Abstract
Aims
Hydrogen sulfide (H2S) is a vasoactive gasotransmitter that is endogenously produced in the vasculature by the enzyme cystathionine γ-lyase (CSE). However, the importance of CSE activity and local H2S generation for ischaemic vascular remodelling remains completely unknown. In this study, we examine the hypothesis that CSE critically regulates ischaemic vascular remodelling involving H2S-dependent mononuclear cell regulation of arteriogenesis.
Methods and results
Arteriogenesis including mature vessel density, collateral formation, blood flow, and SPY angiographic blush rate were determined in wild-type (WT) and CSE knockout (KO) mice at different time points following femoral artery ligation (FAL). The role of endogenous H2S in regulation of IL-16 expression and subsequent recruitment of monocytes, and expression of VEGF and bFGF in ischaemic tissues, were determined along with endothelial progenitor cell (CD34/Flk1) formation and function. FAL of WT mice significantly increased CSE activity, expression and endogenous H2S generation in ischaemic tissues, and monocyte infiltration, which was absent in CSE-deficient mice. Treatment of CSE KO mice with the polysulfide donor diallyl trisulfide restored ischaemic vascular remodelling, monocyte infiltration, and cytokine expression. Importantly, exogenous H2S therapy restored nitric oxide (NO) bioavailability in CSE KO mice that was responsible for monocyte recruitment and arteriogenesis.
Conclusion
Endogenous CSE/H2S regulates ischaemic vascular remodelling mediated during hind limb ischaemia through NO-dependent monocyte recruitment and cytokine induction revealing a previously unknown mechanism of arteriogenesis.
Keywords: Ischaemia, Hydrogen sulfide, Nitric oxide, Cystathionine γ-lyase, Vascular remodelling, Arteriogenesis
1. Introduction
Hydrogen sulfide (H2S) is a gasotransmitter that has many positive cardiovascular effects. H2S has recently been identified as an endogenously produced gaseous signalling molecule. H2S is synthesized by cystathionine γ-lyase (CSE) and 3-mercaptopyruvate sulfur transferase (3-MST) in peripheral vascular tissues.1 CSE is recognized as the major enzyme that produces H2S with l-cysteine serving as the primary substrate. The CSE/H2S pathway mediates various physiological effects including angiogenesis, vasoregulation, neuromodulation, reducing oxidative stress, cytoprotection, and cellular signalling.2 H2S signalling is impaired during regeneration of vascular tissue following ischaemia.3 H2S has also both pro-inflammatory and anti-inflammatory properties, which are crucial for the initiation and development of mature collaterals.4 H2S promotes vascular smooth muscle relaxation and induces vasodilation of isolated blood vessels by opening K-ATP channels and induces angiogenesis by cross talk with nitric oxide (NO).5,6 Furthermore, studies have suggested that H2S is endogenously synthesized by monocytes and macrophages, which in turn regulates the functions of these cells that may play roles in arteriogenesis.4 In addition, recently, it was shown in a study that H2S induces endothelial progenitor cell (EPC) function and improves wound healing in type 2 diabetic mice.7
Peripheral arterial disease (PAD) is a chronic occlusive disorder involving reduction of blood flow in the limbs that may lead to detrimental complications such as critical limb ischaemia and limb amputation, and is associated with increased death due to involvement of other cardiovascular disorders.8,9 Additionally, patients with other cardiovascular disorders experience an increased prevalence of PAD.10 Therapeutic angiogenesis is one of the better treatment options for PAD, but multiple clinical trials have shown limited benefits.11 Neovascularization is a fundamental requirement for many pathophysiological conditions.12 It occurs through three distinct processes that include vasculogenesis,13 angiogenesis, or arteriogenesis3 via several intermediary signalling molecules that enhance these processes.14 The changes in mechanical forces acting on the endothelial cells lining the premature arteries stimulate the inflammatory signals needed for the start of the collateral growth.15
Patients with both PAD and other co-morbid disorders suffer from impaired arteriogenesis due to dysfunction in inflammatory cell monocytes.16 Cytokines such as IL-16 have been shown as a pro-arteriogenic factor in mouse hind limb ischaemia.17 Studies with human subjects have shown that impaired number and function of EPCs was observed in cardiovascular disease patients,18 including those with diabetes mellitus.19 However, mobilization and recruitment of EPCs was observed during ischaemia and inflammation to enhance vasculogenesis.20 Ischaemia can up-regulate various other cytokines, such as VEGF, SDF-1, and MCP-1 or ILs, that induce circulating EPCs and enhance revascularization.21
Thus, H2S and mononuclear cell infiltrates might be important for arteriogenesis and angiogenesis under ischaemic conditions. In the present study, we examined the importance of endogenous CSE/H2S during femoral artery ligation (FAL) and their effects on ischaemic vascular remodelling.
2. Methods
2.1. Animals and experimental procedures
Twelve-week-old male C57BL/6J (WT) and CSE knockout (CSE KO) mice were used in this study. Mice were maintained in strict accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. All animal studies were approved by the LSU Health-Shreveport institutional animal care and use committee (approval # P-12-011).
2.2. Induction of mouse critical limb ischaemia model with treatment profiles
Permanent hind limb ischaemia was induced in WT and CSE KO mice as previously described.3 Mice were anaesthetized with ketamine/xylazine (100 and 8 mg/kg) injection and ligation of the left femoral artery was performed. Experimental cohorts of: control (phosphate-buffered saline, PBS) or diallyl trisulfide (DATS; 100, 200, or 500 µg) were administered retro-orbitally twice daily following the hind limb ischaemia surgery. More detailed methods are described in Supplementary material online.
2.3. Measurement of CSE activity
Tissues from the ischaemic and non-ischaemic hind limb of mice were collected at different time points of study. CSE activity was measured as we previously described.22
2.4. Measurement of plasma H2S
H2S was measured using monobromobimane by RP-HPLC as we previously reported and described in detail in Supplementary material online.23
2.5. Laser Doppler blood flow measurements
Laser Doppler blood flow measurements were made using a Vasamedics Laserflo BPM2 device in the ischaemic limb of the mice pre- and post-ischaemia induction and indicated days post-ischaemia as we have previously described.3
2.6. Novadaq SPY imaging analysis
The SPY imaging was performed to quantify collateral vessel perfusion as previously described.24
2.7. Immunohistochemical staining of skeletal muscle tissues
Immunohistochemistry staining with anti-alpha smooth muscle actin (α-SMA), anti-CD31 antibodies, and anti-hypoxia inducible factor 1- alpha (HIF-1α) was performed with nuclear stain 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI), as we have previously described.3
2.8. Isolation of monocytes
Bone marrow and whole blood from the mice were collected and processed from ischaemic and non-ischaemic hind limb of WT and CSE KO mice to isolate monocytes using a protocol as described elsewhere with minor modifications.25
2.8.1. Isolation of EPCs
EPCs were isolated from blood, bone marrow, and skeletal muscles (ischaemic and non-ischaemic hind limbs) of WT and CSE KO mice (with or without DATS therapy) using protocols as described elsewhere with modifications.26,27
2.9. Measurement of cytokine expression
IL-16, MCP-1, bFGF, and VEGF levels were measured in gastrocnemius tissues using an ELISA kit from R&D Biosciences. Briefly, PBS- or DATS-treated mice were euthanized at Day 3 and gastrocnemius muscle tissues harvested and protein lysates made. ELISAs were performed according to the manufacturer's instructions.
