Abstract
Background:
Weight gain is the most frequent adverse effect of valproic acid (VPA) treatment, resulting in poor compliance and many endocrine disturbances. Similarities in the weight change of monozygotic twins receiving VPA strongly suggests that genetic factors are involved in this effect. However, few studies have been conducted to identify the relevant genetic polymorphisms. Additionally, the causal relationship between the VPA concentration and weight gain has been controversial. Thus, we investigated the effects of single nucleotide polymorphisms (SNPs) in several appetite stimulation and energy homeostasis genes and the steady state plasma concentrations (Css) of VPA on the occurrence of weight gain in patients.
Methods:
A total of 212 epilepsy patients receiving VPA were enrolled. Nineteen SNPs in 11 genes were detected using the Sequenom MassArray iPlex platform, and VPA Css was determined by high-performance liquid chromatography (HPLC).
Results:
After 6 months of treatment, 20.28% of patients were found to gain a significant amount of weight (weight gained ≥7%). Three SNPs in the leptin receptor (LEPR), ankyrin repeat kinase domain containing 1 (ANKK1), and α catalytic subunit of adenosine monophosphate-activated protein kinase (AMPK) showed significant associations with VPA-induced weight gain (p < 0.001, p = 0.017 and p = 0.020, respectively). After Bonferroni correction for multiple tests, the genotypic association of LEPR rs1137101, the allelic association of LEPR rs1137101, and ANKK1 rs1800497 with weight gain remained significant. However, the VPA Css in patents who gained weight were not significantly different from those who did not gain weight (p = 0.121).
Conclusions:
LEPR and ANKK1 genetic polymorphisms may have value in predicting VPA-induced weight gain.
Keywords: concentration, polymorphism, valproic acid, weight gain
Introduction
Valproic acid (VPA) is one of the most frequently prescribed antiepileptic drugs (Löscher, 2002) and is also increasingly used for other indications, such as bipolar psychiatric disorder (Bowden and Singh, 2005), schizophrenia, borderline personality disorder (Haddad et al., 2009), and migraine prophylaxis (Mathew et al., 1995). Antiepileptic therapy often takes years and may even last the entire lifetime of a patient. This highlights the importance of drug safety throughout the course of therapy. It is well known that one side effect of VPA that negatively influences its appeal is a considerable increase in body weight (Biton et al., 2001). Weight gain induced by VPA seems to be associated with many metabolic and endocrine disturbances, the most frequent of which are hyperinsulinemia and insulin resistance and hyperleptinemia and leptin (LEP) resistance, which are associated with long-term vascular complications, such as hypertension and atherosclerosis (Verrotti et al., 2010; Belcastro et al., 2013). Therefore, weight gain is the most common reason for patients to discontinue VPA treatment. Several clinical studies have indicated that the frequency and extent of weight gain induced by VPA are highly variable (Verrotti et al., 2011). El-Khatib et al. (2007) reported a significant weight gain (≥5kg) in 43.6% of women and 23.5% of men on VPA therapy. And Verrotti et al. (2011) suggested that an increase of 2kg of body weight after 1 month of treatment should imply considerations to change antiepileptic drug therapy. However, the mechanism through which VPA may induce weight gain is still unknown.
Currently, research studying the effect of VPA on weight gain has focused on various hypotheses, such as dysregulation of the hypothalamic system (Lakhanpal and Kaur, 2007), hyperleptinaemia, and LEP resistance (Gungor et al., 2007; Hamed et al., 2009; Verrotti et al., 2011; Kanemura et al., 2012), but there is no single mechanism that can explain VPA-induced weight gain. Interestingly, some patients taking VPA do not gain weight, and the concordance of weight gain was found in monozygotic twins exposed to VPA (Klein et al., 2005), suggesting that genetic variation may play an important role in VPA-related weight gain. Over the past decade, research identifying genes associated with antipsychotic drug-induced weight gain focused on factors that influence the molecular pathways involved in energy homeostasis (e.g., insulin receptor signaling pathway, lipid metabolism), appetite stimulation and satiety inhibition (Czerwensky et al., 2013; Kao and Muller, 2013). Neuropeptide Y (NPY; Tiwari et al., 2013), melanocortin4 receptor (MC4R; Chowdhury et al., 2013), LEP, leptin receptor (LEPR; Brandl et al., 2012), brain-derived neurotrophic factor (BDNF; Zai et al., 2012), and serotonergic 2C-receptor (HTR 2C; Hill and Reynolds, 2011) have been extensively studied.
It has been reported that VPA-treated epileptic patients who gained weight developed an increased appetite and thirst and quenched their thirst with calorie-rich beverages (Belcastro et al., 2013). The genetic variations in the appetite stimulation and energy homeostasis genes are important candidates for exploring the genetic factors involved in VPA-induced weight gain. Previous studies demonstrated that patients treated with VPA who develop obesity have been found to have higher levels of serum insulin and LEP compared with those who do not gain weight (Verrotti et al., 1999). Recently, Avery and Bumpus (2014) reported that VPA is a novel activator of adenosine monophosphate-activated protein kinase (AMPK), a key regulator of cellular metabolism, using primary mouse and human hepatocytes. Genetic variants in the dopamine 2 receptor gene (DRD2) and ankyrin repeat and kinase domain containing 1 gene (ANKK1) influence the functioning of the dopamine-mediated reward circuitry in the brain and the risks of overeating and obesity (Chan et al., 2014), and it has been demonstrated that VPA can potentiate DRD2 activity (Lee et al., 2012). Previous literature also reported that VPA can increase NPY, BDNF, and methylenetetrahydrofolate reductase (MTHFR) gene expression (Roy et al., 2008; Farrelly et al., 2013; Almeida et al., 2014), which are related to appetite stimulation and energy homeostasis (Kao and Muller, 2013). Thus, multiple genes are probably involved in VPA-induced weight gain; however, there is a paucity of pharmacogenetic research on VPA-induced weight gain (Chang et al., 2010). Therefore, a comprehensive analysis of genetic polymorphisms that may relate to VPA-induced weight gain is necessary.
