Abstract
Purpose
To evaluate the effect and tolerance of oral mineralocorticoid antagonists, eplerenone and/or spironolactone, in recalcitrant central serous chorioretinopathy.
Methods
Retrospective consecutive observational case series. Primary outcome measures included central macular thickness (CMT, μm), macular volume (MV, mm3), Snellen visual acuity, and prior treatment failures. Secondary outcomes included duration of treatment, treatment dosage, and systemic side effects.
Results
A total of 120 patients with central serous chorioretinopathy were reviewed, of which 29 patients were treated with one or more mineralocorticoid antagonists. The average age of patients was 58.4 years. Sixteen patients (69.6%) were recalcitrant to other interventions prior to treatment with oral mineralocorticoid antagonists, with an average washout period of 15.3 months. The average duration of mineralocorticoid antagonist treatment was 3.9±2.3 months. Twelve patients (52.2%) showed decreased CMT and MV, six patients (26.1%) had increase in both, and five patients (21.7%) had negligible changes. The mean decrease in CMT of all patients was 42.4 μm (range, −136 to 255 μm): 100.7 μm among treatment-naïve patients, and 16.9 μm among recalcitrant patients. The mean decrease in MV of all patients was 0.20 mm3 (range, −2.33 to 2.90 mm3): 0.6 mm3 among treatment-naïve patients, and 0.0 mm3 among recalcitrant patients. Median visual acuity at the start of therapy was 20/30 (range, 20/20–20/250), and at final follow-up it was 20/40 (range, 20/20–20/125). Nine patients (39.1%) experienced systemic side effects, of which three patients (13.0%) were unable to continue therapy.
Conclusion
Mineralocorticoid antagonist treatment had a positive treatment effect in half of our patients. The decrease in CMT and MV was much less in the recalcitrant group compared to the treatment-naïve group. An improvement in vision was seen only in the treatment-naïve group. Systemic side effects, even at low doses, may limit its usage in some patients.
Keywords: central serous chorioretinopathy, mineralocorticoid antagonist, eplerenone, spironolactone, corticosteroids, central macular thickness, macular volume
Introduction
Central serous chorioretinopathy (CSCR) typically causes transitory central vision loss in young adult men, with an incidence of approximately one per 10,000.1,2 It usually causes serous neurosensory detachment of the macula associated with leakage at the level of the retinal pigment epithelium. Analyses of enhanced depth imaging optical coherence tomography (EDI-OCT) images in patients with CSCR have demonstrated diffuse choroidal thickening, which suggests that the pathogenesis of the disease may involve choroidal vascular stress on the retinal pigment epithelium.3–5 Indocyanine green angiography demonstrates choroidal vascular hyperpermeability in affected and contralateral eyes.6,7
CSCR has been associated with exogenous use of corticosteroids (eg, oral, intravenous, inhaled, intranasal, intramuscular, and topical routes) as well as increased endogenous levels (eg, hypercortisolism, type A personality, pregnancy, and stressful life events).8–12 Corticosteroids include cortisol and aldosterone, as well as their agonists (ie, glucocorticoid and mineralocorticoids, respectively), which are synthesized from cholesterol in the adrenal cortex. Glucocorticoids bind to both the glucocorticoid receptor and the mineralocorticoid receptor. The mineralocorticoid receptor has similar high affinity for aldosterone and glucocorticoids that largely prevail in the plasma.13–15 Prior experiments in rodents by Zhao et al14 suggested that intravitreal injection of high dose glucocorticoids induces choroidal vessel dilation and leakage. Interestingly, the same effect was elicited using aldosterone, a specific mineralocorticoid receptor activator. Mineralocorticoid receptor antagonists reversed this effect, and thus it has been hypothesized that CSCR may result from excessive occupancy of mineralocorticoid receptors by glucocorticoids.15
Two mineralocorticoid-specific receptor antagonists are commercially available and US Food and Drug Administration (FDA)-approved for various systemic diseases. The first, spironolactone (Aldactone®, Pfizer, Inc., New York, NY, USA) is commonly used for treating hyperaldosteronism, hypertension, and congestive heart failure. While effective, it may cause unwanted progestational and antiandrogenic side effects, manifested as gynecomastia, abnormal menstrual cycles, and impotence, which may limit its use. The second, eplerenone (Inspra®, Pfizer, Inc.), is similar except that the 17α-thoacetyl group of spironolactone is replaced with a carbomethoxy group, resulting in lower affinity for other steroid receptors (such as those for progesterone and androgen), which may improve tolerability or specificity.16 In vitro receptor-binding studies have revealed that eplerenone has an affinity for the aldosterone receptor that is approximately 10- to 20-fold less than spironolactone; however, both compounds show similar efficacy in blocking the aldosterone-mediated changes in urinary Na:K ratio in rats and humans.16 In this report, we evaluated the effect of both these mineralocorticoid antagonists in patients with CSCR as well as the frequency of their side effects.