2.10. Statistical analysis
Data were reported as mean ± S.E.M. for all groups. Statistical analysis was performed with Mann–Whitney or Kruskal–Wallis analysis of variance with Dunn's multiple-comparison tests. A P-value of <0.05 was required for statistical significance. Statistics were performed with the GraphPad Prism 4.0 software.
3. Results
3.1. H2S levels and CSE activity were increased in plasma and ischaemic tissue
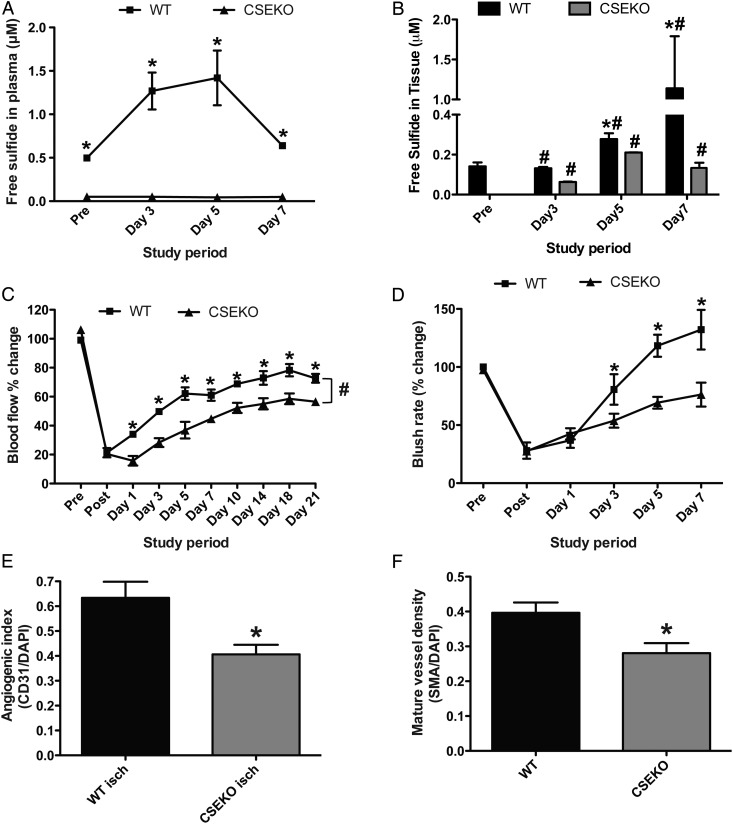
Plasma-free H2S was measured in WT and CSE KO mice, and we found a significant increase at Days 3 and 5 in WT, but not in CSE KO, mice (Figure 1A). Free H2S levels in ischaemic gastrocnemius muscle tissue were also significantly increased at Days 5 and 7 in WT mice (Figure 1B). Although there was no change in free H2S levels in plasma, a significant increase in free H2S levels in ischaemic tissues was observed in CSE KO mice, suggesting other compensatory pathways of H2S generation (cystathionine beta synthase or 3-MST).
Figure 1.
Blood perfusion and blood vessel density is impaired in CSE KO mice under ischaemia. (A and B) Comparison of WT and CSE KO mice plasma- and tissue-free sulfide levels, respectively, at different time points following hind limb ischaemia induction. (C) Per cent change in ischaemic limb blood flow between WT and CSE KO mice as measured by laser Doppler flowmetry. (D) Per cent change in ischaemic limb indocyanine green (ICG) blush rate between WT and CSE KO mice. (E) Immunohistochemical staining for vascular angiogenic index (CD31/DAPI) between WT and CSE KO ischaemic muscle. (F) Immunohistochemical staining for arterial vessels (SMA/DAPI) between WT and CSE KO mice. n = 7 per cohort, *P < 0.05, #P < 0.05 CSEKO vs. WT.
Importantly, we observed a significant increase in CSE activity of plasma and muscle tissues at Days 3 and 5 after ligation in WT mice (see Supplementary material online, Figure S1A and B), which was not observed in CSE KO mice. Increased CSE gene expression was also selectively observed in ischaemic muscle tissues of WT, but not in CSE KO, mice (see Supplementary material online, Figure S1C and D). These results indicate that CSE activity and gene expression are induced during chronic tissue ischaemia.
3.2. Blood flow, blush rate, and mature vessel density were decreased in ischaemic tissues of CSE KO mice
Restoration of blood flow to ischaemic tissues is very important for its survival. We next measured limb blood flow and perfusion rates of WT and CSE KO mice at different time points after FAL. Laser Doppler measurement of blood flow in ischaemic tissue was significantly reduced in CSE KO mice compared with WT mice continuously after establishing tissue ischaemia (Figure 1C). Similarly, acute changes in tissue perfusion (blush rate) by SPY angiogram were significantly reduced in CSE KO mice compared with WT mice at Days 3, 5, and 7 after ligation (Figure 1D).
Formation of mature and functionally stable vessels is important for reperfusion of ischaemic tissue. We next investigated the mature vessel density in WT and CSE KO mice by dual staining with anti-CD31 and anti α-SMA antibodies.3 We observed a significant decrease in mature vessel density and angiogenic indices (Figure 1E and F) in CSE KO mice, suggesting the importance of the role of CSE/H2S during arteriogenesis in ischaemic tissue. Vessel densities were significantly increased in ischaemic tissue vessel densities compared with non-ischaemic controls of WT and CSE KO (see Supplementary material online, Figure S2). Representative images of blush rate and mature vessel density of WT and CSE KO mice are shown in Supplementary material online, Figure S2A and B, respectively.