Aside from genetic factors, the relationship between VPA concentration and VPA-induced weight gain has been being disputed (Henriksen and Johannessen, 1982; Turnbull et al., 1982; Chadwick, 1985; Novak et al., 1999; Demir and Aysun, 2000), so it is necessary to clarify the relationship between steady state plasma concentrations (Css) of VPA and weight change with a large patient group.
Based on these observations, in the present study we carried out a multi-gene analysis to investigate the role of genetic variants in VPA-induced weight gain in epilepsy patients and attempted to analyze the association of VPA concentration with VPA-induced weight gain.
Methods
Study Population
A total of 212 epilepsy patients who received VPA (200–1250mg/day) were enrolled at the Department of Neurology at the First Affiliated Hospital of Sun Yat-sen University. All patients had been diagnosed with epilepsy, and had normal liver and kidney functions based on results from electroencephalograms and biochemical laboratory tests. The exclusion criteria for this study included pregnancy, infancy, severe head injuries, previous medical conditions that required treatment and were not stable (hepatitis C, HIV, thyroid disorder, or diabetes mellitus), substance dependence, clinically-relevant mental retardation, and severe personality disorder. The protocol of this study was approved by the Human Investigation Ethics Committee of the School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou, China (Clinicaltrials.gov Identifier No. NCT01172626), and written consent was obtained from all patients prior to enrollment. The dosing regimen was maintained stably for at least 1 month to ensure that the blood sampling was performed at the VPA Css. At 1, 3, and 6 months after receiving VPA, venous blood samples (2mL) were collected for analysis immediately before taking morning medications. Height and weight for the determination of body mass index (BMI) were measured on the initiation of VPA treatment and at monthly physician visits, and weight gain was determined by a change in BMI over the treatment period. An increase in weight of ≥7% was defined as significant weight gain. From the 212 patients, 121 patients received VPA monotherapy and 91 patients received a combination therapy of VPA with either lamotrigine (n = 72), carbamazepine (n = 12), or oxcarbazepine (n = 7). These other treatments were included in this study because these drugs are known to have little influence on weight change (Pickrell et al., 2013).
Genetic Analyses
Previous literature has reported associations of several factors with VPA-induced weight gain (Verrotti et al., 2011). The single nucleotide polymorphisms (SNPs) studied in our research are the following: NPY: rs16147 and rs3037354; MC4R: rs17782313, rs489693, and rs8087522; LEP: rs10954173 and rs3828942; LEPR: rs1137101 and rs1327120; BDNF: rs6265 and rs1519480; fat mass and obesity associated: rs9939609; ANKK1: rs1800497; DRD2: rs1079598; α2 catalytic subunit of AMPK (PRKAA2): rs10789038; β2 non-catalytic subunit of AMPK (PRKAB2): rs3766522; γ3 non-catalytic subunit of AMPK: rs692243; MTHFR: rs1801133; and HTR 2C: rs3813929. DNA was obtained from peripheral blood (2mL) and was extracted according to a previously described method (Loparev et al., 1991). Genotyping of all polymorphisms was carried out with the Sequenom MassArray technology platform (Sequenom). The DNA absorbance ratio (A260/A280) was greater than 1.8 to ensure high quality, and the concentrations were determined by NanoDrop 2000 (Thermo). For data acquisition and analyses, the MassArray Typer 4.0 software was used. Inspection of the clusters was carried out to ensure a clear cluster separation with satisfactory signal-to-noise cutoff. SpectroChip results with less than 99.5% concordance in duplicate checks along with more than a 10% call rate in a blank check or with more than a 25% call rate in the blank control were considered failed and were repeated.
Quantification of the VPA Plasma Concentration
VPA Css were determined by the high-performance liquid chromatography ultraviolet (HPLC-UV) method (Waters 1525-717-2487 HPLC system; Chen et al., 2012). The calibration curves ranged from 5.0–200 μg/mL. The accuracy and precision data for the intra- and inter-day plasma samples were <15% and could be used to accurately determine VPA concentrations in plasma.
Statistical Analysis
All statistical analyses of the results were performed using SPSS version 21.0. Categorical variables were compared using Pearson χ2 test, and continuous variables were analyzed using the independent t-test for normally-distributed variables or the Mann–Whitney U-test for the non-normally-distributed variables to compare the means between the two subgroups. The patients included in this study varied in gender, age, and baseline BMI. To avoid these confounding factors, analyses of covariance were used for association tests between genotype and BMI change (from baseline) as the dependent variable, with gender, age, and baseline BMI as the covariates. Bonferroni’s corrections were used for multiple comparisons. Haploview 4.2 was used to determine the deviation from the Hardy–Weinberg equilibrium (Barrett et al., 2005). Statistical significance was assumed for p values less than 0.05.
