Abstract
Background:
Neurochemical studies have pointed to a modulatory role in human aggression for a variety of central neurotransmitters and neuromodulators such as cytokines. While animal studies of cytokines suggest an aggression-facilitating role for central cytokines, especially for interleukin-1β and other cytokines, no cerebrospinal fluid studies of cytokines have yet been reported in regard to human aggression.
Methods:
Basal lumbar cerebrospinal fluid samples were obtained from 38 physically healthy subjects with DSM-5 Personality Disorder and assayed for cerebrospinal fluid interleukin-6 (log IL-6) and cerebrospinal fluid soluble IL-1 Receptor II protein in the context of their relationship with measures of aggression.
Results:
Cerebrospinal fluid soluble interleukin-1 Receptor II (r=.35, r2 = .12, P= .03), but not log interleukin-6 (r = -.05, r2 = .00, P= .76), levels were positively correlated with a composite measure of aggression. Adding relevant covariates, including cerebrospinal fluid levels of serotonin and dopamine metabolites, to the statistical model doubled the strength of this relationship (partial r = .54, r2 = .29, P= .002). No relationship was seen with history of suicidal behavior or with any measure of impulsivity, negative affectivity, or of general dimensions of personality.
Conclusion:
These data suggest a positive relationship between at least one inflammatory cytokine in the central nervous system and aggression in human subjects. This finding adds to the complex picture of the central neurochemistry of impulsive aggression in human subjects.
Keywords: CSF, IL-6, sIL-1RII, aggression, impulsivity, personality
Introduction
Cytokines have roles both as mediators of inflammation and as neuromodulators (Dantzer et al., 2008). A modulating role for cytokines in aggressive behavior in mammals is suggested by direct experimental manipulation in lower mammals (Hassanain et al., 2003, 2005; Zalcman and Siegel, 2006; Bhatt et al., 2008), by the effect of inflammatory cytokines on anger and aggression in patients treated with cytokine immunotherapy (McHuthison et al., 1998; Kraus et al., 2003), and by correlative studies of plasma inflammatory cytokines in otherwise healthy humans (Suarez et al., 2002; Suarez, 2003; Kiecolt-Glaser et al., 2005; Marsland et al., 2008; Brummett et al., 2010).
Recently, we reported significant positive correlations between aggression and plasma levels of interleukin-6 (IL-6) in impulsively aggressive subjects and controls (Coccaro et al., 2014). Circulating inflammatory markers in plasma can cross into the central nervous system. These pathways include passage through leaky regions in the blood-brain-barrier, active transport through saturable transporters, activation of cells lining the cerebral vasculature that produce cytokines and binding to receptors on peripheral afferent nerve fibers that can relay cytokine signals to relevant brain regions such as the hypothalamus, and other brain structures (Quan and Banks, 2007; Dantzer et al., 2008). Thus, it is an open question if cytokines assessed in spinal fluid also display a positive relationship with aggression in humans and if so, this may be a more direct way for cytokines to influence behavior.
For this follow-up study of cerebrospinal fluid (CSF) inflammatory markers and aggression, we chose to examine CSF levels of IL-6 and soluble IL-1 Receptor II (sIL-1RII) protein. IL-6 was selected because it is a proinflammatory cytokine (Heinrich et al., 2003) and has been shown to correlate positively with measures of aggression in human subjects (Suarez, 2003; Marsland et al., 2008; Brummet et al., 2010; Coccaro et al., 2014). sIL-1RII protein was selected as a proxy for IL-1β, an inflammatory cytokine that is typically nondetectable in generally healthy subjects, as it was in our preliminary studies. While circulating or CSF levels of IL-1β have yet to be studied in human aggression, we have observed a significant positive relationship between circulating levels of sIL-1RII and aggression (E. F. Coccaro, R. Lee, and M. Coussons-Read, in preparation). In addition, studies in animals demonstrate that injection of IL-1β into medial hypothalamus, or periaqueductal grey, increases aggression in the defensive-rage model in the cat (Hassanain et al., 2003, 2005). sIL-1RII is an IL-1–related protein that serves as a “decoy” IL-1 receptor (Colotta et al., 1994). It acts to bind IL-1β, preventing it from binding to sIL-1RI receptor protein, which is necessary to enable IL-1β to act as an inflammatory cytokine. While sIL-1RII also binds IL-1α, only IL-1β is readily available in the circulation and capable of entering the CNS (Gabay et al., 2010). sIL-RII is not the only protein that modulates IL-1’s proinflammatory activity. IL-1Ra, a competitive inhibitor of IL-1, also blocks IL-1 activity, but only when IL-1Ra is present in great excess of IL-1 (Avend et al., 1990; Fischer et al., 1991). In contrast, IL-1β binds to IL-1RII with much greater affinity than either IL-1α or IL-1Ra, and thus sIL-1RII can serve as “proxy” for IL-1β, in turn, representing a counter-response to inflammatory activity mediated through IL-1β (Gabay et al., 2010).