3.3. DATS therapy rescues arteriogenesis and ischaemic limb blood flow in CSE KO mice
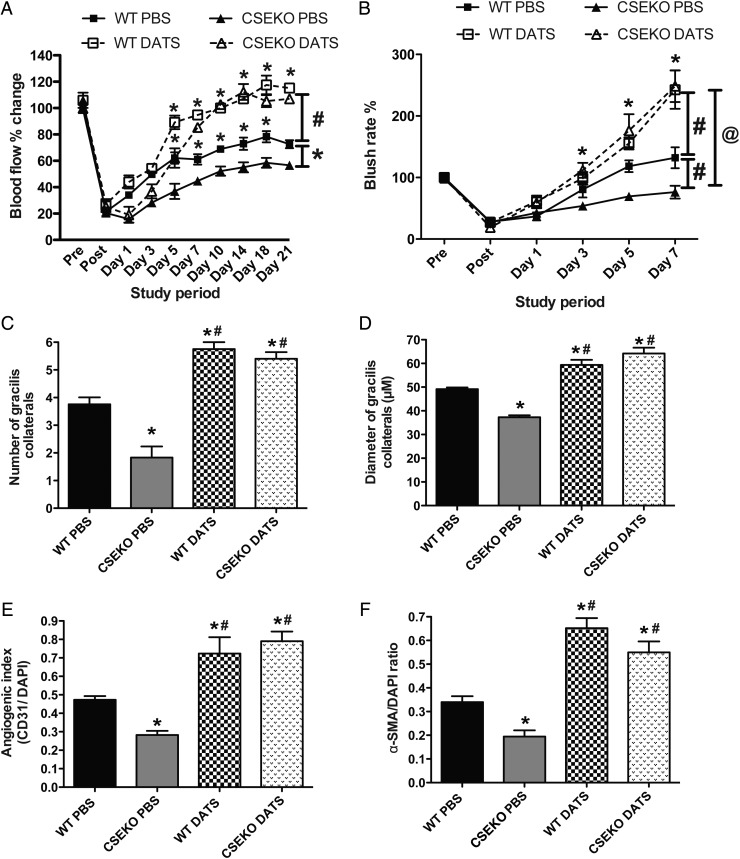
We next investigated the effect of exogenous H2S therapy using DATS. Parameters of arteriogenesis were closely examined since CSE/H2S appeared to significantly affect ischaemic limb arteriogenesis activity. To understand the effect of DATS on ischaemic blood flow recovery, we used 200 µg/kg DATS based on our previous observations.28 We determined the pharmacokinetics of plasma (see Supplementary material online, Figure S3A) and gastrocnemius skeletal muscle tissue (see Supplementary material online, Figure S3B) total sulfide in wild type mice given DATS via intraperitoneal or retro-orbital routes, with retro-orbital administration being found superior. Additionally, we performed therapeutic dose-dependent studies using a range of DATS concentrations administered via RO injection (100, 200, and 500 µg/kg, twice daily; see Supplementary material online, Figure S3C). Hind limb blood flow in ischaemic tissue was dose-dependently increased by DATS in WT mice with a median dose of 200 µg/kg. Therefore, this therapeutic dose regimen of DATS was used for all exogenous sulfide studies reported below. Administration of DATS significantly restored ischaemia blood flow in both WT and CSE KO mice (Figure 2A). Similarly, blush rate detected by SPY angiogram was also augmented by DATS therapy in both WT and CSE KO mice (Figure 2B).
Figure 2.
DATS therapy induces blood flow and collateral formation. (A) Changes in WT or CSE KO mouse ischaemic limb blood flow with PBS or DATS (200 μg/kg, twice daily) over time. (B) ICG blush rate changes between WT or CSE KO mice given PBS or DATS therapy. #P<0.05 vs WT PBS. (C and D) Quantitative measurements of the number and diameter of gracilis collaterals between WT and CSE KO mice with PBS or DATS therapy, respectively. (E and F) Angiogenic index (CD31/DAPI) and arterial vessel staining (SMA/DAPI) between WT and CSE KO mice with PBS or DATS therapy, respectively. n = 5 per cohort, *P < 0.05. #P < 0.05 vs. WT PBS; @P < 0.05 vs. CSE KO PBS.
Collateral arteriolar growth and remodelling are crucial during arteriogenesis.29 We next examined the perfusion and diameter of the gracilis arteries in ischaemic muscles with or without DATS therapy in WT and CSE KO mice. Figure 2C and D reports that the diameter and number of perfused gracilis arteries was significantly less in CSE KO mice compared with WT mice, and was significantly increased after DATS therapy in both CSE KO and WT mice. Moreover, CD31- and α-SMA-positive vessels were significantly increased after DATS therapy in ischaemic tissue of CSE KO and WT mice (Figure 2E and F).
3.4. Effect of CSE expression on EPCs and monocytes during hind limb ischaemia
Bone marrow-derived EPCs (BM-EPCs) in circulation and homed EPCs at the sites of ischaemic event contribute to formation of blood vessel.30 Therefore, to determine cellular mechanisms of H2S/CSE on arteriogenesis, we examined EPCs and monocytes from the circulation, skeletal muscle tissue, and the bone marrow from WT and CSE KO mice. There was a significant increase in BM-derived and circulating EPCs in the ischaemic limb by Day 3, which was further elevated upon DATS treatment (see Supplementary material online, Figure S4A and B). BM-derived EPCs increased in the circulation and the skeletal muscle by Days 5 and 7 post-ligation in WT mice, especially with DATS treatment (see Supplementary material online, Figure S4C). Induction of EPCs was significantly reduced in CSE KO mice (see Supplementary material online, Figure S4D–F). Moreover, a moderate increase in CSE KO mouse EPC induction in bone marrow and blood was observed with DATS treatment, but no increase in skeletal muscle tissues.
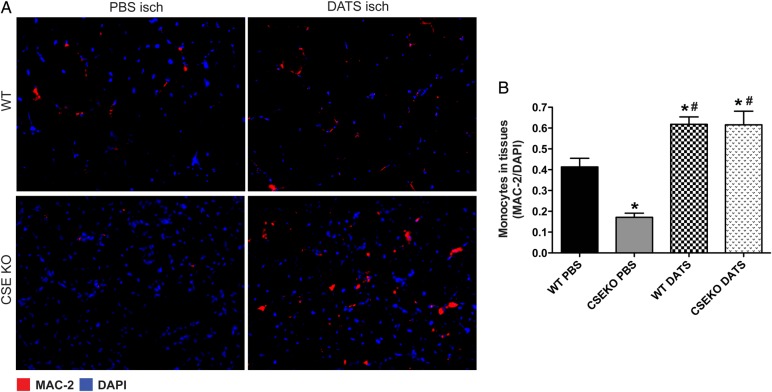
Having observed a moderate effect of CSE/DATS on EPC function in ischaemic tissue, we next examined whether monocyte recruitment was altered in CSE KO mice, as these cells critically regulate arteriogenesis.31 Monocyte infiltration was increased in WT mice, but significantly attenuated in CSE KO mice compared with WT ischaemic tissue. Importantly, monocyte infiltration into ischaemic tissue was increased with DATS therapy in WT mice and rescued in CSE KO mice (Figure 3). Interestingly, ischaemic tissue monocyte infiltration, CSE activity, and H2S levels were significantly increased in circulating (see Supplementary material online, Figure S5A and B) and BM-derived monocytes (see Supplementary material online, Figure S5C and D) of WT mice at Day 3 after FAL compared with sham WT mice. However, these changes were not observed in monocyte populations isolated from CSE KO mice. Moreover, we observed that under hypoxia, expression of CSE though adenoviral-CSE induces monocyte-dependent endothelial cell proliferation in co-culture, while inhibition of CSE with specific CSE-shRNA significantly blunts this response (see Supplementary material online, Figure S5E).
