Abstract
Background:
The noradrenergic nucleus locus coeruleus (LC) has functional relevance in several psychopathologies such as stress, anxiety, and depression. In addition to glutamatergic and GABAergic synaptic inputs, the activation of somatodendritic α2-adrenoceptors is the main responsible for LC activity regulation. The Wistar Kyoto (WKY) rat exhibits depressive- and anxiety-like behaviors and hyperresponse to stressors. Thus, the goal of the present study was to investigate in vitro the sensitivity of α2-adrenoceptors, as well as the glutamatergic and GABAergic synaptic activity on LC neurons of the WKY strain.
Methods:
For that purpose patch-clamp whole-cell recordings were done in LC slices.
Results:
The α2-adrenoceptors of LC neurons from WKY rats were less sensitive to the effect induced by the agonist UK 14 304 as compared to that recorded in the Wistar (Wis) control strain. In addition, the GABAergic input to LC neurons of WKY rats was significantly modified compared to that in Wis rats, since the amplitude of spontaneous GABAergic postsynaptic currents was reduced and the half-width increased. On the contrary, no significant alterations were detected regarding glutamatergic input to LC neurons between rat strains.
Conclusions:
These results point out that in WKY rats the inhibitory control exerted by α2-adrenoceptors and GABAergic input onto LC neurons is dysregulated. Overall, this study supports in this animal model the hypothesis that claims an imbalance between the glutamatergic-GABAergic systems as a key factor in the pathophysiology of depression.
Keywords: depression, GABA, glutamate, patch-clamp
Introduction
Animal models, despite inherent limitations, are critical to achieve substantial progress in understanding the neurobiological bases of psychiatric disorders (Nestler and Hyman, 2010; Overstreet, 2012; Armario and Nadal, 2013). Among these models, the Wistar Kyoto (WKY) rat is frequently used to study the pathophysiology of depression. A substantial amount of work has shown that WKY rats present innate depressive-like behavior in different tests, such as the forced swimming test and the learned helplessness test (Pare, 1989; Pare and Redei, 1993; Lahmame and Armario, 1996; Pare and Tejani-Butt, 1996; Lahmame et al., 1997; Lopez-Rubalcava and Lucki, 2000; Rittenhouse et al., 2002; Tejani-Butt et al., 2003; Will et al., 2003; Malkesman, Braw, et al., 2006; Carr et al., 2010; Yamada et al., 2013; Kyeremanteng et al., 2014; Nam et al., 2014). They also exhibit disturbances in the sleep-wake cycle, including increased rapid-eye-movement sleep and sleep fragmentation (Dugovic et al., 2000), which are prevalent symptoms in depressive patients (Bunney and Bunney, 2013). In addition, hormonal abnormalities of the hypothalamic-pituitary-adrenal axis, often disrupted in depressive patients, have been extensively reported in this rat strain as compared to the Wistar (Wis) control strain (Pare and Redei, 1993; Redei et al., 2001; Solberg et al., 2001; Rittenhouse et al., 2002; De La Garza and Mahoney, 2004; Malkesman, Maayan, et al., 2006). Acute noradrenergic response to stress—measured by tyrosine hydroxylase mRNA levels, noradrenaline (NA) release, or c-fos stimulation—is also attenuated in WKY rats compared to Sprague-Dawley rats (Pardon et al., 2002). By contrast, chronic stress results in a greater sensitization of the hypothalamic-pituitary-adrenal axis and central noradrenergic system in WKY as compared to Sprague-Dawley rats (Morilak et al., 2005). A recent electrophysiological study shows that neuronal basal firing rate of the locus coeruleus (LC) nucleus is augmented and the sensitivity of α2-adrenoceptors reduced in WKY as compared to Wis rats (Bruzos-Cidon et al., 2014).
The LC is the main source of NA in most brain structures, including cortical and limbic regions. It has an important role in mediating behavioral states such as mood, motivation, stress, attention, and arousal (see for review Sara and Bouret, 2012). In fact, this brain structure has been proposed as a part of the central stress circuitry, which has functional relevance in the pathophysiology of several mental disorders, including stress, anxiety, and depression (Itoi and Sugimoto, 2010). The LC basal activity is mainly regulated by the activation of somatodendritic α2-adrenoceptors, which open a G protein–coupled inwardly-rectifying potassium (GIRK) channel leading to a decrease of cell excitability in vivo and in vitro (Cedarbaum and Aghajanian, 1976; Williams et al., 1985; Aghajanian and Wang, 1986; Torrecilla et al., 2008, 2013). Additionally, major glutamatergic input from the paragigantocellularis nucleus influences LC activity (Aston-Jones et al., 1991), acting on postsynaptically located NMDA (Olpe et al., 1989) and non-NMDA receptors (Williams et al., 1991). Glutamate may also be synthesized and released within the LC neurons themselves (Noriega et al., 2007). The inhibitory input to the LC is provided by GABAergic neurons from the nucleus prepositus hypoglossi (Aston-Jones et al., 1991) and a population of small GABAergic interneurons (Iijima and Ohtomo, 1988; Aston-Jones et al., 2004), acting onto pre- and postsynaptically located GABAA receptors (Corteen et al., 2011). Interestingly, dysfunctional glutamatergic and GABAergic systems are also considered to play a relevant role in the pathophysiology of depression (Krystal et al., 2002; Sanacora et al., 2012).
Given the role of the LC in the development of mental disorders, we have investigated the neurophysiology of this brain area in the WKY strain. For that purpose, whole-cell voltage-clamp recordings were carried out in LC brain slices to evaluate the sensitivity of α2-adrenoceptors, as well as the glutamatergic and GABAergic synaptic activity of this noradrenergic nucleus in the WKY rat. As a control group we used the Wis strain, from which WKY rats were derived (Okamoto and Aoki, 1963).
Experimental Procedures
Animals
Male Wis and WKY rats weighing 175–250g were provided by Harlan Interfauna Ibérica. This weight range ensures the use of age-matched animals, according to the animal supplier. Every effort was made to minimize the suffering of the animals and to use the minimum number of animals possible. Experimental protocols were reviewed and approved by the Local Committee for Animal Experimentation at the University of the Basque Country. All the experiments were performed in compliance with the European Community Council Directive on the Protection of Animals Used for Experimental and Other Scientific Purposes (86/609/EEC) and with the Spanish Law (RD 1201/2005) for the care and use of laboratory animals.