Since inflammatory cytokines are elevated in the plasma of human subjects as a function of aggression (Keicolt-Glazer et al., 2005; Marsland et al., 2008; Brommet et al., 2010, Coccaro et al., 2014) and because previous work links inflammatory cytokines with aggressive behavior in lower mammals (Zalcman and Siegel, 2006), we hypothesized that CSF levels of IL-6 and sIL-1RII protein would correlate directly with measures of aggression in personality disordered subjects. In addition, we hypothesized that any relationship between CSF levels of these inflammatory cytokines and aggression would not be accounted for by relationships between aggression and CSF levels of serotonin or dopamine metabolites (Coccaro and Lee, 2010).
Methods
Subjects
Thirty-eight physically healthy adult male and female subjects, between 20 and 50 years of age, participated in this study. All subjects were afebrile and medically healthy and were systematically evaluated in regard to aggressive and other behaviors as part of a larger program designed to study the biological correlates of impulsive aggressive and other personality-related behaviors in human subjects. Subjects were recruited from clinical settings and through newspaper advertisements seeking out aggressive and nonaggressive individuals interested and willing to participate in biological studies of personality traits. All subjects gave informed consent and signed the informed consent document approved by our Committee for the Protection of Human Subjects. Subjects with a life history of bipolar disorder, schizophrenia (or other psychotic disorder), or mental retardation were excluded from this study.
Diagnostic Assessment
Syndromal and personality disorder diagnoses were made according to DSM-5 criteria (American Psychiatric Association, 2013). Research assessments were performed by individuals with masters/doctoral degrees in clinical psychology with inter-rater (kappa) reliability ranging from .79 to 93 (mean ± SD: 84 ± .05) across mood, anxiety, substance use, impulse control, and personality disorders. Final diagnoses were assigned by previously described best-estimate consensus procedures (Bunce et al., 2005) utilizing information from: (1) Structured Clinical Interview for DSM Diagnoses (First et al., 1997), (2) Structured Interview for the Diagnosis of DSM-IV Personality Disorder (Phohl et al., 1997), (3) clinical interview by a research psychiatrist, and (4) review of all available clinical data. The medical health of all subjects was documented by medical history, physical examination, electrocardiogram, and blood hematology, chemistry (including hepatic profile), thyroid function tests, urinalysis, and drug screen (subjects screening positive for illicit drugs were not entered into study). Syndromal and personality disorder diagnoses are listed in Table 1.
Table 1.
Syndromal and Personality Disorder Diagnoses in the Sample
| PD Subjects (N = 38) | |
|---|---|
| Current syndromal disorders | |
| Any depressive disorder | 7 (18.4%) |
| Any anxiety disorder | 2 (5.3%) |
| Any substance use disorder | 0 (0.0%) |
| Intermittent explosive disorder | 10 (26.3%) |
| Stress and trauma disorders | 1 (2.6%) |
| Eating disorders | 1 (2.6%) |
| Somatoform disorders | 2 (5.3%) |
| Lifetime syndromal disorders | |
| Any depressive disorder | 16 (42.1%) |
| Any anxiety disorder | 3 (7.9%) |
| Any substance use disorder | 14 (39.5%) |
| Intermittent explosive disorder | 12 (31.6%) |
| Stress and trauma disorders | 4 (10.5%) |
| Eating disorders | 2 (5.3%) |
| Somatoform disorders | 2 (5.3%) |
| Personality disorder | |
| Cluster A | 10 (26.3%) |
| Cluster B | 13 (34.2%) |
| Cluster C | 9 (23.7%) |
| Personality disorder-NOS | 10 (39.5%) |
Abbreviations: NOS, not otherwise specified; PD, personality disorder.
