Abstract
Pneumonia caused by influenza A virus (IAV) can have devastating effects, resulting in respiratory failure and death. The idea that a new influenza pandemic might occur in the near future has triggered renewed interests in IAV infection. The receptor for advanced glycation end products (RAGE) is expressed on different cell types and plays a key role in diverse inflammatory processes. We here investigated the role of RAGE in the host response to IAV pneumonia using wild-type (wt) and RAGE deficient (−/−) mice. Whereas strong RAGE was constitutively expressed in the lungs of uninfected wt mice, in particular on endothelium, IAV pneumonia was associated with enhanced expression on endothelium and de novo expression on bronchial epithelium. Additionally, the high-affinity RAGE ligand high mobility group box 1 was upregulated during IAV pneumonia. RAGE−/− mice were relatively protected from IAV induced mortality and showed an improved viral clearance and enhanced cellular T cell response and activation of neutrophils. These data suggest that RAGE is detrimental during IAV pneumonia.
Keywords: Influenza A virus, Viral pneumonia, Receptor for advanced glycation end products, High mobility group box 1, Host defense
Introduction
Recent outbreaks of highly pathogenic influenza A virus (IAV) infections have had important economic consequences and the notion that a new influenza pandemic might occur in the near future has triggered renewed interest in influenza infection. Influenza pneumonia develops rapidly and can result in respiratory failure and death. 20,000 people die after influenza infection in the United States each year and in the large pandemic of 1918, over 20 million people died worldwide (Cheung et al., 2002; Palese, 2004). Three types of single stranded RNA influenza viruses that can cause upper respiratory tract infection have been described; A, B and C, of which influenza A is clinically the most important (Wright and Webster, 2001). From a preventive and treatment perspective, IAV is regarded as a problem pathogen. Although vaccines and antiviral molecules to control IAV have been developed recently, the disease is by no means under control since these treatments are not available worldwide and their efficacy is not optimal (Kandel and Hartshorn, 2005; Palese, 2004; Hayden, 2004). Moreover, the segmented genome of influenza virus is subject to antigenic drift and shift, which may result in influenza variants that are highly pathogenic for humans (De Jong et al., 2000). Due to these genetic changes vaccine strategies and antiviral therapy may become ineffective. Therefore, insight in factors involved in host defense during IAV could help identify possible new preventive and/or therapeutic targets in this severe infection.
The receptor for advanced glycation end products (RAGE) is a promiscuous receptor that has been shown to be involved in pulmonary inflammation and infection. RAGE can interact with diverse ligands such as high mobility group box (HMGB)1 (Sorci et al., 2004), some members of the S100 family; S100A12 (Moroz et al., 2002), S100B (Valencia et al., 2004) and S100P (Arumugam et al., 2004), advanced glycation end products (Kislinger et al., 1999), amyloid (Yan et al., 2000) and β-sheet fibrils (Yan et al., 1996). RAGE was first identified in lung tissue (Schmidt et al.,1992) and endothelial cells, alveolar and bronchial epithelial and plasmacytoid dendritic cells have been found to express RAGE. Recent studies have confirmed that RAGE is extensively expressed in normal, healthy lungs and that patients with interstitial and postobstructive pneumonia have an increased pulmonary expression of RAGE (Morbini et al., 2006). Recently, we demonstrated pulmonary RAGE upregulation in murine pneumonia caused by Streptococcus (S.) pneumoniae (van Zoelen et al., 2009).