Drugs
(2S)-3-[(1S)-1-(3,4-Dichlorophenyl )ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid hydrochloride (CGP 55845 hydrochloride), D-(-)-2-Amino-5-phosphonopentanoic acid (D-AP5), 6,7-Dinitroquinoxaline-2,3-dione disodium salt (DNQX disodium salt), 4-Hydroxyquinoline-2-carboxylic acid (kynurenic acid), (5S,10R)-(+)-5-Methyl-10,11-dihydro 5H-dibenzo[a,d] cyclohepten-5,10-imine maleate [(+)-MK-801 maleate], picrotoxin, and 17-Hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride (yohimbine hydrochloride) were obtained from Tocris Bioscience. Chloral hydrate and 5-Bromo-6-(2-imidazolin-2-ylamino) quinoxaline (UK 14 304) were obtained from Sigma-Aldrich and (S)-1-Aminopropane-1,3-dicarboxylic acid (glutamate) from Research Biochemicals International.
Drug stocks, except chloral hydrate, were prepared in distilled water and diluted in artificial cerebrospinal fluid (ACSF) right before use. Chloral hydrate was prepared in 0.9% saline and applied by one intraperitoneal injection to achieve animal anaesthesia.
Patch-Clamp Recordings of Locus Coeruleus Neurons from Rat Brain Slices
Slice preparation and whole-cell patch-clamp recordings were performed as described by Torrecilla et al. (2008).
Brain Slice Preparation
Animals were anaesthetized with chloral hydrate (400mg/kg, i.p) and decapitated. The brain was immediately extracted and placed in cooled ACSF containing (in mM): 125 NaCl, 2.5 KCl, 1.2 MgCl2, 26 NaH2CO3, 1.25 NaH2PO4, 2.4 CaCl2, and 11 D-glucose saturated with 95% O2 and 5% CO2 (pH 7.3–7.4). In order to reduce neuronal damage, (+)-MK-801 maleate (10 µM) was added to ACSF. To record excitatory synaptic activity, kynurenic acid (100 µM) was substituted for (+)-MK-801 maleate. Horizontal brainstem sections (220 μm) containing the LC were cut using a vibrotome (VT1200S; Leica Microsystems). LC slices were incubated in warmed (35ºC) ACSF for at least 30min before recording.
Neuronal Identification and Recording
The slice mounted on a recording chamber was maintained at 35–37 ºC and perfused with ACSF at a flow rate of 1.5–2ml/min, through bath perfusion by gravity. LC neurons were visualized, using an upright microscope with infrared optics (Eclipse E600FN, Nikon), as a dense and compact group of cells located on the lateral border of the central gray and the fourth ventricle, just anterior to the genu of the facial nucleus (Figure 1A).
Electrophysiological parameters, UK 14 304, and glutamate-induced whole-cell currents, as well as spontaneous glutamatergic and GABAergic postsynaptic currents (sEPSC and sIPSC, respectively) and evoked glutamatergic and GABAergic postsynaptic currents (eEPSC and eIPSC, respectively) were recorded in voltage-clamp mode. For that purpose, glass pipettes (2–4 MΏ) prepared from borosilicate glass capillaries (World Precision Instruments) with a micropipette puller (PC-10, Narishige CO., LTD) were filled with an internal solution containing (in mM): 130 K-Gluconate, 5 NaCl, 1 MgCl2, 1 ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA), 10 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), 2 adenosine 5’-triphosphate magnesium salt (Mg-ATP), 0.5 guanosine 5’-triphosphate sodium salt hydrate (Na-GTP), and 10 phosphocreatine disodium salt hydrate (pH: 7.4, 280 mOsm). Currents were recorded with the membrane potential held at -50 mV. LC neurons were identified by the presence of a resting inwardly-rectifying potassium (IRK) conductance by stepping the membrane potential from -40 to -120 mV in -10 mV increments (100ms/step; Figure 1B; Williams et al., 1988). The term inward rectification refers to an increase in slope of the current-voltage (I/V) relationship near the reversal potential (Nernst prediction for potassium, -105 mV for our recording conditions). Immediately after neuron identification membrane capacitance (Cm), series resistance and input resistance (Rm) were measured using Clampex software. In some experiments, resting membrane potential (Vm) was also recorded (I = 0). Two concentrations of UK (0.15 µM and 1.5 µM) were administered to calculate UK ratio. For that purpose, the peak current induced by 0.15 µM UK was divided by the peak current induced by 1.5 µM UK. In addition, activation of GIRK currents by UK were detected using the above described I/V protocol before and during drug application. Activation of G-protein coupled receptors opens a GIRK conductance, evidenced by an increase in the resting I/V (Williams et al., 1988). The amplitude of UK-induced currents in LC neurons was also normalized to the capacitance of the neuron, resulting in a current density. Series resistance was monitored throughout the experiment and the cell was discarded if it exceeded 15 MΩ.
To evaluate sEPSCs and eEPSCs, voltage-clamp recordings were performed with 100 µM picrotoxin in the ACSF to block GABAA receptors. The glass pipettes were filled with an internal solution containing (in mM): 70 CsSO4, 20 CsCl, 20 NaCl, 1.5 MgCl2, 5 HEPES, 1 EGTA, 2 Mg ATP, and 0.5 Na-GTP (pH: 7.4, 280 mOsm). To trigger both α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptors-mediated responses, eEPSCs were recorded at a holding potential of +40 mV. By passing constant current pulses (15–50 µA, at every 20 s, using the Iso-Flex stimulus isolator (A.M.P.I)) through a glass pipette situated into the LC and filled with external solution, 30 eEPSCs were evoked. After events stabilization, NMDA receptors antagonist D-AP5 (100 µM) was applied in order to isolate AMPA receptors-mediated eEPSCs. Glutamatergic total response and AMPA receptors-mediated eEPSCs were calculated from the average of at least seven consecutive events. NMDA receptors-mediated eEPSCs were calculated by subtraction of AMPA receptors-mediated response to total eEPSCs. Peak currents of isolated AMPA and NMDA receptors-mediated eEPSC were used to calculate the AMPA/NMDA ratio.