Psychometric Measures: Aggression
Aggression, the primary outcome variable, was assessed with the Aggression Scale from Life History of Aggression (LHA; Coccaro et al., 1997) and the Aggression Factor from the Buss-Durkee Hostility Inventory (BDHI; Buss and Durkee, 1957) before the lumbar puncture procedure. In healthy controls, LHA Aggression scores run from 0 to 11, while BDHI Aggression scores run from 0 to 25.
Psychometric Measures: Related and Other Behavioral Variables
Life history of suicidal behavior, a related variable, was assessed during the SCID interviews described above. Impulsivity was assessed using the Barratt Impulsivity Scale (BIS-11; Patton et al., 2005) and the Impulsiveness Scale from the Eysenck Personality Questionnaire-2 (EPQ-2; Eysenck and Eysenck, 1977). Other assessments included the Hamilton Depression Rating Scale (Hamilton, 1961) to assess current state depressive symptomatology, Speilberger State Anxiety Inventory (Speilberger et al., 1999) to assess current state levels of anxiety, Life Experiences Survey (Sarason et al., 1978) for both positive and negative stressful psychosocial life events over the previous 6 months, neuroticism from the Eysenck Personality Questionnaire (EPQ-N; Eysenck and Eysenck, 1975) to assess the relationship between the CSF inflammatory markers and “negative affectivity,” and novelty seeking, harm avoidance, and reward dependence scales from the Tri-Dimensional Personality Questionnaire (Cloninger, 1988) as general measures of personality function. The Global Assessment of Function (American Psychiatric Association, 1994) scale was used to assess current psychosocial functioning, and socioeconomic status was estimated using the method of Hollingshead (Edwards-Hewitt and Gray, 1995). All measures were assessed before the lumbar puncture procedure.
General Preparation for Study
No subject was taking any medical or psychotropic agents for at least 4 weeks at the time of study, typically much longer, and only 7 subjects (18%) had any lifetime exposure to psychotropic agents. Subjects were informed that initial and follow-up urine toxicology would be performed randomly just prior to study; illicit drug use was not detected in any subject reported herein.
Lumbar Puncture
The evening before the lumbar puncture, subjects reported to the Clinical Procedures Lab at approximately 8:00 pm. At approximately 11:00 pm subjects had a snack and were placed at rest in a supine position in a hospital bed. Lumbar punctures were performed by a research neurologist in the morning hours after no less than 8 hours of fasting and rest; all subjects were observed by staff to be sleeping through the night prior to lumbar puncture. The procedure was performed under sterile technique with the subject in the lateral decubitus position. A total of 20 cc of CSF was obtained in 6 aliquots: aliquots 1, 2, and 4, 5, 6 each consisted of 1 cc of CSF and were set aside for future analyses. Aliquot 3 was composed of 1 pooled 15-cc sample of CSF, subsequently subdivided into fifteen 1-cc subaliquots for later analysis. One pooled aliquot was used for assay of IL-6 and sIL-1RII. All CSF samples were placed in polypropylene tubes and were frozen immediately at -80oC until assay in the laboratory of one of the coauthors (M.C.R.).
Assay of CSF Inflammatory Cytokines
All laboratory assays were conducted blind to any diagnostic or psychometric data. Commercially available enzyme-linked immunosorbent assay kits (R&D Systems) were used to quantify CSF IL-6 and CSF sIL-1RII; preliminary studies revealed no detectable levels of IL-1β, and thus analysis of these data were not possible. Undiluted CSF samples were tested in duplicate and according to the directions provided by the manufacturer. Optical density at 450nm was assessed using an automatic microplate reader (LabSystems MultiSkan), and the amount of IL-1RII in each sample was determined using the standard curve generated with each assay according to the manufacturer’s instructions. Samples were run together to avoid problems with assay drift and interassay variability. Enzyme-linked immunosorbent assay kits from the same manufacturer’s lot were used for all assays for all measures. In practice, these assays show minimal variability between the standard curves (<6% variability) in our laboratory. The mean of the duplicates was used as the unit of analysis for statistical evaluation of these data. In addition to CSF IL-6 and CSF sIL-1RII, CSF levels of 5-hydroxyindolacetic acid (5-HIAA) and homovanillic acid (HVA) were also available in these subjects, as previously described (Coccaro and Lee, 2010).