Ligand binding to RAGE leads to sustained receptor-dependent signaling and activation of nuclear factor-κB and mitogen-activated protein kinase pathways. In line, inhibition of RAGE signaling has been found to reduce inflammatory responses in several models, including models of hepatic injury (Cataldegirmen et al., 2005; Zeng et al., 2004; Ekong et al., 2006), diabetic atherosclerosis (Park et al., 1998), type II collagen-induced arthritis (Hofmann et al., 2002) and sepsis (Liliensiek et al., 2004). Given the ubiquitous expression of RAGE in the lung and its upregulation during inflammatory processes and S. pneumoniae pneumonia, we hypothesized that this receptor plays a role in the regulation of lung inflammation during viral infection of the respiratory tract. Therefore, we here sought to determine the role of RAGE in pneumonia caused by IAV. For this RAGE deficient (RAGE−/− mice) were intranasally infected with a mouse adapted IAV and the course of the infection in these mice was compared with that in concurrently infected wild-type (wt) mice.
Results
Influenza A pneumonia results in enhanced RAGE expression in the lungs
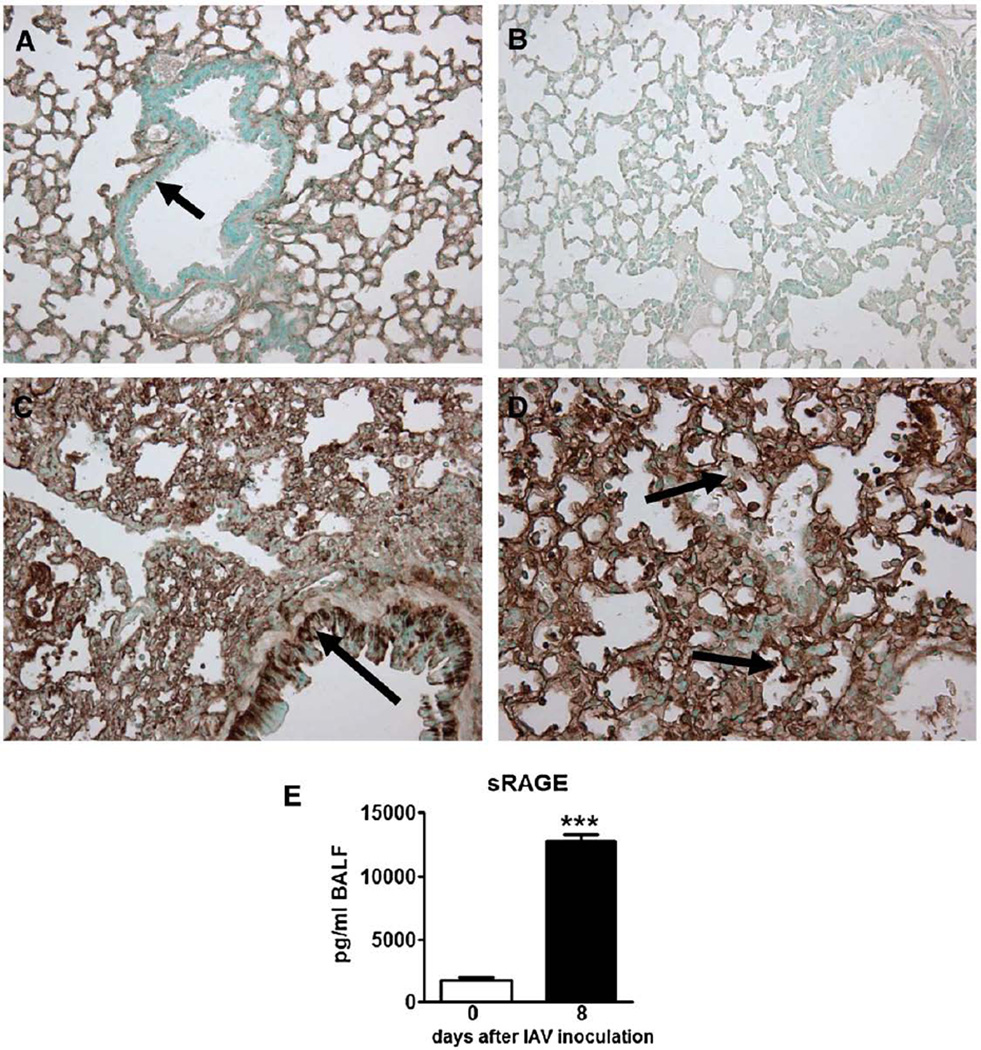
Earlier studies indicated that normal, healthy lungs express RAGE (Cheng et al., 2005; Morbini et al., 2006; Uchida et al., 2006; Wittkowski et al., 2007) and that expression of RAGE in the lungs is increased in patients with interstitial and postobstructive pneumonia (Morbini et al., 2006). We recently demonstrated that RAGE is upregulated during S. pneumoniae pneumonia (van Zoelen et al., 2009). To study whether RAGE expression alters in our IAV pneumonia model, we performed immunohistochemical stainings of RAGE of lung tissue from wt mice after inoculation with IAV. In accordance with the literature (Cheng et al., 2005; Morbini et al., 2006; Uchida et al., 2006; Wittkowski et al., 2007), normal healthy mice displayed broad RAGE staining in their lungs (Fig. 1A). RAGE was predominantly present in the interalveolar septae, showing an endothelial pattern, whereas RAGE was clearly absent in the bronchial epithelium (Fig. 1A, arrow). Immunohistochemical analysis of lungs obtained from RAGE−/− mice, used as negative controls, confirmed the specificity of the RAGE staining (Fig. 1B). Lungs from IAV infected mice showed an upregulation of RAGE expression on endothelial cells (Fig. 1C). Moreover, bronchial epithelial cells displayed de novo expression of RAGE (Fig. 1C, arrow), as did some inflammatory cells (Fig. 1D, arrows). In addition to immunohistochemistry, we measured soluble (s)RAGE in bronchoalveolar lavage fluid (BALF) from healthy wt mice and mice inoculated with IAV. sRAGE levels increased after IAV infection (Fig. 1E, P<0.005).
Fig. 1.
Expression of pulmonary receptor for advanced glycation end products (RAGE) during influenza A virus (IAV) pneumonia. Shown are representative RAGE stainings (original magnification, × 10) of lung tissue (A–D). Normal, uninfected wild-type (wt) mouse (A) displaying extensive RAGE expression in the interalveolar septae with an endothelial pattern. Arrow indicates bronchial epithelium being negative for RAGE staining. Absence of RAGE positivity in the lung of a RAGE−/− mouse (B). Lungs form a wt mouse 8 days after the inoculation of IAV (C and D). Arrow in C indicates bronchial epithelium being positive for RAGE staining (compared with A) and arrows in D indicate inflammatory cells being positive for RAGE staining. Soluble (s)RAGE levels in bronchoalveolar lavage fluid (BALF) from normal, uninfected wt mice (open bar) and from mice 8 days after IAV inoculation (E) (filled bar, n=5–6 mice per group). ***P<0.005, vs. healthy, uninfected mice (Mann–Whitney U test).
HMGB1 is increased during influenza A virus pneumonia
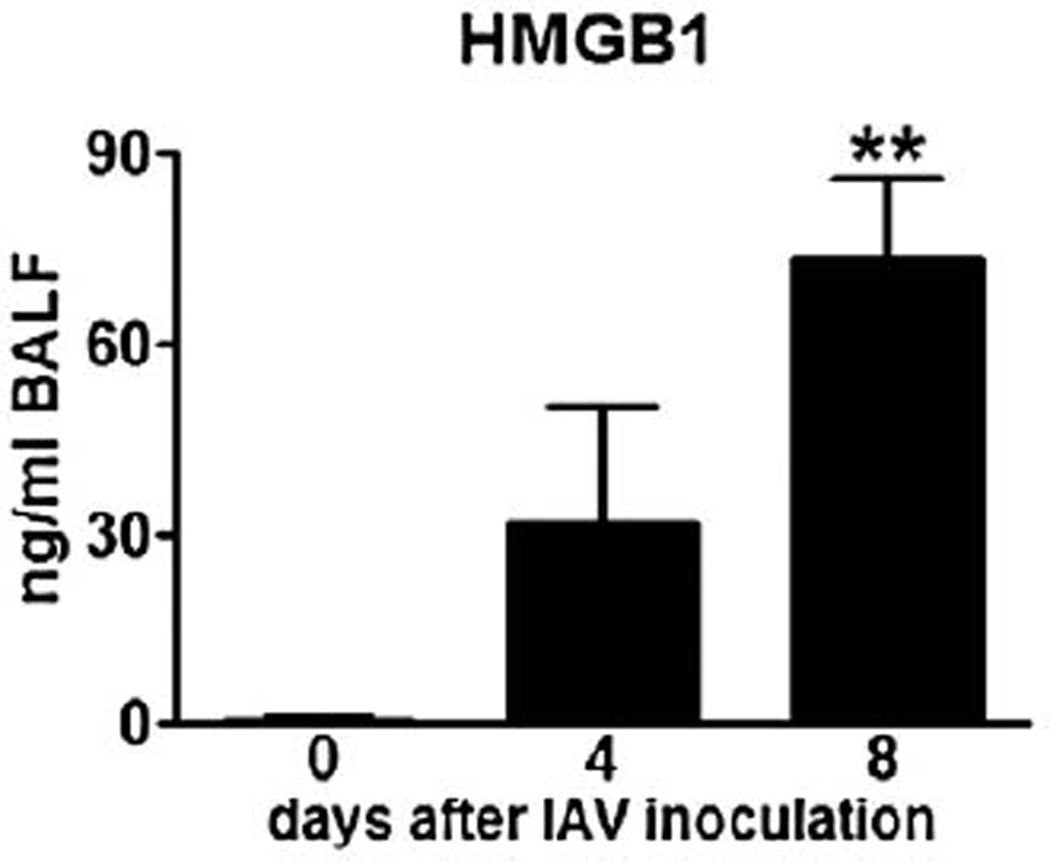
After having shown that RAGE expression is enhanced during IAV pneumonia, we next investigated whether IAV pneumonia is associated with release of its high-affinity ligand HMGB1 (Sorci et al., 2004). We previously demonstrated increased HMGB1 concentrations in BALF from the infected site of patients with pneumonia (van Zoelen et al., 2007), with S. pneumoniae being isolated in 3 out of 4 cases. Relative to healthy controls, mice with pneumonia induced by IAV had elevated HMGB1 levels in BALF at day 4 and day 8 (Fig. 2, P<0.01 at day 8), the former time point not being statistically significant using a small number of mice.
Fig. 2.