To record sIPSCs and eIPSCs, we added 1 μM CGP55845, 50 µM D-AP5 and 20 µM DNQX to the ACSF to block GABAB, NMDA and AMPA/kainate receptors, respectively. The internal solution contained (in mM): 115 KCl, 20 NaCl, 1.5 MgCl2, 5 HEPES, 10 1,2-bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA), 2 Mg-ATP, and 0.5 Na-GTP (pH: 7.4, 280 mOsm). eIPSCs were recorded at a holding potential of -50 mV and one (1–10 mA) or five pulse trains (1–10 mA) were trigged at 10 Hz using a bipolar tungsten electrode situated posterior to the LC. GABAA receptors-mediated eIPSCs were calculated by averaging at least seven consecutive events. Changes in the probability of GABA release were investigated by measuring the IPSCn/IPSC 1 ratio. To do that the amplitude of a consecutive IPSC (IPSCn) was divided by the amplitude of the first IPSC (IPSC1).
Data Acquisition and Analysis
Recordings were detected with an Axopatch-200B (Axon Instruments), filtered at 5kHz and digitized with a Digidata 1322A (Axon Instruments). Data were sampled at 10kHz and analyzed with Clampex 10.2 software (Axon Instruments).
Recordings were filtered post hoc at 1kHz and visually inspected to select manually synaptic events. Template detection was used to select spontaneous synaptic currents (Clampfit 10.3 software), with deflections <7 pA excluded from analysis. The template was generated from an average of multiple spontaneous events, the selection was fitted to the 3.5 threshold of the template, and each peak was visually inspected. The duration of synaptic events was determined by measuring the width at 50% of the peak amplitude (half-width). In all cases, spontaneous activity from each neuron was measured for 1 minute. All data were extracted using Clampfit 10.3 software.
Graph Prism (v.5.01; GraphPad Software, Inc.) was used for statistical evaluations. Non-repeated two-way ANOVA was used to compare the outward current induced by two different concentrations of UK in both rat strains, Wis and WKY. The amplitude of spontaneous synaptic currents was analyzed by the Kolmogorov-Smirnov test (Clampfit 10.3 software). The rest of the electrophysiological parameters evaluated in the present work were statistically compared through strains using Student’s t-test. The level of significance was considered as p < 0.05.
Results
Electrophysiological Findings
Intrinsic Electrophysiological Properties of the Locus Coeruleus Neurons from Wistar Kyoto Rats
To study whether LC neurons from WKY and Wis rats had substantial differences in their intrinsic electrical properties, besides visual localization, we first identify LC neurons by the presence of a resting IRK conductance (see in Experimental Procedures, the Neuronal Identification section and Figure 1B). A total of 138 LC neurons were recorded: 65 neurons from the Wis strain (n = 12 animals) and 73 neurons from the WKY strain (n = 12 animals). The basal properties of these LC cells, such as Cm, Rm, and Vm, did not statistically differ between strains (Table 1).

Figure 1. Localization and electrophysiological identification of noradrenergic locus coeruleus (LC) neurons in vitro. (A) Representative picture of LC localization in the horizontal plane (Paxinos and Watson, 1997). (B) Representative traces of inwardly-rectifying potassium currents in a LC neuron triggered by stepping the membrane potential in -10 mV increments for 100ms.
Table 1.
In vitro intrinsic electrophysiological properties of locus coeruleus neurons recorded in Wistar and Wistar Kyoto rats.
| Cm (pF) | Rm (MΩ) | Vm (mV) | |
|---|---|---|---|
| Wistar | 142.9±6.6 | 260.8±13.7 | -56.38±2.81 |
| Wistar Kyoto | 153.0±7.5 | 237.8±12.2 | -52.14±1.72 |
All values represent the mean ± standard error of the mean of experiments. To calculate Cm and Rm, Wis n = 65 and WKY n = 74. For Vm Wis n = 13 and WKY n = 25.
No statistical significances were found between strains using unpaired two-tailed t-tests.
Cm, membrane capacitance; Rm, membrane resistance; Vm, resting membrane potential.
In Vitro Noradrenergic Transmission in Locus Coeruleus Neurons of Wistar Kyoto Rats
To evaluate the noradrenergic transmission on LC neurons from Wis and WKY strains, we recorded the whole-currents induced by two different concentrations of the α2-adrenoceptor agonist UK (0.15 µM and 1.5 µM). In LC neurons of both Wis and WKY rats, 0.15 µM and 1.5 µM UK application caused a concentration-dependent outward current at a holding potential of -50 mV (p < 0.001, two-way ANOVA; Figure 2A and B). The amplitude of the peak current induced by each concentration was not statistically different between strains (0.15 µM UK, Wis: 124.9±7.79 pA, n = 7 and WKY: 110.1±9.02 pA n = 5; 1.5 µM UK, Wis: 152.1.9±7.61 pA, n = 7 and WKY: 154.9±14.52 pA, n = 5; Figure 2B, upper panel). Accordingly, the current densities induced by UK perfusion were not different between Wis and WKY rats (0.15 µM UK, Wis: 0.65±0.06 pA/pF, n = 7 and WKY: 0.61±0.07 pA/pF, n = 5; 1.5 µM UK, Wis: 0.82±0.06 pA/pF, n = 7 and WKY: 0.94±0.08 pA/pF, n = 5; Figure 2B, lower panel). However, when we analyzed the UK ratio of the peak current induced by both concentrations of UK, data showed that LC neurons from WKY rats were significantly less sensitive than those from the Wis strain (WKY: 0.64±0.03, n = 5; Wis: 0.79±0.04, n = 7; p = 0.028, unpaired two-tailed t-test; Figure 2C). The UK-induced outward current was completely reversed by α2-adrenoceptor antagonist yohimbine (10 µM; Figure 2A).
Figure 2.

Whole-cell currents induced by UK 14 304 in locus coeruleus (LC) neurons of Wistar (Wis) and Wistar Kyoto (WKY) rats. (A) Representative recordings of the outward current induced by UK (0.15µM and 1.5µM) in LC neurons of Wis and WKY rats. Administration of the α2-antagonist yohimbine (10 µM) completely reversed the UK-induced current. (B) Summary bar graphs displaying the mean amplitudes of the UK-induced outward currents and mean of current densities in a concentration-dependent manner (*p < 0.01 and **p < 0.001, unpaired two-tailed t-test). (C) Summary data of UK ratio (*p = 0.028, unpaired two-tailed t-test). (D) Whole-cell current-voltage relationship in LC neurons of Wis (grey symbols) and WKY (black symbols) rats at rest (circles) and during (squares) UK perfusion (n = 6–7). (E) Current-voltage relationship in LC neurons of Wis (grey symbols) and WKY (black symbols) rats for the data concerning basal (circles) and induced current densities (pA/pF) during (squares) UK perfusion (n = 6–7).