Patients and methods
We first used mineralocorticoid antagonists for the treatment of CSCR in January 2012. We therefore conducted a consecutive retrospective observational case series in patients who were diagnosed with CSCR between January 1, 2012 and September 1, 2014 at our institution. Prior to data collection, the study received University of Iowa institutional review approval. All investigations of this study adhered to the tenets of the Declaration of Helsinki. A comparison between treatment-naïve patients and those previously treated for a prior exacerbation of CSCR (deemed “recalcitrant”) was made. The washout period was defined as the time (months) between prior alternative treatment and initiation of oral mineralocorticoid antagonists. Only patients treated with oral mineralocorticoid antagonists (eplerenone and/or spironolactone) were included, and a minimum of 1 month of mineralocorticoid antagonist treatment was required. Patients who were lost to follow-up or who had comorbid macular disease were excluded. Patients who did not have imaging using the same OCT device at follow-up were also excluded. A review of the patients’ past medical history, past ocular history, medications, ophthalmologic examination, fundus photos, fundus fluorescein angiograms (when available), and OCT imaging were reviewed to exclude known conditions, such as renal failure, pregnancy, uncontrolled idiopathic systemic hypertension, or other causes of macular disease, that may have caused serous detachments. The patient tolerability of the oral mineralocorticoid therapy was evaluated based on history and documentation available throughout treatment.
The central macular thickness (CMT) and macular volume (MV) were determined by the Spectralis® Heidelberg OCT. All images were reviewed to ensure that the segmentation software was accurate and of adequate quality. The change associated with therapy was defined as the difference in measurement at the start of therapy minus either treatment cessation or most recent follow-up while on therapy at the time of analysis. Negligible change was defined as a difference of zero, or when one value (ie, CMT or MV) was positive and the other was negative. The best-corrected visual acuity (BCVA, Snellen), average duration of treatment (months), treatment dosage (mg), tolerable and intolerable systemic side effects, and prior treatment failures were reviewed. In cases of bilateral disease, only the more severe eye was included as the study eye for statistical analysis. Results of age, follow-up, and duration of treatment are given as mean ± standard deviation.
Results
A total of 120 patients were diagnosed with CSCR between January 1, 2012 and September 1, 2014, of which 29 patients were treated with oral mineralocorticoid antagonists. Three patients were excluded because they were lost to follow-up or were seen by their local referring ophthalmologist. Two patients were excluded because OCT measurements done at initial presentation were with a device other than the Spectralis® Heidelberg machine (Heidelberg Engineering, Carlsbad, CA, USA). One patient was excluded because of an alternative diagnosis of idiopathic polypoidal choroidal vasculopathy.
