Abstract
Aims
Post-infarction remodelled failing hearts have reduced metabolic efficiency. Paradoxically, they have increased tolerance to further ischaemic injury. This study was designed to investigate the metabolic mechanisms that may contribute to this phenomenon and to examine the relationship between ischaemic tolerance and metabolic efficiency during post-ischaemic reperfusion.
Methods and results
Male C57BL/6 mice were subjected to coronary artery ligation (CAL) or SHAM surgery. After 4 weeks, in vivo mechanical function was assessed by echocardiography, and then isolated working hearts were perfused in this sequence: 45 min aerobic, 15 min global no-flow ischaemia, and 30 min aerobic reperfusion. Left ventricular (LV) function, metabolic rates, and metabolic efficiency were measured. Relative to SHAM, both in vivo and in vitro CAL hearts had depressed cardiac function under aerobic conditions (45 and 36%, respectively), but they had a greater recovery of LV function during post-ischaemic reperfusion (67 vs. 49%, P < 0.05). While metabolic efficiency (LV work per ATP produced) was 50% lower during reperfusion of SHAM hearts, metabolic efficiency in CAL hearts did not decrease. During ischaemia, glycogenolysis was 28% lower in CAL hearts, indicative of lower ischaemic proton production. There were no differences in mitochondrial abundance, calcium handling proteins, or key metabolic enzymes.
Conclusion
Compared with SHAM, remodelled CAL hearts are more tolerant to ischaemic injury and undergo no further deterioration of metabolic efficiency during reperfusion. Less glycogen utilization in CAL hearts during ischaemia may contribute to increased ischaemic tolerance by limiting ischaemic proton production that may improve ion homeostasis during early reperfusion.
Keywords: Ischaemic tolerance, Remodelled hearts, Cardiac metabolism, Glycolysis, Glucose oxidation
1. Introduction
Hearts that are remodelled following ischaemia exhibit impaired mechanical function during both in vivo and ex vivo aerobic perfusion.1 Previously, we investigated what energy metabolic mechanisms may contribute to the observed mechanical dysfunction and demonstrated that hearts remodelled following coronary artery ligation (CAL) are not energetically starved as they maintain comparable rates of energy substrate oxidation, rates of ATP production, and ATP contents despite lower mechanical function. Instead, post-infarction remodelled hearts are metabolically inefficient,1 that is, remodelled hearts have adequate rates of energy substrate metabolism and ATP production, but this energy is not translated into mechanical function as efficiently as in normal hearts. Although many factors may influence cardiac metabolic efficiency (reviewed in Masoud et al.2), metabolic inefficiency in remodelled hearts may be related to higher rates of proton production from glucose metabolism. This increased proton production can increase the amount of energy used for the correction of acidosis-induced dysregulation of ion homeostasis that includes Na+ and Ca2+ overload.
Based on this metabolic inefficiency, it might be expected that remodelled hearts would be more susceptible to injury following a subsequent episode of ischaemia compared with normal healthy hearts. However, several studies have provided evidence that remodelled hearts actually have a greater tolerance to ischaemia.3–5 In a comparison of normal and remodelled hearts perfused in the Langendorff mode, Kalkman et al.4 reported that remodelled hearts have a lower ischaemia-induced release of lactate and purines. A similar improved tolerance to ischaemic injury in remodelled hearts was confirmed by Pantos et al.3 who showed that a lower release of lactate dehydrogenase was accompanied by less ischaemic contracture, a higher expression of heat shock protein 70, as well as an improved recovery of mechanical function during post-ischaemic reperfusion. Both studies employed non-working Langendorff preparations, so energy metabolic mechanisms were not investigated. Less dysregulation of ion homeostasis has also been noted in remodelled hearts by Sharikabad and colleagues,5 who reported that cardiomyocytes derived from post-infarction remodelled rat hearts exhibit less Na+ and Ca2+ accumulation than cells from normal hearts. These authors also found lower release of lactate dehydrogenase and less depletion of ATP content during a hypoxic challenge of remodelled cardiomyocytes, but the mechanistic basis of the increased tolerance has not yet been elucidated.
Energy substrate metabolic rates and energy substrate preference during reperfusion of ischaemic hearts affect recovery of LV mechanical function.6–9 For example, acceleration of fatty acid oxidation during reperfusion inhibits glucose oxidation (the ‘Randle effect’), thereby increasing the uncoupling of glycolysis and glucose oxidation leading to increased proton production. This slows the rate of recovery of intracellular pH and contributes to further accumulation of Na+ and Ca2+ overload.6 Moreover, inhibition of glycolysis during early reperfusion limits Ca2+ overload and improves post-ischaemic recovery of LV function.7
This study was designed to compare post-ischaemic mechanical function in normal and remodelled hearts and to investigate the associated changes in cardiac energy substrate metabolism and metabolic efficiency. Studies were performed in mouse hearts that had undergone remodelling following CAL or a sham procedure. Hearts were isolated and perfused in working heart mode in the presence of relevant energy substrates (fatty acids, glucose, and lactate) and energy demand (workload), to assess LV mechanical work, rates of energy substrate metabolism, and metabolic efficiency.
2. Methods
2.1. Remodelled mouse hearts
All experimental procedures were approved by the Animal Care and Use Committee, University of Alberta. They also conform to the guidelines of the Canadian Council of Animal Care and to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, Revised 2011). Male C57BL/6 mice (aged 12 weeks) were subjected to either a CAL (n = 17) or a sham procedure (SHAM, n = 33), as described previously.10,11 Briefly, animals were anaesthetized with pentobarbital sodium (60 mg/kg, ip), intubated, and ventilated with 100% oxygen. A left thoracotomy incision was made to expose the heart, and the left anterior descending coronary artery was permanently ligated with a 7-0 prolene suture. The chest wall, muscle, and skin were closed in layers using 6-0 silk. Mice in the CAL and SHAM groups were maintained for a 4-week post-surgical period prior to functional and metabolic evaluation.