Results
Clinical Characteristics and Genotype Results
The clinical characteristics of the study population (n = 212) are summarized in Table 1. The allelic distributions of all of the SNPs detected in this study were consistent with the Hardy–Weinberg equilibrium (p > 0.05), except for HTR 2C rs 3813929, which is a promoter polymorphism of the X-linked HTR 2C gene. Table 2 summarizes all 19 SNP polymorphisms, with the allele frequencies and p-values of the allelic association of each polymorphism with the BMI change. Of these, we observed three polymorphisms—LEPR rs1137101, ANKK1 rs1800497, and PRKAA2 rs10789038—that were associated with a BMI increase within 6 months after initiation of VPA treatment (p < 0.001, p = 0.017, and p = 0.020, respectively; Table 3). After correcting for Bonferroni multiple tests (19 SNPs, p corrected = 0.0026), the difference observed in LEPR rs1137101 remained significant. For allelic association analyses, all of the allele frequencies were close to the allele frequency data of the reference HapMap population (http://www.ncbi.nlm.nih.gov/snp/). Among these alleles, we found that patients who were carriers of the A allele of LEPR rs1137101 gained significantly more weight than those with the GG genotype (AA+AG vs GG, p < 0.001). Patients who were carriers of the C allele of ANKK1 rs1800497 gained significantly more weight than those with the TT genotype (CC+CT vs TT, p = 0.0021). Similarly, for PRKAA2 rs10789038, the G allele carriers had greater changes in BMI than AA genotype carriers (GG+AG vs AA, p = 004). The statistical significance under the dominant model of LEPR rs1137101 and the recessive model of ANKK1 rs1800497 were also found after correction for Bonferroni multiple testing (19 SNPs, p corrected = 0.0026; Figure 1). Except for these, other alleles were found to have no relationship with BMI change in our study.
Table 1.
Clinical and Demographic Characteristics of the Samples (n = 212)
| Characteristics | Sample (n = 212) |
|---|---|
| Age (years) | 24.9±10.0 |
| Gender | |
| Male/female | 126/86 |
| Baseline weight (kg) | 58.13±12.85 |
| Weight after 6 months (kg) | 60.38±13.74 |
| Baseline BMI (kg/m2) | 21.42±3.38 |
| BMI after 6 months (kg/m2) | 22.17±3.73 |
| Drug prescribed | |
| VPA monotherapy | 121 |
| VPA+LTG | 72 |
| VPA+CBZ | 12 |
| VPA+OXC | 7 |
| Dosage of VPA (mg/day) | 200–1250 |
| VPA Css (μg/mL) | 58.4±25.4 |
CBZ, carbamazepine; Css, steady state plasma concentrations; LTG, lamotrigine; OXC, oxcarbazepine; VPA, valproic acid.
Table 2.
Genotypes and the Association with BMI Gain for the Total Sample (n = 212)
| Gene | SNP | BMI gain (kg/m2) (n) | p-valuea | Gene | SNP | BMI gain (kg/m2) (n) | p-valuea |
|---|---|---|---|---|---|---|---|
| NPY | rs16147 | AA 0.79±1.21 (29) | 0.227 | BDNF | rs1519480 | CC 0.87±1.3 (19) | 0.906 |
| AG 0.57±0.89 (92) | CT 0.69±1.22 (94) | ||||||
| GG 0.93±1.43 (91) | TT 0.79±1.18 (99) | ||||||
| rs3037354 | TG/TG 0.89±1.23 (91) | 0.105 | FTO | rs9939609 | AA 1.2±1.3 (4) | 0.780 | |
| TG/- 0.55±0.91 (99) | AT 0.83±1.17 (40) | ||||||
| -/- 1.05±1.86 (22) | TT 0.72±1.21 (168) | ||||||
| MC4R | rs17782313 | CC 0.41±0.6 (9) | 0.429 | ANKK1 | rs1800497 | CC 1.01±1.37 (73) | 0.017b |
| CT 0.92±1.47 (66) | CT 0.76±1.22 (91) | ||||||
| TT 0.69±1.07 (137) | TT 0.35±0.69 (48) | ||||||
| rs489693 | AA 0.87±0.81 (9) | 0.617 | DRD2 | rs1079598 | CC 0.55±0.83 (42) | 0.583 | |
| AC 0.62±0.99 (78) | CT 0.76±1.27 (113) | ||||||
| CC 0.83±1.34 (125) | TT 0.89±1.29 (57) | ||||||
| rs8087522 | AA 0.45±0.83 (4) | 0.868 | PRKAA2 | rs10789038 | AA 0.69±1.27 (150) | 0.020b | |
| AG 0.74±1.0 (53) | AG 0.87±0.99 (54) | ||||||
| GG 0.76±1.27 (155) | GG 1.23±1.08 (8) | ||||||
| LEP | rs10954173 | AA 0.61±0.91 (12) | 0.110 | PRKAB2 | rs3766522 | AA 0.67±0.85 (5) | 0.794 |
| AG 0.57±1.25 (73) | AT 0.66±0.89 (56) | ||||||
| GG 0.87±1.15 (127) | TT 0.79±1.31 (151) | ||||||
| rs3828942 | AA 0.8±1.16 (103) | 0.562 | PRAKG3 | rs692243 | CC 0.76±1.1 (52) | 0.334 | |
| AG 0.75±1.3 (91) | CG 0.81±1.26 (117) | ||||||
| GG 0.5±0.88 (18) | GG 0.59±1.16 (43) | ||||||
| LEPR | rs1137101 | AA 1.71±1.49 (4) | < 0.001c | MTHFR | rs1801133 | CC 0.85±1.33 (119) | 0.357 |
| AG 1.19±1.18 (46) | CT 0.62±1.04 (76) | ||||||
| GG 0.6±1.17 (162) | TT 0.65±0.9 (17) | ||||||
| rs1327120 | AA 0.74±1.21 (176) | 0.574 | HTR 2C | rs3813929 | C/CC 0.74±1.20 (181) | 0.508 (Total) | |
| AG 0.73±1.13 (32) | CT 0.68±1.04 (12) | ||||||
| GG 1.37±1.69 (4) | T/TT 0.94±1.34 (19) | ||||||
| BDNF | rs6265 | AA 0.78±1.53 (43) | 0.850 | rs3813929 | CC 0.62±1.01 (72) | 0.774 (Female)d | |
| AG 0.78±1.09 (111) | CT/TT 0.57±0.95 (14) | ||||||
| GG 0.68±1.13 (58) | rs3813929 | C 0.82±1.32 (109) T 1.07±1.42 (17) |
0.204 (Male)d |
a Analyses of covariance with gender, age, and baseline BMI (kg/m2) as covariates.
b p < 0.05.
c The association of rs1137101 with BMI change remained significant after the Bonferroni correction, which was set at p < 0.0026 (0.05/19 SNPs).
d Sex-specific analyses for HTR 2C rs3813929 (on the X chromosome); p-values calculated using Mann–Whitney U.