Figure 3.
Monocyte recruitment is inhibited in CSE KO hind limb ischaemia. (A) Representative photomicrographs of MAC-2 and DAPI counterstained ischaemic skeletal muscle sections of PBS- or DATS-treated WT and CSE KO mice at Day 3. (B) Quantitative image analysis of ischaemic muscle MAC-2/DAPI staining between WT and CSE KO mice treated with PBS or DATS. n = 5 per cohort, *P < 0.05 vs. WT, #P < 0.05 vs. CSE KO PBS.
3.5. CSE regulates ischaemia and inflammatory gene expression
Changes in mechanical forces after occlusion of the artery acts on endothelial cells lining the vascular walls and promotes the inflammatory signals required to start collateral formation.32,33 We first evaluated the expression of HIF-1α in skeletal muscle tissue, which was elevated under ischaemia in WT but not in CSE KO mice and with DATS treatment in both WT and CSE KO mice (see Supplementary material online, Figure S6A). We next investigated the MCP-1 and IL-16 expression in ischaemic tissues of WT and CSE KO mice at Day 3 after FAL as they participate in arteriogenesis.17,34 IL-16 levels were significantly increased in ischaemic tissue of WT mice, but not of CSE KO mice; however, DATS therapy augmented IL-16 levels in both WT and CSE KO mice (see Supplementary material online, Figure S6B). No significant difference was observed in MCP-1 levels of CSE KO mice compared with WT mice (data not shown). These data indicate the role of CSE/H2S on IL-16 expression during ischaemia, which modulates arteriogenesis via recruitment of monocytes.
Growth factors including VEGF and bFGF play significant roles during arteriogenesis and angiogenesis under ischaemic conditions.35 VEGF and bFGF expression were attenuated in ischaemic limbs of CSE KO mice compared with WT mice, but were augmented on DATS therapy in both WT and CSE KO mice (see Supplementary material online, Figure S6C and D). Taken together, data here demonstrate that CSE/H2S increased IL-16, VEGF, and bFGF expression levels that are known to participate in ischaemic vascular remodelling.
3.6. CSE/H2S stimulates ischaemic vascular remodelling and growth via NO-dependent pathway
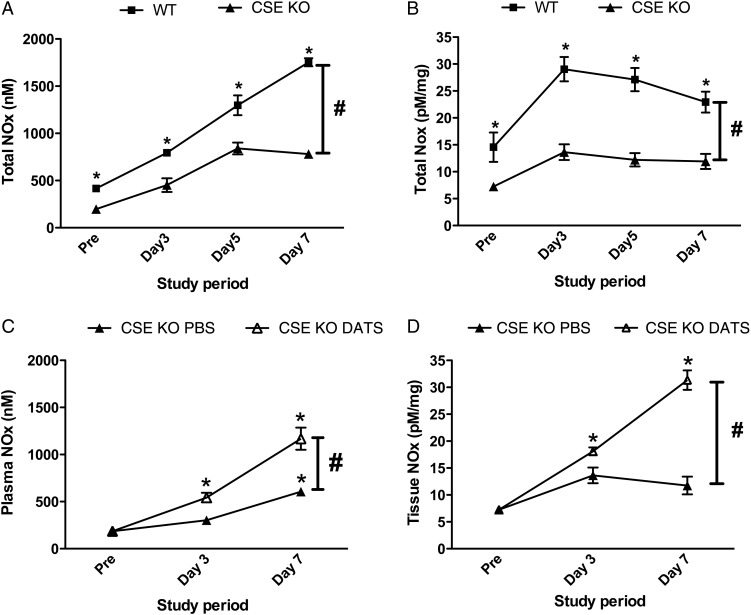
NO is a prime mediator of vascular remodelling in ischaemic tissue.36 Our previous work has shown that exogenous H2S stimulates ischaemic revascularization in a NO-/HIF-1α-dependent pathway.3 Based on these findings, we sought to quantify plasma and tissue levels of NO in WT and CSE KO mice with DATS treatment (Figure 4A and B). We observed a significant decrease in total nitric oxides (NOX) levels of plasma and muscle tissues at pre-ligation, Days 3, 5, and 7, and after ligation in CSE KO mice compared with WT mice. This indicates a role of CSE/H2S in modulating NO bioavailability that corroborates our earlier report. Importantly, this defect was recovered by DATS therapy in CSE KO mice, where a significant increase in NOx levels was observed in plasma and ligated muscle tissues (Figure 4C and D).
Figure 4.
CSE KO mice have reduced NOx levels that are restored by DATS treatment. (A) Levels of total plasma NOx at different time points between WT and CSE KO mice. (B) Ischaemic muscle tissue total NOx at different time points between WT and CSE KO mice. (C) Plasma total NOx levels in CSE KO mice treated with PBS or DATS therapy over time. (D) Ischaemic muscle tissue total NOx levels in CSE KO mice with PBS or DATS therapy over time. n = 5 per genotype or treatment cohort. *P < 0.05 vs. pre-ligation value, #P < 0.05 WT vs. CSE KO in A and B and PBS vs. DATS in C and D.