To further characterize the agonist-induced outward current in the LC neurons, I/V relationships were obtained before and during UK perfusion, by hyperpolarizing the membrane potential (see Experimental Procedures). While bath perfusion of 0.15 µM UK increased the I/V relationship recorded in LC neurons of Wis rats to a greater extent than that in WKY rats (Figure 2D), perfusion of 1.5 µM UK induced a similar increment in both strains (Figure 2D). The reversal potential of the I/V obtained during UK perfusion was similar to that predicted by Nernst for a potassium conductance (-105 mV) (Williams et al., 1988). Current densities of the basal and UK-induced I/V were also provided, and were all similar in both strains (Figure 2E).
Study of the Glutamatergic Input to Locus Coeruleus Neurons of Wistar Kyoto Rats
Bath perfusion of glutamate (100 µM) onto LC neurons induced an inward current of similar amplitude in both Wis and WKY strains (238.1±47.66 pA, n = 6; 164.4±32.00 pA, n = 5, respectively; Figure 3A and B).
Figure 3.

Whole-cell currents induced by glutamate in locus coeruleus (LC) neurons of Wistar (Wis) and Wistar Kyoto (WKY) rats. (A) Representative traces and (B) summary data of the mean amplitude of the inward current induced by a brief application of glutamate (100 µM) in LC neurons of Wis and WKY rats. The holding potential was -50 mV. Bars represent the mean ± standard error of the mean of experiments (n = 5–6).
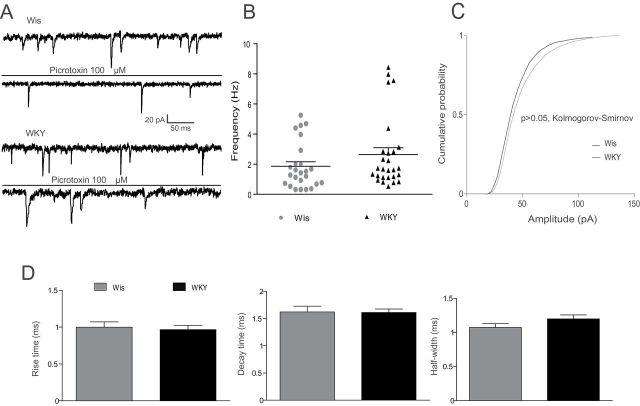
Next, we examined the spontaneous glutamatergic synaptic input to LC neurons in the presence of picrotoxin (100 µM) to ensure the blockade of GABAA receptors (Figure 4A). Thus, frequency (Figure 4B) and amplitude (Figure 4C) of sEPSCs measured in LC neurons were indistinguishable between Wis (1.86±0.31 Hz and 47.52±0.38 pA, n = 25) and WKY rats (2.64±0.46 Hz and 41.32±0.23 pA, n = 27). Kinetic of the sEPSCs was also evaluated by measuring their rise and decay time and half-width parameter, and showed no differences between strains (Figure 4D).
Figure 4.
Spontaneous excitatory input to locus coeruleus (LC) neurons in Wistar (Wis) and Wistar Kyoto (WKY) rats. (A) Representative current traces of spontaneous glutamatergic postsynaptic currents (sEPSCs) before and after picrotoxin perfusion. (B) Dot graphs showing the frequency of sEPSCs in LC neurons of Wis and WKY rats. (C) Cumulative histogram of sEPSCs amplitude showing no differences between Wis and WKY rats. (D) Bar graphs showing rise time, decay time, and half-width of sEPSCs recorded on LC neurons from Wis (n = 16) and WKY (n = 23) rats. Bars represent the mean ± standard error of the mean of experiments.
Secondly, glutamate release was evoked by electrical single stimulus (5–15 µA) at a holding potential of +40 mV to simultaneously trigger both AMPA and NMDA receptor-dependent eEPSCs (Figure 5B). No differences were observed in the eEPSC amplitudes (Wis: 236.9±44.16 pA, n = 8; WKY: 359.5±73.46 pA, n = 12) nor in the parameters associated with neurotransmitter kynetics (rise time for Wis: 1.54±0.10ms, n = 8 and WKY: 1.88±0.46ms, n = 10; decay time for Wis: 40.63±5.30ms, n = 8 and WKY: 39.61±8.60ms, n = 10; half-width for Wis: 10.77±0.9ms, n = 8 and WKY: 11.37±1.55ms, n = 12; Figure 5A). In addition, we further analyzed possible strain differences regarding the roles of AMPA and NMDA glutamatergic receptors on the excitatory activity of LC neurons by calculating the AMPA/NMDA ratio in each rat strain (see Experimental Procedures). Both Wis and WKY rats showed similar AMPA/NMDA ratios regarding the amplitude and the half-width of eEPSC in LC neurons (amplitude for Wis: 2.24±0.55, n = 7 and WKY: 1.71±0.27, n = 10; half-width for Wis: 0.44±0.05, n = 7 and WKY: 0.32±0.03, n = 10; Figure 5A and B).
Figure 5.

Evoked glutamatergic synaptic activity in locus coeruleus (LC) noradrenergic neurons of Wistar and Wistar Kyoto rats. (A) Bar graphs showing rise time, decay time and half-width of evoked glutamatergic postsynaptic currents (eEPSCs) recorded in LC neurons of Wis (n = 8) and WKY (n = 12) rats. (B) Illustration of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and NMDA receptors mediated eEPSC at holding potential of +40 mV on LC neurons of Wis and WKY rats. Bar graphs showing the amplitude and half-width of the AMPA/NMDA ratio recorded from both rat strains (n = 7–10).