High mobility group box (HMGB)1 levels in bronchoalveolar lavage fluid (BALF) from normal, uninfected wt mice (open bar) and from mice 4 and 8 days after influenza A virus inoculation (filled bars, n=4–6 mice per group). **P<0.01, vs. healthy, uninfected mice (Mann–Whitney U test).
RAGE−/− mice demonstrate a delayed mortality after infection with high dose influenza A
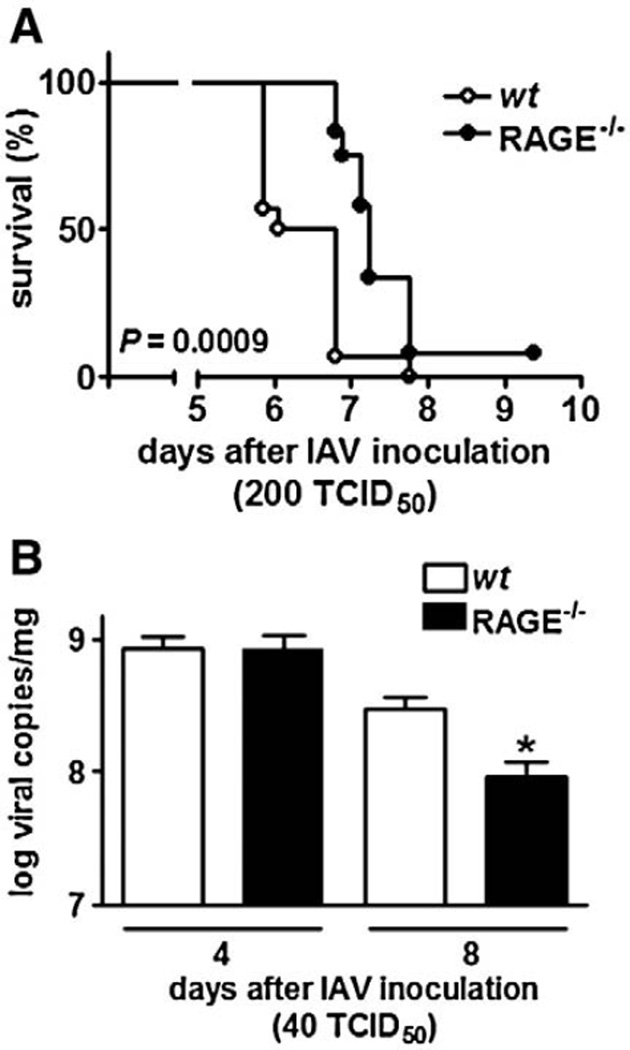
To obtain a first insight in the role of RAGE in the host response to severe influenza infection, RAGE−/− and wt mice were intranasally infected with 200 TCID50 IAV and followed for 10 days (Fig. 3A). This viral dose was uniformly lethal in all mice. However, mortality was significantly delayed in RAGE−/− mice (P=0.0009 vs. wt mice). Indeed, at day 6.5 post infection already 50% of wt mice had succumbed, whereas at this time point all RAGE−/− mice were still alive. At day 7.5 after infection 93% of the wt mice vs. only 67% of RAGE−/− mice were dead. Hence, RAGE deficiency is associated with a relative protection against influenza induced lethality.
Fig. 3.
(A) Receptor for advanced glycation end product deficient (RAGE−/−) mice have a prolonged survival during influenza A virus (IAV) pneumonia. Wild-type (wt; open circles) and RAGE−/− (filled circles) mice (n=12–14 mice/genotype) received 200 TCID50 influenza virus intranasally on day 0. Mice were monitored at least twice a day after inoculation. (B) Receptor for advanced glycation end product (RAGE) deficiency diminishes viral load in the lungs. Viral load in lungs in wild-type (wt; open bars) and RAGE deficient (RAGE−/−; filled bars) mice at 4 and 8 days after 40 TCID50 intranasal oculation with influenza A virus (IAV). Data are means ± SEs (n=6–8 mice/genotype). *P<0.05, vs. wt mice (Kaplan–Meier analysis by log rank test).
RAGE−/− mice have a reduced viral load 8 days after infection with lower dose influenza A
To obtain insight into the role of RAGE in the clearance of IAV from the lungs, wt and RAGE−/− mice were intranasally infected with a lower dose (40 TCID50) to avoid bias due to the accelerated mortality of wt mice and viral loads were determined in lung homogenates obtained 4 and 8 days post infection (Fig. 3B). At day 4, viral loads were similar in the lungs of wt and RAGE−/− mice. At day 8, the number of viral copies had decreased in both mouse strains, but significantly more in RAGE−/− mice (P<0.05 vs. wt mice). Thus, RAGE deficiency is associated with an increased clearance of influenza A from the respiratory tract.
RAGE−/− mice demonstrate more activation of CD4+ and CD8+ T cells in lung infiltrates
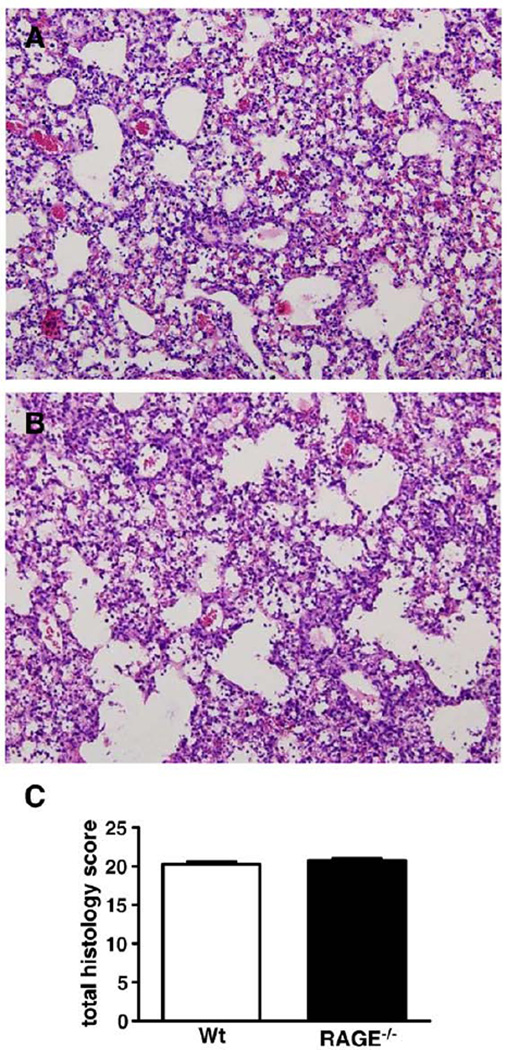
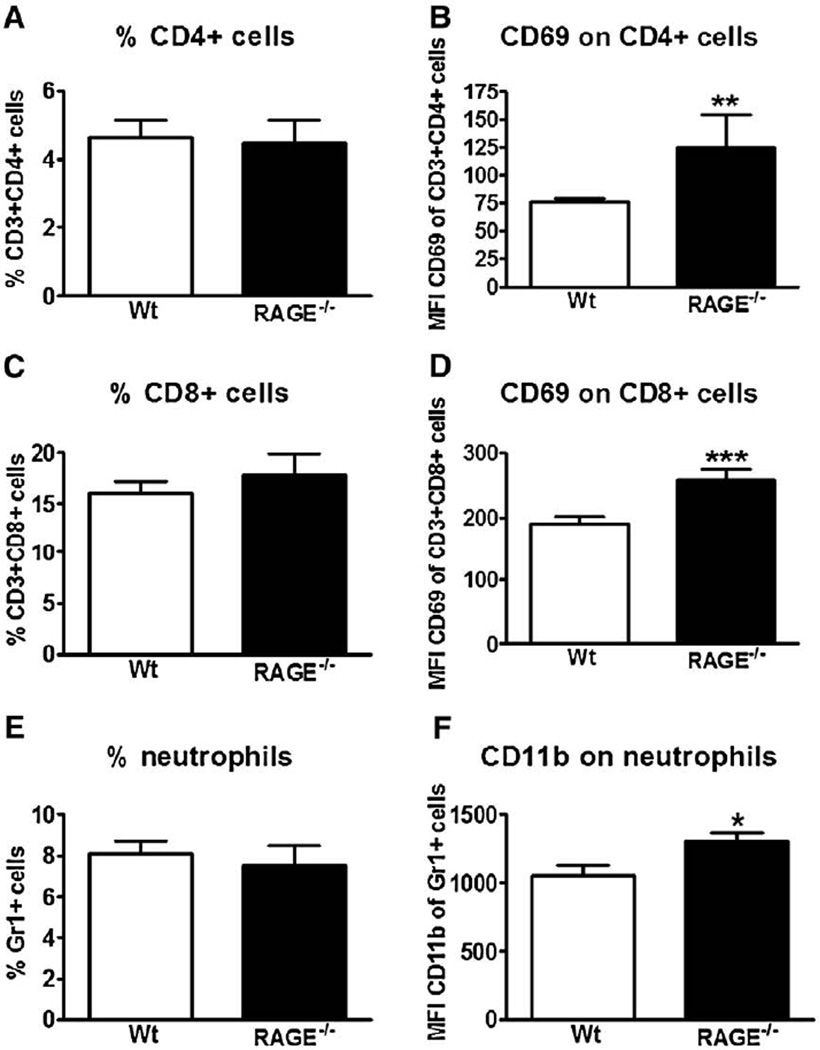