The demographics of the 23 patients who met our inclusion criterion are summarized in Table 1. Prior treatment failures are outlined in Table 1, and the average washout period for the recalcitrant group was 15.3 months. The average duration of treatment of oral mineralocorticoid antagonists was 3.9±2.3 months (range, 1–8.5 months). Treatment dose for both eplerenone and spironolactone ranged from 25 to 50 mg twice-daily. The BCVA and OCT findings (ie, CMT and MV) for all patients at the start of therapy and at final follow-up can be found in Table 2. In the treatment-naïve group, the median BCVA at baseline was 20/30 and at final follow-up was 20/20. In the recalcitrant group, the median BCVA at baseline was 20/30 and at final follow-up was 20/45.
Table 1.
Summary of patient demographics and history
| Number of patients treated with mineralocorticoid antagonists | N=23 |
| Average age (years) | 58.4±10.5 |
| Number of males | 15 (65.2%) |
| Bilateral disease | 7 (30.4%) |
| Prior treatment failure(s): | 16 (69.6%) |
| • Intravitreal anti-VEGF agents | 7 |
| • Ketoconazole | 4 |
| • Photodynamic therapy | 5 |
| • Focal thermal laser | 3 |
| • Rifampin | 2 |
| History of steroid usage (oral, intravenous, topical, inhaled) | 9 (39.1%) |
| History of systemic hypertension | 12 (52.2%) |
Abbreviation: VEGF, vascular endothelial growth factor.
Table 2.
Visual and anatomical outcome measures of the study eyea
| Treatment | Number of patients | Median BCVA at baseline | Median BCVA at final follow-up | Mean CMT (μm) at baseline | Mean CMT (μm) at final follow-up | Mean MV (mm3) at baseline | Mean MV (mm3) at final follow-up |
|---|---|---|---|---|---|---|---|
| Eplerenone only | 15 | 20/30 | 20/30 | 387.5 | 352.5 | 8.7 | 8.6 |
| Spironolactone only | 3 | 20/30 | 20/40 | 359.3 | 274.3 | 7.6 | 7.2 |
| Eplerenone, followed by spironolactone | 5 | 20/50 | 20/50 | 373.0 | 333.8 | 8.7 | 8.4 |
| All patients | 23 | 20/30 | 20/40 | 376.2 | 335.2 | 8.6 | 8.4 |
Notes:
Study eye was the eye with most severe OCT findings when bilateral disease was present. Final follow-up is defined as time of cessation of therapy, or at the soonest follow-up if ongoing therapy.
Abbreviations: BCVA, best-corrected visual acuity (Snellen); CMT, central macular thickness; MV, macular volume; OCT, optical coherence tomography.
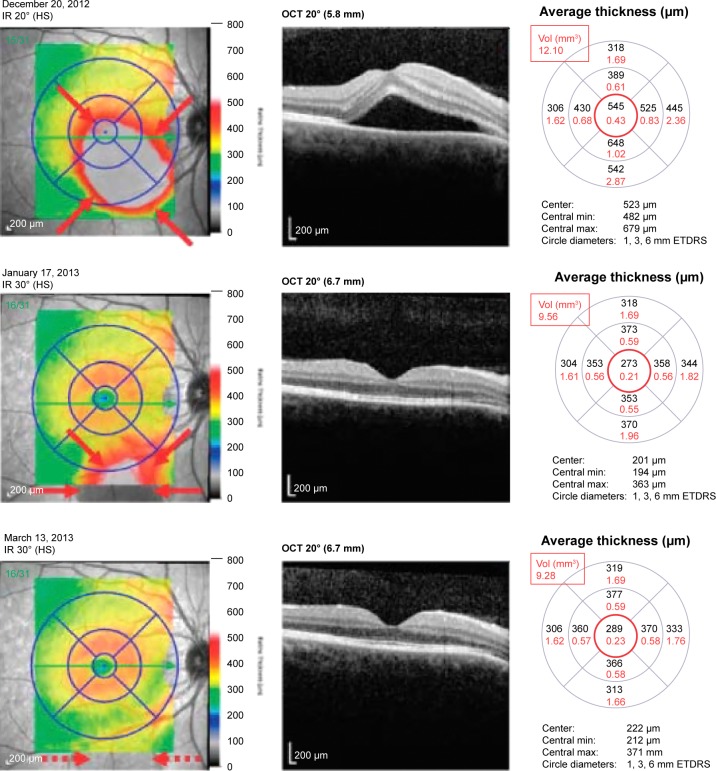