Study of the GABAergic Input to Locus Coeruleus Neurons of Wistar Kyoto Rats
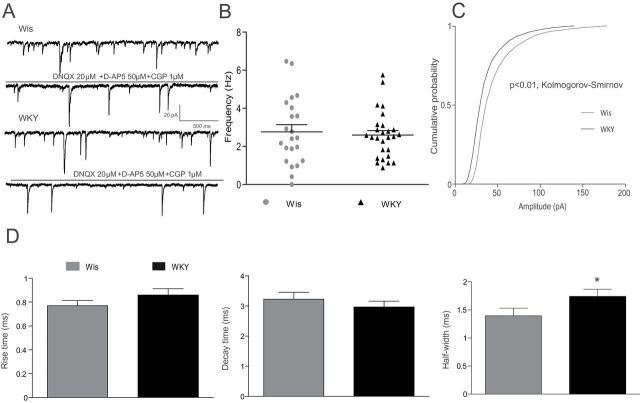
To evaluate fast inhibitory synaptic activity in LC neurons, GABAA transmission was pharmacologically isolated by adding the following AMPA, NMDA, and GABAB receptor blockers to the external bath solution (Figure 6A): DNQX disodium salt (20 µM), D-AP5 (50 µM), and CGP 55845 hydrochloride (1 µM). The frequency of sIPSCs in LC neurons was not statistically different between strains (Wis: 2.76±0.38 Hz, n = 22; WKY: 2.59±0.24 Hz, n = 27; Figure 6B). However, in the WKY rats the mean amplitude of sIPSCs was significantly reduced as compared to that recorded in Wis rats (Wis: 45.27±0.0.44 pA, n = 22; WKY: 34.33±0.33 pA, n = 27; p < 0.01, Kolmogorov-Smirnov test; Figure 6C). Regarding the kinetic of sIPSC, no differences were observed in rise time (Wis: 0.77±0.04ms, n = 18; WKY: 0.85±0.05ms, n = 25) or in decay time of the spontaneous events (Wis: 3.22±0.23ms, n = 18; WKY: 2.94±0.18ms, n = 25). However, as shown in Figure 6D, the half-width of sIPSCs in WKY rats was significantly increased compared to that recorded in Wis rats (WKY: 1.74±0.13ms, n = 18; Wis: 1.35±0.14ms, n = 25; p < 0.05, unpaired one-tailed t-test).
Figure 6.
Spontaneous inhibitory input to locus coeruleus (LC) neurons in Wistar (Wis) and Wistar Kyoto (WKY) rats. (A) Representative current traces of spontaneous GABAergic postsynaptic currents (sIPSC) before and after pharmacological isolation. (B) Dot graphs showing the frequency of sIPSCs in LC neurons of Wis and WKY rats. (C) Cumulative histograms of sIPSCs amplitude in LC neurons of Wis and WKY rats, showing that in WKY rats the amplitude of sIPSCs was reduced (p < 0.01, Kolmogorov-Smirnov test). (D) Summary bar graphs of sIPSCs kinetics parameters in LC neurons, showing that half-width is greater in WKY as compared to in Wis strain (*p < 0.05, unpaired t-test) Bars represent the mean ± standard error of the mean of experiments (n = 22–27).
Secondly, GABA release was induced by electrical stimulation (1–10 mA) of LC neurons with a membrane potential held at -50 mV. No differences were observed in the amplitude (Wis: 231.5±34.29 pA, n = 12; WKY: 218.7±27.08 pA, n = 12) or in the parameters associated to the kinetics of eIPSC between WKY and Wis rats (rise time: 1.13±0.17ms and 1.60±0.30ms; decay time: 13.60±0.81ms and 15.02±0.86ms; and half-width: 6.89±0.73ms and 8.64±0.75ms, for Wis [n = 12] and WKY [n = 12] respectively; Figure 7A). In order to evaluate whether GABA probability release was altered in LC neurons of WKY rats, an electric stimulus train of five pulses at 10 Hz was trigged to evoke consecutive eIPSCs (Figure 7B). No statistical differences were observed in the amplitude of consecutive eIPSC or in the IPSCn/IPSC1 ratio between WKY and Wis rats.
Figure 7.

Evoked inhibitory synaptic activity in locus coeruleus (LC) neurons of Wistar (Wis) and Wistar Kyoto (WKY) rats. (A) Bar graphs showing the amplitude, rise time, decay time, and half-width of evoked GABAergic postsynaptic currents (eIPSCs) in LC neurons of Wis and WKY rats. (B) Representative eIPSC traces induced by a train of five pulses at 10 Hz on LC neurons of Wis and WKY rats. (C) Curve graphs showing the amplitude and IPSCn/IPSC1 ratio on LC neurons of Wis and WKY rats (n = 11–12). IPSCn is the amplitude of a subsequent IPSC, and IPSC1 is the amplitude of the first IPSC.
It is important to note that while the frequency of inhibitory synaptic events was statistically greater in the LC of Wis rats than the frequency of the excitatory events (sIPSCs: 2.76±0.38 Hz, n = 22; sEPSCs: 1.86±0.31 Hz, n = 25; p < 0.05, unpaired t-test; see Figures 4B and 6B), in WKY rats both frequencies were similar. Interestingly, a previous patch-clamp study showed that sEPSCs frequencies were lower than sIPSCs frequencies in the LC of Sprague-Dawley rats (Sugiyama et al., 2012), in line with what we observed in the Wis strain. This suggests that synaptic activity might be dysregulated in LC neurons of WKY rats.
Discussion
A recent in vivo study from our group reveals significant alterations in LC activity of the WKY rat (Bruzos-Cidon et al., 2014) in a proposed animal model of anxiety/depression (Lahmame et al., 1997; Lopez-Rubalcava and Lucki, 2000; Pare, 2000; Pardon et al., 2002; Tejani-Butt et al., 2003; De La Garza and Mahoney, 2004; Ma and Morilak, 2004; Tizabi et al., 2012). To further investigate the origin of those adaptations, the main goal of the present study was to determine whether WKY rats display a differential modulation of LC neuronal activity compared to that of Wis rats. For that purpose, intrinsic sensitivity of α2-adrenoceptors and extrinsic synaptic inputs to LC neurons were evaluated in Wis and WKY rats in vitro. Our results indicate that in LC neurons of WKY rats, α2-adrenoceptors are desensitized and, while spontaneous GABAergic synaptic activity is reduced, the glutamatergic activity is similar in both rat strains. The latter data may reflect an imbalance between the glutamatergic and GABAergic transmissions, which has been previously described in depressive patients (Krystal et al., 2002; Luscher et al., 2011) and WKY rats .