To obtain insight into the role of RAGE in the lung inflammatory response during influenza infection, lungs were harvested from wt and RAGE−/− mice 8 days after inoculation for histopathology. Both mouse strains showed extensive inflammation characterized by severe bronchitis, alveolar inflammation and edema (Figs. 4A and B show representative slides of wt and RAGE−/− mice, respectively). The extent of lung inflammation, as determined by the semiquantitative histology score described in the Materials and methods section, did not differ between wt and RAGE−/− mice (Fig. 4C). Next we compared the cellular composition of pulmonary infiltrates at day 8 by flow cytometry analysis. The percentages of CD4+ and CD8+ T cells, which both are considered important for host defense against influenza infection, were similar in wt and RAGE−/− mice (Figs. 5A and C, respectively). Remarkably, however, RAGE−/− mice displayed a higher expression of the activation marker CD69 on both CD4+ and CD8+ T cells (Figs. 5B and D, both P<0.01 vs. wt mice), indicating that RAGE deficiency resulted in an enhanced activation of T cells in infected lungs. Moreover, whereas the percentages of neutrophils were similar in lungs of wt and RAGE−/− mice, pulmonary neutrophils in RAGE−/− mice demonstrated a relatively enhanced expression of the activation marker CD11b (Figs. 5E and F, P<0.05 vs. wt mice).
Fig. 4.
Lung histopathology. Shown are representative hematoxylin–eosin stainings (original magnification, × 10) of lung tissue of wt (A) and receptor for advanced glycation end product deficient (RAGE−/−; B) mice obtained 8 days after inoculation with influenza A virus (IAV). Total histology scores of the lungs were determined in wt and RAGE−/− mice 8 days after influenza virus inoculation (C) as described in the Materials and methods section. Data are means ± SEs (n=8–9 mice/genotype). *P<0.05, vs. wt mice (Mann–Whitney U test).
Fig. 5.
Receptor for advanced glycation end product deficient (RAGE−/−) mice show enhanced activation of pulmonary T lymphocytes and neutrophils. Wild-type (wt) and RAGE−/− mice were intranasally inoculated with influenza A virus (IAV). After 8 days, lung cell suspensions were collected and flow cytometry was performed as described in the Materials and methods section. Results are represented as percentage of CD4+ (A), CD8+ (C) cells and neutrophils (E) in the lungs and as the mean fluorescence intensity of CD69 surface expression within the CD4+ (B) and CD8+ (D) population and of CD11b surface expression within the Gr1+ population (F). Data are means ± SEs of 8–9 mice/genotype. *P<0.05, vs. wt mice. **P<0.01, vs. wt mice. ***P<0.005, vs. wt mice (Mann–Whitney U test).
Cytokine and chemokine responses
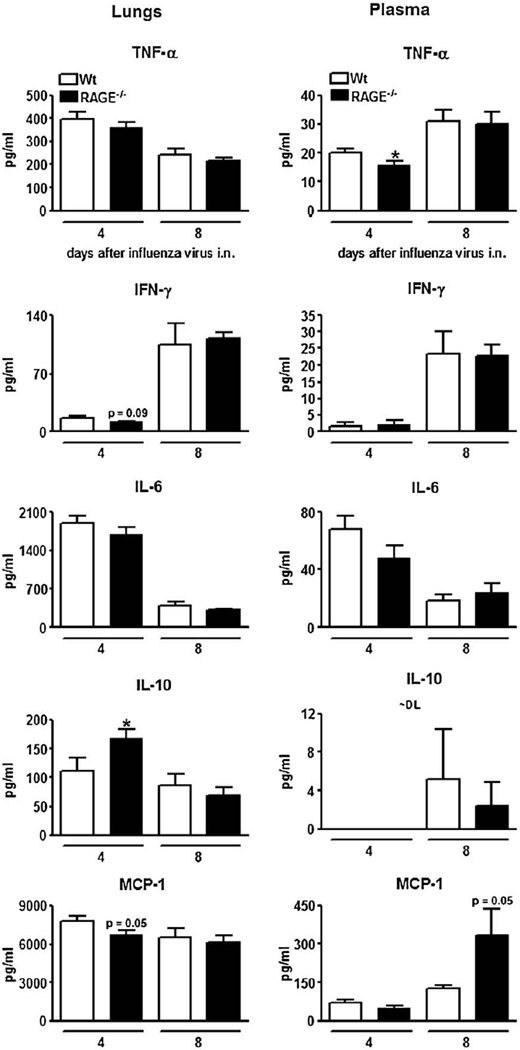
To establish the contribution of RAGE to cytokine and chemokine production during influenza, lung (left panels) and plasma (right panels) concentrations of TNF-α, IFN-γ, IL-6, IL-10 and MCP-1 were measured in wt and RAGE−/− mice 4 and 8 days after infection (Fig. 6). Overall, the levels of these mediators did not differ between the two mouse strains, with the exception of elevated lung IL-10 concentrations and reduced plasma TNF-α concentrations in RAGE−/− mice relative to wt mice at day 4 post infection (both P<0.05). IL-10was not or barely detectable in plasma in both mouse strains.