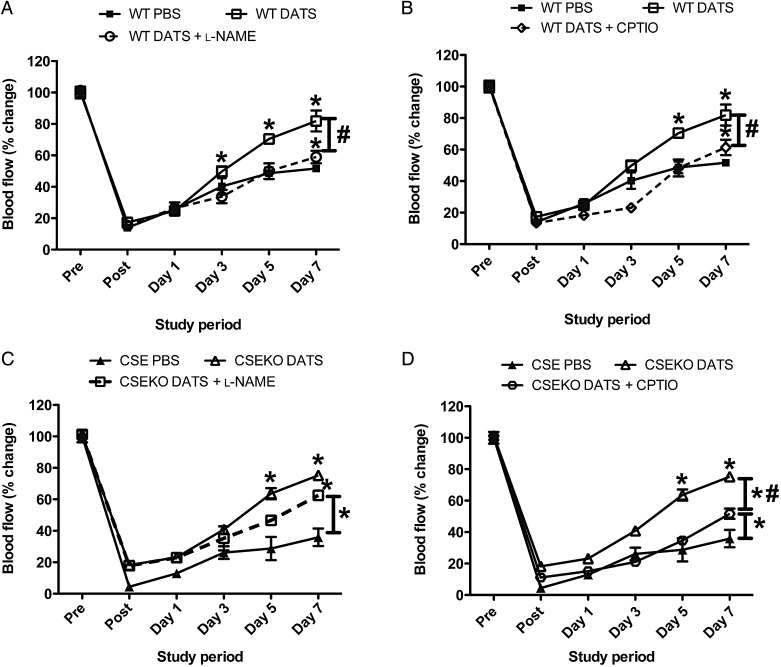
We next examined whether DATS therapy restored blood flow and revascularization in WT and CSE KO mice via NO. Either the nitric oxide synthase (NOS) inhibitor l-NAME, which potently inhibits nNOS, endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS)37–39 activity in this order, or the NO scavenger cPTIO was used for these studies. Both l-NAME and cPTIO blunted DATS restoration of ischaemic hind limb reperfusion in WT mice (Figure 5A and B). However, l-NAME treatment with DATS in CSE KO mice did not attenuate ischaemic limb reperfusion (Figure 5C), whereas cPTIO treatment did significantly inhibit DATS-mediated ischaemic limb reperfusion in CSE KO mice (Figure 5D), indicating that the effects of DATS in CSE KO mice are dependent on NO signalling, but not necessarily dependent on eNOS (or other NOS) activity. Supplementary material online, Figure S7 further reports the effect of these treatments on DATS vascular remodelling as determined by CD31 vs. α-SMA staining. Inhibition of NO bioavailability via these agents significantly blunted tissue staining for these vascular remodelling markers in CSE KO and WT mice.
Figure 5.
DATS-mediated restoration of blood flow is NO-dependent. (A and B) DATS-mediated changes in ischaemic limb blood flow of WT mice with l-NAME (LN) or NO scavenger (CPTIO), respectively. (C and D) DATS-mediated changes in ischaemic limb blood flow of CSE KO mice with LN or CPTIO, respectively. n = 6 per cohort, *P < 0.05 vs. PBS treatment, #P < 0.05 DATS vs. DATS plus either L-NAME or CPTIO.
3.7. Nitrite therapy increases NO bioavailability, ischaemic limb blood flow, and vascular density in CSE KO ischaemic tissues
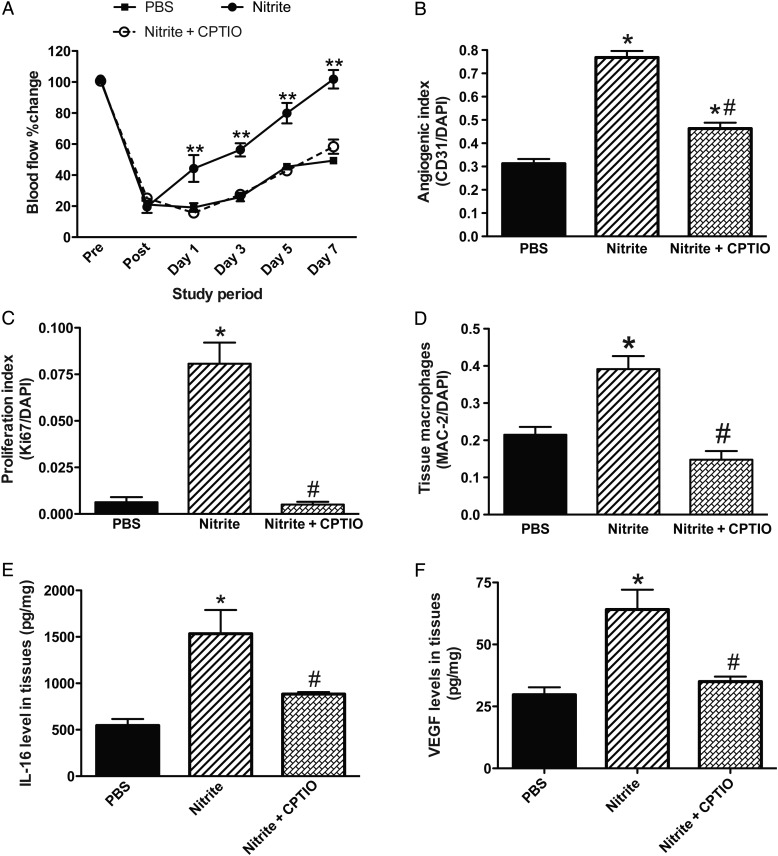
Our group and others have previously demonstrated that nitrite therapy selectively restores ischaemic limb blood flow via its conversion back to NO that stimulates ischaemic vascular remodelling.24,40,41 Given the significant deficiency of NO bioavailability in CSE KO mice, we sought to examine whether ischaemic vascular remodelling in these animals was primarily due to reduced NO. Thus, exogenous nitrite or control PBS was therapeutically administered to CSE KO mice with FAL. We found in CSE KO mice that 165 μg/kg nitrite robustly stimulates ischaemic vascular remodelling by augmenting blood flow (Figure 6A) and vascular density and proliferation (Figure 6B and C) in ischaemic tissues. Importantly, these effects of nitrite on ischaemic vascular remodelling and proliferation were significantly inhibited by the NO scavenger cPTIO (Figure 6B and C).
Figure 6.
Nitrite restores blood flow in CSE KO mice. (A) Per cent change in ischaemic limb blood flow of CSE KO mice given PBS, nitrite (165 μg/kg), or nitrite + cPTIO (1 mg/kg) twice daily therapy. (B) Quantitative measurement of angiogenic index (CD31/DAPI) staining of CSE KO ischaemic muscle treated with nitrite or nitrite + cPTIO therapy. (C) Quantitative measurement of cell proliferation index (Ki67/DAPI) staining of CSE KO ischaemic muscle treated with nitrite or nitrite + cPTIO therapy. (D) Tissue macrophage (MAC-2/DAPI) staining of CSE KO ischaemic muscle treated with nitrite or nitrite + cPTIO therapy. (E and F) IL-16 and VEGF levels in CSE KO ischaemic tissues with nitrite or nitrite + cPTIO therapy, respectively. n = 5 per cohort, **P < 0.01 nitrite vs. PBS therapy, *P < 0.05 vs. PBS, #P < 0.05 nitrite vs. nitrite + CPTIO.
Beneficial effects of nitrite and the inhibitory action by cPTIO were also observed with MAC-2 ischaemic tissue staining for macrophages (Figure 6D). We next examined whether the effect of macrophage recruitment was through a NO/IL-16 axis. We found that IL-16 levels were significantly increased in ischaemic tissue of CSE KO with nitrite therapy, which was significantly reduced by cPTIO (Figure 6E). Likewise, VEGF levels were significantly increased with nitrite therapy in CSE KO mice and inhibited by cPTIO co-treatment (Figure 6F). These data clearly demonstrate that defective NO bioavailability is a primary mediator of defective ischaemic vascular remodelling in CSE KO mice.