A detailed analysis of the change (Δ, final – initial) in CMT and MV for all patients can be found in Table 3. There was a modest decrease in the mean CMT and MV in all groups, although it was greatest in the group treated with only spironolactone. Twelve patients (52.2%) had a decrease in both CMT and MV measurements, six patients (26.1%) had an increase in both, and five patients (21.7%) had negligible changes. For the entire group of treated patients, there was a mean decrease of 42.4 μm in CMT and 0.20 mm3 in MV. Those patients who were treatment-naïve for CSCR are indicated by an asterisk in Table 3; the mean decrease in CMT in this group was 100.7 μm and the mean decrease in MV was 0.6 mm3. Among the recalcitrant group, the mean decrease in CMT was only 16.9 μm, and the mean change in MV was 0.0 mm3. Figures 1 and 2 demonstrate two patients who had a positive and negligible response to treatment, respectively. The patient tolerability of the oral mineralocorticoid therapy can be found in Table 4. None of these were organ or life threatening; however, two patients on eplerenone and two patients on spironolactone required cessation of treatment due to systemic side effects. Any patient-concern or side effect(s) that required drug cessation was declared “intolerable”, whereas those that did not require drug cessation was “tolerable”.
Table 3.
Decrease (Δ, initial – final) in CMT and MV for all patients treated with oral mineralocorticoid antagonists, sorted primarily by CMT, and secondarily by MV
| Patient number | Treatment | Duration of treatment (months) | ΔCMT (μm) | ΔMV (mm3) |
|---|---|---|---|---|
| 1a | E | 7 | 255 | 2.90 |
| 2 | S | 1.5 | 232 | 0.84 |
| 3 | ES | 6.5 | 221 | 1.38 |
| 4a | E | 2 | 180 | 1.18 |
| 5a | E | 1 | 164 | 0.43 |
| 6a | E | 1 | 85 | 0.11 |
| 7 | E | 3.5 | 53 | 0.05 |
| 8a | ES | 2 | 45 | 0.29 |
| 9 | E | 6 | 30 | 0.13 |
| 10 | E | 2.5 | 28 | 0.51 |
| 11 | S | 8 | 22 | 0.24 |
| 12 | E | 6 | 9 | 0.01 |
| 13 | ES | 3 | 9 | −0.07 |
| 14 | E | 3 | 8 | −0.03 |
| 15 | E | 1 | 3 | 0 |
| 16 | S | 2 | 1 | 0 |
| 17a | E | 3 | −2 | −0.03 |
| 18a | E | 4 | −22 | −0.17 |
| 19 | E | 3 | −22 | −0.69 |
| 20 | ES | 7 | −36 | 0.02 |
| 21 | ES | 8.5 | −43 | 0.23 |
| 22 | E | 4 | −109 | −2.33 |
| 23 | E | 3 | −136 | −0.35 |
| Mean ± SD | 3.9±2.3 | 42.4±103.3 | 0.20±0.91 |
Notes:
Patients who had no prior treatment for CSCR (ie, treatment-naïve). Green denotes positive decrease in both CMT and MV, yellow denotes equivocal with negligible change in CMT and/or MV, red denotes increase in both CMT and MV.
Abbreviations: CMT, central macular thickness; MV, macular volume; E, eplerenone; S, spironolactone; ES, eplerenone, followed by spironolactone; SD, standard deviation; CSCR, central serous chorioretinopathy.
Figure 1.
Patient with CSCR, treated with eplerenone 50 mg twice-daily.
