zhu et al., 1995; Bersell et al., 2009; Wadugu and Kühn, 2012). Peripheral NRG1 treatment might therefore directly affect muscle cells, masking possible central nervous system responses. Independent open field experiments with i.c.v. and i.p. NRG1 administration were therefore performed.
Intracerebroventricular Cannula Implantation
Mice destined for i.c.v. treatment underwent surgical implantation of an i.c.v. cannula one week prior to experiments. Animals were anaesthetized with an inhalation 1.5% isoflurane (Delvet)/98.5% oxygen mix. Using a stereotactic frame (Kopf Instruments), a 21 G guide cannula was implanted into the brain, targeting the right lateral ventricle (Bregma -0.25mm rostral, -1mm medial, -1.5mm from top of skull; Paxinos and Franklin, 2001). The guide cannula was fixed to the skull with anchor screws (2mm, Microbiotech) and dental acrylic (Ketac Cem, Halas Dental Limited). All animals were injected with 0.1ml of a depot-antibiotic substance (Medicam, Boehriner Ingelheim) and additionally received it in their drinking water (0.1%) for three days following surgery. Dummy cannula (28 G), were inserted into the guide to keep it clean and prevent occlusion. Following surgery, mice were individually housed and their recovery was monitored daily until the behavioral experiments commenced.
Locomotion After Treatment
Mice were habituated to a round open field arena (40cm diameter) for 30min before i.p. and/or i.c.v. injections, and re-exposed to the open field for 30min thereafter. For the i.p. experiments, all animals (n = 8/group) received an i.p. injection of NRG1 (5, 25, or 50 µg/kg), PCP (3mg/kg), PCP + NRG1 (5, 25, or 50 µg/kg), or vehicle. For the i.c.v. experiments, all animals (n = 6–8/group) received an i.c.v. injection of NRG1 (3, 30, or 300ng/kg) or ACSF and simultaneously an i.p. injection of PCP (3mg/kg) or vehicle. NRG1 concentrations were selected below reported sedative levels (Snodgrass-Belt et al., 2005). Distance traveled was recorded before and after injections using the EthoVision tracking system (Noldus). Activity data were collected in 5min intervals, with individual post-treatment distance traveled normalized to the habituation performance.
Sensorimotor Gating
Sensorimotor gating (i.e. pre-pulse inhibition of startle response) was assessed as previously published (Karl et al., 2011). Briefly, one week before testing, mice (n = 8/group) were allowed 30min habituation to the prepulse inhibition (PPI) chambers at background noise levels (70 dB; SR-LAB; San Diego Instruments). Animals received an i.p. injection of NRG1 (5, 25, or 50 µg/kg), PCP (3mg/kg), PCP + NRG1 (5, 25, or 50 µg/kg), or vehicle directly following a 5min re-acclimatization period in the PPI chambers. Sensorimotor gating was tested in 100 trials over 25min, consisting of 3 startle response blocks and PPI response trails (pre-pulse intensities: 74 dB, 82 dB, and 86 dB),
Microdialysis and Amino Acid Quantification
Microdialysis was performed as described previously (Landgraf et al., 2003). Briefly, similar to the i.c.v. cannula implantation, a microdialysis guide cannula (MAB 6.14.IC Microbiotech/SE) was surgically implanted into either the medial PFC or dorsal CA1 to accommodate a microdialysis probe. Mice were individually housed in purpose-built microdialysis cages (25 x 25 x 40cm, grey Polyvinyl chloride (PVC) with clear front) for all experimental procedures, which allowed free access to food and water. After seven days of recovery, the microdialysis probe (1mm membrane length, 15kDa cut off, MAB 6.14.1, Microbiotech) was inserted into the guide cannula and lowered into the respective target area of the right mPFC/dCA1 2.5h before the first treatment. It was connected via low volume in- and outflow tubing (FEP-tubing, 4001004, Microbiotech; fluid swivel 375/D/22QM, Instechlabs) via a counter-balanced lever arm to a syringe pump (1 µl/min, Harvard Instruments). Microdialysis samples were collected in 10min intervals 2.5h after probe insertion. Animals received 10min infusions with NRG1 (10nM), PCP (10 µM), and NRG1 + PCP, separated by 2.5h of ACSF each. Potassium-stimulated neurotransmitter release (Stanford et al., 2000) and probe placement were confirmed before data analysis.
Gas Chromatography Mass Spectrometry Sample Processing and Analysis
Derivatization followed a previously published method (Eckstein et al., 2008). Briefly, samples were supplemented with heavy isotopes (20 pmole GABA-d6, 200 pmole Glycine-C13-2, N15, 2 pmole Glutamate-d5) and diluted in acetonitrile/methanol (1:1) before centrifugation. The supernatant was dried off at 37°C under nitrogen before derivatization with pentafluoropropionic acetic anhydride and 2,2,3,3,3-pentafluoro-1-propanol (PM Separations PTY). Following derivatization, samples were resuspended in toluene for analysis. Samples were analyzed using negative chemical ionization in a gas chromatograph with triple quad mass spectrometry (GC-MS/MS, 7000B & 7890A, Agilent Technologies). Specific multiple-reaction monitoring transitions were performed to monitor the glutamate ion transition m/z 379.0 → m/z 188.0, qualifier transitions (m/z 537.0 → m/z 313.0 and m/z 537.0 → m/z 217.0), and m/z 542 → m/z 221 for the corresponding isotopic d 5 labeled internal standard. GABA ion transition was detected at m/z 191.1 → 109.0, qualifier transitions (m/z 321.0 → m/z 301.0 and m/z 341.0 → m/z 208.0), and m/z 327 → m/z 306 for the corresponding isotopic d 6 labeled internal standard. Glycine ion transition was detected at m/z 333.0 → m/z 313.0, qualifier transitions at m/z 333.0 → 200.0 and m/z 365.0 → 232.0, and m/z 336.0 → m/z 316.0 for the corresponding isotopic C13-2 labeled internal standard. The area under the curve for peaks of GABA, glutamate, and glycine were normalized by their respective internal standard peaks, followed by normalization to the relevant baseline samples.
Statistical Analysis
Pre- and post-injection locomotor activity and startle following pulse-only trails were assessed by a one-way analysis of variance (ANOVA), followed by a Bonferroni’s multiple comparison post hoc analysis when required. Single time point differences in distance traveled were assessed by one-way ANOVA for repeated measurements, followed by Bonferroni’s multiple comparison post hoc analysis. Differences in PPI were assessed using two-way repeated-measures ANOVA (treatment x pre-pulse intensity), followed by Bonferroni’s multiple comparison post hoc analysis. Treatment effects on neurotransmitter levels were assessed using Linear Mixed Model (LMM) on repeated measurements for each brain area and transmitter following baseline normalization. Overall differences in extracellular neurotransmitter levels were assessed by calculating the area under the curve (AUC) for the entire sampling period using the trapezoidal rule. AUC and single time point differences were assessed by one-way ANOVA for repeated measurements, followed by Tukey’s post hoc analysis where appropriate. Analyses were performed using SPSS 19.0 (IBM). Statistical significance was accepted at p < 0.05 and data are presented as the mean ± standard error of the mean.