2.2. Assessment of cardiac function in vivo by echocardiography
Three days before ex vivo heart perfusions, animals were randomly selected from CAL and SHAM groups and anaesthetized with isoflurane (3% for induction and 1.5% for maintenance). Transthoracic M-mode echocardiography (Vevo 770, Visualsonics, Toronto, Canada) was performed to measure chamber volumes, wall thickness, and cardiac systolic and diastolic function.12–15
2.3. Assessment of cardiac function ex vivo
Animals were deeply anaesthetized with pentobarbital (480 mg/kg, ip), and hearts were rapidly excised and immediately placed in ice-cold Krebs–Henseleit solution. The aorta was cannulated, and the heart was perfused for 10 min in the Langendorff mode (37°C and 60 mmHg pressure). Following cannulation of the left atrium, hearts were perfused aerobically in the working mode16,17 (11.5 mmHg preload and 50 mmHg afterload) using a recirculating perfusate (100 mL) composed of (in mM) 1.2 MgSO4 7H2O, 1.2 KH2PO4, 4.7 KCl, 25 NaHCO3, 2.5 CaCl2.2H2O, and 118 NaCl. Glucose (11 mM), palmitate (1.2 mM, prebound to 3% fatty acid-free BSA), and lactate (1 mM) were added as energy substrates along with insulin (100 µU/mL). The perfusate pH (7.4) and O2 saturation were maintained using a thin-film oxygenator gassed with carbogen (95% O2 and 5% CO2). All hearts were perfused aerobically for 45 min and then subjected to 15 min global no-flow ischaemia followed by 30 min of aerobic reperfusion. Cardiac output (CO), aortic flow, heart rate, and systolic and diastolic aortic pressures were monitored continuously throughout the perfusion protocol. LV work was derived from the equation: LV work (J/min/g dry wt) = [(peak systolic pressure − preload pressure) × CO × 0.133]/viable ventricular dry wt, where 0.133 is a factor used to convert mmHg mL/min to J/min. Functional recovery is calculated as the percentage of post-ischaemic LV work to pre-ischaemic LV work. At the end of reperfusion, infarct areas were identified visually and excised. The remaining viable heart tissue was immediately frozen using a Wollenberger clamp cooled in liquid nitrogen and then stored at −80°C for subsequent biochemical and immunoblot assays. Additional subgroups of hearts were perfused under identical conditions and frozen at the end of the 15-min ischaemic period.
2.4. Measurement of rates of energy substrate metabolism
Metabolic rates (fatty acid oxidation, glucose oxidation, glycolysis, and lactate oxidation) were assessed (µmol/min/g dry wt) by measuring rates of 3H2O and 14CO2 production from radiolabeled substrates ([9,10-3H]palmitate, [U-14C]glucose, [5-3H]glucose and [U-14C]lactate, respectively) as described previously.18 Metabolic rates were calculated based on the grams of dry weight, which was the weight of viable tissue in CAL hearts (total ventricular weight excluding the infarct weight) and total ventricular tissue in SHAM hearts. Rates of overall ATP production were calculated from rates of metabolism of each substrate (104 for palmitate oxidation, 31 for glucose oxidation, 2 for glycolysis, and 17 for lactate oxidation).
2.5. Calculation of proton production from glucose metabolism
Hydrolysis of glycolytic ATP produces one proton per ATP, while mitochondria utilize one proton for each pyruvate molecule oxidized. As two ATPs are produced per glucose molecule and so produces two protons, the rate of proton production from glucose metabolism can be determined as [2 × (glycolysis rate – glucose oxidation rate)].19
2.6. Measurements of cardiac triacylglycerol content and turnover
Frozen powdered tissues (15–30 mg) from hearts frozen at the end of ischaemia or at the end of reperfusion were extracted in 2:1 chloroform–methanol (20-fold volume). Then methanol (0.2 volume) was added and the extract was vortexed for 30 s. The mixture was then centrifuged at 1500 g for 10 min, and the supernatant was collected. Calcium chloride (0.04%, 0.2 volume) was added to the supernatant, which was then centrifuged at 700 g for 20 min. The upper phase was removed and the interface was washed with 150 µL of pure solvent upper phase consisting of 24 mL methanol, 1.5 mL chloroform, and 23.5 mL water. This step was repeated two times and the final wash was removed. Methanol (50 µL) was then added to form one phase. The samples were then dried under N2 at 60°C and re-dissolved in 50 µL of 3:2 tert-butyl alcohol-Triton X-100/methanol. This was followed by a colorimetric quantification of cardiac triacylglycerol (TG, represented as TG fatty acid content) with an enzymatic assay kit (Wako Pure Chemical Industries).20 The net change in TG content (degradation or accumulation) was measured from the change in the myocardial TG content during the 15-min period of ischaemia or during the 30-min period of reperfusion and expressed as µmol TG fatty acid/min/g dry wt.
2.7. Measurements of glycogen content and turnover
Frozen powdered heart tissues (25–30 mg) were extracted in 30% KOH and subjected to ethanol precipitation and acid hydrolysis (2 N H2SO4). Glycogen content was expressed as µmol glucose units/g dry wt.19 The net change in glycogen content (degradation or accumulation) was measured from the change in myocardial glycogen content during the 15-min period of ischaemia or during the 30-min period of reperfusion.21
2.8. Immunoblot analyses
Frozen heart ventricular tissues were homogenized, and samples were subjected to SDS–PAGE followed by transfer to nitrocellulose membranes.21 The membranes were then immunoblotted with rabbit antibodies against phospho-AMP-dependent kinase (p-AMPKThr172), AMP-dependent kinase (AMPK), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), glucose transporter-4 (GLUT4), lactate dehydrogenase (LDH), pyruvate dehydrogenase (PDH), hexokinase, glyceraldehyde 3 phosphate dehydrogenase (GAPDH), voltage-dependent anion channel (VDAC), glycogen synthase kinase 3β (GSK3β), phospho-glycogen synthase kinase 3β (pGSK3β), peroxisome proliferator-activated receptor α (PPARα), and sarco/endoplasmic reticulum Ca2+-ATPase 2 (SERCA2) (Cell Signaling Technology Inc., Danvers, MA, USA). Rabbit antibodies were used against phospho Ca2+/calmodulin-dependent protein kinase (p-CaMKIIThr286), Ca2+/calmodulin-dependent protein kinase (CaMKII), long-chain acyl-CoA dehydrogenase (LCAD), β-hydroxy acyl-CoA dehydrogenase (β-HAD), and complex I subunit (NADH dehydrogenase [ubiquinone] 1β subcomplex subunit 6 (NDUFB6)). Mouse antibodies were used against phospholamban (ABCAM Inc., Cambridge, MD, USA) and GLUT1 (FabGennix Inc.). After the membranes were extensively washed, they were incubated with a peroxidase-conjugated goat anti-rabbit secondary antibody (Cell Signaling Technology Inc.) or goat anti-mouse (Bio-Rad) as appropriate. Antibodies were visualized using the Pharmacia-enhanced chemiluminescence western blotting and detection system (Amersham, UK). Densitometric analyses of immunoblots (n = 3–4 per experimental group) were performed using Image J software. The most consistently expressed band in each of the Ponceau-stained membranes was used as loading control22 since the abundance of the otherwise house-keeping proteins (actin) was altered in CAL hearts (unpublished data).
2.9. Measurement of adenine nucleotide and creatine contents
Adenine nucleotides (ATP, ADP, AMP), GTP, inosine, creatine phosphate, and creatine were separated using ultra-performance liquid chromatography (UPLC) as described previously1 using a 2.1 × 100 mm ACQUITY UPLC HSS T3 column packed with 1.7 µm particles (Waters, Milford, MA, USA) and quantified by recording the optical density at 254 nm for adenine nucleotides and 210 nm for creatine and phosphocreatine. Calibration stock solution (0.1 M, pH 7) was prepared in 0.2 M Na2HPO4 and stored at −40°C for a maximum of 3 days to minimize degradation of phosphocreatine and ATP.