4. Discussion
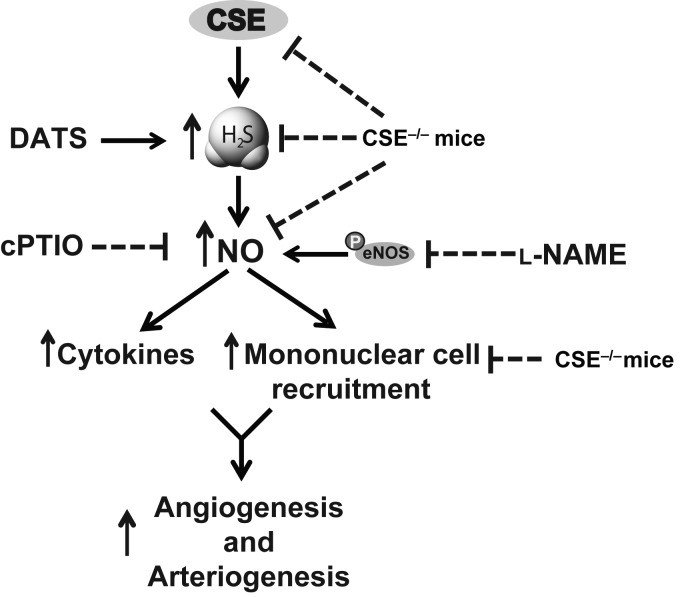
Studies from ours and other laboratories have shown that exogenous H2S augments blood perfusion under ischaemic conditions by stimulating vascular remodelling and angiogenesis.3,42,43 However, the role of CSE and endogenous H2S generation on collateral vessel growth and remodelling during hind limb ischaemia has not been elucidated. In the present study, we have made several important findings, as illustrated in Figure 7, including (i) tissue ischaemia quickly induces CSE activity and H2S production necessary for arteriogenesis and angiogenesis, (ii) DATS therapy in CSE-mutant mice rescues defective NO bioavailability, cytokine production, and mononuclear cell infiltration in ischaemic tissue, and (iii) nitrite-mediated NO therapy rescues CSE-mutant deficiencies in tissue perfusion and ischaemic vascular remodelling, cytokine production, and myeloid cell infiltration in a NO-dependent manner.
Figure 7.
Summary of CSE/H2S regulation of ischaemic vascular remodelling pathways. FAL and subsequent limb chronic ischaemia lead to increased CSE expression, activity, and H2S generation. Genetic deficiency of CSE inhibits ischaemia-mediated induction of H2S-dependent stimulation of NO bioavailability and downstream activation of cytokine and cellular responses regulating arteriogenesis and angiogenesis pathways.
H2S regulates many endothelial functions and the metabolism of signalling molecules that participate in angiogenesis and arteriogenesis under ischaemic conditions.42,44,45 In the current study, we have reported using CSE genetically deficient mice with an endogenous defect in H2S and also NO bioavailability, leading to impaired ischaemic vascular remodelling and blood flow. Currently, several donors of H2S including DATS, Na2S, and NaHS have been reported in the literature to have cardiovascular therapeutic effects under different experimental settings.3,46,47 In the present study, we have demonstrated that the exogenous H2S donor, DATS, augments the number and diameter of mature arterial collaterals and CD31-positive vessel density upon treatment following ischaemia. These changes mediated by H2S involve NO that induces ischaemic arteriogenesis and angiogenesis that subsequently rescue perfusion of ischaemic tissue of CSE KO and WT mice following FAL.
We have previously reported that exogenous H2S therapy stimulates ischaemic vascular remodelling moderately altering eNOS phosphorylation or expression of other NOSs (iNOS and nNOS).3 However, we uniquely observed that exogenous H2S-mediated recovery of ischaemic limb blood flow through angiogenesis was significantly mediated by nitrite reduction to NO via xanthine oxidase vs. changes in NOS expression or function, revealing a crosstalk between H2S and NO production pathways.3 Those findings in a chronic ischaemia model differ from studies of acute ischaemia–reperfusion, which indicate that the therapeutic effects of H2S are dependent on eNOS signalling pathway.45 This is likely due to the differential nature of ischaemic responses (i.e. chronic ischaemia vs. acute ischaemia and reperfusion) coupled with differences in organ tissues studied (e.g. the heart45 vs. skeletal muscle). Findings in this current study regarding the role of endogenous sulfide reveals that CSE activity and H2S bioavailability are rapidly induced under ischaemic conditions where NOS enzyme activities would be least effective. This reinforces our earlier discovery and the importance of H2S–NO crosstalk for ischaemic vascular remodelling, suggesting that H2S-mediated NO production via non-NOS pathways could be an archaic adaptive response to maintain NO bioavailability necessary for tissue perfusion and protection. This is supported by the fact that DATS therapy clearly restored blood flow in CSE KO mice that was completely blunted by cPTIO, but unaffected by l-NAME indicating that NO formation from other non-NOS sources during chronic ischaemia in CSE KO mice are pathophysiologically important for adaptive responses, as we have proposed.3
NO serves dichotomous roles for vascular remodelling, which is also referred as the NO paradox.48 At early stages of remodelling, NO stimulates recruitment of monocytes and expression of growth factors to promote vascular remodelling.49 However, in this study, we have shown for the first time that CSE/H2S is important for stimulating arteriogenesis. Our data reveal that formation of collaterals is significantly attenuated in CSE KO compared with WT mice. Importantly, direct stimulation of smooth muscle cells and endothelial cells is pivotal to initiate arteriogenesis.15,50 We also demonstrated that collateral arterial density and microvascular vessel density were significantly less in CSE KO mice subjected to hind limb ischaemia, suggesting that loss of CSE may influence arteriogenesis stimulation responses in vascular cells, which will require further study. Finally, CSE KO defects in vessel density and blood flow changes were completely restored by exogenous DATS therapy, confirming the importance of CSE/H2S for adaptive vascular remodelling.
Different cytokines and signalling molecules including NO are involved in the initiation of arteriogenesis.16,24,51 With this study, we determined that in CSE KO mice, apart from reduced NO bioavailability, levels of cytokines, such as IL-16, bFGF, and VEGF, were severely blunted after FAL. However, restoration of NO through nitrite (NO prodrug) therapy restores IL-16 expression, and increases VEGF expression in ischaemic tissues of CSE KO mice, indicating an important, yet poorly understood relationship between sulfide bioavailability and NO metabolites. Further study is required to better understand the mechanistic interactions between sulfide and NO metabolite bioavailability for vascular remodelling in response to ischaemia.