Statistical Analysis and Data Reduction
Data analysis involved correlational analyses including Pearson correlation and multiple regression analysis. An alpha level of 0.05 denoted statistical significance. CSF IL-6 levels were not normally distributed and were log-transformed so that parametric statistical procedures could be employed. CSF sIL-1RII data were normally distributed and thus were analyzed without any transformation. Follow-up analyses were also conducted adding the effect of body mass index, current depression scores from the Hamilton Depression Rating Scale, positive and negative stressful life events during the previous 6 months from the Life Experiences Survey, and current alcohol consumption status (yes/no) and current smoking status (yes/no); quantitative data regarding alcohol/smoking consumption were not available for this study. The primary behavioral outcome variable was a composite of the LHA and BDHI Aggression scores. These source variables were significantly correlated (r = .51, P < .001), and the composite variable was created by taking the Z-score for each, and dividing by 2, in a data-reduction step as previously described (Coccaro et al., 2010). The same composite variable was created for a composite impulsivity variable (BIS-11/EPQ-2).
Results
Demographic, Behavioral, and CSF Data
Data regarding the demographic, behavioral characteristics, and CSF levels for the sample are displayed in Table 2. Neither CSF IL-6 nor CSF sIL-1RII levels were significantly correlated with demographic variables such as age, gender, race, or socioeconomic status.
Table 2.
Demographic, Behavioral, and CSF Inflammatory Cytokine Data for the Sample
| Demographic Variables | PD Subjects (N = 38) |
|---|---|
| Age (y) | 32.5±7.7 |
| Gender (M/F) | 29/9 |
| Race (white/nonwhite) | 26/12 |
| SES class (I/II-IV/V) | 1/26/11 |
| GAF score | 58.7±9.7 |
| Covariate variables | |
| Body mass index | 24.2±3.8 |
| Hamilton depression rating score | 5.7±4.2 |
| Life experiences survey | 0.8±14.2 |
| Current alcohol use status (yes) | 68.4% |
| Current smoking status (yes) | 28.9% |
| Aggression behavioral variables | |
| LHA aggression score | 9.5±6.4 |
| BDHI aggression score | 23.3±10.0 |
| Aggression-related variables | |
| History of suicide attempt (yes) | 23.7% |
| Other behavioral variables | |
| BIS-11 impulsivity score | 66.3±13.8 |
| EPQ-2 impulsivity score | 7.4±5.2 |
| State anxiety score | 38.6±14.8 |
| EPQ neuroticism score | 11.2±6.3 |
| TPQ novelty seeking | 16.4±6.0 |
| TPQ harm avoidance | 12.4±7.5 |
| TPQ reward dependence | 20.2±5.0 |
| CFS inflammatory cyokine levels | |
| CSF IL-6 (pg/mL) | 3.0±1.9 |
| CSF sIL-1RII protein (pg/mL) | 334.0±64.7 |
Abbreviations: BDHI, Buss-Durkee Hostility Inventory; BIS, Barratt Impulsivity Scale; CSF, cerebrospinal fluid; EPQ, Eysenck Personality Questionnaire; GAF, Global Assessment of Function; LHA, Life History of Aggression; SES, socioeconomic status; TPQ, Tridimensional Personality Questionnaire.
CSF Inflammatory Cytokine Levels and Composite Aggression
CSF sIL-1RII (r = .35, r2 = .12, P= .03), but not log CSF IL-6 (r = -.05, r2 = .00, P= .76), levels were correlated with composite aggression. Adding the relevant covariates to the statistical model did not alter this result (partial r = .38, r2 = .14, P= .029) for CSF sIL-RII levels. Using multiple regression analysis to break down composite aggression into separate LHA and BDHI scores revealed nearly equal contributions for LHA (partial r = .16, r2 = .03) and BDHI (partial r = .20, r2 = .04) to CSF sIL-1Rll levels. Adding the relevant covariates (body mass index, current depression scores, recent psychosocial stress score, current alcohol/smoking status) either did not change (BDHI: partial r = .19, r2 = .04) or modestly increased (LHA: partial r = .22, r2 = .06) the magnitude of these correlations.