2.10. Statistical analysis
Normality of data distribution was tested using the Shapiro–Wilk normality test. Normally distributed data are expressed as mean ± SEM. Unpaired Student's t-test was used to compare differences between two groups, and one-way ANOVA was used to compare three or more groups. Two-way repeated-measures ANOVA was used to compare time-dependent measurements. Bonferroni post hoc test was used to provide comparison between selected pairs of data. If data are not normally distributed, they are presented as median ± inter-quartile ranges. Statistical analysis of differences was then made using non-parametric methods (Mann–Whitney test to compare two groups, or Kruskal–Wallis ANOVA to compare three or more groups followed by Dunn's post hoc test). Differences were considered significant when P < 0.05. Statistical analyses were performed using GraphPad Prism 5 (Graphpad Software Inc., CA, USA).
3. Results
3.1. CAL hearts have lower in vivo function
In accordance with our previously published data,1 echocardiographic evaluation of CAL mice revealed impaired LV function relative to the age-matched SHAM group (Figure 1). CAL mice have lower systolic function as indicated by reduced LV% ejection fraction (%EF) and LV% fractional area change (%FAC). CAL hearts have co-existent diastolic dysfunction (higher Tei index, E/E′, and IVRT) and marked dilatation (as indicated by the significant increases in LV diastolic and systolic volumes and internal diameters). CAL hearts are also mildly hypertrophied [higher diastolic interventricular septal thickness (IVSd) but unaltered LV posterior wall thickness] (see Supplementary material online, Table S1).
Figure 1.
In vivo echocardiographic functional assessment. (A) A parasternal long-axis view of a SHAM heart. (B) A parasternal long-axis view of a CAL heart. Notice the dilatation of the LV. (C) An m-Mode capture of a SHAM heart. (D) An m-Mode capture of a CAL heart. Notice the increased LV volume (B) and reduced wall motion (D) in CAL hearts compared with SHAM hearts (A and C).
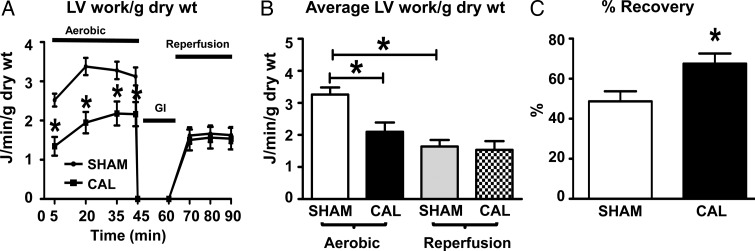
Ex vivo mechanical function (average LV work during aerobic perfusion, J/min/g dry wt) is 36% lower in CAL hearts than in SHAM hearts, which confirms the in vivo findings of lower systolic function. However, CAL hearts recovered to aerobic levels of LV work during post-ischaemic reperfusion, in contrast to SHAM hearts that show significant deterioration of LV work during post-ischaemic reperfusion (Figure 2). When LV work during reperfusion is expressed as a percent recovery of pre-ischaemic values (Figure 2C), there is a significantly higher recovery of LV work in CAL hearts compared with SHAM hearts (67 ± 5 vs. 49 ± 5%, P < 0.05). A similar recovery of other haemodynamic parameters was also seen in CAL hearts post-ischaemia (see Supplementary material online, Table S2).
Figure 2.
Ex vivo assessment of mechanical function and recovery. (A) Time-dependent changes in LV work and (B) average LV work for SHAM (n = 33) and CAL (n = 17) hearts during aerobic perfusion, global ischaemia, and reperfusion. (C) % Recovery of mechanical function during reperfusion. *Significant difference (P < 0.05) from aerobic SHAM parameters. Data are expressed as means ± SEM.
3.2. CAL hearts are not energy-starved during post-ischaemic reperfusion
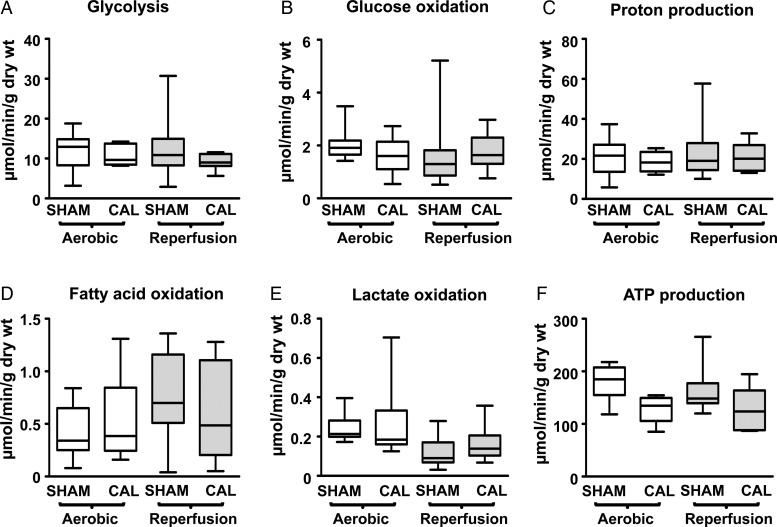
As we reported previously,1 CAL hearts, relative to SHAM hearts, are not energy-starved during aerobic perfusion, as indicated by similar rates of glycolysis, glucose oxidation, fatty acid oxidation, and lactate oxidation in the two groups (Figure 3A–E). Thus, the rate of ATP production from these energy substrates is similar in CAL and SHAM hearts, indicating that CAL hearts are not deficient in energy availability (Figure 3F). Moreover, we now show that post-ischaemic reperfused CAL hearts maintain similar rates of energy substrate metabolism to SHAM hearts, which indicates that they are also not energy-starved during reperfusion.
Figure 3.
Rates of energy substrate metabolism in SHAM and CAL hearts. Glycolysis, glucose oxidation, and calculated proton production are shown in (A–C) (SHAM, n = 16; CAL, n = 7). Fatty acid oxidation (D; SHAM, n = 15; CAL, n = 10) and lactate oxidation (E; SHAM, n = 15; CAL, n = 9) are also shown. Calculated ATP production rates are shown in F. Since data are not normally distributed, they are represented by box and whisker graphs. Medians are shown as a transverse line crossing the boxes. The upper and lower ends of the box represent the 75 and 25% quartiles, respectively. The upper and lower whiskers represent the 95th and 5th percentiles, respectively. Differences are considered significant when P < 0.05. End aerobic nucleotide content is presented in the online supplement of Masoud et al.1
Similarly, CAL hearts maintain comparable ATP and creatine phosphate contents to SHAM hearts during post-ischaemic reperfusion (see Supplementary material online, Table S3), also indicative of a lack of energy starvation.