Results
NRG1 Treatment Does Not Alter Locomotor Activity, But Prevents PCP-Induced Hyperlocomotion
Increased spontaneous locomotor activity in the open field arena is considered to be an indirect measure of hyperdopaminergic and hypoglutamateric tone experienced by patients with schizophrenia during a psychotic episode (Adell et al., 2012) and reported from several NRG1 genetic animal models (Lu et al., 2011).
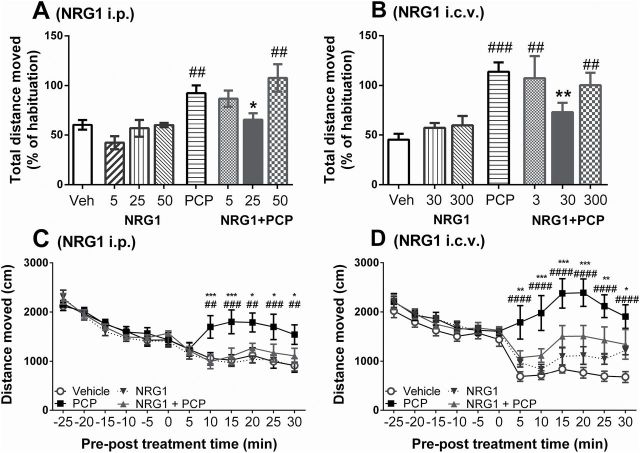
Locomotor activity differed significantly between treatment groups (F 7, 54 = 7.35, p < 0.001), with activity of i.p. NRG1-treated mice being similar to vehicle-treated mice (5 µg/kg: p = 0.22, 25 µg/kg and 50 µg/kg: p = 0.99 vs vehicle; Figure 1A). Mice treated with PCP showed an increase in locomotor activity (p = 0.003 vs vehicle; Figure 1A), consistent with previous studies (Gleason and Shannon, 1997; Mouri et al., 2011). Combining PCP with NRG1 i.p. altered the PCP-induced increase in locomotor activity in a concentration-dependent manner. NRG1 at 25 μg/kg, but not at 5 or 50 µg/kg, prevented the PCP hyperlocomotive effect (p = 0.02 vs PCP, 5 μg/kg: p = 0.99, 50 μg/kg: p = 0.79 vs PCP; Figure 1A).
Figure 1.
Peripheral and central administered neuregulin 1 (NRG1) prevents PCP-induced hyperlocomotion in mice. (A & B) Post-treatment distance moved as percentage of habituation locomotion for varying doses of i.p. NRG1 (A, µg/kg) or i.c.v. NRG1 (B, ng/kg), administered in combination with PCP (3mg/kg) or vehicle (A, 0.9% NaCl; B, artificial cerebrospinal fluid + 0.9% NaCl). Data is presented as mean percentage difference to habituation locomotion + standard error of the mean (n = 8/group). (C & D) Mean distance moved within 5min windows pre- and post-treatment ± standard error of the mean (n = 8/group) after (C, i.p.) vehicle, NRG1 (25 μg/kg), PCP, or NRG1 + PCP (25 μg/kg + 3mg/kg); (D, i.c.v. + i.p.) vehicle, NRG1 (30ng/kg + 0.9% NaCl), PCP (artificial cerebrospinal fluid + 3mg/kg), or NRG1 + PCP (30ng/kg + 3mg/kg). *p < 0.05, **p < 0.01 vs PCP; #p < 0.01, ##p < 0.001, ###p < 0.0001 vs vehicle.
One-way ANOVA also revealed a significant treatment effect on locomotor activity of i.c.v. treated mice (F 6, 43 = 6.6, p < 0.001). Locomotor activity following i.c.v. NRG1 treatment was similar to vehicle-treated animals (p = 0.99 for 30ng/kg and 300ng/kg vs vehicle; Figure 1B). PCP treatment again increased locomotor activity (p < 0.001 vs vehicle; Figure 1B). Similar to i.p. treatment, NRG1 i.c.v. altered the PCP effect in a concentration-dependent manner. NRG1 at 30ng/kg, but not at 3 or 300ng/kg, prevented PCP-induced hyperlocomotion (p = 0.024 vs PCP; 3ng/kg and 300ng/kg: p = 0.99 vs PCP; Figure 1B).
Comparing the distance traveled for each post-treatment interval via repeated-measure two-way ANOVA (i.p. NRG1 Treatment F 3, 28 = 2.93, p = 0.05; i.c.v. NRG1 Treatment F 3, 28 = 8.88, p < 0.001) showed that PCP treatment triggered hyperlocomotion within 10min post-treatment (p = 0.002 vs vehicle, Figure 1C and D). Combined treatment with either i.p. 25 μg/kg NRG1 (Figure 1C) or i.c.v. 30ng/kg NRG1 (Figure 1D) prevented the increase in locomotor activity from the treatment onset (Figure 1C and D), while NRG1 treatment in the absence of PCP did not alter locomotor activity.
NRG1 Treatment Prevents PCP-Induced Deficit of Pre-Pulse Inhibition of Startle
Patients with schizophrenia show impaired sensorimotor gating (Braff et al., 2001), which can be tested in rodents through inhibition of startle response experiments (Geyer et al., 2001). In our sensorimotor gating experiment, startle response to a 120 dB pulse was not affected by any of the treatments (F 7, 58 = 1.5, p = 0.18; Figure 2A). Pre-pulse intensity had a significant effect on PPI for vehicle-treated animals (F 1.2, 8.5 = 69.52, p < 0.001; Figure 2B). Furthermore, PPI differed significantly between treatment groups (F 6, 45 = 5.55, p < 0.001). NRG1 treatment at 25 μg/kg and 50 μg/kg had no effect on PPI performance (p > 0.8 vs vehicle), while 5 µg/kg NRG1 impaired the PPI effect at 74 and 82 dB pre-pulse intensity (p = 0.01 and p = 0.03 vs vehicle, respectively; Figure 2B). PCP treatment impaired PPI for all three pre-pulse intensities (74 dB: p = 0.01, 82 dB: p < 0.001, and 86 dB: p = 0.002 vs vehicle; Figure 2B), which is consistent with previous studies (Bakshi and Geyer, 1995; Yee et al., 2004). NRG1 prevented the PCP-induced disruption of PPI for pre-pulses of 82 and 86 dB in a dose-dependent manner (25 µg/kg NRG1 at 82 dB: p = 0.006; at 86 dB: p = 0.04; 50 µg/kg NRG1 at 82 dB: p = 0.02; Figure 2B). The ability of NRG1 to prevent PCP-induced sensorimotor gating impairment indicates an acute influence over several schizophrenia-relevant neurotransmitter systems.