AMPK, AMP-activated protein kinase; ANKK1, ankyrin repeat kinase domain containing 1; BDNF, brain-derived neurotrophic factor; DRD2, dopamine 2 receptor gene; FTO, fat mass and obesity associated; HTR 2C, serotonergic 2C-receptor; LEP, leptin; LEPR, leptin receptor; MC4R, melanocortin4 receptor; MTHFR, methylenetetrahydrofolate reductase; NPY, neuropeptide Y; PRKAA2, α2 catalytic subunit of AMPK; PRKAB2, β2 non-catalytic subunit of AMPK; PRAKG3, γ3 non-catalytic subunit of AMPK; SNP, single nucleotide polymorphism.
Table 3.
The Association Between LEPR rs1137101, ANKK1 rs1800497, and PRKAA2 rs10789038 Genotypes with BMI Gain Within 6 Months After Initiation of VPA Treatment (n = 212)
| 1 month | 2 months | 3 months | 4 months | 5 months | 6 months | |
|---|---|---|---|---|---|---|
| LEPR rs1137101 | ||||||
| AA | 0.08±0.09 | 0.40±0.30 | 0.89±0.66 | 1.23±1.01 | 1.45±1.23 | 1.71±1.49 |
| AG | 0.08±0.14 | 0.28±0.34 | 0.63±0.64 | 0.86±0.87 | 1.05±1.06 | 1.19±1.18 |
| GG | 0.05±0.18 | 0.22±0.42 | 0.34±0.68 | 0.45±0.87 | 0.55±1.06 | 0.6±1.17 |
| p-valuea | 0.448 | 0.184 | 0.004b | 0.002c | 0.002c | < 0.001c |
| ANKK1 rs1800497 | ||||||
| CC | 0.07±0.17 | 0.28±0.42 | 0.57±0.78 | 0.76±1.03 | 0.90±1.22 | 1.01±1.37 |
| CT | 0.07±0.22 | 0.24±0.49 | 0.42±0.70 | 0.55±0.91 | 0.68±1.12 | 0.76±1.22 |
| TT | 0.02±0.04 | 0.10±0.18 | 0.17±0.34 | 0.25±0.47 | 0.32±0.60 | 0.35±0.69 |
| p-valuea | 0.199 | 0.055 | 0.004b | 0.007b | 0.011b | 0.017b |
| PRKAA2 rs10789038 | ||||||
| AA | 0.05±0.13 | 0.19±0.36 | 0.37±0.66 | 0.50±0.91 | 0.61±1.13 | 0.69±1.27 |
| AG | 0.09±0.28 | 0.31±0.56 | 0.52±0.73 | 0.67±0.85 | 0.79±0.95 | 0.87±0.99 |
| GG | 0.06±0.56 | 0.26±0.25 | 0.65±0.62 | 0.93±0.92 | 1.09±1.06 | 1.23±1.08 |
| p-valuea | 0.194 | 0.049b | 0.080 | 0.085 | 0.089 | 0.020b |
a p-values calculated using analyses of covariance with gender, age, and baseline BMI (kg/m2) as covariates.
b p < 0.05.
c The difference remained significant after the Bonferroni correction, which was set at p < 0.0026 (0.05/19 SNPs).
ANKK1, ankyrin repeat kinase domain containing 1; LEPR, leptin receptor; PRKAA2, α2 catalytic subunit of AMPK; SNP, single nucleotide polymorphism ; VPA, valproic acid.
Figure 1.
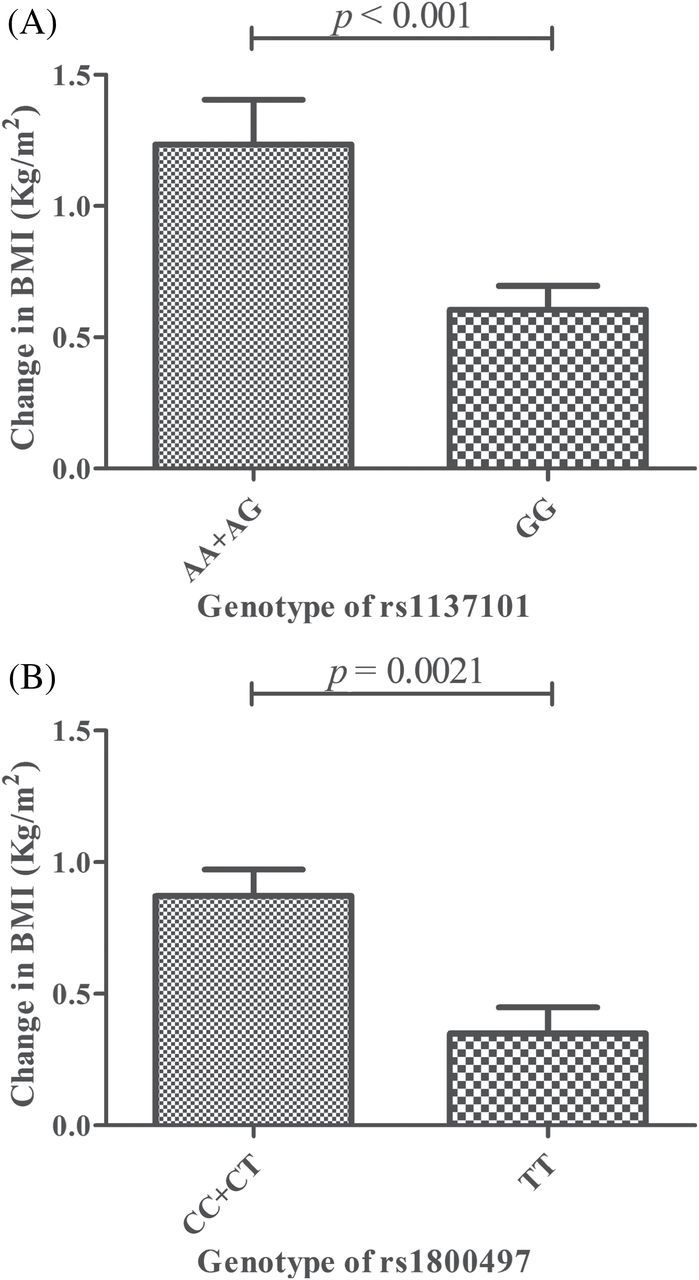
The allelic association of (A) LEPR rs1137101 and (B) ANKK1 rs1800497 with BMI change in patients with epilepsy (n = 212). Data are expressed as the mean ± standard error of the mean.