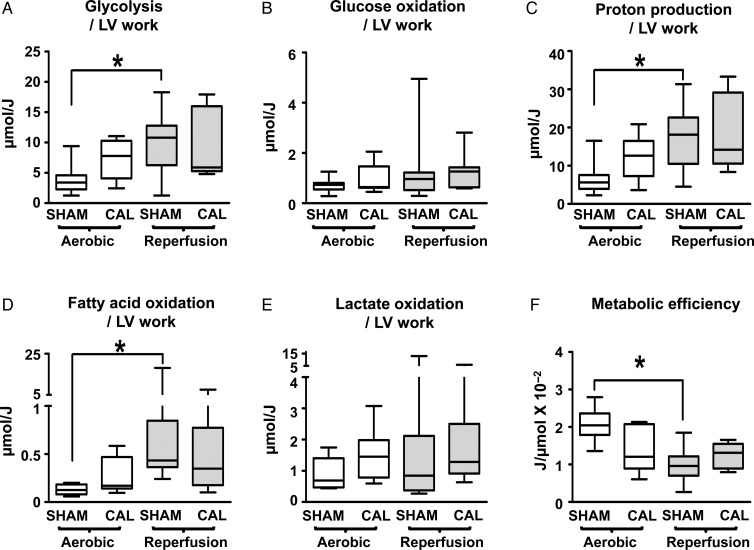
3.3. CAL hearts undergo less deterioration in metabolic efficiency during reperfusions
During aerobic perfusion, when normalized per unit LV work (µmol/J), CAL hearts have higher rates of glycolysis (Figure 4A, P < 0.05, t-test) and similar rates of glucose oxidation (Figure 4B), a finding confirming our previous study.1 The greater mismatch in the rates of glycolysis and glucose oxidation in CAL hearts results in a higher rate of proton production (Figure 4C, P < 0.05). Fatty acid oxidation and lactate oxidation rates were similar in SHAM and CAL hearts (Figure 4D–E). CAL hearts have lower metabolic efficiency (LV work done per ATP produced, J/µmol) (Figure 4F, P < 0.05).
Figure 4.
Metabolic rates per LV work of SHAM and CAL hearts. Glycolysis, glucose oxidation, and calculated proton production are shown in A–C (SHAM, n = 16; CAL, n = 7). Fatty acid oxidation (D; SHAM, n = 15; CAL, n = 10) and lactate oxidation (E; SHAM, n = 15; CAL, n = 9) are also shown. Values for calculated metabolic efficiency are shown in F. Since data are not normally distributed, they are represented by box and whisker graphs. Medians are shown as a transverse line crossing the boxes. The upper and lower ends of the box represent the 75 and 25% quartiles, respectively. The upper and lower whiskers represent the 95th and 5th percentiles, respectively. *Differences are considered significant when P < 0.05.
During reperfusion, compared with aerobic perfusion, SHAM hearts exhibit a significantly higher glycolysis per LV work (Figure 4A) while glucose oxidation per LV work is unchanged (Figure 4B), yielding a significantly higher rate of proton production per LV work (Figure 4C). Rates of fatty acid oxidation per LV work significantly increase (Figure 4D), while lactate oxidation per LV work remains similar to aerobic rates (Figure 4E). Accordingly, metabolic efficiency is significantly lower in reperfused SHAM hearts (Figure 4F).
In contrast, reperfused CAL hearts exhibit no further changes in glucose metabolism (Figure 2A and B). Thus, proton production per LV work remains at similar levels to aerobic perfusion (Figure 4C). Similarly, fatty acid oxidation and lactate oxidation per LV work are comparable to values during aerobic perfusion (Figure 4D and E). Thus, in CAL hearts, energy substrate preference is unaltered and metabolic efficiency does not further deteriorate during post-ischaemic reperfusion (Figure 4F).
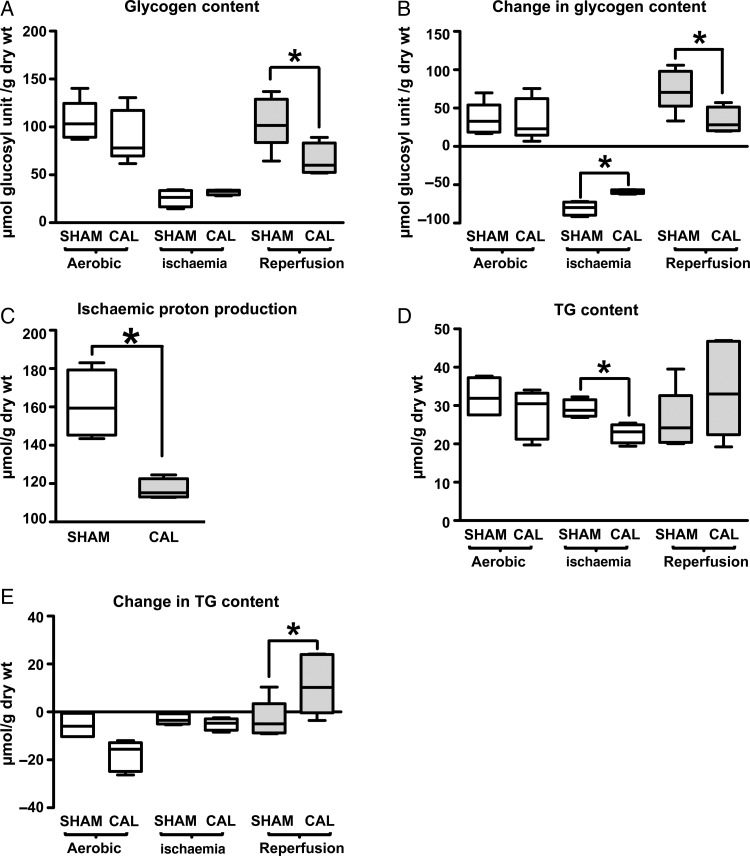
3.4. Endogenous substrate utilization during ischaemia and reperfusion
Compared with SHAM hearts, CAL hearts utilize less glycogen during ischaemia but accumulate less glycogen during reperfusion (Figure 5A and B). As a result, CAL hearts produce less protons during ischaemia compared with SHAM hearts (Figure 5C). TG utilization is similar in CAL and SHAM hearts during ischaemia. While SHAM hearts continue to utilize TGs during reperfusion, CAL hearts accumulate TG (Figure 5D and E), which, together with comparable LV work levels, indicates a higher metabolic efficiency in reperfused CAL hearts.
Figure 5.