Fig. 6.
Pulmonary and plasma cytokine concentrations. Cytokine levels in lungs (left panels) and plasma (right panels) from wild-type (wt; open bars) and receptor for advanced glycation end product deficient (RAGE−/−; filled bars) mice 4 and 8 days after inoculation with influenza A virus (IAV). Data are means ± SEs (n=6–8 mice/genotype). *P<0.05, vs. wt mice.
Discussion
IAV is a common cause of upper respiratory tract infection and pneumonia. Host defense against influenza pneumonia is orchestrated by a complex interaction between immune cells and regulatory cytokines. RAGE is a multiligand receptor of the immunoglobulin superfamily that is expressed in all tissues on a wide range of cell types, including cells involved in the innate immune system, e.g. neutrophils, monocytes, macrophages, endothelial and plasmacytoid dendritic cells (Bierhaus et al., 2006; Dumitriu et al., 2005b, 2005a, 2007). Considering its ubiquitous expression in lungs and its established role in inflammation, we here investigated the role of RAGE during pulmonary infection with IAV. Our main finding is that RAGE−/− mice had an increased resistance against influenza pneumonia, as reflected by a delayed mortality associated with an accelerated viral clearance from the lungs.
To the best of our knowledge only one study has been published investigating the role of RAGE in pneumonia. We recently found that RAGE deficiency is beneficial during murine pneumonia induced by S. pneumoniae (van Zoelen et al., 2009). In this report, RAGE−/− mice had a better survival in combination with lower bacterial loads. Part of these results could be explained by a better killing performance of S. pneumoniae by RAGE−/− macrophages. This is the first study focusing on the role of RAGE in viral pneumonia.
Our data on expression of RAGE in the lungs extend earlier reports in finding broad RAGE expression in normal, healthy lungs (Cheng et al., 2005; Morbini et al., 2006; Uchida et al., 2006; Wittkowski et al., 2007) and an upregulation of pulmonary RAGE expression during interstitial and postobstructive pneumonia (Morbini et al., 2006; van Zoelen et al., 2009). In two other studies it was found that full length RAGE expression is not increased during lung inflammation. Acute lung injury in rats induced by intratracheal instillation of LPS did not alter the distribution of RAGE-expressing cells (Uchida et al., 2006). Furthermore, patients with the acute respiratory distress syndrome did not display an upregulation of RAGE expression (Wittkowski et al., 2007). In the present study, we found that IAV pneumonia is associated with an upregulation of RAGE expression on endothelial cells and with de novo expression of RAGE on bronchial epithelial cells.
RAGE can interact with several different ligands such as HMGB1 (Hori et al., 1995; Sorci et al., 2004), advanced glycation end products (Kislinger et al., 1999), amyloid (Yan et al., 2000), β-sheet fibrils (Yan et al., 1996) and some members of the S100 family (Valencia et al., 2004; Arumugam et al., 2004; Moroz et al., 2002). From these ligands, HMGB1 and S100 family members are likely to be released during pneumonia. We here showed that HMGB1 levels are elevated in BALF during IAV pneumonia. From the S100 family members, definitive evidence for binding to RAGE has been deduced for S100A12, S100B and S100P (Valencia et al., 2004; Arumugam et al., 2004; Moroz et al., 2002). Evidence that a functional S100A12 gene is not present in the murine genome (Fuellen et al., 2003) implies that RAGE-S100A12 ligation does not attribute to the host response to pneumonia in mice. Until now, there are no data suggesting that S100B and S100P are likely to play an important in pneumonia. By far the brain is the richest source of S100B, and astrocytes represent the cell type with the highest expression. S100P, initially identified in placenta, is expressed in a number of cells and tissues and is significantly upregulated in highly metastatic cancer cells suggesting an involvement in tumor cell migration. Nevertheless, future research is warranted to investigate whether RAGE-S100B and/or RAGE-S100P ligation play a role during pneumonia and other infectious diseases. In addition, it would be of interest to study the possible therapeutic effects of RAGE (ligand) inhibitors in IAV pneumonia.
Several studies have investigated the contribution of specific immune cells during viral airway infection. Both CD4+ and CD8+ T cells have been implicated in host defense against IAV. The CD4+ T cell subset has been suggested to be the primary inducer of inflammatory processes during IAV infection. However, depletion of CD4+ T cells in normal mice had little effect on the clearance of IAV or the cell composition and the localization of CD8+ T cells in the lungs of mice (Allan et al.,1990). In contrast, depletion of CD8+ T cells did affect IAV titers in the lungs. Therefore, CD8+ T cells are regarded as primary effector cells involved in the clearance of IAV (Eichelberger et al.,1991; Scherle et al., 1992). In our study, the RAGE−/− mice did not show differences in the number of either CD4+ or CD8+ cells. However, the cellular immune response against IAV infection was enhanced in the RAGE−/− mice, as reflected by a higher expression of CD69 on the surface of the CD4+ and CD8+ T lymphocytes when compared with wt mice. Furthermore, pulmonary neutrophils from RAGE−/− mice displayed an increased capacity to upregulate their CD11b expression during IAV. These data indicate that the improved survival and lower viral loads at 8 days after inoculation with IAV could at least partially be explained by an increased activation status of CD4+,CD8+Tcells and/or neutrophils (neutrophils see also further).
In addition to an increased activation of CD4+ and CD8+ T cells, we also found that RAGE deficiency is associated with an enhanced capacity to upregulate CD11b on neutrophils. Neutrophils may play a direct role in viral clearance. First of all, they are recruited to the respiratory tract early in the course of IAV infection (White et al., 2007; LeVine et al., 2001; Sakai et al., 2000; Sweet and Smith, 1980) and they can bind to and take up IAV (Hartshorn et al., 1990, 1995). Furthermore, IAV stimulates various activation signals and H2O2 generation by neutrophils (Hartshorn et al., 1990, 1995). Previously it has been shown that (immature) Gr1+ CD11b+ cells are responsible for augmentation of the Th2 cell-dependent and depression of Th1 cell-dependent responses during severe infection (sepsis) (Delano et al., 2007). In general this phenomenon is associated with sepsis-associated morbidity and worsened outcome. Data about this myeloid-derived suppressor cell-induced Th shift during IAV pneumonia are lacking. In our model we did find enhanced CD11b expression on Gr1+ cells and higher IL-10 levels in the lungs of the RAGE−/− mice. However, it remains to be elucidated whether this is due to a Th shift. The Gr1+ CD11b+ cells in our lung cell suspensions could very well be mature neutrophils originating from the blood being infiltrated into the lungs. In addition, in sepsis, this shift is generally associated with enhanced morbidity and mortality and in our influenza model the elevated CD11b expression is associated with decreased mortality. Therefore, future studies should indicate whether this phenomenon is induced during IAV infection and whether it can be of benefit for the host.