CSF Inflammatory Cytokines and Past History of Suicide Attempt
Despite the observation that composite aggression scores were significantly higher among subjects with a past history of a suicide attempt [SA+ (n = 9): 0.63±0.95 vs SA- (n = 29): -.20±0.75; t36 = 2.71, P= .01], neither CSF log IL-6 nor CSF sIL-RII levels differed as a function of a past history of a suicide attempt.
CSF Inflammatory Cytokines and Nonaggressive Behavioral Variables
In contrast to composite aggression, neither of the CSF inflammatory cytokine levels correlated significantly with composite impulsivity, state anxiety, or any of the other personality trait variables (Table 3).
Table 3.
Correlations between CSF Inflammatory Cytokines and Nonaggression Variables
| CSF Log IL-6 | CSF sIL-1RII | |
|---|---|---|
| Composite impulsivity | r = -.01 (P= .94) | r = .04 (P= .81) |
| State anxiety | r = .08 (P= .63) | r = .05 (P= .75) |
| Neuroticism | r = .00 (P= .98) | r = .05 (P= .76) |
| Novelty seeking | r = .09 (P= .60) | r = .09 (P= .58) |
| Harm avoidance | r = .02 (P= .90) | r = -.06 (P= .71) |
| Reward dependence | r = -.19 (P= .26) | r = -.26 (P= .11) |
CSF Inflammatory Cytokines and Diagnostic Variables and Other Personality Variables
Neither CSF log IL-6 nor IL-RII levels differed as a function of current or lifetime history of depressive disorder, anxiety disorder, intermittent explosive disorder, or substance use disorder.
Relationship between CSF Inflammatory Cytokines, CSF 5-HIAA/CSF HVA Levels, and Aggression
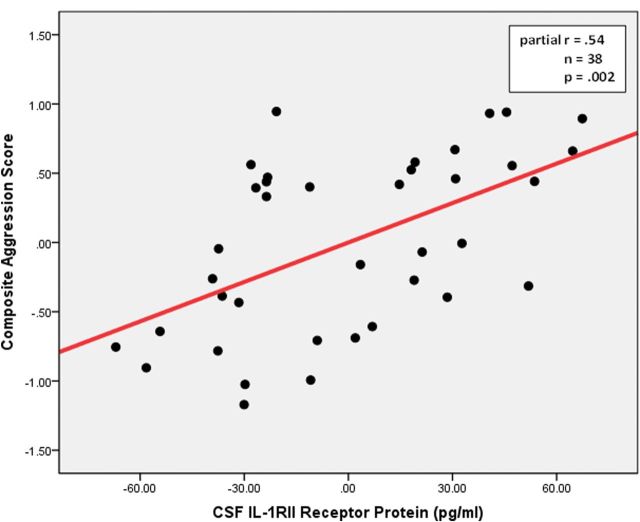
Significant relationships between CSF 5-HIAA, CSF HVA, and measures of aggression have been documented in several reports (eg, Coccaro and Lee, 2010). Accordingly, it was of interest to determine if there was a relationship between CSF 5-HIAA, and/or HVA, and the CSF marker/mediators in this study. Multiple regression with composite aggression as the dependent variable, and CSF 5-HIAA, CSF HVA, and CSF sIL-1RII as independent variables (along with the other relevant covariates), revealed a significant relationship between composite aggression and CSF sIL-1RII levels (partial r = .54, r2 = .29, P= .002) even with the presence of CSF 5-HIAA/HVA in the statistical model (Figure 1).
Fig. 1.
Partial regression plot between cerebrospinal fluid (CSF) soluble IL-1 Receptor II (sIL-1RII) receptor protein and composite aggression in personality disordered subjects (includes covariates: CSF 5-hydroxyindolacetic acid [5-HIAA], CSF homovanillic acid [HVA], body mass index, current depressive score, recent psychosocial stress score, and current drinking/smoking status).
Discussion
This is the first study to investigate the relationship of central nervous system inflammatory cytokines and aggression in human subjects. The positive correlation between a history of aggressive acts, and between a personality measure of aggression, and CSF sIL-1RII level suggests a role for inflammatory activity in the modulation of aggressive behavior and is consistent with data from lower mammals that IL-1β is involved in the positive modulation of aggression (Hassanain et al., 2003, 2005; Zalcman and Siegel, 2006; Bhatt et al., 2008). The absence of a similar observation with CSF IL-6, however, given a positive correlation observed between aggression and plasma levels of IL-6 in a separate group of subjects (Coccaro et al., 2014) may be due to differences in the 2 subject groups and/or differences in circulating and CSF in regards to IL-6.