Contents and change of endogenous energy substrates in SHAM and CAL hearts. Glycogen content is shown in A at the end of aerobic perfusion (SHAM, n = 5 and CAL, n = 5), end of ischemia (SHAM, n = 4 and CAL, n = 4), and end of reperfusion (SHAM, n = 6 and CAL, n = 4). Changes in glycogen content during these intervals are presented in B. Ischaemic proton production from glycogen utilization is presented in C. Triglyceride (TG) content is shown in D at the end of aerobic perfusion (SHAM, n = 4 and CAL, n = 5), end of ischaemia (SHAM, n = 4 and CAL, n = 4), and end of reperfusion (SHAM, n = 6 and CAL, n = 5). Changes in TG content during these intervals are presented in E. Since data are not normally distributed, they are represented by box and whisker graphs. Medians are shown as a transverse line crossing the boxes. The upper and lower ends of the box represent the 75 and 25% quartiles, respectively. The upper and lower whiskers represent the 95th and 5th percentiles, respectively. *Differences are considered significant when P < 0.05.
3.5. Abundance of mitochondrial markers and key metabolic enzymes
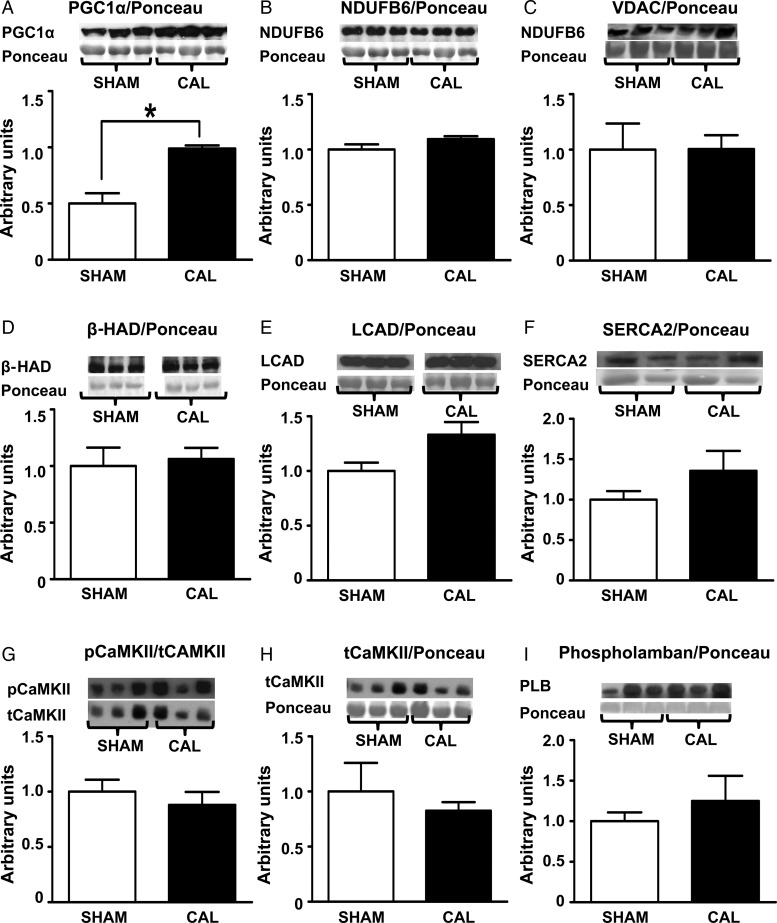
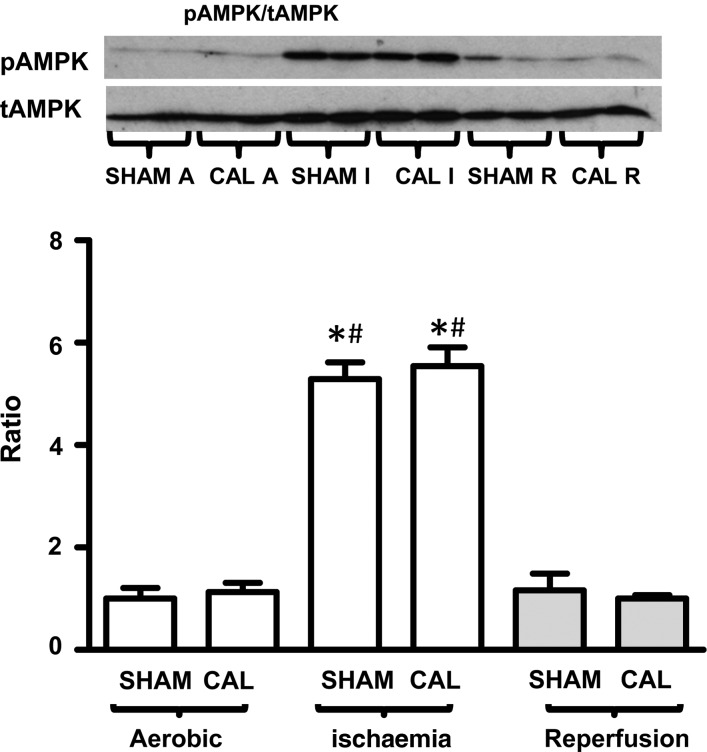
CAL hearts have a significantly higher PGC-1α protein expression (see Supplementary material online, Figure S1A), indicative of a higher stimulus for mitochondrial biogenesis. However, the expression of markers of mitochondrial abundance, Complex 1 subunit (NDUFB6), VDAC, β-HAD, and LCAD, is comparable in CAL and SHAM hearts (Figure 6B–E), indicative of an unchanged mitochondrial content in CAL hearts. Expression levels of SERCA2, CaMKII, pCaMKII, and phospholamban are also similar in CAL and SHAM hearts (Figure 6F–H), suggesting the absence of abnormalities in Ca2+ handling. Similarly, the expression of PPARα, PDH, hexokinase, GLUT1, GLUT4, GSK3β, pGSK3β, LDH, and GAPDH is similar in CAL hearts, supporting the lack of major changes in metabolic rates in CAL hearts compared with that in SHAM hearts (see Supplementary material online, Figure S1A–I). Similarly, AMPK activity (pAMPK/tAMPK) is similar in CAL and SHAM hearts at each time point (Figure 7A). As expected, AMPK activity is higher in both SHAM and CAL hearts at the end of ischaemia (Figure 7A), but it recovered during reperfusion.
Figure 6.
Protein expression of markers of stimulus for mitochondrial biogenesis (PGC-1α—A), markers of mitochondrial abundance [complex I subunit, NADH dehydrogenase (ubiquinone) 1 beta subcomplex (NDUFB6), VDAC, β-hydroxy acyl-CoA dehydrogenase (β-HAD), and long-chain acyl-CoA dehydrogenase (LCAD)—B–E], and calcium handling proteins (SERCA2, pCaMKII, tCaMKII, and phospholamban—F–I). Data are expressed as mean ± SEM, n = 3–6. *Differences are considered significant when P < 0.05.
Figure 7.