Regarding noradrenergic transmission in WKY rats, we have previously shown that in vivo LC neurons of this rat strain are less sensitive to the inhibitory effect of the α2-adrenoceptor agonist clonidine to the basal firing rate (Bruzos-Cidon et al., 2014). The present in vitro study indicates that WKY rats display lower sensitivity to α2-adrenoceptor agonist UK compared to the Wis strain, since the UK ratios of the peak current induced by two submaximal concentrations of the drug are significantly smaller in WKY rats compared to in Wis rats. These results confirm that noradrenergic transmission in LC neurons from WKY rats is intrinsically altered, since α2-adrenoceptors are desensitized in vitro. This could be due to a number of reasons, including both different functionality/density of α2-adrenoceptor and/or GIRK channels in this rat strain. Although so far expression of GIRK channels has not been investigated in the WKY rat, an early study using in situ hybridization showed that expression of α2-adrenoceptor mRNA in the LC of WKY rats is not different to that obtained in Wis rats (Sands et al., 2000). In line with this, specific binding to α2- adrenoceptors in the LC of WKY rats is similar to that in the control Sprague-Dawley strain (Tejani-Butt et al., 1994). All in all, dysregulation of this signaling pathway in the LC of WKY rats could be due to altered expression levels or function of other downstream components such as Gi/o proteins and/or the GIRK channels.
Evidence indicates that noradrenergic transmission is altered in WKY rats. Thus, deficient noradrenergic reactivity to acute stress (Pardon et al., 2002), low levels of NA in the LC and several of its terminal sites (De La Garza and Mahoney, 2004; Scholl et al., 2010), as well as reduced cortical NA reuptake (Jeannotte et al., 2009) have been described in this rat strain. Accordingly, it has been recently reported that acute administration of the noradrenergic antidepressant reboxetine is less potent at inhibiting in vivo basal activity of LC neurons in the WKY rats than that recorded in the Wis strain (Bruzos-Cidon et al., 2014). Overall, a dysregulation of the noradrenergic transmission, including the inhibitory control that α2-adrenoceptors exert onto neuronal activity of LC neurons, could be related to the in vivo LC hyperactivity observed in the WKY strain. Interestingly, chronic exposure to cold stress, which resembles alterations observed on central noradrenergic transmission of patients with mood and anxiety disorders, alters electrophysiological properties of LC neurons not only in vivo but also in vitro (Mana and Grace, 1997; Jedema and Grace, 2003).
Preclinical and clinical studies support a relevant role of the glutamatergic transmission in the pathology of psychiatric illnesses such as depression (Krystal et al., 2002; Pittenger et al., 2007). Our results show that postsynaptic sensitivity of LC neurons to glutamate is not different between the WKY and Wis strains. Regarding glutamatergic synaptic input to LC, no significant alterations have been detected in WKY rats. In the present study we separately evaluated AMPA and NMDA receptors-mediated eEPSC and we did not observe differences between the two strains. Although lower or similar NMDA receptor binding has been observed in the hippocampus, as well as in other brain areas of WKY compared to Wis rats (Lei et al., 2009), the ratio of AMPA/NMDA receptor densities is similar in both strains and is selectively increased in the WKY strain after chronic treatment with ketamine (Tizabi et al., 2012). Further experiments would be of interest to determine the electrophysiological response of noradrenergic neurons to antidepressant drugs that affect glutamatergic transmission in WKY rats. Despite lacking data about NMDA and AMPA receptor densities in LC of WKY rats, our data indicate that the synaptic activity of both NMDA and AMPA receptors is similar in the WKY and Wis strains.
The current study indicates that the frequency of sIPSC in LC neurons is similar in both strains, which suggests that release probability of GABA is not significantly altered in LC neurons of WKY strain. On the contrary, sIPSCs amplitude, which is also determined by postsynaptic factors such as receptor sensitivity, is reduced in LC neurons of WKY rats as compared with those of Wis rats. In addition, the half-width of sIPSCs is significantly greater in LC neurons of WKY rats, indicating that in this rat strain GABAergic spontaneous events are slower than in the Wis strain. Interestingly, several studies identify a GABAergic deficit as a possible cause of mood disorders. On one hand, reduced plasma and brain concentrations of GABA have been reported in depressed patients (Gerner and Hare, 1981; Petty and Sherman, 1984; Honig et al., 1988; Hasler et al., 2007). On the other, animal studies demonstrate that GABAergic transmission is dysregulated in several animal models of depression (Dennis et al., 1994; Zink et al., 2009; Veeraiah et al., 2014; Venzala et al., 2013) and that chronic administration of antidepressants markedly changes GABAergic transmission (Wabno and Hess, 2013). As previously described in Sprague-Dawley rats (Sugiyama et al., 2012), the frequency of sEPSCs and sIPSCs are uneven in LC neurons of Wis rats. Conversely, LC neurons of WKY rats showed similar frequencies of both spontaneous synaptic events, suggesting synaptic dysregulation. Since the LC spontaneous firing rate is regulated by GABAergic input (Szabo and Blier, 2001; Belujon et al., 2009), the deficient inhibitory GABAergic transmission in this brain area may be underlying the in vivo hyperactivity of LC neurons in WKY rats (Bruzos-Cidon et al., 2014). Corticotropin-releasing factor fails to increase GABAergic activity in dorsal raphe neurons of WKY rats, as is normally seen in Sprague-Dawley rats (Lemos et al., 2011). Altogether, these results support the GABAergic deficit hypothesis as a cause of depressive disorder in the WKY strain. Importantly, reduced GABAergic transmission in LC of WKY rats could account for the disruption of the balance between glutamatergic and GABAergic systems that has been reported as an important factor in the pathophysiology of mood disorders, including depression (Krystal et al., 2002; Luscher et al., 2011).
Our data indicate that LC neurons of WKY are differentially regulated as compared to those of Wis rats, since the noradrenergic and GABAergic systems are altered. Thus, both intrinsic and extrinsic inhibitory mechanisms are diminished in LC neurons of WKY rats. In summary, this study supports the utility of the WKY rat as a useful animal model to characterize the neurobiological bases of pathologies related to dysfunctions of multiple neurotransmitter systems, such as anxiety and depression.
Statement of Interest
None
Acknowledgements
This work was supported by grants from the Basque Government (S-PE11UN055); the University of the Basque Country, UPV/EHU (UFI 11/32); and the Spanish Government (FIS PI12/00613). Dr Bruzos-Cidón had a postdoctoral fellowship from the University of the Basque Country, UPV/EHU, and Dr Llamosas had a predoctoral fellowship from the University of the Basque Country, UPV/EHU.
These institutions had no further role in the present study. The authors were entirely responsible for the scientific content of the manuscript and the decision to submit it for publication. We thank The Advanced Research Facilities (SGIker) of the University of the Basque Country, UPV/EHU for animal care.