Figure 2.
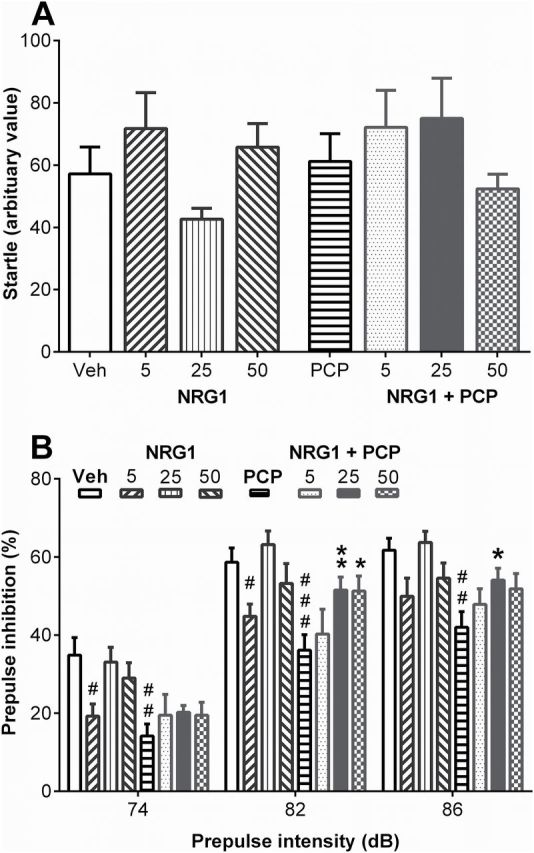
Peripheral neuregulin 1 (NRG1) injection prevents PCP-induced prepulse inhibition (PPI) impairment. Mice (n = 7–8/group) treated intraperitoneally with vehicle, NRG1 (5, 25, or 50 μg/kg), PCP (3mg/kg), or NRG1 + PCP (5, 25, or 50 μg/kg NRG1 + 3mg/kg PCP). (A) Treatments had no effect on startle response to 120 dB pulses (one-way analysis of variance, F 7, 58 = 1.502, p = 0.18). Values are presented as mean of startle intensity + standard error of the mean. (B) Treatment effect on PPI following different prepulse intensities. Values are presented as mean percentage of PPI + standard error of the mean. #p < 0.05, ##p < 0.01, ###p < 0.001 vs vehicle (veh); *p < 0.05, **p < 0.01 vs PCP.
NRG1 Reduces Glutamate and Glycine Levels in the Mouse PFC In Vivo
The microdialysis experiment was designed to assess the consequence of increased NRG1 signaling on schizophrenia-relevant neurotransmission under healthy and impaired conditions. We first measured GABA, glutamate, and glycine levels in the PFC following NRG1 retrodialysis and combined with PCP. The retrodialysis treatments had a significant overall effect on extracellular glutamate and glycine levels (LMM: glutamate: F 3, 145.8 = 11.41, p < 0.001; glycine: F 3, 145.1 = 6.85, p < 0.001), without affecting overall GABA levels (GABA: F 3, 123.9 = 0.55, p = 0.64). By comparing single time point GABA levels, we identified an increase 10min after NRG1 treatment (194.0±47.0% of baseline, p = 0.03; Figure 3A), which returned to baseline levels for the remaining sampling period. PCP infusion reduced extracellular GABA levels 20min after treatment (37.6±8.0% of baseline, p = 0.005; Figure 3A). Combined infusion of NRG1 + PCP prevented this reduction of extracellular GABA levels (104.2±45%, p > 0.99 vs baseline; Figure 3A). GABA levels expressed as AUC revealed that none of the three treatments affected overall GABA concentration across the sampling period (F 3, 18.6 = 0.9, p = 0.46; Figure 3B).
Figure 3.
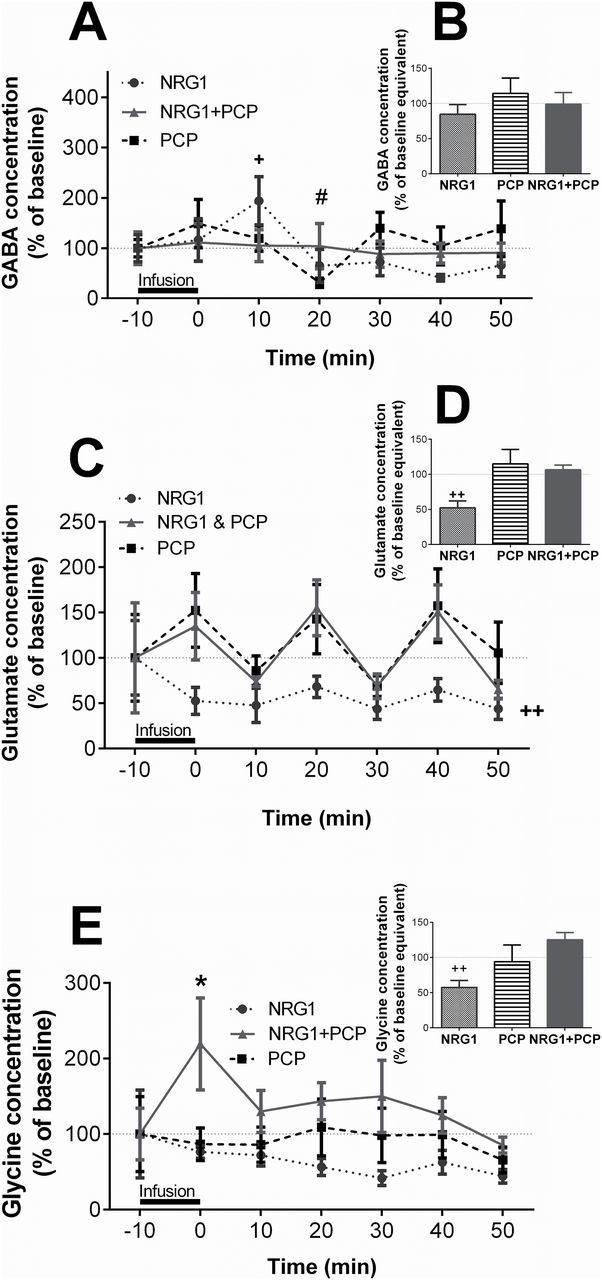
Neuregulin 1 (NRG1) reduces extracellular glutamate and glycine in the prefrontal cortex (PFC) in vivo. Mice (n = 8–10/treatment) received a local infusion of NRG1 (10nM), PCP (10 µM), or NRG1 + PCP for 10min in the PFC by retrodialysis. Extracellular GABA (A & B), glutamate (C & D), and glycine (E & F) levels are expressed as a percentage of the respective mean baseline value ± standard error of the mean for each time point (A, C, E) or baseline equivalent for area under the curve analysis (B, D, F). Dotted lines indicate baseline levels before each treatment. *p < 0.05 NRG1 + PCP vs baseline; # p < 0.05 PCP vs baseline; + p < 0.05, ++ p < 0.01 NRG1 vs baseline.