Phosphorylation status (pAMPK/tAMPK) of AMPK, indicative of activity, at end of aerobic perfusion, at end of ischaemia, and at end of reperfusion. Data are expressed as mean ± SEM, n = 4. *Differences are considered significant when P < 0.05. *Significantly different from aerobic; #Significantly different from reperfusion counterparts.
4. Discussion
A number of major findings are demonstrated in this study. Confirming previous studies, we demonstrate that hearts remodelled following a CAL are not energy deficient, but rather have a lower metabolic efficiency. Of importance, we provide the novel observation that hearts remodelled following CAL exhibit less deterioration of mechanical function during post-ischaemic reperfusion. Despite lower metabolic efficiency during aerobic (pre-ischaemic) conditions, remodelled hearts maintain similar levels of metabolic efficiency during reperfusion. In contrast, normal hearts exhibit a marked deterioration of mechanical function and metabolic efficiency during reperfusion following ischaemia. Recovery of cardiac energy metabolism was not compromised in either normal or remodelled hearts following ischaemia. However, there is a significant attenuation of glycogen utilization in remodelled hearts during ischaemia, resulting in a lower rate of proton production during ischaemia in the remodelled hearts. This decrease in proton production in remodelled hearts compared with normal may explain the improved tolerance to ischaemic injury in the remodelled hearts.
While there are numerous studies of energy substrate metabolism and reperfusion injury in normal hearts, few have addressed the relationships between metabolism and post-ischaemic function in hearts remodelled following myocardial infarction. To create an experimental model of the remodelled heart, we produced a permanent ligation of the coronary artery in mice, followed by a 4-week post-surgical period during which a mature scar formed and LV dysfunction developed. The examination of LV mechanical and metabolic function during isolated working heart perfusion enabled comparisons of normal and remodelled hearts under controlled conditions of energy substrate availability and workload and so provided an appropriate assessment of metabolic efficiency, a measure of LV work produced per unit ATP produced (J/µmol). In our model, LV mechanical dysfunction during the 60-min period of reperfusion following global ischaemia (18 min) is mainly due to reversible injury (stunning) as the release of LDH or troponin I, indicative of irreversible injury (infarction), is not detectable under these conditions (unpublished data).
Post-infarction remodelled hearts exhibit a marked deterioration of mechanical function.1 We previously showed that CAL hearts are not energy-starved since they maintain comparable adenine nucleotide contents to SHAM hearts, and the reduction in ATP production rates from fatty acid, glucose, and lactate metabolism in these hearts does not match the deterioration of mechanical function. Thus, there is a significant reduction of metabolic efficiency (i.e. inefficient utilization of ATP for generation of external mechanical work) in the CAL hearts during aerobic perfusion.1 We also showed that this may be due to increased proton production per LV work as a result of increased mismatch between glycolytic flux and glucose oxidation rates1 that may lead to a diversion of ATP from contractile work towards correction of ion homeostasis. The demonstration in this study that CAL hearts maintain similar metabolic rates to SHAM hearts during aerobic perfusion confirms our previous finding that CAL hearts are not energy-starved1 but instead develop metabolic inefficiency. In addition to confirming our previously published data, we now demonstrate that following ischaemia and reperfusion, CAL hearts maintain comparable rates of energy substrate metabolism to reperfused SHAM hearts. This excludes the possibility of a change in energy substrate preference as a contributor to the observed lower functional deterioration in reperfused CAL hearts. Indeed, the finding that CAL hearts maintain similar metabolic rates per LV work during reperfusion as during aerobic perfusion indicates that there is no further deterioration of metabolic efficiency in reperfused CAL hearts. The demonstration that CAL hearts maintain similar reperfusion nucleotide and creatine content to their aerobic values (published in Masoud et al.1) while SHAM hearts develop a significant decrease of their reperfusion ATP content compared with their aerobic values (published in Masoud et al.1) confirms better ischaemic tolerance and maintained reperfusion metabolic efficiency in CAL hearts. These findings are in accordance with previously published reports highlighting a better ischaemic tolerance in remodelled post-infarction hearts as indicated by reduced release of purines and lactate,4 less ischaemic contracture, and less lactate dehydrogenase release.3 Similarly, cardiomyocytes derived from post-infarction remodelled hearts exhibit lower decreases in ATP levels and less release of lactate dehydrogenase following hypoxia re-oxygenation.5
In contrast to CAL hearts, reperfused SHAM hearts exhibit a significant increase in glycolytic flux per LV work that is not matched by a corresponding increase in the rate of glucose oxidation, resulting in increased proton production per LV work. This finding, together with a significant increase in reperfusion fatty acid oxidation rate per LV work, contributes to the deteriorating metabolic efficiency during reperfusion of SHAM hearts. These findings are also in accordance with previous published data showing that during post-ischaemic reperfusion, normal mouse hearts exhibit a marked deterioration of oxidative metabolism. This augments the mismatch between glycolytic flux and glucose oxidation rates, resulting in lactate accumulation.8,23–26 Meanwhile, protons derived from the hydrolysis of glycolytically produced ATP accumulate, increasing intracellular acidosis.6 The subsequent activation of the Na+–H+ exchanger causes Na+ accumulation,27 which, in turn, activates reverse mode Na+–Ca2+ exchange leading to Ca2+ overload.28 To correct Na+ and Ca2+ overload, more ATP is diverted towards ionic homeostasis than to mechanical function. This is expected to lead to deterioration of metabolic efficiency associated with a significant deterioration of mechanical function (reviewed in Ref. 2).
A higher abundance of mitochondria in CAL hearts is a potential mechanism for the finding that CAL hearts, compared with SHAM hearts, do not show further deterioration of metabolic efficiency during reperfusion. Although higher expression of PGC-1α (which stimulates mitochondrial biogenesis) is observed in CAL hearts, this did not translate into increase in mitochondrial abundance of enzymes such as Complex 1 subunit (NDUFB6), VDAC, βHAD, or LCAD. Moreover, protein expression of key enzyme regulators of oxidative metabolism is similar in CAL and SHAM hearts. Nevertheless, the increased level of PGC-1α expression in CAL hearts may have contributed to the maintenance of mitochondrial mass and oxidative capacity in the remodelled viable tissue. Thus, it is unlikely that the better functional recovery and maintained metabolic efficiency during reperfusion of remodelled hearts are due to alterations in mitochondrial abundance.
Improved calcium handling is another potential mechanism for the absence of functional deterioration during reperfusion of CAL hearts. If present, it may contribute to reduced post-ischaemic contracture and more efficient utilization of ATP for external mechanical work. However, the absence of changes in the calcium handling proteins, SERCA2, CaMKII, and phospholamban, suggests the presence of other contributing factors to the observed absence of post-ischaemic functional deterioration in CAL hearts.