Association of VPA Css with Weight Change
After 6 months of treatment, 20.28% of patients were found to significantly gain weight (defined as an increase of weight ≥7%), and the VPA Css of all patients were determined. However, the VPA Css in patents who gained significant weight were not different from those who did not gain weight (p = 0.121, Mann–Whitney U-test Figure 2).
Figure 2.
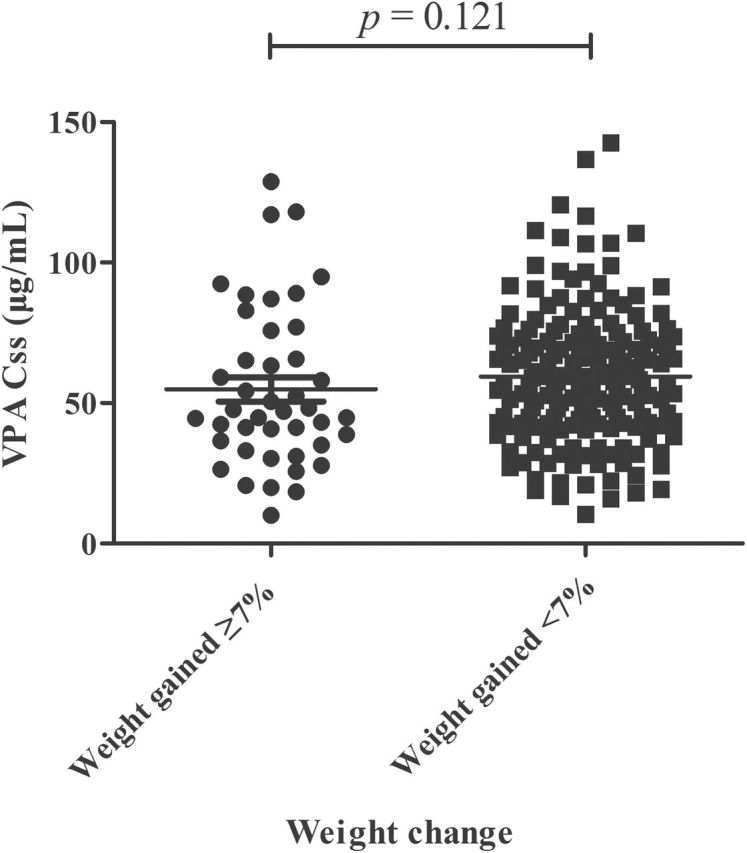
Valproic acid (VPA) steady state plasma concentrations (Css) in epilepsy patients who gained (weight gained ≥7%) and did not gain weight (weight gained <7%; n = 212).
Discussion
VPA-induced weight gain is the most frequent adverse effect of VPA treatment, resulting in various serious chronic diseases, but the underlying mechanism of the effect is still unknown. In the present study, we carried out a multi-gene analysis to study the role of genetic variants in VPA-induced weight gain, and we found, for the first time, that polymorphisms of LEPR, ANKK1, and PRKAA2 were associated with weight changes in Chinese Han epilepsy patients undergoing therapy with VPA. The genotypic association of LEPR rs1137101 and the allelic association of LEPR rs1137101 and ANKK1 rs1800497 with weight gain remained significant after correcting for Bonferroni multiple testing (19 SNPs, p corrected = 0.0026). These results may deepen the understanding of the potential mechanisms in VPA-related weight gain and provide further evidence implicating genes involved in the regulation of food intake and energy homeostasis in this highly relevant adverse effect.
As LEP and LEPR play an important role in the regulation of body weight and maintenance of energy homeostasis, the influence of the variation LEP and LEPR genes on body weight, whether or not in combination with antipsychotic treatment, is currently being investigated (Yiannakouris et al., 2001; Gregoor et al., 2009). Of interest is the LEPR rs1137101 polymorphism (G allele mutated to A allele), leading to a single amino acid change—a glutamine (Q) for an arginine (R)—which could affect the functionality of the receptor and alter its signaling capacity (Yiannakouris et al., 2001). Early reports have demonstrated that after treatment with VPA, patients who became obese showed increased serum LEP levels compared to patients who did not gain weight (Verrotti et al., 1999). Our analyses indicate that carriers of the A-allele of LEPR rs1137101 have a higher risk of weight gain and BMI increase under VPA therapy. This result is in line with previous reports in which the A allele was associated with an increased risk of weight gain and metabolic syndrome in schizophrenia patients who received antipsychotics (Gregoor et al., 2009; Roffeei et al., 2014). Although the G allele appears to be protective against weight gain or metabolic syndrome, some studies found that the A allele was associated with a lower prevalence of obesity (Yiannakouris et al., 2001; Gregoor et al., 2011). Therefore, the function of the SNP should be investigated in the future research.