Notes: Rapid resolution of SRF (borders highlighted with red arrows) is measured by a decrease in central macular thickness (CMT, red circle) and macular volume (MV, red rectangle) measurements from December 20, 2012 to January 17, 2013. Ongoing improvement of the eccentric SRF (inferiorly along the arcades, dashed red arrows) on March 13, 2013 can best be followed via MV.
Abbreviations: Vol, volume; min, minimum; max, maximum; ETDRS, Early Treatment Diabetic Retinopathy Study; IR, infrared radiation; OCT, optical coherence tomography; HS, high-sensitivity; CSCR, central serous chorioretinopathy; CMT, central macular thickness; MV, macular volume; SRF, subretinal fluid.
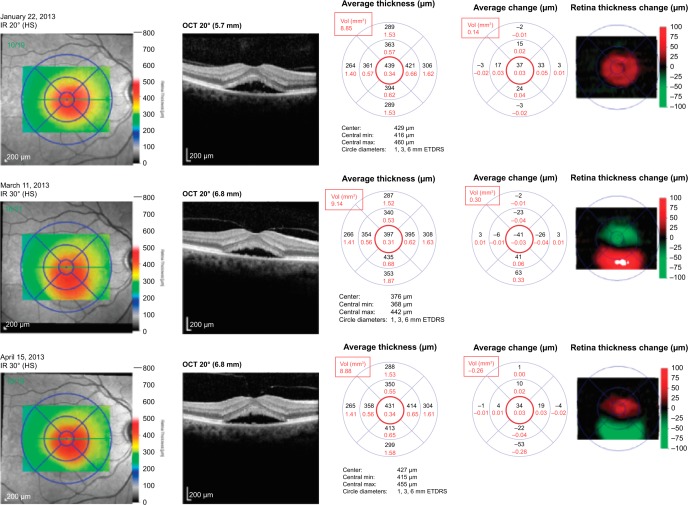
Figure 2.
Patient with CSCR, treated with eplerenone 25 mg twice-daily from January to March 2013.
Notes: The dosage was increased to eplerenone 50 mg twice-daily from March to April 2013, with overall negligible change in CMT (red circle) and MV (red rectangle) throughout the course of treatment.
Abbreviations: Vol, volume; min, minimum; max, maximum; ETDRS, Early Treatment Diabetic Retinopathy Study; IR, infrared radiation; OCT, optical coherence tomography; HS, high-sensitivity; CSCR, central serous chorioretinopathy; CMT, central macular thickness; MV, macular volume.
Table 4.
Tolerable and intolerable side effects of mineralocorticoid antagonists in our cohort
| Treatment | Tolerable side effects | Intolerable side effects |
|---|---|---|
| Eplerenone | N=3 (13.0%) | N=2 (8.7%) |
| • Fatigue/malaise | • Constipation | |
| • Leg cramps | • Thirst/dehydration | |
| Spironolactone | N=2 (8.7%) | N=2 (8.7%) |
| • Fatigue/malaise | • Fatigue/malaise | |
| • Gynecomastia | • Libido | |
| • Orthostatic hypotension | • Cough and emesis |
Discussion
Oral mineralocorticoid antagonists have been proposed as a treatment for CSCR. In our cohort, we found an average decrease in CMT of only 42 μm at an average follow-up of 3.9 months. We found that approximately half of our patients responded positively to treatment based on both CMT and MV from the Spectralis® Heidelberg OCT as shown in Figures 1 and 2. More specifically, those patients who were treatment-naïve had a much greater decrease in CMT compared to the group of patients that were recalcitrant to other therapies. Given that seven patients (30.4%) had bilateral disease, and 16 patients (69.6%) had prior treatment failures at our tertiary care center, our cohort represents recalcitrant cases of CSCR that may be unresponsive to other therapies.