A third potential explanation of the observed better functional recovery following ischaemia and maintained metabolic efficiency in CAL hearts is a decrease in intracellular acidosis and Na+ and Ca2+ overload during ischaemia. A major contributor to the development of acidosis during ischaemia is the incorporation of glycogenolysis-derived glucose-6-phosphate into anaerobic glycolysis producing lactate and protons. The finding that CAL hearts exhibit significantly lower ischaemic glycogenolysis compared with SHAM hearts suggests that CAL hearts have a reduced metabolic demand during ischaemia. Moreover, this suggests that there is lower ischaemic production of lactate and protons in CAL hearts. Evidence to support this hypothesis comes from the finding that recovery of intracellular pH in reperfused hearts is delayed if the rates of glycolytic flux are not matched with glucose oxidation and that improving the coupling enhances the recovery of intracellular pH and improves both mechanical function and cardiac efficiency.6 In fact, many interventions that inhibit glycolytic flux during early reperfusion are cardioprotective. Omar et al.7 showed that diverting glucose metabolism towards glycogen synthesis rather than to glycolytic flux reduces proton production, limits Ca2+ overload, and improves recovery of post-ischaemic mechanical function. Similarly, other cardioprotective interventions such as adenosine16 or ischaemic preconditioning29,30 reduce glycogen utilization and inhibit glycolytic flux. The demonstration that CAL hearts utilize less glycogen than SHAM hearts during ischaemia and the fact that glycogen utilization feeds into anaerobic glycolysis suggest that CAL hearts have lower proton accumulation at the end of ischaemia. This lower stimulus for Na+ and Ca2+ accumulation during early reperfusion is expected to contribute to the lack of further deterioration in mechanical function and the lower deterioration in metabolic efficiency.
Attenuation of rates of glycogenolysis during ischaemia has been noted with some cardioprotective interventions such as ischaemic preconditioning and adenosine29,31 as well as with lower rates of ATP catabolism.32 Indeed, the findings that CAL hearts have lower rates of glycogenolysis during ischaemia and preserved ATP contents during reperfusion suggest that the improved ischaemic tolerance of remodelled hearts may mimic some of the mechanisms involved in ischaemic preconditioning. Furthermore, the demonstration that preconditioning mechanisms are already activated in remodelled hearts may explain the failure of additional preconditioning interventions to achieve functional benefits in remodelled hearts.33–35
AMPK is a known regulator of glycogen turnover.36,37 Previous study by Jaswal et al.36 showed that AMPK inhibition at post-ischaemic reperfusion of rat hearts shifts glucose metabolism towards glycogen synthesis. The inhibition of glycogenolysis and subsequent reduction of glycolytic flux rates improve coupling between glycolytic flux and glucose oxidation rates. This, in turn, reduces reperfusion proton production and is associated with a better functional recovery. We studied the expression and activation of AMPK at different perfusion time points. The demonstration that CAL hearts exhibit similar pattern of ischaemic AMPK activation indicates that changes in AMPK are unlikely to explain the observed reduction of ischaemic glycogen utilization in CAL hearts.
In conclusion, remodelled CAL hearts exhibit impaired LV mechanical function and metabolic efficiency during baseline aerobic conditions. Following exposure to an acute ischaemic episode, remodelled hearts exhibit higher recovery of LV function compared with normal hearts, with no further deterioration of metabolic efficiency. Lower glycogenolysis during ischaemia with subsequent less intracellular acidosis and ion dysregulation may contribute to the greater tolerance to ischaemic injury in the remodelled heart.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work is supported by operating grants from the Canadian Institutes of Health Research (MOP-115055 for A.S.C. and MOP-10865 for G.D.L.). W.G.T.M. received QEII Doctoral Studentship from the University of Alberta.
Acknowledgements
We thank Donna Becker from the Cardiovascular Research Centre, University of Alberta, for performing echocardiographic examinations.
Conflict of interest: none declared.
References
- 1.Masoud WG, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, Lygate CA, Neubauer S, Clanachan AS, Lopaschuk GD. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Cardiovasc Res 2014;101:30–38. [DOI] [PubMed] [Google Scholar]
- 2.Masoud WT, Clanachan A, Lopaschuk G. The failing heart: is it an inefficient engine or an engine out of fuel? In: Jugdutt BI, Dhalla NS, eds. Cardiac Remodeling. New York: Springer; 2013. p65–84. [Google Scholar]
- 3.Pantos C, Mourouzis I, Dimopoulos A, Markakis K, Panagiotou M, Xinaris C, Tzeis S, Kokkinos AD, Cokkinos DV. Enhanced tolerance of the rat myocardium to ischemia and reperfusion injury early after acute myocardial infarction. Basic Res Cardiol 2007;102:327–333. [DOI] [PubMed] [Google Scholar]
- 4.Kalkman EA, Saxena PR, Schoemaker RG. Sensitivity to ischemia of chronically infarcted rat hearts; effects of long-term captopril treatment. Eur J Pharmacol 1996;298:121–128. [DOI] [PubMed] [Google Scholar]
- 5.Sharikabad MN, Aronsen JM, Haugen E, Pedersen J, Moller AS, Mork HK, Aass HC, Sejersted OM, Sjaastad I, Brors O. Cardiomyocytes from postinfarction failing rat hearts have improved ischemia tolerance. Am J Physiol Heart Circ Physiol 2009;296:H787–H795. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol 2002;39:718–725. [DOI] [PubMed] [Google Scholar]
- 7.Omar MA, Wang L, Clanachan AS. Cardioprotection by GSK-3 inhibition: role of enhanced glycogen synthesis and attenuation of calcium overload. Cardiovasc Res 2010;86:478–486. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Arslan F, Ren Y, Adav SS, Poh KK, Sorokin V, Lee CN, de Kleijn D, Lim SK, Sze SK. Metabolic adaptation to a disruption in oxygen supply during myocardial ischemia and reperfusion is underpinned by temporal and quantitative changes in the cardiac proteome. J Proteome Res 2012;11:2331–2346. [DOI] [PubMed] [Google Scholar]
- 9.Jaswal JS, Keung W, Wang W, Ussher JR, Lopaschuk GD. Targeting fatty acid and carbohydrate oxidation - a novel therapeutic intervention in the ischemic and failing heart. Biochim Biophys Acta 2011;1813:1333–1350. [DOI] [PubMed] [Google Scholar]