These results were not due to variability in other relevant factors. First, subjects were all physically healthy, free of any medication treatment for at least 4 weeks (typically much longer), and not dependent on alcohol or other drugs of any kind. Second, even after accounting for body mass index and other variables that might affect CSF levels of inflammatory markers (eg, current depressive symptoms, recent psychosocial stress, and current alcohol/smoking status), the results remained the same. This does not mean these variables are not relevant in the expression of CSF sIL-1RII levels, because these variables can be associated with elevations in a variety of inflammatory markers (Suarez, 2003; Howren et al., 2009; Dowlati et al., 2010) in human subjects. Instead, our analysis simply shows that even after accounting for these variables, there is a significant relationship between CSF sIL-1RII level and composite aggression. Third, these results are not due to the presence of other Axis I or II conditions, impulsivity, negative affectivity, or to other dimensions of general personality other than the dimension of aggression. In this way, these data tend to support the recently proposed RDoc approach to the study of dimensions of behavior and neurobiology rather than simply examining diagnostic entities (Insel et al., 2010).
Plasma levels of inflammatory cytokines are reported to correlate positively with measures of aggression in human subjects (Suarez, 2003; Kiecolt-Glaser et al., 2005; Suarez et al., 2006; Marsland et al., 2008; Coccaro et al., 2014). Thus, it was surprising to observe a relationship between aggression measures and CSF sIL-1RII but not with CSF IL-6. This may be due to fundamental differences between the inflammatory cytokines and/or type II error. While IL-6 is a direct marker, sIL-1RII is an indirect marker of inflammation. This is because sIL-1RII becomes a high affinity IL-1β receptor once bound to its accessory protein (Gabay et al., 2010). In this high affinity state, the degree of IL-1β available to bind to IL-1RI receptors on target cells is diminished, in turn reducing the propagation of a IL-1β–mediated proinflammatory response (Gabay et al., 2010).
The inflammatory cytokines studied are present in human CSF both because of the presence of inflammatory processes in the CNS and because of transfer of such cytokines from the circulation to the CNS. Circulating inflammatory markers in plasma can cross the blood-brain-barrier through a number of pathways, including, but not limited to, binding to receptors on peripheral afferent nerve fibers that can relay cytokine signals to relevant brain regions such as the hypothalamus, and other brain structures (Quan and Banks, 2007; Dantzer et al., 2008;). While we cannot know how much of the sIL-1RII detected in the CSF of these subjects comes from within the CNS or from the circulation, cytokines such as IL-1β (which bind well to sIL-1RII and less well to sIL-1RI, receptors) can modulate aggressive behaviors in animals when centrally administered (Hassanain et al., 2003, 2005). The effect of IL-1β may be mediated by its stimulation of 5-HT-2 receptors in the medial hypothalamus (Hassanain et al., 2005). If so, it is noteworthy that CSF sIL-1RII levels correlated with composite aggression, even when CSF sIL-1RII and CSF 5-HIAA levels are in the same statistical model. These data suggest that, despite the known relationship between CSF 5-HIAA levels and aggression (Coccaro and Lee, 2010), CSF sIL-1RII levels appear related to aggression above and beyond the explanatory nature of relevant monoamine metabolites.
It is possible that central inflammatory markers are elevated in response to the stress of repeated aggressive interactions. Clinical study of healthy individuals demonstrates an increase inflammatory cytokines after anger recall interviews (Brummett et al., 2010). However, these changes were small and dependent on the subject having high self-assessed depression scores. In the current study, subjects were examined in the morning after sleeping overnight in a bed in the Clinical Procedures Suite, with no subject reporting physical injury of any notable psychosocial stressor on the day of, or during the day before, sample collection. On the other hand, undergoing a lumbar puncture can be perceived as anxiety-provoking, if not stressful, and there may have been some variability among subjects along this dimension. Despite this, we observed no relationship between any of the CSF inflammatory markers and state levels of anxiety suggesting that inter-subject variability along this dimension cannot account for the present findings. Finally, statistical control for differences in recent psychosocial stress with the Life Experiences Survey did not alter the results, further suggesting that this variable cannot account for these findings.