Our results are comparable to a prospective nonrandomized pilot study by Bousquet et al17 in France, which administered eplerenone 25 mg daily for 1 week, followed by twice-daily dosing (or 50 mg per day) for 1 or 3 months. Their study included 13 patients, of whom nine had prior steroid usage, one had hypertension, and only one patient had congenital kidney anomalies. In contrast to our cohort, patients with prior treatment with photodynamic therapy or anti-vascular endothelial growth factor (VEGF) therapy were excluded from the Bousquet et al study.17 They found a greater improvement in visual acuity and reduction in CMT and subretinal fluid (SRF), most likely because their population did not include recalcitrant cases of CSCR. Specifically, the mean CMT in their study decreased from 352 μm at baseline to 246 and 189 μm at 1 and 3 months, respectively, while on oral eplerenone 50 mg per day. In our cohort, the subset of patients who responded positively (ie, a decrease in both CMT and MV) had an average decrease in CMT of 110 μm; however, the mean CMT at the start of therapy was only 295 μm in this group, which may have limited the potential decrease in CMT. Our study is also distinct in that spironolactone was used and evaluated as an alternative mineralocorticoid antagonist, which has a 10- to 20-fold greater binding affinity in comparison to eplerenone.16 For both agents, we advanced the dosage as tolerated, typically with a starting dose of 25 mg twice-daily and up to 50 mg twice-daily.
Despite prior reports,15,17,18 our patients had both tolerable and intolerable side effects seen with both eplerenone and spironolactone (Table 4). Although there is a difference in selectivity for the mineralocorticoid receptor and mechanisms outlined previously, we found no significant difference in tolerability between eplerenone and spironolactone. One patient had intolerable cough that was seen only while on spironolactone therapy. This resolved with cessation of this medication, and interestingly the patient did not have this side effect while taking losartan–hydrochlorothiazide for hypertension. Results of routine, basic metabolic panels were not readily available for review; however, we suspect that leg cramps or fatigue may partly relate to mild electrolyte imbalances. Throughout treatment, frequent correspondence was made with the patients’ outside primary care provider and/or subspecialists, such that other comorbidities such as renal function and hypertension (which affected 52.2% of our cohort) could be monitored and adjusted appropriately. If a patient also had hypertension, we would routinely check the blood pressure in the clinic and correspond with the primary team regarding the initiation of a mineralocorticoid antagonist as either a replacement or supplement to their current oral antihypertensive agents.
Our study has limitations, including its retrospective nature, small sample size, absence of choroidal thickness measurements via EDI-OCT, and lack of a control group. Our study did not distinguish acute versus chronic disease; however, prior treatment failures with various oral and invasive modalities (such as laser and injections) allude to the chronic and recurrent nature that this cohort represents. For some patients, either a lower dose of one drug may have been used, or the alternative mineralocorticoid antagonist may have not been trialed. We discourage labeling patients as either complete “success” or “failures” of this therapy, as potentially higher doses could have been attempted. Additionally, a dry fovea center (and therefore negligible change in CMT) may not necessarily reflect the positive response to therapy that is best represented by MV in cases of multifocal or eccentric areas of SRF (Figure 1). It is important to look at both the CMT and MV together because the former may correspond to a patient’s visual acuity, whereas the latter may more accurately reflect the total response to this novel treatment.
In summary, both spironolactone and eplerenone may be efficacious in reducing SRF and MV in some patients with CSCR. Although spontaneous resolution or regression may occur in presumed “acute” CSCR,19 it is possible that mineralocorticoid antagonists may show more rapid resolution of SRF, or serve as a prophylactic or adjuvant for recurrent or chronic disease. Since half of our patient responded positively to treatment, perhaps CSCR is a multifactorial disease in which mineralocorticoid receptors play an important part in some but not all patients. A majority of the patients in this study were recalcitrant to other treatments, and the change in CMT and MV was far less than those who were treatment-naïve. In these difficult patients, the drugs rarely achieved the ultimate goal of improving vision or completely resolving subfoveal SRF. Taking into account that the drugs are well tolerated, however, they should be considered as a potential first-line treatment. Further prospective, randomized control studies should be designed to determine the optimal dose and duration of treatment with these agents.