NRG1 infusion reduced extracellular glutamate levels in the PFC throughout the sampling period (LMM: 50.8±10.2% of baseline, p < 0.001; AUC: 52.2±9.8% of baseline, p = 0.005; Figure 3C and D). However, neither PCP nor NRG1 + PCP affected glutamate levels at single time points or during the entire sampling period (LMM: p = 0.69 and p > 0.99 vs baseline, AUC: p = 0.81 and p = 0.65 vs baseline, respectively; Figure 3C and D). Overall, extracellular glycine levels were reduced following NRG1 infusion (AUC: 57.5±9.8% of baseline, p = 0.008; Figure 3E and F). Glycine levels were not affected by PCP (AUC: p = 0.98 vs baseline). Combined NRG1 + PCP treatment led to a marked increase in glycine levels during the infusion period (219.0±61.0% of baseline, p = 0.02; Figure 3E). This increase did not persist for the remaining sampling period (AUC: 125.1±10.4% of baseline, p = 0.11). The PFC microdialysis experiment shows that NRG1 has an immediate influence on extracellular neurotransmitter levels with potential relevance to schizophrenia.
NRG1 Reduces Glutamate and Glycine Levels and Prevents PCP-Induced GABA Increase in the Hippocampus In Vivo
Impaired signaling between the hippocampus and PFC has been implicated in the pathophysiology of schizophrenia (Godsil et al., 2013). We therefore further investigated the effect of NRG1 increase on hippocampal neurotransmitter levels. In the dorsal CA1 of the hippocampus, the retrodialysis treatments had a significant effect on overall extracellular glutamate and glycine levels (glutamate: F 3, 158.9 = 16.85, p < 0.001; glycine: F 3, 159.1 = 6.68, p < 0.001), but only affected GABA levels at single time points (F 3, 147.4 = 1.39, p = 0.248). NRG1 showed a tendency to increase GABA levels during the infusion period (212.0±66.0% of baseline, p = 0.1), with post-infusion samples remaining close to baseline (Figure 4A). PCP significantly increased extracellular GABA concentration during the 10min infusion period (313.0±82.8% of baseline, p = 0.01; Figure 4A). Co-infusion of NRG1 prevented the PCP effect (103.0±16.0% of baseline, p = 0.96; Figure 4A). AUC analysis showed a trend in overall treatment effect on GABA levels (F 3,19.9 = 2.8, p = 0.07), with a trend towards reduced overall GABA levels following NRG1 + PCP (NRG1: p = 0.31; PCP: p > 0.99; NRG1 + PCP: p = 0.07 vs baseline; Figure 4B).
Figure 4.
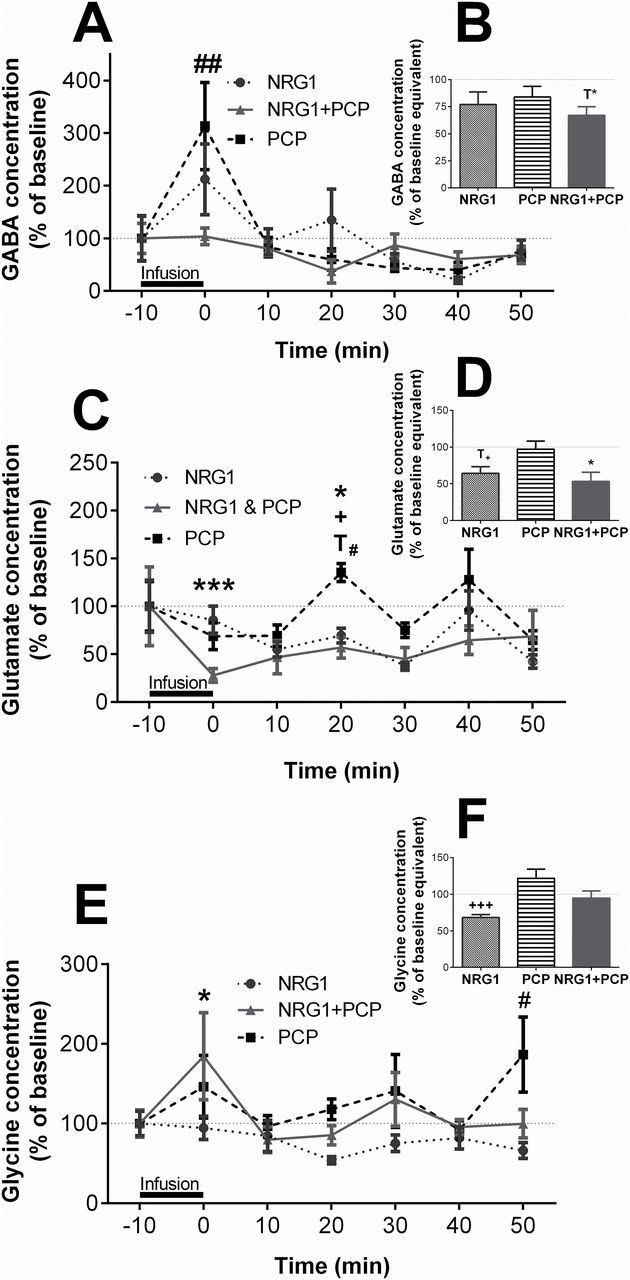
Neuregulin 1 (NRG1) prevents PCP-induced increase in GABA levels and reduces extracellular glutamate in the presence of PCP in the dorsal hippocampus CA1 (dCA1) in vivo. Mice (n = 8–10/treatment) received a local infusion of NRG1 (10nM), PCP (10 µM), or NRG1 + PCP for 10min in the dCA1 by retrodialysis. Extracellular GABA (A & B), glutamate (C & D), and glycine (E & F) levels are expressed as a percentage of the respective mean baseline value ± standard error of the mean for each time point (A, C, E) or baseline equivalent for area under the curve analysis (B, D, F). Dotted lines indicate baseline levels before each treatment. T*p = 0.07, *p < 0.05, **p < 0.001 NRG1 + PCP vs baseline; T# p = 0.06, # p < 0.05, ## p < 0.01 PCP vs baseline; T+ p = 0.07, + p < 0.05, ++ p < 0.001 NRG1 vs baseline.
Glutamate levels were reduced 10min after NRG1 treatment for 20min (10 min: 54.9±8.9% of baseline, p = 0.006; 20 min: 61.7±7.9%, p < 0.007; 30 min: 37.7±4.3%, p < 0.001; Figure 4C). PCP increased extracellular glutamate levels 20min after treatment (135.3±10.8% of baseline, p = 0.06; Figure 4C). Combined treatment of NRG1 + PCP reduced glutamate levels during the infusion period (28.0±9.0% of baseline, p < 0.001), with levels remaining below baseline until 50min after treatment (Figure 4C). The combined treatment therefore prevented the 20min PCP peak (70.0±10.2% of baseline, p < 0.001 vs PCP). The AUC analysis (F 3, 23.2 = 5.92, p = 0.004) confirmed this observation, with NRG1 and NRG1 + PCP leading to glutamate levels below baseline values (64.3±9.2%, p = 0.07 and 53.2±12.6%, p = 0.01 vs baseline, respectively; Figure 4D).