The strengths of this study include a well-characterized patient sample, multiple validated measures of aggression and related measures, a standardized approach to drug-free status, and subject activity to minimize the effect of extraneous factors on CSF inflammatory marker levels. Limitations to this include the fact that this is a cross-sectional study, that no causal conclusions can be made from associative and correlational analyses, and that the magnitude of these findings may be smaller, or non-existent, in a larger sample. An additional limitation is that only categorical assessments of current alcohol consumption and current cigarette smoking were available for analysis. While adding these categorical measures did not change the results, it is possible that adding the number of alcoholic drinks per day and the number of cigarettes per day could have reduced or enhanced these results. Finally, the ascertainment of subjects may limit the generalizability of these findings in that these involved subjects who volunteered for research study rather than for clinical treatment. However, nearly all subjects (84%) reported a past history of psychiatric treatment (63%) or of having episodes of behavioral disturbance for which they or others thought they should have sought mental health services but did not (23%). If so, at least this group of subjects may be similar to those who would have been recruited from a clinical setting.
In summary, we report a positive relationship between CSF sIL-1RII protein levels and aggression in human subjects, particularly in those with a personality disorder. This relationship was not accounted for by any factors studied such as demographic/physical correlates, psychiatric disorder, body mass index, current depressive symptoms, recent psychosocial stress, current alcohol or smoking status, negative affectivity, or other personality traits other than aggression. These data are in line with the hypothesized central role IL-1β plays in regulation of aggression as reported in animal models of aggression (Zalcman and Seigel, 2006). However, only, experimental studies examining aggressive responding in the laboratory, with and without pretreatment with antiinflammatory agents, can shed light on whether IL-1β or other inflammatory cytokines are related to aggressive behavior in any meaningful causal fashion. Given that a disorder of aggression, intermittent explosive disorder displays, a 2% to 3% 1-year prevalence rate in the US (Kessler et al., 2006), and that currently available psychotropic treatments bring <50% of those treated into remission (Coccaro et a., 2009), additional strategies for the examination and intervention of aggression in human subjects is needed.
Interest Statement
Dr. Coccaro reports that he is on the Scientific Advisory Board of Azevan Pharmaceuticals., Inc. and that he has stock options in Azivan Pharmaceuticals, Inc., through this role. Dr. Lee reports that he has received a research grant from Azivan Pharmaceuticals, Inc. Dr. Coussons-Read reports no real or apparent conflicts of interest.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (RO1 MH 66984, RO1 MH 60836; RO1 MH 63262 to Dr. Coccaro) and a Project Pilot grant from the University of Colorado Denver (to Dr. Coussons-Read).
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (5th edition). Washington, DC: American Psychiatric Association Press. [Google Scholar]
- Arend WP, Welgus HG, Thompson RC, Eisenberg SP. (1990). Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Inv 85:1694–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Bhatt R, Zalcman SS, Siegel A. (2008). Role of IL-1 beta and 5-HT2 receptors in midbrain periaqueductal gray (PAG) in potentiating defensive rage behavior in cat. Brain Behav Immun 22:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Boyle SH, Ortel TL, Becker RC, et al. (2010). Associations of depressive symptoms, trait hostility, and gender with C-reactive protein and interleukin-6 response after emotion recall. Psychosom Med. 72:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunce SC, Noblett KL, McCloskey MS, Coccaro EF. (2005). High prevalence of personality disorders among healthy volunteers for research: implications for control group bias. J Psychiatr Res 39:421–430. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. (1957). An inventory for assessing different kinds of hostility. J Consult Psychol 21:343–348. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. (1988). A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psych 44:573–588. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. (2012). Intermittent explosive disorder as a disorder of impulsive aggression for DSM-5. Am J Psych 69:577–588. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R. (2010). Cerebrospinal fluid 5-hydroxyindolacetic acid and homovanillic acid: reciprocal relationships with impulsive aggression in human subjects. J Neural Trans 117:241–248. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Berman ME, Kavoussi RJ. (1997). Assessment of life history of aggression: development and psychometric characteristics. Psych Res 73:147–157. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee RJ, Kavoussi RJ. (2009). A double-blind, randomized, placebo-controlled trial of fluoxetine in patients with intermittent explosive disorder. J Clin Psych 70:653–662. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Kavoussi RJ. (2010). Aggression, suicidality, and intermittent explosive disorder: serotonergic correlates in personality disorder and healthy control subjects. Neuropsychopharm 35:435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Coussons-Read M. (2014). Plasma markers of inflammation are elevated in subjects with Intermittent Explosive Disorder and correlate directly with aggression in human subjects. JAMA Psychiatry 71:158–165. [DOI] [PubMed] [Google Scholar]