Dopamine receptors are involved in midbrain reward circuit activation (Roth et al., 2013), and polymorphisms of DRD2 have been found to be associated with altered perception of food reward and weight gain (Muller et al., 2012; Roth et al., 2013). Whether these variants are associated with drug-induced weight gain is unclear. The most commonly tested and referred to DRD2 polymorphism is ANKK1 rs1800497 (known as Taq IA), which has a single nucleotide change [C(A2)/T(A1)], that was shown to lie within the adjacent gene ANKK1 (Dubertret et al., 2004). Taq IA is located downstream of the termination codon of DRD2, has traditionally been considered to be a gene marker for DRD2, and is known to be a restriction fragment length polymorphism located in the coding region of ANKK1. It was reported that the Taq IA A1(T) allele was associated with a 7% weight gain in patients of European ancestry taking clozapine or olanzapine, but a negative association of Taq IA variation with weight change after treatment of dopamine agonists (Prolactinomas) was observed in a recent study (Athanasoulia et al., 2014). However, the C-allele of ANKK1 rs1800497 was found to be associated with a greater weight gain in our study. Thus, our observation is distinct from the previous reports. Possible explanations for the inconsistent findings with this SNP include heterogeneity with respect to the effects of these drugs to DRD2, sample size, population ethnicity, and study design. Future research should not only consider additional gene variants and functional analyses, but also analyze patients from multiple ethnic groups.
In the present study, the intronic SNP rs10789038 in the gene coding for PRKAA2, the α2 catalytic regulatory subunit of AMPK, was found to be associated with VPA-induced weight gain. Our result is similar to a previous report in which the G allele of rs10789038 was associated with an increased risk of weight gain after receiving antipsychotic drugs (Souza et al., 2012). Although this result was not significant after taking into account multiple testing, further study is recommended to confirm this result. AMPK is expressed throughout the brain, and its activation in the hypothalamus is associated with increased food intake (Lim et al., 2010). The literature has reported that VPA is an activator of AMPK (Avery and Bumpus, 2014). Thus, studies in larger sample sets with SNPs covering the regulatory region of these genes coupled with functional analyses may shed light into their role in VPA-induced weight gain and other related metabolic disorders.
In the early literature, it was reported that the incidences of hair loss and weight gain increased with VPA Css at or above 100 μg/mL, and this is one of the reasons that the upper limit of the therapeutic range of VPA was defined at 100 μg/mL (Henriksen and Johannessen, 1982; Turnbull et al., 1982; Chadwick, 1985). However, our study (p = 0.121, Figure 2) and many others also demonstrated that there is no correlation between the degree of weight gain and the daily VPA dosage and serum VPA concentrations (Novak et al., 1999; Demir and Aysun, 2000). Therefore, it is necessary to clarify the relationship between VPA Css and weight change with a large patient group.
Nevertheless, owing to the well-known confounding factors of pharmacogenetic studies, independent studies with larger patient groups and longer observation periods are needed to confirm these results.
In conclusion, we found evidence of an association between LEPR rs1137101and ANKK1 rs1800497 with the occurrence of weight gain in Chinese epilepsy patients after treatment of VPA. The replication of these observations in independent samples is necessary before drawing any firm conclusions on the utility of these results for personalized medicine.
Statement of Interest
The authors declare no conflict of interests.
Acknowledgments
This work was supported by the Natural Major Projects for science and technology development from Science and Technology Ministry of China (Grant No.2012ZX09506001-004), the National Science Foundation of China (Grant Nos. 81320108027, 81173131, and 81473283), and the Major Scientific and Technological Project of Guangdong Province (Grant No. 2011A080300001).
The trial registry name is “Exploration of Genotype Based Personalized Prescription of Valproate Sodium in Anti-epileptic Treatment (EGBPPVPA)” and the
ClinicalTrials.gov Identifier is NCT01172626 (http://clinicaltrials.gov/show/NCT01172626).