Hippocampal glycine levels were unaffected by NRG1 infusion throughout the sampling period (LMM: p = 0.17; Figure 4E). PCP infusion caused an increase in extracellular glycine levels 50min after treatment (186.5±47.0% of baseline, p < 0.05; Figure 4E). However, combined infusion of NRG1 + PCP increased glycine levels during the infusion period (196.0±58.0% of baseline, p = 0.047, Figure 4E). None of the treatments affected overall glycine levels compared to baseline (Figure 4F).
Discussion
In the present study, we showed that NRG1 treatment: (1) did not affect spontaneous behavior, but prevented PCP-induced behavioral impairments in the open field and prepulse inhibition paradigms; (2) reduced extracellular glutamate and glycine levels in the PFC and hippocampus; and (3) prevented PCP-induced changes to extracellular GABA levels in the hippocampus. With these results we provide the first compelling in vivo evidence for the involvement of NRG1 in schizophrenia-relevant behavior and neurotransmission and its treatment potential.
NRG1 Treatment Does Not Affect Spontaneous Behavior, But Prevents PCP-Induced Impairments
NRG1 administration did not adversely affect performance in the behavioral paradigms tested. Mice receiving central or peripheral NRG1 injections traveled a similar distance as vehicle-treated mice, and no difference in spontaneous behavior was observed. This finding is in line with a published study which reports no difference in locomotor activity 24h and 28d after two days of subcutaneous NRG1 (166 µg/kg) treatment in mice (Mahar et al., 2011). Experiments with genetic animals have shown that reduced NRG1 (e.g. reducing ERBB or NRG1 expression) or increased NRG1 signaling (e.g. overexpression of NRG1 type I) leads to hyperactivity in the open field arena (Karl et al., 2007; Kato et al., 2010; Wen et al., 2010; Luo et al., 2013; Yin et al., 2013). While acute application of NRG1 had no effect on locomotion, chronically altering the NRG1 signaling pathway likely caused the locomotor differences in genetically-modified animals, instead of short-term effects of NRG1 on related neurotransmission.
Several NRG1 transgenic animal models show sensorimotor gating impairments (Chen et al., 2008; Deakin et al., 2009; Wen et al., 2010; Karl et al., 2011). We therefore assessed the acoustic startle response following NRG1 treatment in wild type mice. Neither startle response nor PPI performance was affected by 25 or 50 μg/kg NRG1 compared to vehicle, while 5 μg/kg reduced the PPI effect. These results suggest that sensorimotor gating differences caused by genetically modifying the NRG1 signaling pathway are not related to acute NRG1 signaling. Furthermore, the dose-dependent behavioral response indicates that minor imbalances in NRG1 signaling potentially contribute to the disorder phenotype.
To explore the consequence of increased NRG1 signaling on schizophrenia-relevant behavioral impairments, we administered NRG1 to mice also receiving PCP. Consistent with previous publications, PCP treatment induced hyperlocomotion and impaired PPI (Bakshi and Geyer, 1995; Gleason and Shannon, 1997; Mouri et al., 2011). We subsequently discovered that NRG1 treatment dose-dependently prevented the PCP-induced hyperlocomotion and PPI impairment (Figures 1 and 2B). The PCP-rodent model is commonly used for identifying substances with antipsychotic-like potential (Adell et al., 2012). Current antipsychotics, including olanzapine, clozapine, and haloperidol, have been shown to prevent PCP-induced hyperlocomotion and PPI impairments (Bakshi and Geyer, 1995; Gleason and Shannon, 1997; Geyer et al., 2001; Linn et al., 2003; Mutlu et al., 2011). By preventing PCP-induced behavior impairments, NRG1 treatment shows antipsychotic-like potential. Remarkably, NRG1 i.c.v. and i.p. administrations had comparable outcomes against PCP-induced hyperlocomotion, which suggests that peripheral NRG1 administration has neuromodulatory properties on central receptors. This observation supports previous studies, which have shown that peripherally-administered recombinant NRG1 can readily cross the blood-brain barrier (Kastin et al., 2004; Kato et al., 2011; Rösler et al., 2011).
NRG1 Reduces Glutamate and Glycine Levels and Prevents PCP-Induced GABA Increase In Vivo
In the brain, the NRG1 receptor ERBB4 is predominantly located on GABAergic interneurons, with high expression in the PFC and hippocampus (Fazzari et al., 2010; Neddens et al., 2011), which are associated with the behavioral symptoms of schizophrenia (Inan et al., 2013). This expression pattern, together with the reported effects of NRG1 on neurotransmission in vitro, suggests that NRG1 signaling may influence GABAergic and glutamatergic neurotransmission in the PFC and hippocampus. We therefore assessed the effects of local NRG1 application on extracellular GABA, glutamate, and glycine levels in the PFC and hippocampus in vivo. Glutamate and glycine levels in both brain regions were reduced following NRG1 retrodialysis (Figure 3B and C and Figure 4B and C). This effect potentially contributes to the reported reduction in NMDA receptor currents and subunit internalization following NRG1 application in PFC slice preparations (Gu et al., 2005). While recent work shows that NRG1 can reduce glutamate release via the pre-synaptic LIMK1 protein (Yin et al., 2013), the exact mechanism underlying the present finding is worthy of further investigation.
NRG1 application in vitro has been shown to increase depolarization-evoked GABA release in the PFC by activating ERBB4 receptors (Woo et al., 2007; Wen et al., 2010). Prolonged NRG1 treatment has been shown to reduce surface levels of GABA-A receptors (Okada and Corfas, 2004), potentially compensating for the reported increase in GABA release. We observed an increase in GABA levels during the infusion period in the hippocampus and, slightly delayed, in the PFC. Our in vivo findings suggest that GABA-releasing cells in the adult nervous system respond to acute NRG1 signaling, adding support to the existing in vitro studies.
Deficits in the GABAergic and glutamatergic system have been identified in the PFCs and hippocampi of patients with schizophrenia (Gonzalez-Burgos et al., 2011; Nakazawa et al., 2012). We therefore investigated whether the response to NRG1 administration would differ when combined with PCP. PCP treatment caused a short-term increase in GABA levels in the hippocampus. While reduced GABA levels in the PFCs of rodents following PCP infusion have been reported previously (Yonezawa et al., 1998; Zhu et al., 2004), these studies used higher PCP concentrations (10 vs 50 and 100 μM) coupled with longer treatment durations (10 vs 40min), which are likely responsible for the effect difference. Combined NRG1 and PCP infusion completely prevented the PCP-induced increase in GABA levels. The observed effect on GABA by combined NRG1 and PCP treatment occurred during the retrodialysis period, suggesting a direct action on local neurotransmitter release or uptake mechanisms. Notably, a recent study identified an interaction between ERBB4 and GABA-A receptors in hippocampal interneurons, which possibly contributed to the observed NRG1 treatment effect (Mitchell et al., 2013).