Our data point towards an adverse effect of RAGE on viral clearance, activation of CD4+ and CD8+ T cells and mortality during IAV infection. The underlying mechanism of RAGE-mediated immunosuppression is unclear. Previously, Dumitriu et al. (2005b, 2005a, 2007) found that RAGE mediates the maturation of plasmacytoid dendritic cells (pDCs) through autocrine release of HMGB1. Antigen presentation by pDC is associated with immunotolerance and may explain the adverse effect of RAGE on T cell activation (de Heer et al., 2004; Derks et al., 2007). However, the contribution of pDCs during viral infection is largely unknown. In addition, HMGB1 and RAGE have also been implicated in the maturation of monocyte-derived dendritic cells (Yang et al., 2007). Further research is required to identify the role of RAGE and/or HMGB1 in DC maturation during viral airway infection.
Summarized, our data suggest that RAGE−/− mice have an improved host defense during IAV pneumonia, possibly by an increased activation of pulmonary neutrophils and thereby a better antiviral clearance.
Considering the role of HMGB1 and RAGE in the maturation of pDCs, the observed effects in the RAGE−/− mice could at least be partly explained by interaction of HMGB1 with RAGE. Of note, negative-stranded RNA viruses such as RSV and Sendai virus have been shown to induce maturation of pDCs (Hornung et al., 2004). In addition to HMGB1, advanced glycation end products (AGEs) – another group of known RAGE ligands – could also play a role during IAV infection; IAV infection is associated with the production of proinflammatory cytokines and the recruitment of inflammatory cells of the innate and adaptive immune system, which contribute to oxidative stress (Han and Meydani, 2000; Peterhans, 1997) and may give rise to the formation of AGEs. Further research is warranted to address the individual contribution of these RAGE ligands.
Cytokines play an important role in the host defense during IAV infection via e.g. activation of T cells or via their more direct antiviral properties. IFN-γ plays an important role in the antiviral host defense at least in part by its promotion of the activation of CD8+ cells, which are responsible for specific lysis of virus-infected cells (Micallef et al., 1996; Gherardi et al., 2003; Nguyen et al., 1998). In addition, its involvement in the antiviral immune defense is suggested by the finding that several viruses encode proteins designed to interfere with IFN-γ signaling (Alcami and Smith, 1996). Other cytokines such as TNF-α, IL-6, IL-10 and MCP-1 are released during IAV as well (Dessing et al., 2007). To determine whether the delayed mortality and the reduced viral load at 8 days after inoculation could (at least partially) be due to an altered release of cytokines, we measured IFN-γ, TNF-α, IL-6, IL-10 and MCP-1. There were no differences in cytokines between the two mouse strains, except for lower plasma levels of TNF-α at 4 days and elevated IL-10 levels at 8 days after inoculation in the RAGE−/− mice. These data suggest that the delayed mortality and the increased clearance of the viral load were not likely the consequence of an effect of RAGE deficiency on IAV induced cytokine production.
Our key finding was that RAGE deficiency enhances the resistance against IAV pneumonia as reflected by an increased survival and an enhanced viral clearance. The current study is the first to establish that RAGE plays a detrimental role in the antiviral defense against IAV. The delayed mortality and decreased viral loads in the RAGE−/− mice were associated with an enhanced cellular T cell response, implicating that endogenous RAGE impairs the cellular immunity against respiratory tract infection with IAV. Hence, these data suggest that along with inhibitors of viral replication, RAGE inhibitors might be useful in the management of severe IAV infection.
Materials and methods
Mice
Pathogen-free 8 to 10 week old female wt C57Bl/6 mice were purchased from Harlan Sprague Dawley Inc. (Horst, The Netherlands). RAGE−/− mice, backcrossed ten times to a C57Bl/6 background, were generated as described previously (Liliensiek et al., 2004). The Institutional Animal Care and Use Committee of the Academic Medical Center, University of Amsterdam, approved all experiments.
Experimental infection
Influenza infection was induced as described previously using influenza A strain A/PR/8/34 (ATCC no. VR-95; Rockville, MD) (Keller et al., 2006). Mice were anesthetized by inhalation with isoflurane (Abott Laboratories Ltd., Kent, United Kingdom) and infected intranasally with 40 TCID50 (5600 viral copies) influenza A in 50 µl PBS. After 4 or 8 days, mice were anesthetized with ketamine (Eurovet Animal Health BV, Bladel, The Netherlands) and medetomidine (Pfizer Animal Health BV, Capelle aan de IJssel, The Netherlands) and killed for the measurements described below. Additionally, in a separate survival study mice were infected with 200 TCID50 (28,000 viral copies) influenza A.
Preparation of blood samples and lung homogenates
Blood was collected by heart puncture in heparin containing tubes and centrifuged at 1500 ×g for 10 min, after which plasma was collected and frozen at −20 °C until assayed. Lungs were harvested and weighed to obtain relative organ weight. Additionally, lungs were homogenized at 4 °C in 4 volumes of sterile isotonic saline with a tissue homogenizer (Biospect Products, Bartlesville, OK) which was carefully cleaned and disinfected with 70% ethanol after each homogenization. Lung homogenates were lysed in 1 volume of lysis buffer (300 mM NaCl, 15 mM Tris [tris(hydroxymethyl)aminomethane], 2 mM MgCl2, 2 mM Triton X-100, pepstatin A, leupeptin and aprotinine [20 ng/ml], pH 7.4) on ice for 30 min and centrifuged at 1500 ×g at 4 °C for 10 min. The supernatants were frozen at −20 °C until assayed.
Bronchoalveolar lavage
In separate mice, not used for pathology or preparation of lung homogenates, BALF was obtained and differential counts were carried out as described earlier (Rijneveld et al., 2001). Briefly, the trachea was exposed through a midline incision and BALF was harvested by instilling and retrieving two 0.5-ml aliquots of sterile isotonic saline. Cell counts were determined using an automated counter (Beckham Coulter, Coulter ZF, Mijdrecht, The Netherlands).
Assays
Viral load was determined using real-time quantitative PCR exactly as described (Keller et al., 2006; Dessing et al., 2007). Tumor necrosis factor (TNF)-α, interferon-gamma (IFN-γ), interleukin (IL)-6, IL-10 and monocyte chemoattractant protein (MCP)-1 levels were determined using a cytometric beads array (CBA) multiplex assay (BD Biosciences, San Jose, CA) in accordance with the manufacturer's recommendations. sRAGE levels were measured by ELISA (R&D, Minneapolis, MN, USA) as described before (Kalea et al., 2009). HMGB1 was measured by Western immunoblotting as described before (Wang et al., 1999; Yang et al., 2004).