References
- Aghajanian GK, Wang YY. (1986). Pertussis toxin blocks the outward currents evoked by opiate and alpha 2-agonists in locus coeruleus neurons. Brain Res 371:390–394. [DOI] [PubMed] [Google Scholar]
- Armario A, Nadal R. (2013). Individual differences and the characterization of animal models of psychopathology: A strong challenge and a good opportunity. Front Pharmacol 4:–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, Ennis M, van Bockstaele E, Pieribone V, Shiekhattar R, Akaoka H, Drolet G, Astier B. (1991). Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Prog Brain Res 88:47–75. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Zhu Y, Card JP. (2004). Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: Possible interneuronal pool. J Neurosci 24:2313–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belujon P, Baufreton J, Grandoso L, Boue-Grabot E, Batten TF, Ugedo L, Garret M, Taupignon AI. (2009). Inhibitory transmission in locus coeruleus neurons expressing GABAA receptor epsilon subunit has a number of unique properties. J Neurophysiol 102:2312–2325. [DOI] [PubMed] [Google Scholar]
- Bruzos-Cidon C, Miguelez C, Rodriguez JJ, Gutierrez-Lanza R, Ugedo L, Torrecilla M. (2014). Altered neuronal activity and differential sensitivity to acute antidepressants of locus coeruleus and dorsal raphe nucleus in wistar kyoto rats: A comparative study with sprague dawley and wistar rats. Eur Neuropsychopharmacol 24:1112–1122 [DOI] [PubMed] [Google Scholar]
- Bunney BG, Bunney WE. (2013). Mechanisms of rapid antidepressant effects of sleep deprivation therapy: Clock genes and circadian rhythms. Biol Psychiatry 73:1164–1171. [DOI] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I. (2010). Antidepressant-like effects of kappa-opioid receptor antagonists in wistar kyoto rats. Neuropsychopharmacology 35:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Aghajanian GK. (1976). Noradrenergic neurons of the locus coeruleus: Inhibition by epinephrine and activation by the alpha-antagonist piperoxane. Brain Res 112:413–419. [DOI] [PubMed] [Google Scholar]
- Corteen NL, Cole TM, Sarna A, Sieghart W, Swinny JD. (2011). Localization of GABA-A receptor alpha subunits on neurochemically distinct cell types in the rat locus coeruleus. Eur J Neurosci 34:250–262. [DOI] [PubMed] [Google Scholar]
- De La Garza R, 2nd, Mahoney JJ., 3rd (2004). A distinct neurochemical profile in WKY rats at baseline and in response to acute stress: Implications for animal models of anxiety and depression. Brain Res 1021:209–218. [DOI] [PubMed] [Google Scholar]
- Dennis T, Beauchemin V, Lavoie N. (1994). Antidepressant-induced modulation of GABAA receptors and beta-adrenoceptors but not GABAB receptors in the frontal cortex of olfactory bulbectomised rats. Eur J Pharmacol 262:143–148. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. (2000). Sleep in the wistar-kyoto rat, a putative genetic animal model for depression. Neuroreport 11:627–631. [DOI] [PubMed] [Google Scholar]
- Gerner RH, Hare TA. (1981). CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. Am J Psych 138:1098–1101. [DOI] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. (2007). Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Honig A, Bartlett JR, Bouras N, Bridges PK. (1988). Amino acid levels in depression: A preliminary investigation. J Psychiatr Res 22:159–164. [DOI] [PubMed] [Google Scholar]
- Iijima K, Ohtomo K. (1988). Immunocytochemical study using a GABA antiserum for the demonstration of inhibitory neurons in the rat locus ceruleus. Am J Anat 181:43–52. [DOI] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. (2010). The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol 22:355–361. [DOI] [PubMed] [Google Scholar]
- Jeannotte AM, McCarthy JG, Redei EE, Sidhu A. (2009). Desipramine modulation of alpha-, gamma-synuclein, and the norepinephrine transporter in an animal model of depression. Neuropsychopharmacology 34:987–998. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. (2003). Chronic exposure to cold stress alters electrophysiological properties of locus coeruleus neurons recorded in vitro. Neuropsychopharmacology 28:63–72. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. (2002). Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry 7 (Suppl 1):71–80. [DOI] [PubMed] [Google Scholar]
- Kyeremanteng C, MacKay JC, James JS, Kent P, Cayer C, Anisman H, Merali Z. (2014). Effects of electroconvulsive seizures on depression-related behavior, memory and neurochemical changes in wistar and wistar-kyoto rats. Prog Neuropsychopharmacol Biol Psychiatry 54C:170–178. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Armario A. (1996). Differential responsiveness of inbred strains of rats to antidepressants in the forced swimming test: Are wistar kyoto rats an animal model of subsensitivity to antidepressants? Psychopharmacology (Berl) 123:191–198. [DOI] [PubMed] [Google Scholar]
- Lahmame A, del Arco C, Pazos A, Yritia M, Armario A. (1997). Are wistar-kyoto rats a genetic animal model of depression resistant to antidepressants? Eur J Pharmacol 337:115–123. [DOI] [PubMed] [Google Scholar]
- Lei Y, Yaroslavsky I, Tejani-Butt SM. (2009). Strain differences in the distribution of N-methyl-d-aspartate and gamma (gamma)-aminobutyric acid-A receptors in rat brain. Life Sci 85:794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JC, Zhang G, Walsh T, Kirby LG, Akanwa A, Brooks-Kayal A, Beck SG. (2011). Stress-hyperresponsive WKY rats demonstrate depressed dorsal raphe neuronal excitability and dysregulated CRF-mediated responses. Neuropsychopharmacology 36:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. (2000). Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology 22:191–199. [DOI] [PubMed] [Google Scholar]
- Luscher B, Shen Q, Sahir N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 16:383–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Morilak DA. (2004). Induction of FOS expression by acute immobilization stress is reduced in locus coeruleus and medial amygdala of wistar-kyoto rats compared to sprague-dawley rats. Neuroscience 124:963–972. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Braw Y, Maayan R, Weizman A, Overstreet DH, Shabat-Simon M, Kesner Y, Touati-Werner D, Yadid G, Weller A. (2006). Two different putative genetic animal models of childhood depression. Biol Psychiatry 59:17–23. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Maayan R, Weizman A, Weller A. (2006). Aggressive behavior and HPA axis hormones after social isolation in adult rats of two different genetic animal models for depression. Behav Brain Res 175:408–414. [DOI] [PubMed] [Google Scholar]
- Mana MJ, Grace AA. (1997). Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience 81:1055–1064. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. (2005). Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 29:1214–1224. [DOI] [PubMed] [Google Scholar]