- Colotta F, Dower SK, Sims JE, Mantovani A. (1994). The type II ‘decoy’ receptor: a novel regulatory pathway for interleukin 1. Immun Today 15:562–566. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, et al. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, et al. (2010). A meta-analysis of cytokines in major depression. Bio Psych 67:446–457. [DOI] [PubMed] [Google Scholar]
- Edwards-Hewitt T, Gray JJ. (1995). Comparison of measures of socioeconomic status between ethnic groups. Psychol Reports 77:699–702. [Google Scholar]
- Eysenck HJ, Eysenck SBG. (1975). Manual of the Eysenck personality questionnaire (junior and adult). London: Hodder and Stoughton. [Google Scholar]
- Eysenck SBG, Eysenck HJ. (1977). The place of impulsiveness in a dimensional system of personality description. Br J Soc Clin Psychol 16:57–68. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I Disorders (SCID). New York: Psychiatric Institute, Biometrics Research, 1997. [Google Scholar]
- Fischer E, Marano MA, Barber AE. (1991). Comparison between effects of interleukin-1 alpha administration and sublethal endotoxemia in primates. Am J Physiol 261:R442–R452. [DOI] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. (2010). IL-1 pathways in inflammation and human diseases. Nat Rev Rheum 6:232–241. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Zalcman S, Bhatt S, Siegel A. (2003). Interleukin-1 beta in the hypothalamus potentiates feline defensive rage: role of serotonin-2 receptors. Neurosci 120:227–233. [DOI] [PubMed] [Google Scholar]
- Hassanain M, Bhatt S, Zalcman S, Siegel A. (2005). Potentiating role of interleukin-1beta (IL-1beta) and IL-1beta type 1 receptors in the medial hypothalamus in defensive rage behavior in the cat. Brain Res 1048:1–11. [DOI] [PubMed] [Google Scholar]
- Heinrich PC, Behrmann I, Haan S, Hermanns H, et al. (2003). Principles of interleukin-6-type cytokine signalling and its regulation. Biochem J 374:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71:171–186. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, et al. (2010). Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psych 167:748–751. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Coccaro EF, Fava M, Jaeger S, et al. (2006). The prevalence and correlates of DSM-IV intermittent explosive disorder in the National Comorbidity Survey Replication. Arch Gen Psych 63:669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, et al. (2005). Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psych 62:1377–1384. [DOI] [PubMed] [Google Scholar]
- Kraus MR, Schafer A, Faller H, Csef H, et al. (2003). Psychiatric symptoms in patients with chronic hepatitis C receiving interferon alpha-2b therapy. J Clin Psych 64:708–714. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Prather AA, Petersen KL, Cohen S, et al. (2008). Antagonistic characteristics are positively associated with inflammatory markers independently of trait negative emotionality. Brain Behav Immun 22:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHuthison JG, Gordon SC, Schiff ER. (1998). Interferon alpha-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. NEJM 339:1485–1492. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. (1995). Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Pfohl B, Blum N, Zimmerman M.University of Iowa Department of Psychiatry (1997). Structured interview for DSM-IV Personality: SIDP-IV. Washington, DC: American Psychiatric Press. [Google Scholar]
- Quan N, Banks WA. (2007). Brain-immune communication pathways. Brain Behav Immun 21:727–735. [DOI] [PubMed] [Google Scholar]
- Sarason I, Johnson J, Siegel J. (1978). Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol 1978 46:932–946. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ. (1999). Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: The use of psychological testing for treatment planning and outcomes assessment (Maruish ME, ed; 2nd edition), pp993–1021. Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Suarez EC. (2003). Joint effect of hostility and severity of depressive symptoms on plasma interleukin-6 concentration. Psychosom Med 65:523–527. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn C. (2002). The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun 16:675–684. [DOI] [PubMed] [Google Scholar]
- Zalcman SS, Siegel A. (2006). The neurobiology of aggression and rage: role of cytokines. Brain Behav Immun 20:507–514. [DOI] [PubMed] [Google Scholar]