- 10.Kolk MV, Meyberg D, Deuse T, Tang-Quan KR, Robbins RC, Reichenspurner H, Schrepfer S. LAD-ligation: a murine model of myocardial infarction. J Vis Exp 2009; doi:10.3791/1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virag JA, Lust RM. Coronary artery ligation and intramyocardial injection in a murine model of infarction. J Vis Exp 2011; doi:10.3791/2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang XP, Liu YH, Rhaleb NE, Kurihara N, Kim HE, Carretero OA. Echocardiographic assessment of cardiac function in conscious and anesthetized mice. Am J Physiol 1999;277:H1967–H1974. [DOI] [PubMed] [Google Scholar]
- 13.Benavides-Vallve C, Corbacho D, Iglesias-Garcia O, Pelacho B, Albiasu E, Castano S, Munoz-Barrutia A, Prosper F, Ortiz-de-Solorzano C. New strategies for echocardiographic evaluation of left ventricular function in a mouse model of long-term myocardial infarction. PLoS One 2012;7:e41691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan LJ, Wang T, Kahn ML, Ferrari VA. High-resolution echocardiographic assessment of infarct size and cardiac function in mice with myocardial infarction. J Am Soc Echocardiogr 2011;24:219–226. [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Bu L, Gong H, Jiang G, Li L, Ma H, Zhou N, Lin L, Chen Z, Ye Y, Niu Y, Sun A, Ge J, Zou Y. Effects of heart rate and anesthetic timing on high-resolution echocardiographic assessment under isoflurane anesthesia in mice. J Ultrasound Med 2010;29:1771–1778. [DOI] [PubMed] [Google Scholar]
- 16.Fraser H, Lopaschuk GD, Clanachan AS. Alteration of glycogen and glucose metabolism in ischaemic and post-ischaemic working rat hearts by adenosine A1 receptor stimulation. Br J Pharmacol 1999;128:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyck JR, Barr AJ, Barr RL, Kolattukudy PE, Lopaschuk GD. Characterization of cardiac malonyl-CoA decarboxylase and its putative role in regulating fatty acid oxidation. Am J Physiol 1998;275:H2122–H2129. [DOI] [PubMed] [Google Scholar]
- 18.Barr RL, Lopaschuk GD. Direct measurement of energy metabolism in the isolated working rat heart. J Pharmacol Toxicol Methods 1997;38:11–17. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi M, Finegan BA, Clanachan AS. Role of glucose metabolism in the recovery of postischemic LV mechanical function: effects of insulin and other metabolic modulators. Am J Physiol Heart Circ Physiol 2008;294:H2576–H2586. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson LL, Kozak R, Kelly SE, Onay Besikci A, Russell JC, Lopaschuk GD. Potential mechanisms and consequences of cardiac triacylglycerol accumulation in insulin-resistant rats. Am J Physiol Endocrinol Metab 2003;284:E923–E930. [DOI] [PubMed] [Google Scholar]
- 21.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Effects of adenosine on myocardial glucose and palmitate metabolism after transient ischemia: role of 5′-AMP-activated protein kinase. Am J Physiol Heart Circ Physiol 2006;291:H1883–H1892. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in western blots. Anal Biochem 2010;401:318–320. [DOI] [PubMed] [Google Scholar]
- 23.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol 2011;301:H1723–H1741. [DOI] [PubMed] [Google Scholar]
- 24.Ofir M, Arad M, Porat E, Freimark D, Chepurko Y, Vidne BA, Seidman CE, Seidman JG, Kemp BE, Hochhauser E. Increased glycogen stores due to gamma-AMPK overexpression protects against ischemia and reperfusion damage. Biochem Pharmacol 2008;75:1482–1491. [DOI] [PubMed] [Google Scholar]
- 25.Rosano GM, Fini M, Caminiti G, Barbaro G. Cardiac metabolism in myocardial ischemia. Curr Pharm Des 2008;14:2551–2562. [DOI] [PubMed] [Google Scholar]
- 26.Barillas R, Friehs I, Cao-Danh H, Martinez JF, del Nido PJ. Inhibition of glycogen synthase kinase-3beta improves tolerance to ischemia in hypertrophied hearts. Ann Thorac Surg 2007;84:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi S, Hisamitsu T, Nakamura TY. Regulation of the cardiac Na(+)/H(+) exchanger in health and disease. J Mol Cell Cardiol 2013;61:68–76. [DOI] [PubMed] [Google Scholar]
- 28.Baartscheer A, Schumacher CA, Coronel R, Fiolet JW. The driving force of the Na/Ca-exchanger during metabolic inhibition. Front Physiol 2011;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finegan BA, Lopaschuk GD, Gandhi M, Clanachan AS. Ischemic preconditioning inhibits glycolysis and proton production in isolated working rat hearts. Am J Physiol 1995;269:H1767–H1775. [DOI] [PubMed] [Google Scholar]
- 30.Weiss RG, de Albuquerque CP, Vandegaer K, Chacko VP, Gerstenblith G. Attenuated glycogenolysis reduces glycolytic catabolite accumulation during ischemia in preconditioned rat hearts. Circ Res 1996;79:435–446. [DOI] [PubMed] [Google Scholar]
- 31.Finegan BA, Lopaschuk GD, Gandhi M, Clanachan AS. Inhibition of glycolysis and enhanced mechanical function of working rat hearts as a result of adenosine A1 receptor stimulation during reperfusion following ischaemia. Br J Pharmacol 1996;118:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogt AM, Elsasser A, Pott-Beckert A, Ackermann C, Vetter SY, Yildiz M, Schoels W, Fell DA, Katus HA, Kubler W. Myocardial energy metabolism in ischemic preconditioning and cardioplegia: a metabolic control analysis. Mol Cell Biochem 2005;278:223–232. [DOI] [PubMed] [Google Scholar]
- 33.Miki T, Miura T, Tanno M, Sakamoto J, Kuno A, Genda S, Matsumoto T, Ichikawa Y, Shimamoto K. Interruption of signal transduction between G protein and PKC-epsilon underlies the impaired myocardial response to ischemic preconditioning in postinfarct remodeled hearts. Mol Cell Biochem 2003;247:185–193. [DOI] [PubMed] [Google Scholar]
- 34.Miki T, Miura T, Tsuchida A, Nakano A, Hasegawa T, Fukuma T, Shimamoto K. Cardioprotective mechanism of ischemic preconditioning is impaired by postinfarct ventricular remodeling through angiotensin II type 1 receptor activation. J Cardiol 2001;37:112–113. [PubMed] [Google Scholar]
- 35.Miki T, Miura T, Tanno M, Nishihara M, Naitoh K, Sato T, Takahashi A, Shimamoto K. Impairment of cardioprotective PI3K-Akt signaling by post-infarct ventricular remodeling is compensated by an ERK-mediated pathway. Basic Res Cardiol 2007;102:163–170. [DOI] [PubMed] [Google Scholar]
- 36.Jaswal JS, Gandhi M, Finegan BA, Dyck JR, Clanachan AS. Inhibition of p38 MAPK and AMPK restores adenosine-induced cardioprotection in hearts stressed by antecedent ischemia by altering glucose utilization. Am J Physiol Heart Circ Physiol 2007;293:H1107–H1114. [DOI] [PubMed] [Google Scholar]
- 37.Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes (Lond) 2008;32(Suppl. 4):S29–S35. [DOI] [PubMed] [Google Scholar]