- Nam H, Clinton SM, Jackson NL, Kerman IA. (2014). Learned helplessness and social avoidance in the wistar-kyoto rat. Front Behav Neurosci 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. (2010). Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriega NC, Garyfallou VT, Kohama SG, Urbanski HF. (2007). Glutamate receptor subunit expression in the rhesus macaque locus coeruleus. Brain Res 1173:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Aoki K. (1963). Development of a strain of spontaneously hypertensive rats. Jpn Circ J 27:282–293. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Steinmann MW, Brugger F, Pozza MF. (1989). Excitatory amino acid receptors in rat locus coeruleus. an extracellular in vitro study. Naunyn Schmiedebergs Arch Pharmacol 339:312–314. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. (2012). Modeling depression in animal models. Methods Mol Biol 829:125–144. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. (2002). Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: Implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience 115:229–242. [DOI] [PubMed] [Google Scholar]
- Pare WP. (1989). Stress ulcer susceptibility and depression in wistar kyoto (WKY) rats. Physiol Behav 46:993–998. [DOI] [PubMed] [Google Scholar]
- Pare WP. (2000). Investigatory behavior of a novel conspecific by wistar kyoto, wistar and sprague-dawley rats. Brain Res Bull 53:759–765. [DOI] [PubMed] [Google Scholar]
- Pare WP, Redei E. (1993). Depressive behavior and stress ulcer in wistar kyoto rats. J Physiol Paris 87:229–238. [DOI] [PubMed] [Google Scholar]
- Pare WP, Tejani-Butt SM. (1996). Effect of stress on the behavior and 5-HT system in sprague-dawley and wistar kyoto rat strains. Integr Physiol Behav Sci 31:112–121. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (1997). The Rat Brain in Stereotaxic Coordinates, Compact. San Diego, CA: Academic Press. [Google Scholar]
- Petty F, Sherman AD. (1984). Plasma GABA levels in psychiatric illness. J Affect Disord 6:131–138. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Sanacora G, Krystal JH. (2007). The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol Disord Drug Targets 6:101–115. [DOI] [PubMed] [Google Scholar]
- Redei EE, Solberg LC, Kluczynski JM, Pare WP. (2001). Paradoxical hormonal and behavioral responses to hypothyroid and hyperthyroid states in the wistar-kyoto rat. Neuropsychopharmacology 24:632–639. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. (2002). Amplified behavioral and endocrine responses to forced swim stress in the wistar-kyoto rat. Psychoneuroendocrinology 27:303–318. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. (2012). Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62:63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands SA, Strong R, Corbitt J, Morilak DA. (2000). Effects of acute restrain stress on tyrosine hydroxylase mRNA expression in Locus coeruleus of Wistar and Wistar-Kyoto rats. Mol Brain Res 75:1–7. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. (2012). Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 76:130–141. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Renner KJ, Forster GL, Tejani-Butt S. (2010). Central monoamine levels differ between rat strains used in studies of depressive behavior. Brain Res 1355:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Olson SL, Turek FW, Redei E. (2001). Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol 281:R786–794. [DOI] [PubMed] [Google Scholar]
- Sugiyama D, Hur SW, Pickering AE, Kase D, Kim SJ, Kawamata M, Imoto K, Furue H. (2012). In vivo patch-clamp recording from locus coeruleus neurones in the rat brainstem. J Physiol 590:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo ST, Blier P. (2001). Serotonin (1A) receptor ligands act on norepinephrine neuron firing through excitatory amino acid and GABA(A) receptors: A microiontophoretic study in the rat locus coeruleus. Synapse 42:203–212. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Pare WP, Yang J. (1994). Effect of repeated novel stressor on depressive behavior and brain norepinephrine receptor system in Sprague-Dawley and Wistar Kyoto (WKY) rats. Brain Res 649:27–35. [DOI] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Pare WP. (2003). Strain-dependent modification of behavior following antidepressant treatment. Prog Neuropsychopharmacol Biol Psychiatry 27:7–14. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. (2012). Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female wistar-kyoto rats. Neuroscience 213:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Quillinan N, Williams JT, Wickman K. (2008). Pre- and postsynaptic regulation of locus coeruleus neurons after chronic morphine treatment: A study of GIRK-knockout mice. Eur J Neurosci 28:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrecilla M, Fernandez-Aedo I, Arrue A, Zumarraga M, Ugedo L. (2013). Role of GIRK channels on the noradrenergic transmission in vivo: An electrophysiological and neurochemical study on GIRK2 mutant mice. Int J Neuropsychop 16:1093–1104. [DOI] [PubMed] [Google Scholar]
- Veeraiah P, Noronha JM, Maitra S, Bagga P, Khandelwal N, Chakravarty S, Kumar A, Patel AB. (2014). Dysfunctional glutamatergic and gamma-aminobutyric acidergic activities in prefrontal cortex of mice in social defeat model of depression. Biol Psychiatry 76(3):231–8. [DOI] [PubMed] [Google Scholar]
- Venzala E, Garcia-Garcia AL, Elizalde N, Tordera RM. (2013). Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur Neuropsychopharmacol 23:697–708. [DOI] [PubMed] [Google Scholar]
- Wabno J, Hess G. (2013). Repeated administration of imipramine modifies GABAergic transmission in rat frontal cortex. J Neural Transm 120:711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE. (2003). Selectively bred wistar-kyoto rats: An animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry 8:925–932. [DOI] [PubMed] [Google Scholar]
- Williams JT, Henderson G, North RA. (1985). Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience 14:95–101. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Tokimasa T. (1988). Inward rectification of resting and opiate-activated potassium currents in rat locus coeruleus neurons. J Neurosci 8:4299–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, Bobker DH, Harris GC. (1991). Synaptic potentials in locus coeruleus neurons in brain slices. Prog Brain Res 88:167–172. [DOI] [PubMed] [Google Scholar]
- Yamada M, Kawahara Y, Kaneko F, Kishikawa Y, Sotogaku N, Poppinga WJ, Folgering JH, Dremencov E, Kawahara H, Nishi A. (2013). Upregulation of the dorsal raphe nucleus-prefrontal cortex serotonin system by chronic treatment with escitalopram in hyposerotonergic wistar-kyoto rats. Neuropharmacology 72:169–178. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA. (2009). Reduced expression of GABA transporter GAT3 in helpless rats, an animal model of depression. Neurochem Res 34:1584–1593. [DOI] [PubMed] [Google Scholar]