In line with existing reports (Zhu et al., 2004), local PCP infusion in the PFC and hippocampus did not change glutamate or glycine levels. However, glycine levels were markedly increased during the infusion period of NRG1 and PCP in both brain regions, while glutamate levels remained below baseline levels for the sampling period. Since the effect duration differs for both neurotransmitters, separate mechanisms are likely involved. Furthermore, there appears to be no correlation between the treatment effects on the extracellular levels of GABA, glutamate, and glycine. PCP and NRG1 have been reported to alter extracellular dopamine levels in vivo (Yonezawa et al., 1998; Zhu et al., 2004; Kwon et al., 2008). Assessing the effect of the combined treatment on dopamine levels will aid the understanding of mechanisms underlying the effect of NRG1 administration by itself and in the presence of PCP on neurotransmission.
The effect of antipsychotics on local PCP-induced neurotransmitter alterations has not been published. However, local clozapine and haloperidol administration have been reported to reduce GABA levels, with clozapine, but not haloperidol, also increasing glutamate levels in the PFCs of rats (Bourdelais and Deutch, 1994; López-Gil et al., 2007). NRG1 infusion changed GABA, glutamate, and glycine levels in the presence of PCP, indicating a potential for NRG1 signaling to acutely alter impaired neurotransmission.
The Potential Implications of Altered NRG1 Signaling in Schizophrenia
Existing in vitro experiments have indicated that NRG1 administration is more likely to affect neurotransmitter receptor availability than neurotransmitter release in PFC and hippocampus slice preparations (Liu et al., 2001; Okada and Corfas, 2004; Chang and Fischbach, 2006; Pitcher et al., 2011; Shamir et al., 2012). The observed rapid neurotransmitter level changes following NRG1 and the combined NRG1 and PCP infusion are therefore noteworthy. However, since each sample covers a 10min window, we cannot determine the exact order of neurochemical events, which is of particular relevance since GABA and glycine have been reported to influence glutamate levels (Tanaka et al., 2003; Lee et al., 2009). Furthermore, the behavioral response to i.c.v. NRG1 suggests that neurotransmission in other brain regions, such as the striatum, are also rapidly affected.
The effects of NRG1-ERBB4 signaling have been suggested to follow an “inverted-U model” when studying neuronal activity (Role and Talmage, 2007). The dose-dependent response of NRG1 against PCP-induced behavioral impairments indicates a non-linear concentration response. U-shaped behavioral responses have been associated with PFC dopamine levels (Husain and Mehta, 2011), and are considered critical for the development of pharmacological agents targeting cognitive symptoms of schizophrenia (Millan et al., 2012). However, the bi-phasic dose-response of NRG1 in the present study requires further exploration using a wider concentration range to determine the exact NRG1-ERBB complex relationship. Broadening the concentration spectrum may also shed light on the consequence of altered NRG1 signaling in humans, since both increased and decreased NRG1 expression has been observed in patients with schizophrenia (Deng et al., 2013). Furthermore, chronically increased or decreased NRG1 signaling in mice has led to behavioral and structural differences (Lu et al., 2011). This highlights the need to assess the NRG1 signaling system at different stages during development. Such investigations will also provide critical information about the onset and duration of a possible NRG1 treatment strategy following our identification of the antipsychotic-like treatment potential. The suitability of NRG1 administration to humans has recently been explored in successful phase II clinical trials in patients with chronic heart failure (Gao et al., 2010; Jabbour et al., 2011). Thus, a combination of pre-clinical and clinical data suggests that NRG1 has a therapeutic system-dependent effect, likely within a specific concentration range.
Conclusion
In the present study, we showed that acute treatment with NRG1 has little effect on spontaneous behavior, while significantly reducing glutamatergic neurotransmission in the PFC and hippocampus. Importantly, our results demonstrate that NRG1 administration prevents schizophrenia-relevant behavior impairments and disruptions of the GABAergic system caused by PCP. The ability of NRG1 treatment to alter GABA, glutamate, and glycine levels in the presence of PCP also suggests that NRG1 signaling has the potential to alter disrupted neurotransmission in patients with schizophrenia. Our findings are therefore the first step towards exploring the therapeutic potential of NRG1 for the treatment of schizophrenia.
Statement of Interest
All authors declare no competing financial interest in relation to the work described.
Acknowledgements
This work was supported by the Schizophrenia Research Institute, utilizing infrastructure funding from New South Wales Health, a Illawarra Health and Medical Research Institute senior research fellowship to Dr Jenner and a Motor Neuron Disease Research Institute of Australia grant (Mick Rodger Benalla), a career development fellowship (1045643), and a project grant (1003886) from the National Health and Medical Research Council to Dr Karl. The microdialysis setup was funded by the Clive and Vera Ramaciotti Foundation.
We thank Dr Tracy Maddocks, Jerzy Zieba, and Zhixiang Wu for their technical assistance and Dr Angelique Hoolahan, Dr Lezanne Ooi, and Dr Simon Kaja for scientific discussion.