Stimulation of the monocytic pathway has shown promising results for formation of collaterals after occlusion of major arteries.52,53 Studies from different laboratories have indicated that recruitment of monocytes and their transformation into macrophages in the site of injury is important during arteriogenesis.52–54 Our data show that monocyte recruitment into ischaemic tissue is impaired in CSE KO mice, indicating that a functional CSE/H2S system is required for monocyte recruitment for arteriole remodelling. We also made the discovery that CSE activity and H2S generation in circulating and bone marrow-derived monocytes following ischaemia were significantly increased in WT mice. In the hind limb ischaemia model, circulating monocytes are recruited into the tissue and converted to macrophages with two functional phenotypes classified as M1 and M2.55 Based on the varied expression levels of the chemokine receptors CCR2 and CXCR1, they exhibit either pro-inflammatory or anti-inflammatory and pro-angiogenic functions.56 However, the involvement of M1 vs. M2 macrophages in relation to H2S-mediated responses in CSE KO mice has yet to be determined and is a prime target for future investigation. Taken together, it is clear that H2S may play an important role in monocyte/macrophage-mediated arteriogenesis, and that other mononuclear cell responses such as EPC-induced vasculogenesis are less involved.
In conclusion, the findings of the present study indicate that dysregulation or deficiency of CSE as well as endogenous H2S generation have a significant impact on cytokines/growth factors affecting vascular remodelling and blood perfusion in the mouse ischaemic hind limb model. However, exogenous H2S rescue therapy in CSE KO mice elicits downstream NO bioavailability alleviating impaired vascular remodelling and blood perfusion via up-regulation of mononuclear cell recruitment (monocytes/macrophages) and cytokine/growth factor expression that includes IL-16/bFGF/VEGF signalling pathway. As NO plays a critical role in this response, our study highlights important interactions between these two molecules to co-ordinately regulate arteriogenesis and restore blood flow in the mouse ischaemic limb. Thus, it is possible that endogenous H2S bioavailability and metabolism may be important in the setting of PAD or critical limb ischaemia.
5. Significance
The role of endogenous H2S generation and bioavailability for ischaemic vascular growth and remodelling has not been known. Our study provides critical new insights that increased endogenous cystathionine gamma lyase expression, activity, and H2S generation leading to augmentation of NO bioavailability are required for ischaemic vascular remodelling that may be useful for future therapeutic revascularization approaches.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
C.G.K. is the recipient of NIH Grant HL113303. G.K.K., S.C.B., and S.Y. were funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center-Shreveport.
Acknowledgments
Conflict of interest: C.G.K. has intellectual property regarding nitrite therapy, and is a founder and scientific advisor for Theravasc, Inc. and Innolyzer LLC.
References
- 1.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev 2012;92:791–896. [DOI] [PubMed] [Google Scholar]
- 2.Kolluru GK, Shen X, Bir SC, Kevil CG. Hydrogen sulfide chemical biology: pathophysiological roles and detection. Nitric Oxide 2013;35:5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bir SC, Kolluru GK, McCarthy P, Shen X, Pardue S, Pattillo CB, Kevil CG. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J Am Heart Assoc 2012;1:e004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhi L, Ang AD, Zhang H, Moore PK, Bhatia M. Hydrogen sulfide induces the synthesis of proinflammatory cytokines in human monocyte cell line U937 via the ERK-NF-kappaB pathway. J Leukoc Biol 2007;81:1322–1332. [DOI] [PubMed] [Google Scholar]
- 5.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 2002;16:1792–1798. [DOI] [PubMed] [Google Scholar]
- 6.Kolluru GK, Shen X, Kevil CG. A tale of two gases: NO and H2S, foes or friends for life? Redox Biol 2013;1:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu F, Chen DD, Sun X, Xie HH, Yuan H, Jia W, Chen AF. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes 2014;63:1763–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eberhardt RT, Coffman JD. Cardiovascular morbidity and mortality in peripheral arterial disease. Curr Drug Targets Cardiovasc Haematol Disord 2004;4:209–217. [DOI] [PubMed] [Google Scholar]
- 9.Schaper NC, Nabuurs-Franssen MH, Huijberts MS. Peripheral vascular disease and type 2 diabetes mellitus. Diabetes Metab Res Rev 2000;16(Suppl. 1):S11–S15. [DOI] [PubMed] [Google Scholar]
- 10.Ness J, Aronow WS, Newkirk E, McDanel D. Prevalence of symptomatic peripheral arterial disease, modifiable risk factors, and appropriate use of drugs in the treatment of peripheral arterial disease in older persons seen in a university general medicine clinic. J Gerontol A Biol Sci Med Sci 2005;60:255–257. [DOI] [PubMed] [Google Scholar]
- 11.Shimamura M, Nakagami H, Koriyama H, Morishita R. Gene therapy and cell-based therapies for therapeutic angiogenesis in peripheral artery disease. Biomed Res Int 2013;2013:186215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Weel V, van Tongeren RB, van Hinsbergh VW, van Bockel JH, Quax PH. Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann Vasc Surg 2008;22:582–597. [DOI] [PubMed] [Google Scholar]
- 13.Dimmeler S. ATVB in focus: novel mediators and mechanisms in angiogenesis and vasculogenesis. Arterioscler Thromb Vasc Biol 2005;25:2245. [DOI] [PubMed] [Google Scholar]
- 14.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res 2001;49:507–521. [DOI] [PubMed] [Google Scholar]
- 15.van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res 2001;49:543–553. [DOI] [PubMed] [Google Scholar]
- 16.Ruiter MS, van Golde JM, Schaper NC, Stehouwer CD, Huijberts MS. Diabetes impairs arteriogenesis in the peripheral circulation: review of molecular mechanisms. Clin Sci (Lond) 2010;119:225–238. [DOI] [PubMed] [Google Scholar]
- 17.Stabile E, Kinnaird T, la Sala A, Hanson SK, Watkins C, Campia U, Shou M, Zbinden S, Fuchs S, Kornfeld H, Epstein SE, Burnett MS. CD8+ T lymphocytes regulate the arteriogenic response to ischemia by infiltrating the site of collateral vessel development and recruiting CD4+ mononuclear cells through the expression of interleukin-16. Circulation 2006;113:118–124. [DOI] [PubMed] [Google Scholar]
- 18.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 2005;353:999–1007. [DOI] [PubMed] [Google Scholar]
- 19.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation 2002;106:2781–2786. [DOI] [PubMed] [Google Scholar]
- 20.Ribatti D, Nico B, Crivellato E, Vacca A. Endothelial progenitor cells in health and disease. Histol Histopathol 2005;20:1351–1358. [DOI] [PubMed] [Google Scholar]
- 21.Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, Imada T, Iwasaka T, Matsubara H. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res 2003;93:980–989. [DOI] [PubMed] [Google Scholar]