References
- Almeida LE, Roby CD, Krueger BK. (2014). Increased BDNF expression in fetal brain in the valproic acid model of autism. Mol Cell Neurosci 59:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasoulia AP, Sievers C, Uhr M, Ising M, Stalla GK, Schneider HJ. (2014). The effect of the ANKK1/DRD2 Taq1A polymorphism on weight changes of dopaminergic treatment in prolactinomas. Pituitary 17:240–245. [DOI] [PubMed] [Google Scholar]
- Avery LB, Bumpus NN. (2014). Valproic acid is a novel activator of AMP-activated protein kinase and decreases liver mass, hepatic fat accumulation, and serum glucose in obese mice. Mol Pharmacol 85:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265. [DOI] [PubMed] [Google Scholar]
- Belcastro V, D’Egidio C, Striano P, Verrotti A. (2013). Metabolic and endocrine effects of valproic acid chronic treatment. Epilepsy Res 107:1–8. [DOI] [PubMed] [Google Scholar]
- Biton V, Mirza W, Montouris G, Vuong A, Hammer AE, Barrett PS. (2001). Weight change associated with valproate and lamotrigine monotherapy in patients with epilepsy. Neurology 56:172–177. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Singh V. (2005). Valproate in bipolar disorder: 2000 onwards. Acta Psychiatr Scand Suppl 426: 13–20. [DOI] [PubMed] [Google Scholar]
- Brandl EJ, Frydrychowicz C, Tiwari AK, Lett TA, Kitzrow W, Buttner S, Ehrlich S, Meltzer HY, Lieberman JA, Kennedy JL, Muller DJ, Puls I. (2012). Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog Neuropsychopharmacol Biol Psychiatry 38:134–141. [DOI] [PubMed] [Google Scholar]
- Chadwick DW. (1985). Concentration-effect relationships of valproic acid. Clin Pharmacokinet 10:155–163. [DOI] [PubMed] [Google Scholar]
- Chan TW, Bates JE, Lansford JE, Dodge KA, Pettit GS, Dick DM, Latendresse SJ. (2014). Impulsivity and genetic variants in DRD2 and ANKK1 moderate longitudinal associations between sleep problems and overweight from ages 5 to 11. Int J Obes (Lond) 38:404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Gean PW, Chou CH, Yang YK, Tsai HC, Lu RB, Chen PS. (2010). C825T polymorphism of the GNB3 gene on valproate-related metabolic abnormalities in bipolar disorder patients. J Clin Psychopharmacol 30:512–517. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, Wang XD, Wang HS, Chen SD, Zhou LM, Li JL, Shu WY, Zhou JQ, Fang ZY, Zhang Y, Huang M. (2012). Simultaneous determination of valproic acid and 2-propyl-4-pentenoic acid for the prediction of clinical adverse effects in Chinese patients with epilepsy. Seizure 21:110–117. [DOI] [PubMed] [Google Scholar]
- Chowdhury NI, Tiwari AK, Souza RP, Zai CC, Shaikh SA, Chen S, Liu F, Lieberman JA, Meltzer HY, Malhotra AK, Kennedy JL, Muller DJ. (2013). Genetic association study between antipsychotic-induced weight gain and the melanocortin-4 receptor gene. Pharmacogenomics J 13:272–279. [DOI] [PubMed] [Google Scholar]
- Czerwensky F, Leucht S, Steimer W. (2013) Association of the common MC4R rs17782313 polymorphism with antipsychotic-related weight gain. J Clin Psychopharmacol 33:74–79. [DOI] [PubMed] [Google Scholar]
- Demir E, Aysun S. (2000). Weight gain associated with valproate in childhood. Pediatr Neurol 22:361–364. [DOI] [PubMed] [Google Scholar]
- Dubertret C, Gouya L, Hanoun N, Deybach JC, Ades J, Hamon M, Gorwood P. (2004). The 3’ region of the DRD2 gene is involved in genetic susceptibility to schizophrenia. Schizophr Res 67:75–85. [DOI] [PubMed] [Google Scholar]
- El-Khatib F, Rauchenzauner M, Lechleitner M, Hoppichler F, Naser A, Waldmann M, Trinka E, Unterberger I, Bauer G, Luef GJ. (2007). Valproate, weight gain and carbohydrate craving: a gender study. Seizure 16:226–232. [DOI] [PubMed] [Google Scholar]
- Farrelly LA, Savage NT, O’Callaghan C, Toulouse A, Yilmazer-Hanke DM. (2013). Therapeutic concentrations of valproate but not amitriptyline increase neuropeptide Y (NPY) expression in the human SH-SY5Y neuroblastoma cell line. Regul Pept 186:123–130. [DOI] [PubMed] [Google Scholar]
- Gregoor JG, van der Weide J, Mulder H, Cohen D, van Megen HJ, Egberts AC, Heerdink ER. (2009). Polymorphisms of the LEP- and LEPR gene and obesity in patients using antipsychotic medication. J Clin Psychopharmacol 29:21–25. [DOI] [PubMed] [Google Scholar]
- Gregoor JG, van der Weide J, Loovers HM, van Megen HJ, Egberts TC, Heerdink ER. (2011). Polymorphisms of the LEP, LEPR and HTR2C gene: obesity and BMI change in patients using antipsychotic medication in a naturalistic setting. Pharmacogenomics 12:919–923. [DOI] [PubMed] [Google Scholar]
- Gungor S, Yucel G, Akinci A, Tabel Y, Ozerol IH, Yologlu S. (2007). The role of ghrelin in weight gain and growth in epileptic children using valproate. J Child Neurol 22:1384–1388. [DOI] [PubMed] [Google Scholar]
- Haddad PM, Das A, Ashfaq M, Wieck A. (2009). A review of valproate in psychiatric practice. Expert Opin Drug Metab Toxicol 5:539–551. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Fida NM, Hamed EA. (2009). States of serum leptin and insulin in children with epilepsy: risk predictors of weight gain. Eur J Paediatr Neurol 13:261–268. [DOI] [PubMed] [Google Scholar]
- Henriksen O, Johannessen SI. (1982). Clinical and pharmacokinetic observations on sodium valproate – a 5-year follow-up study in 100 children with epilepsy. Acta Neurol Scand 65: 504–523. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Reynolds GP. (2011). Functional consequences of two HTR2C polymorphisms associated with antipsychotic-induced weight gain. Pharmacogenomics 12:727–734. [DOI] [PubMed] [Google Scholar]