References
- Adell A, Jiménez-Sánchez L, López-Gil X, Romón T. (2012). Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr Bull 38:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agim ZS, Esendal M, Briollais L, Uyan O, Meschian M, Martinez LAM, Ding Y, Basak AN, Ozcelik H. (2013). Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. PLOS ONE 8:e53042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison JG, Das PM, Ma J, Inglis FM, Jones FE. (2011). The ERBB4 intracellular domain (4ICD) regulates NRG1-induced gene expression in hippocampal neurons. Neurosci Res 70:155–163. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. (1995). Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology (Berl) 122:198–201. [DOI] [PubMed] [Google Scholar]
- Barakat A, Dean B, Scarr E, Evin G. (2010). Decreased Neuregulin 1 C-terminal fragment in Brodmann’s area 6 of patients with schizophrenia. Schizophr Res 124:200–207. [DOI] [PubMed] [Google Scholar]
- Bennett M. (2009). Positive and negative symptoms in schizophrenia: the NMDA receptor hypofunction hypothesis, neuregulin/ErbB4 and synapse regression. Aus New Zeal J Psychiatry 43:711–721. [DOI] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kühn B. (2009). Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138:257–270. [DOI] [PubMed] [Google Scholar]
- Bertram I, Bernstein H-G, Lendeckel U, Bukowska A, Dobrowolny H, Keilhoff G, Kanakis D, Mawrin C, Bielau H, Falkai P, Bogerts B. (2007). Immunohistochemical evidence for impaired neuregulin-1 signaling in the prefrontal cortex in schizophrenia and in unipolar depression. Ann NY Acad Sci 1096:147–156. [DOI] [PubMed] [Google Scholar]
- Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, Sigmundsson A, Firth DR, Nielsen B, Stefansdottir R, Novak TJ, Stefansson K, Gurney ME, Andresson T. (2007). Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/- knock-outs compared with wild-type mice. J Neurosci 27:4519–4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdelais AJ, Deutch AY. (1994). The effects of haloperidol and clozapine on extracellular GABA levels in the prefrontal cortex of the rat: an in vivo microdialysis study. Cereb Cortex 1991 4:69–77. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. (2001). Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 156:234–258. [DOI] [PubMed] [Google Scholar]
- Buonanno A. (2010). The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull 83:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Zhu N, Tsantoulas C, Ma Z, Grist J, Loeb JA, Bennett DLH. (2010). Neuregulin-ErbB signaling promotes microglial proliferation and chemotaxis contributing to microgliosis and pain after peripheral nerve injury. J Neurosci 30:5437–5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Fischbach GD. (2006). An acute effect of neuregulin 1 beta to suppress alpha 7-containing nicotinic acetylcholine receptors in hippocampal interneurons. J Neurosci 26:11295–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-JJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, Rosoklija G, Liu R-C, Gingrich JA, Small S, Moore H, Dwork AJ, Talmage DA, Role LW. (2008). Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci 28:6872–6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-J, Zhang M, Yin D-M, Wen L, Ting A, Wang P, Lu Y-S, Zhu X-H, Li S-J, Wu C-Y, Wang X-M, Lai C, Xiong W-C, Mei L, Gao T-M. (2010). ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci USA 107:21818–21823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. (2008). Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res 100:270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. (2009). Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. Neuroreport 20:1523–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Pan B, Engel M, Huang X-F. (2013). Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway. Psychopharmacology (Berl) 226:201–215. [DOI] [PubMed] [Google Scholar]
- Du Bois TM, Newell KA, Huang X-F. (2012). Perinatal phencyclidine treatment alters neuregulin 1/erbB4 expression and activation in later life. Eur Neuropsychopharmacol 22:356–363. [DOI] [PubMed] [Google Scholar]
- Eckstein JA, Ammerman GM, Reveles JM, Ackermann BL. (2008). Simultaneous profiling of multiple neurochemical pathways from a single cerebrospinal fluid sample using GC/MS/MS with electron capture detection. J Mass Spectrom 43:782–790. [DOI] [PubMed] [Google Scholar]
- Fazzari P, Paternain AV, Valiente M, Pla R, Luján R, Lloyd K, Lerma J, Marín O, Rico B. (2010). Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464:1376–1380. [DOI] [PubMed] [Google Scholar]
- Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, Li T, Liu X, Xu Y, Li X, Zhou M. (2010). A Phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol 55:1907–1914. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. (2001). ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol 433:86–100. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. (2001). Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 156:117–154. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. (1997). Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 129:79–84. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Kiss JP, Spedding M, Jay TM. (2013). The hippocampal-prefrontal pathway: the weak link in psychiatric disorders? Eur Neuropsychopharmacol 23:1165–1181. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Fish KN, Lewis DA. (2011). GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast 2011:723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Fu AKY, Ip NY, Yan Z. (2005). Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci 25:4974–4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C-G, Wang H-Y, Cho D-S, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. (2006). Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med 12:824–828. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. (2004). Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9:299–307. [DOI] [PubMed] [Google Scholar]
- Husain M, Mehta MA. (2011). Cognitive enhancement by drugs in health and disease. Trends Cogn Sci 15:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Petros TJ, Anderson SA. (2013). Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. Neurobiol Dis 53:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y, Nawa H. (2013). ErbB1-4-dependent EGF/neuregulin signals and their cross talk in the central nervous system: pathological implications in schizophrenia and Parkinson’s disease. Front Cell Neurosci 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, Amor R, Liu X, Li XY, Zhou MD, Graham RM, Macdonald PS. (2011). Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 13:83–92. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D. (2012). Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl T, Duffy L, Scimone A, Harvey RP, Schofield PR. (2007). Altered motor activity, exploration and anxiety in heterozygous neuregulin 1 mutant mice: implications for understanding schizophrenia. Genes Brain Behav 6:677–687. [DOI] [PubMed] [Google Scholar]
- Karl T, Burne THJ, Van den Buuse M, Chesworth R. (2011). Do transmembrane domain neuregulin 1 mutant mice exhibit a reliable sensorimotor gating deficit? Behav Brain Res 223:336–341. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V, Pan W. (2004). Neuregulin-1-beta1 enters brain and spinal cord by receptor-mediated transport. J Neurochem 88:965–970. [DOI] [PubMed] [Google Scholar]
- Kato T, Kasai A, Mizuno M, Fengyi L, Shintani N, Maeda S, Yokoyama M, Ozaki M, Nawa H. (2010). Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLOS ONE 5:e14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Abe Y, Sotoyama H, Kakita A, Kominami R, Hirokawa S, Ozaki M, Takahashi H, Nawa H. (2011). Transient exposure of neonatal mice to neuregulin-1 results in hyperdopaminergic states in adulthood: implication in neurodevelopmental hypothesis for schizophrenia. Mol Psychiatry 16:307–320. [DOI] [PubMed] [Google Scholar]
- Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, Buonanno A. (2008). Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci USA 105:15587–15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. (2003). Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci 18:403–411. [DOI] [PubMed] [Google Scholar]
- Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. (2006). Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5’ SNPs associated with the disease. Proc Natl Acad Sci USA 103:6747–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E-A, Cho J-H, Choi I-S, Nakamura M, Park H-M, Lee J-J, Lee M-G, Choi B-J, Jang I-S. (2009). Presynaptic glycine receptors facilitate spontaneous glutamate release onto hilar neurons in the rat hippocampus. J Neurochem 109:275–286. [DOI] [PubMed] [Google Scholar]