- 22.Shen X, Carlstrom M, Borniquel S, Jadert C, Kevil CG, Lundberg JO. Microbial regulation of host hydrogen sulfide bioavailability and metabolism. Free Radic Biol Med 2013;60:195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen X, Pattillo CB, Pardue S, Bir SC, Wang R, Kevil CG. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic Biol Med 2011;50:1021–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bir SC, Pattillo CB, Pardue S, Kolluru GK, Docherty J, Goyette D, Dvorsky P, Kevil CG. Nitrite anion stimulates ischemic arteriogenesis involving NO metabolism. Am J Physiol Heart Circ Physiol 2012;303:H178–H188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francke A, Herold J, Weinert S, Strasser RH, Braun-Dullaeus RC. Generation of mature murine monocytes from heterogeneous bone marrow and description of their properties. J Histochem Cytochem 2011;59:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi L, Rossi F. Purification of progenitors from skeletal muscle. J Vis Exp 2011;2476 doi:10.3791/2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozuyaman B, Ebner P, Niesler U, Ziemann J, Kleinbongard P, Jax T, Godecke A, Kelm M, Kalka C. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thromb Haemost 2005;94:770–772. [DOI] [PubMed] [Google Scholar]
- 28.Polhemus DJ, Kondo K, Bhushan S, Bir SC, Kevil CG, Murohara T, Lefer DJ, Calvert JW. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ Heart Fail 2013;6:1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol 2003;23:1143–1151. [DOI] [PubMed] [Google Scholar]
- 30.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol 2002;157:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoefer IE, Grundmann S, van Royen N, Voskuil M, Schirmer SH, Ulusans S, Bode C, Buschmann IR, Piek JJ. Leukocyte subpopulations and arteriogenesis: specific role of monocytes, lymphocytes and granulocytes. Atherosclerosis 2005;181:285–293. [DOI] [PubMed] [Google Scholar]
- 32.Tang GL, Chang DS, Sarkar R, Wang R, Messina LM. The effect of gradual or acute arterial occlusion on skeletal muscle blood flow, arteriogenesis, and inflammation in rat hindlimb ischemia. J Vasc Surg 2005;41:312–320. [DOI] [PubMed] [Google Scholar]
- 33.Tressel SL, Kim H, Ni CW, Chang K, Velasquez-Castano JC, Taylor WR, Yoon YS, Jo H. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler Thromb Vasc Biol 2008;28:1989–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shireman PK. The chemokine system in arteriogenesis and hind limb ischemia. J Vasc Surg 2007;45(Suppl. A):A48–A56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang HT, Yan Z, Abraham JA, Terjung RL. VEGF(121)- and bFGF-induced increase in collateral blood flow requires normal nitric oxide production. Am J Physiol Heart Circ Physiol 2001;280:H1097–H1104. [DOI] [PubMed] [Google Scholar]
- 36.Kumar D, Branch BG, Pattillo CB, Hood J, Thoma S, Simpson S, Illum S, Arora N, Chidlow JH Jr, Langston W, Teng X, Lefer DJ, Patel RP, Kevil CG. Chronic sodium nitrite therapy augments ischemia-induced angiogenesis and arteriogenesis. Proc Natl Acad Sci U S A 2008;105:7540–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Soud HM, Feldman PL, Clark P, Stuehr DJ. Electron transfer in the nitric-oxide synthases. Characterization of l-arginine analogs that block heme iron reduction. J Biol Chem 1994;269:32318–32326. [PubMed] [Google Scholar]
- 38.Furfine ES, Harmon MF, Paith JE, Garvey EP. Selective inhibition of constitutive nitric oxide synthase by l-NG-nitroarginine. Biochemistry 1993;32:8512–8517. [DOI] [PubMed] [Google Scholar]
- 39.Garvey EP, Tuttle JV, Covington K, Merrill BM, Wood ER, Baylis SA, Charles IG. Purification and characterization of the constitutive nitric oxide synthase from human placenta. Arch Biochem Biophys 1994;311:235–241. [DOI] [PubMed] [Google Scholar]
- 40.Amin A, Choi SK, Osman-Elazeik Y, Badr El-Din NK, Kevil CG, Navar LG, Kadowitz P, Trebak M, Matrougui K. Sodium nitrite therapy rescues ischemia-induced neovascularization and blood flow recovery in hypertension. Pflugers Arch 2012;464:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kevil CG, Kolluru GK, Pattillo CB, Giordano T. Inorganic nitrite therapy: historical perspective and future directions. Free Radic Biol Med 2011;51:576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai WJ, Wang MJ, Moore PK, Jin HM, Yao T, Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res 2007;76:29–40. [DOI] [PubMed] [Google Scholar]
- 43.Coletta C, Papapetropoulos A, Erdelyi K, Olah G, Modis K, Panopoulos P, Asimakopoulou A, Gero D, Sharina I, Martin E, Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A 2012;109:9161–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res 2014;114:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King AL, Polhemus DJ, Bhushan S, Otsuka H, Kondo K, Nicholson CK, Bradley JM, Islam KN, Calvert JW, Tao YX, Dugas TR, Kelley EE, Elrod JW, Huang PL, Wang R, Lefer DJ. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc Natl Acad Sci U S A 2014;111:3182–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, Grinsfelder DB, Condit ME, Lefer DJ. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol 2012;302:H2410–H2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 2013;18:1906–1919. [DOI] [PubMed] [Google Scholar]
- 48.Cirino G, Distrutti E, Wallace JL. Nitric oxide and inflammation. Inflamm Allergy Drug Targets 2006;5:115–119. [DOI] [PubMed] [Google Scholar]
- 49.Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric Oxide 2002;7:1–10. [DOI] [PubMed] [Google Scholar]
- 50.Bergmann CE, Hoefer IE, Meder B, Roth H, van Royen N, Breit SM, Jost MM, Aharinejad S, Hartmann S, Buschmann IR. Arteriogenesis depends on circulating monocytes and macrophage accumulation and is severely depressed in op/op mice. J Leukoc Biol 2006;80:59–65. [DOI] [PubMed] [Google Scholar]
- 51.Sager HB, Middendorff R, Rauche K, Weil J, Lieb W, Schunkert H, Ito WD. Temporal patterns of blood flow and nitric oxide synthase expression affect macrophage accumulation and proliferation during collateral growth. J Angiogenes Res 2010;2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fung E, Helisch A. Macrophages in collateral arteriogenesis. Front Physiol 2012;3:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Francke A, Weinert S, Strasser RH, Braun-Dullaeus RC, Herold J. Transplantation of bone marrow derived monocytes: a novel approach for augmentation of arteriogenesis in a murine model of femoral artery ligation. Am J Transl Res 2013;5:155–169. [PMC free article] [PubMed] [Google Scholar]
- 54.Scholz D, Ito W, Fleming I, Deindl E, Sauer A, Wiesnet M, Busse R, Schaper J, Schaper W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch 2000;436:257–270. [DOI] [PubMed] [Google Scholar]
- 55.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional fifferentiation. Front Immunol 2014;5:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 2014;17:109–118. [DOI] [PubMed] [Google Scholar]