Histology
Lungs for histologic examination were harvested after 8 days, fixed in 4% formaldehyde, embedded in paraffin and cut in 4-µm thick sections for staining procedures. Immunostaining for RAGE was performed on paraffin slides after deparaffinization and rehydration using standard procedures. Endogenous peroxidase activity was quenched using 1.5% H2O2 in PBS. Primary antibodies used were goat anti-mouse RAGE polyclonal antibodies (Neuromics, Edina, MN) and secondary antibodies were biotinlylated rabbit anti-goat antibodies (DakoCytomation, Glostrup, Denmark). ABC solution (Dako-Cytomation, Glostrup, Denmark) was used as the detection enyzme. DAB peroxidase (Sigma, St. Louis, MO) was used as substrate for visualization. Counterstaining was performed with methylgreen (Sigma Aldrich, St. Louis, MO). Hematoxylin–eosin stainings were performed as described (Leemans et al., 2002) and analyzed by a pathologist who had no knowledge of the genotype of the mice. To score lung inflammation and damage, the lung samples were screened for the following parameters: interstitial inflammation, alveolar inflammation, vasculitis, bronchitis, edema, and pleuritis. Each parameter was graded on a scale of 0 to 4 (0, absent; 1, very mild; 2, mild; 3, moderate; and 4, severe). The total “lung inflammation score” was expressed as the sum of the scores for each parameter, the maximum being 24 (Leemans et al., 2002).
Flow cytometry
Lung cell suspensions were obtained from infected mice by grinding lung tissue through nylon sieves and analyzed by flow cytometry using FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA) in essence as described previously (Dessing et al., 2007). Cells were brought to a concentration of 4 × 106/ml in FACS buffer (PBS supplemented with 0.5% BSA, 0.01% NaN3 and 0.35 mM EDTA). Immunostaining of cell surface molecules was performed for 30 min and 4 °C with anti-CD4-allophycocyanin (APC), anti-CD8-peridinin chlorophyl protein (PerCP), anti-CD3-biotin, anti-CD69-phycoerythrin (PE), anti-Gr1-phycoerythrin (PE, clone RB6-8C5) and anti-CD11b-allophycocyanin (APC). All antibodies were from the same manufacturer (BD Pharmingen, San Diego, CA) and used in concentrations recommended. CD69 surface molecules were analyzed on CD3+ cells within the lymphocyte gate and CD11b on Gr1+ cells.
Statistical analysis
All values are expressed as means ± SEs. Comparisons were done with Mann–Whitney U tests. Survival curves were compared by log rank test. Values of P<0.05 were considered statistically significant.
Acknowledgments
We thank J. Daalhuisen and M.S. ten Brink for expert technical assistance. This work was in part supported by a grant from the Deutsche Forschungsgemeinschaft (SFB405 to BA, PPN).
Footnotes
This work has been performed at the Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands.
Contributor Information
Marieke A.D van Zoelen, Email: M.A.vanZoelen@amc.uva.nl.
Koenraad F. van der Sluijs, Email: K.F.vandersluijs@amc.uva.nl.
Ahmed Achouiti, Email: A.Achouiti@amc.uva.nl.
Sandrine Florquin, Email: S.F.Florquin@amc.uva.nl.
Jennie M. Braun-Pater, Email: J.M.Pater@amc.amc.nl.
Huan Yang, Email: hyang@nshs.edu.
Peter P. Nawroth, Email: Peter_Nawroth@med.uni-heidelberg.de.
Kevin J. Tracey, Email: kjtracey@sprynet.com.
Angelika Bierhaus, Email: angelika_bierhaus@med.uni-heidelberg.de.
Tom van der Poll, Email: T.vanderPoll@amc.uva.nl.
References
- Alcami A, Smith GL. Receptors for gamma-interferon encoded by poxviruses: implications for the unknown origin of vaccinia virus. Trends Microbiol. 1996;4:321–326. doi: 10.1016/0966-842x(96)10051-2. [DOI] [PubMed] [Google Scholar]
- Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+ T cells. J. Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE) J. Biol. Chem. 2004;279:5059–5065. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr. Opin. Investig. Drugs. 2006;7:985–991. [PubMed] [Google Scholar]
- Cataldegirmen G, Zeng S, Feirt N, Ippagunta N, Dun H, Qu W, Lu Y, Rong LL, Hofmann MA, Kislinger T, Pachydaki SI, Jenkins DG, Weinberg A, Lefkowitch J, Rogiers X, Yan SF, Schmidt AM, Emond JC. RAGE limits regeneration after massive liver injury by coordinated suppression of TNF-alpha and NF-kappaB. J. Exp. Med. 2005;201:473–484. doi: 10.1084/jem.20040934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Tsuneyama K, Kominami R, Shinohara H, Sakurai S, Yonekura H, Watanabe T, Takano Y, Yamamoto H, Yamamoto Y. Expression profiling of endogenous secretory receptor for advanced glycation end products in human organs. Mod. Path. 2005;18:1385–1396. doi: 10.1038/modpathol.3800450. [DOI] [PubMed] [Google Scholar]
- Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J. Exp. Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong JC, Rimmelzwaan GF, Fouchier RA, Osterhaus AD. Influenza virus: a master of metamorphosis. J. Infect. 2000;40:218–228. doi: 10.1053/jinf.2000.0652. [DOI] [PubMed] [Google Scholar]
- Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derks RA, Jankowska-Gan E, Xu Q, Burlingham WJ. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J. Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- Dessing MC, van der Sluijs KF, Florquin S, van der Poll T. CD14 plays a limited role during influenza A virus infection in vivo. Immunol. Lett. 2007;113:47–51. doi: 10.1016/j.imlet.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur. J. Immunol. 2005a;35:2184–2190. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 2005b;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Bianchi ME, Bacci M, Manfredi AA, Rovere-Querini P. The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 2007;81:84–91. doi: 10.1189/jlb.0306171. [DOI] [PubMed] [Google Scholar]
- Eichelberger M, Allan W, Zijlstra M, Jaenisch R, Doherty PC. Clearance of influenza virus respiratory infection in mice lacking class I major histocompatibility complex-restricted CD8+ T cells. J. Exp. Med. 1991;174:875–880. doi: 10.1084/jem.174.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekong U, Zeng S, Dun H, Feirt N, Guo J, Ippagunta N, Guarrera JV, Lu Y, Weinberg A, Qu W, Ramasamy R, Schmidt AM, Emond JC. Blockade of the receptor for advanced glycation end products attenuates acetaminophen-induced hepatotoxicity in mice. J. Gastroenterol. Hepatol. 2006;21:682–688. doi: 10.1111/j.1440-1746.2006.04225.x. [DOI] [PubMed] [Google Scholar]
- Fuellen G, Foell D, Nacken W, Sorg C, Kerkhoff C. Absence of S100A12 in mouse: implications for RAGE-S100A12 interaction. Trends Immunol. 2003;24:622–624. doi: 10.1016/j.it.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Gherardi MM, Ramirez JC, Esteban M. IL-12 and IL-18 act in synergy to clear vaccinia virus infection: involvement of innate and adaptive components of the immune system. J. Gen. Virol. 2003;84:1961–1972. doi: 10.1099/vir.0.19120-0. [DOI] [PubMed] [Google Scholar]
- Han SN, Meydani SN. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J. Infect. Dis. 2000;182(Suppl. 1):S74–S80. doi: 10.1086/315915. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, Collamer M, White MR, Schwartz JH, Tauber AI. Characterization of influenza A virus activation of the human neutrophil. Blood. 1990;75:218–226. [PubMed] [Google Scholar]