- Linn GS, Negi SS, Gerum SV, Javitt DC. (2003). Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology (Berl) 169:234–239. [DOI] [PubMed] [Google Scholar]
- Liu X, Bates R, Yin D-M, Shen C, Wang F, Su N, Kirov SA, Luo Y, Wang J-Z, Xiong W-C, Mei L. (2011). Specific Regulation of NRG1 Isoform Expression by Neuronal Activity. J Neurosci 31:8491–8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ford B, Mann MA, Fischbach GD. (2001). Neuregulins increase alpha7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J Neurosci 21:5660–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longart M, Chatani-Hinze M, Gonzalez CM, Vullhorst D, Buonanno A. (2007). Regulation of ErbB-4 endocytosis by neuregulin in GABAergic hippocampal interneurons. Brain Res Bull 73:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. (2007). Clozapine and Haloperidol Differently Suppress the MK-801-Increased Glutamatergic and Serotonergic Transmission in the Medial Prefrontal Cortex of the Rat. Neuropsychopharmacology 32:2087–2097. [DOI] [PubMed] [Google Scholar]
- Luo X, He W, Hu X, Yan R. (2013). Reversible Overexpression of Bace1-Cleaved Neuregulin-1 N-Terminal Fragment Induces Schizophrenia-Like Phenotypes in Mice. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Yin D-M, Xiong W-C, Mei L. (2011). Modeling Schizophrenia in Neuregulin 1 and ErbB4 Mutant Mice. In: Animal Models of Schizophrenia and Related Disorders (O’Donnell P, ed), pp261–277. Totowa, NJ: Humana Press. [Google Scholar]
- Mahar I, Tan S, Davoli MA, Dominguez-Lopez S, Qiang C, Rachalski A, Turecki G, Mechawar N. (2011). Subchronic peripheral neuregulin-1 increases ventral hippocampal neurogenesis and induces antidepressant-like effects. PLOS ONE 6:e26610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Nave K-A. (2014). Neuregulin-ERBB Signaling in the Nervous System and Neuropsychiatric Diseases. Neuron 83:27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, et al. (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11:141–168. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makusky A, Markey SP, Buonanno A. (2013). ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity. Proc Natl Acad Sci USA 110:19603–19608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. (2012). From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 37:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Krystal JH. (2012). Capturing the angel in angel dust: Twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophr Bull 38:942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouri A, Koseki T, Narusawa S, Niwa M, Mamiya T, Kano S-I, Sawa A, Nabeshima T. (2011). Mouse strain differences in phencyclidine-induced behavioural changes. Int J Neuropsychop 15:767–779. [DOI] [PubMed] [Google Scholar]
- Mutlu O, Ulak G, Celikyurt IK, Akar FY, Erden F, Tanyeri P. (2011). Effects of olanzapine, sertindole and clozapine on MK-801 induced visual memory deficits in mice. Pharmacol Biochem Behav 99:557–565. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. (2012). GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 62:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, Lewis DA, McBain CJ, Buonanno A. (2011). Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biol Psychiatry 70:636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Corfas G. (2004). Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus 14:337–344. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. (2001). The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press. [Google Scholar]
- Petryshen TL, et al. (2005). Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry 10:366–374, 328. [DOI] [PubMed] [Google Scholar]
- Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. (2011). Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med 17:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt J, Winchester C, Dawson N, Morris B. (2012). Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nat Rev Drug Discov 11:560–579. [DOI] [PubMed] [Google Scholar]
- Radonjić NV, Jakovcevski I, Bumbaširević V, Petronijević ND. (2013). Perinatal phencyclidine administration decreases the density of cortical interneurons and increases the expression of neuregulin-1. Psychopharmacology (Berl) 227:673–683. [DOI] [PubMed] [Google Scholar]
- Rhein M, Muschler M-R, Krauss JK, Bleich S, Frieling H, Schwabe K. (2013). Hypomethylation of neuregulin in rats selectively bred for reduced sensorimotor gating. Schizophr Res 150:262–265. [DOI] [PubMed] [Google Scholar]
- Role LW, Talmage DA. (2007). Neurobiology: new order for thought disorders. Nature 448:263–265. [DOI] [PubMed] [Google Scholar]
- Rösler TW, Depboylu C, Arias-Carrión O, Wozny W, Carlsson T, Höllerhage M, Oertel WH, Schrattenholz A, Höglinger GU. (2011). Biodistribution and brain permeability of the extracellular domain of neuregulin-1-β1. Neuropharmacology 61:1413–1418. [DOI] [PubMed] [Google Scholar]
- Shamir A, Kwon O-B, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, Buonanno A. (2012). The Importance of the NRG-1/ErbB4 Pathway for Synaptic Plasticity and Behaviors Associated with Psychiatric Disorders. J Neurosci 32:2988–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass-Belt P, Gilbert JL, Davis FC. (2005). Central administration of transforming growth factor-alpha and neuregulin-1 suppress active behaviors and cause weight loss in hamsters. Brain Res 1038:171–182. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Giardina K, Gerhardt GA. (2000). In vivo microdialysis studies of age-related alterations in potassium-evoked overflow of dopamine in the dorsal striatum of Fischer 344 rats. Int J Dev Neurosci 18:411–416. [DOI] [PubMed] [Google Scholar]
- Stefansson H, et al. (2002). Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71:877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Powell SB, Breier MR, Hines SR, Light GA. (2013). Coupling of gene expression in medial prefrontal cortex and nucleus accumbens after neonatal ventral hippocampal lesions accompanies deficits in sensorimotor gating and auditory processing in rats. Neuropharmacology 75:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Tsuchida A, Kiuchi Y, Oguchi K, Numazawa S, Yoshida T. (2003). GABAergic modulation of hippocampal glutamatergic neurons: an in vivo microdialysis study. Eur J Pharmacol 465:61–67. [DOI] [PubMed] [Google Scholar]
- Wadugu B, Kühn B. (2012). The role of neuregulin/ErbB2/ErbB4 signaling in the heart with special focus on effects on cardiomyocyte proliferation. Am J Physiol Heart Circ Physiol 302:H2139––H2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu Y-S, Zhu X-H, Li X-M, Woo R-S, Chen Y-J, Yin D-M, Lai C, Terry AV, Jr, Vazdarjanova A, Xiong W-C, Mei L. (2010). Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA 107:1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo R-S, Li X-M, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong X-P, Wu J, Gassmann M, Lai C, Xiong W-C, Gao T-M, Mei L. (2007). Neuregulin-1 enhances depolarization-induced GABA release. Neuron 54:599–610. [DOI] [PubMed] [Google Scholar]
- Yee BK, Chang DLT, Feldon J. (2004). The effects of dizocilpine and phencyclidine on prepulse inhibition of the acoustic startle reflex and on prepulse-elicited reactivity in C57BL6 mice. Neuropsychopharmacology 29:1865–1877. [DOI] [PubMed] [Google Scholar]
- Yin D-M, Chen Y-J, Lu Y-S, Bean JC, Sathyamurthy A, Shen C, Liu X, Lin TW, Smith CA, Xiong W-C, Mei L. (2013). Reversal of behavioral deficits and synaptic dysfunction in mice overexpressing neuregulin 1. Neuron 78: 644–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa Y, Kuroki T, Kawahara T, Tashiro N, Uchimura H. (1998). Involvement of γ-aminobutyric acid neurotransmission in phencyclidine-induced dopamine release in the medial prefrontal cortex. Eur J Pharmacol 341:45–56. [DOI] [PubMed] [Google Scholar]
- Zhu G, Okada M, Uchiyama D, Ohkubo T, Yoshida S, Kaneko S. (2004). Hyperactivity of endoplasmic reticulum associated exocytosis mechanism contributes to acute phencyclidine intoxication. J Pharmacol Sci 95:214–227. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lai C, Thomas S, Burden SJ. (1995). Neuregulin receptors, erbB3 and erbB4, are localized at neuromuscular synapses. EMBO J 14:5842–5848. [DOI] [PMC free article] [PubMed] [Google Scholar]