Footnotes
Disclosure
This project was supported by an unrestricted grant from the VitreoRetinal Surgery Foundation (VRSF). The authors report no other conflicts of interest in this work.
References
- 1.Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980–2002. Ophthalmology. 2008;115(1):169–173. doi: 10.1016/j.ophtha.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol. 2008;86(2):126–145. doi: 10.1111/j.1600-0420.2007.00889.x. [DOI] [PubMed] [Google Scholar]
- 3.Imamura Y, Fujiwara T, Margolis R, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in central serous chorioretinopathy. Retina. 2009;29(10):1469–1473. doi: 10.1097/IAE.0b013e3181be0a83. [DOI] [PubMed] [Google Scholar]
- 4.Jirarattanasopa P, Ooto S, Tsujikawa A, et al. Assessment of macular choroidal thickness by optical coherence tomography and angiographic changes in central serous chorioretinopathy. Ophthalmology. 2012;119(8):1666–1678. doi: 10.1016/j.ophtha.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 2011;31(8):1603–1608. doi: 10.1097/IAE.0b013e31820f4b39. [DOI] [PubMed] [Google Scholar]
- 6.Spaide RF, Hall L, Haas A, et al. Indocyanine green videoangiography of older patients with central serous chorioretinopathy. Retina. 1996;16(3):203–213. doi: 10.1097/00006982-199616030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Iida T, Kishi S, Hagimura N, Shimizu K. Persistent and bilateral choroidal vascular abnormalities in central serous chorioretinopathy. Retina. 1999;19(6):508–512. doi: 10.1097/00006982-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24(12):1743–1756. doi: 10.1038/eye.2010.130. [DOI] [PubMed] [Google Scholar]
- 9.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47(5):431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho-Recchia CA, Yannuzzi LA, Negrão S, et al. Corticosteroids and central serous chorioretinopathy. Ophthalmology. 2002;109(10):1834–1837. doi: 10.1016/s0161-6420(02)01117-x. [DOI] [PubMed] [Google Scholar]
- 11.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol. 1999;128(1):63–68. doi: 10.1016/s0002-9394(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 12.Haimovici R, Koh S, Gagnon DR, et al. Risk factors for central serous chorioretinopathy: a case-control study. Ophthalmology. 2004;111(2):244–249. doi: 10.1016/j.ophtha.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 13.Farman N, Rafestin-Oblin ME. Multiple aspects of mineralocorticoid selectivity. Am J Physiol Renal Physiol. 2001;280(2):F181–F192. doi: 10.1152/ajprenal.2001.280.2.F181. [DOI] [PubMed] [Google Scholar]
- 14.Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Müller glial cells. FASEB J. 2010;24(9):3405–3415. doi: 10.1096/fj.09-154344. [DOI] [PubMed] [Google Scholar]
- 15.Zhao M, Célérier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest. 2012;122(7):2672–2679. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delyani JA. Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int. 2000;57(4):1408–1411. doi: 10.1046/j.1523-1755.2000.00983.x. [DOI] [PubMed] [Google Scholar]
- 17.Bousquet E, Beydoun T, Zhao M, et al. Mineralcorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013;33(10):2096–2102. doi: 10.1097/IAE.0b013e318297a07a. [DOI] [PubMed] [Google Scholar]
- 18.Gruszka A. Potential involvement of mineralocorticoid receptor activation in the pathogenesis of central serous chorioretinopathy: case report. Eur Rev Med Pharmacol Sci. 2013;17(10):1369–1373. [PubMed] [Google Scholar]
- 19.Quin G, Liew G, Ho I-V, et al. Diagnosis and interventions for central serous chorioretinopathy: review and update. Clin Experiment Ophthalmol. 2013;41(2):187–200. doi: 10.1111/j.1442-9071.2012.02847.x. [DOI] [PubMed] [Google Scholar]