- Kanemura H, Sano F, Maeda Y, Sugita K, Aihara M. (2012). Valproate sodium enhances body weight gain in patients with childhood epilepsy: a pathogenic mechanisms and open-label clinical trial of behavior therapy. Seizure 21:496–500. [DOI] [PubMed] [Google Scholar]
- Kao AC, Muller DJ. (2013). Genetics of antipsychotic-induced weight gain: update and current perspectives. Pharmacogenomics 14:2067–2083. [DOI] [PubMed] [Google Scholar]
- Klein KM, Hamer HM, Reis J, Schmidtke J, Oertel WH, Theisen FM, Hebebrand J, Rosenow F. (2005). Weight change in monozygotic twins treated with valproate. Obes Res 13:1330–1334. [DOI] [PubMed] [Google Scholar]
- Lakhanpal D, Kaur G. (2007). Valproic acid alters GnRH-GABA interactions in cycling female rats. Cell Mol Neurobiol 27:1069–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Jeong J, Park YU, Kwak Y, Lee SA, Lee H, Son H, Park SK. (2012). Valproate alters dopamine signaling in association with induction of Par-4 protein expression. PLOS ONE 7:e45618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CT, Kola B, Korbonits M. (2010). AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44:87–97. [DOI] [PubMed] [Google Scholar]
- Loparev VN, Cartas MA, Monken CE, Velpandi A, Srinivasan A. (1991). An efficient and simple method of DNA extraction from whole blood and cell lines to identify infectious agents. J Virol Methods 34:105–112. [DOI] [PubMed] [Google Scholar]
- Löscher W. (2002). Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs 16:669–694. [DOI] [PubMed] [Google Scholar]
- Mathew NT, Saper JR, Silberstein SD, Rankin L, Markley HG, Solomon S, Rapoport AM, Silber CJ, Deaton RL. (1995). Migraine prophylaxis with divalproex. Arch Neurol 52:281–286. [DOI] [PubMed] [Google Scholar]
- Muller DJ, Zai CC, Sicard M, Remington E, Souza RP, Tiwari AK, Hwang R, Likhodi O, Shaikh S, Freeman N, Arenovich T, Heinz A, Meltzer HY, Lieberman JA, Kennedy JL. (2012). Systematic analysis of dopamine receptor genes (DRD1-DRD5) in antipsychotic-induced weight gain. Pharmacogenomics J 12:156–164. [DOI] [PubMed] [Google Scholar]
- Novak GP, Maytal J, Alshansky A, Eviatar L, Sy-Kho R, Siddique Q. (1999). Risk of excessive weight gain in epileptic children treated with valproate. J Child Neurol 14:490–495. [DOI] [PubMed] [Google Scholar]
- Pickrell WO, Lacey AS, Thomas RH, Smith PE, Rees MI. (2013). Weight change associated with antiepileptic drugs. J Neurol Neurosurg Psychiatry 84:796–799. [DOI] [PubMed] [Google Scholar]
- Roffeei SN, Mohamed Z, Reynolds GP, Said MA, Hatim A, Mohamed EH, Aida SA, Zainal NZ. (2014). Association of FTO, LEPR and MTHFR gene polymorphisms with metabolic syndrome in schizophrenia patients receiving antipsychotics. Pharmacogenomics 15:477–485. [DOI] [PubMed] [Google Scholar]
- Roth CL, Hinney A, Schur EA, Elfers CT, Reinehr T. (2013). Association analyses for dopamine receptor gene polymorphisms and weight status in a longitudinal analysis in obese children before and after lifestyle intervention. BMC Pediatr 13:197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Leclerc D, Wu Q, Gupta S, Kruger WD, Rozen R. (2008). Valproic acid increases expression of methylenetetrahydrofolate reductase (MTHFR) and induces lower teratogenicity in MTHFR deficiency. J Cell Biochem 105:467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RP, Tiwari AK, Chowdhury NI, Ceddia RB, Lieberman JA, Meltzer HY, Kennedy JL, Muller DJ. (2012). Association study between variants of AMP-activated protein kinase catalytic and regulatory subunit genes with antipsychotic-induced weight gain. J Psychiatr Res 46:462–468. [DOI] [PubMed] [Google Scholar]
- Tiwari AK, Brandl EJ, Weber C, Likhodi O, Zai CC, Hahn MK, Lieberman JA, Meltzer HY, Kennedy JL, Muller DJ. (2013). Association of a functional polymorphism in neuropeptide y with antipsychotic-induced weight gain in schizophrenia patients. J Clin Psychopharmacol 33:11–17. [DOI] [PubMed] [Google Scholar]
- Turnbull DM, Rawlins MD, Weightman D, Chadwick DW. (1982). A comparison of phenytoin and valproate in previously untreated adult epileptic patients. J Neurol Neurosurg Psychiatry 45:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrotti A, Basciani F, Morresi S, de Martino M, Morgese G, Chiarelli F. (1999). Serum leptin changes in epileptic patients who gain weight after therapy with valproic acid. Neurology 53:230–232. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Manco R, Agostinelli S, Coppola G, Chiarelli F. (2010). The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia 51:268–273. [DOI] [PubMed] [Google Scholar]
- Verrotti A, D’Egidio C, Mohn A, Coppola G, Chiarelli F. (2011). Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev 12:e32– e43. [DOI] [PubMed] [Google Scholar]
- Yiannakouris N, Yannakoulia M, Melistas L, Chan JL, Klimis-Zacas D, Mantzoros CS. (2001). The Q223R polymorphism of the leptin receptor gene is significantly associated with obesity and predicts a small percentage of body weight and body composition variability. J Clin Endocrinol Metab 86:4434–4439. [DOI] [PubMed] [Google Scholar]
- Zai GC, Zai CC, Chowdhury NI, Tiwari AK, Souza RP, Lieberman JA, Meltzer HY, Potkin SG, Muller DJ, Kennedy JL. (2012). The role of brain-derived neurotrophic factor (BDNF) gene variants in antipsychotic response and antipsychotic-induced weight gain. Prog Neuropsychopharmacol Biol Psychiatry 39:96–101. [DOI] [PubMed] [Google Scholar]