- Hartshorn KL, Liou LS, White MR, Kazhdan MM, Tauber JL, Tauber AI. Neutrophil deactivation by influenza A virus. Role of hemagglutinin binding to specific sialic acid-bearing cellular proteins. J. Immunol. 1995;154:3952–3960. [PubMed] [Google Scholar]
- Hayden FG. Pandemic influenza: is an antiviral response realistic? Pediatr. Infect. Dis. J. 2004;23:S262–S269. doi: 10.1097/01.inf.0000144680.39895.ce. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Hudson BI, Gleason MR, Qu W, Lu Y, Lalla E, Chitnis S, Monteiro J, Stickland MH, Bucciarelli LG, Moser B, Moxley G, Itescu S, Grant PJ, Gregersen PK, Stern DM, Schmidt AM. RAGE and arthritis: the G82S polymorphism amplifies the inflammatory response. Genes Immun. 2002;3:123–135. doi: 10.1038/sj.gene.6363861. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J. Biol. Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Hornung V, Schlender J, Guenthner-Biller M, Rothenfusser S, Endres S, Conzelmann KK, Hartmann G. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23(6):1766–1774. doi: 10.1096/fj.08-117739. (Electronic publication ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel R, Hartshorn KL. Novel strategies for prevention and treatment of influenza. Expert. Opin. Ther. Targets. 2005;9:1–22. doi: 10.1517/14728222.9.1.1. [DOI] [PubMed] [Google Scholar]
- Keller TT, van der Sluijs KF, de Kruif MD, Gerdes VE, Meijers JC, Florquin S, van der PT, van Gorp EC, Brandjes DP, Buller HR, Levi M. Effects on coagulation and fibrinolysis induced by influenza in mice with a reduced capacity to generate activated protein C and a deficiency in plasminogen activator inhibitor type 1. Circ. Res. 2006;99:1261–1269. doi: 10.1161/01.RES.0000250834.29108.1a. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, DU YS, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl) lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am. J. Respir. Crit Care Med. 2002;165:1445–1450. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Whitsett JA, Hartshorn KL, Crouch EC, Korfhagen TR. Surfactant protein D enhances clearance of influenza A virus from the lung in vivo. J. Immunol. 2001;167:5868–5873. doi: 10.4049/jimmunol.167.10.5868. [DOI] [PubMed] [Google Scholar]
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J. Clin. Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef MJ, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur. J. Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod. Path. 2006;19:1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- Moroz OV, Antson AA, Dodson EJ, Burrell HJ, Grist SJ, Lloyd RM, Maitland NJ, Dodson GG, Wilson KS, Lukanidin E, Bronstein IB. The structure of S100A12 in a hexameric form and its proposed role in receptor signalling. Acta Crystallogr. D. Biol. Crystallogr. 2002;58:407–413. doi: 10.1107/s0907444901021278. [DOI] [PubMed] [Google Scholar]
- Nguyen HH, Boyaka PN, Moldoveanu Z, Novak MJ, Kiyono H, McGhee JR, Mestecky J. Influenza virus-infected epithelial cells present viral antigens to antigen-specific CD8+ cytotoxic T lymphocytes. J. Virol. 1998;72:4534–4536. doi: 10.1128/jvi.72.5.4534-4536.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P. Influenza: old and new threats. Nat. Med. 2004;10:S82–S87. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Peterhans E. Reactive oxygen species and nitric oxide in viral diseases. Biol. Trace Elem. Res. 1997;56:107–116. doi: 10.1007/BF02778986. [DOI] [PubMed] [Google Scholar]
- Rijneveld AW, Florquin S, Branger J, Speelman P, van Deventer SJ, van der Poll T. TNF-alpha compensates for the impaired host defense of IL-1 type I receptor-deficient mice during pneumococcal pneumonia. J. Immunol. 2001;167:5240–5246. doi: 10.4049/jimmunol.167.9.5240. [DOI] [PubMed] [Google Scholar]
- Sakai S, Kawamata H, Mantani N, Kogure T, Shimada Y, Terasawa K, Sakai T, Imanishi N, Ochiai H. Therapeutic effect of anti-macrophage inflammatory protein 2 antibody on influenza virus-induced pneumonia in mice. J. Virol. 2000;74:2472–2476. doi: 10.1128/jvi.74.5.2472-2476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle PA, Palladino G, Gerhard W. Mice can recover from pulmonary influenza virus infection in the absence of class I-restricted cytotoxic T cells. J. Immunol. 1992;148:212–217. [PubMed] [Google Scholar]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, Esposito C, Hegarty H, Hurley W, Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Sorci G, Riuzzi F, Arcuri C, Giambanco I, Donato R. Amphoterin stimulates myogenesis and counteracts the antimyogenic factors basic fibroblast growth factor and S100B via RAGE binding. Mol. Cell. Biol. 2004;24:4880–4894. doi: 10.1128/MCB.24.11.4880-4894.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet C, Smith H. Pathogenicity of influenza virus. Microbiol. Rev. 1980;44:303–330. doi: 10.1128/mr.44.2.303-330.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am. J. Respir. Crit. Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia JV, Mone M, Zhang J, Weetall M, Buxton FP, Hughes TE. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes. 2004;53:743–751. doi: 10.2337/diabetes.53.3.743. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Laterre PF, van Veen SQ, van Till JW, Wittebole X, Bresser P, Tanck MW, Dugernier T, Ishizaka A, Boermeester MA, van der Poll T. Systemic and local high mobility group box 1 concentrations during severe infection. Crit. Care Med. 2007;35:2799–2804. doi: 10.1097/01.CCM.0000287588.69000.97. [DOI] [PubMed] [Google Scholar]
- van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J. Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- White MR, Tecle T, Crouch EC, Hartshorn KL. Impact of neutrophils on antiviral activity of human bronchoalveolar lavage fluid. Am. J. Physiol., Lung Cell Mol. Physiol. 2007;293:L1293–L1299. doi: 10.1152/ajplung.00266.2007. [DOI] [PubMed] [Google Scholar]
- Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, Roth J, Foell D. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit. Care Med. 2007;35:1369–1375. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- Wright PF, Webster R. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th edition. Philadelphia: Lippincott-Raven; 2001. pp. 1533–1579. [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt AM, Stern D, Kindy M. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat. Med. 2000;6:643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J. Leukoc. Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, Czura CJ, Wang H, Roth J, Warren HS, Fink MP, Fenton MJ, Andersson U, Tracey KJ. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. U. S. A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng S, Feirt N, Goldstein M, Guarrera J, Ippagunta N, Ekong U, Dun H, Lu Y, Qu W, Schmidt AM, Emond JC. Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology. 2004;39:422–432. doi: 10.1002/hep.20045. [DOI] [PubMed] [Google Scholar]