Abstract
Objective:
The purpose of this study was to examine the role of multiple pathologies in the expression of dementia in the oldest-old.
Methods:
A total of 183 participants of The 90+ Study with longitudinal follow-up and autopsy were included in this clinical-pathologic investigation. Eight pathologic diagnoses (Alzheimer disease [AD], microinfarcts, hippocampal sclerosis, macroinfarcts, Lewy body disease, cerebral amyloid angiopathy, white matter disease, and others) were dichotomized. We estimated the odds of dementia in relation to each individual pathologic diagnosis and to the total number of diagnoses. We also examined dementia severity in relation to number of pathologic diagnoses.
Results:
The presence of multiple pathologic diagnoses was common and occurred more frequently in those with dementia compared with those without dementia (45% vs 14%). Higher numbers of pathologic diagnoses were also associated with greater dementia severity. Participants with intermediate/high AD pathology alone were 3 times more likely to have dementia (odds ratio = 3.5), but those with single non-AD pathologies were 12 times more likely to have dementia (odds ratio = 12.4). When a second pathology was present, the likelihood of dementia increased 4-fold in those with intermediate/high AD pathology but did not change in those with non-AD pathologies, suggesting that pathologies may interrelate in different ways.
Conclusions:
In the oldest-old, the presence of multiple pathologies is associated with increased likelihood and severity of dementia. The effect of the individual pathologies may be additive or perhaps synergistic and requires further research. Multiple pathologies will need to be targeted to reduce the burden of dementia in the population.
The oldest-old are the fastest growing segment of the population and the age group with the highest prevalence and incidence of dementia.1,2 Although the prevalence of Alzheimer disease (AD), Lewy bodies, and other brain pathologies appears to plateau in the tenth decade and beyond,3 the risk of dementia continues to double with every 5 years of age reaching a staggering 40% per year in centenarians.2
The causes of dementia and cognitive loss in extreme aging have been difficult to discern. Although AD continues to be the most frequent pathology in the oldest-old, many clinical pathologic studies have suggested a weakening of the association between dementia in this age group and individual pathologies including AD, vascular disease, and Lewy bodies.4–6 The presence of mixed cerebral pathologies, however, becomes more frequent with advancing age.3,7 Using The 90+ Study, we investigated the contribution of 8 individual neuropathologic diagnoses and the role of multiple neuropathologies in the expression of dementia in the oldest-old.
METHODS
Participants.
Participants were members of The 90+ Study, a population-based cohort study of aging and dementia in people aged 90 years and older initiated in 2003.8,9 All longitudinally followed 90+ Study participants were invited to be part of the autopsy program. As of December 31, 2012, a total of 303 participants, about 40% of those with in-person longitudinal evaluations, had enrolled in the autopsy program. Autopsies were performed on 188 of the 207 participants (90%) who died. The current study excluded 4 participants with incomplete pathologic evaluation and one participant on whom a cognitive diagnosis could not be determined for a total of 183 participants.
Standard protocol approvals, registrations, and patient consents.
All participants or their designated informants provided consent to participate in the study. Procedures were reviewed and approved by the University of California, Irvine (UCI) institutional review board.
Pathologic evaluations.
The UCI Alzheimer's Disease Research Center Pathology Core performed all procedures to procure and prepare tissue samples. The evaluation included analysis of the following brain regions: middle frontal, superior temporal, inferior parietal, occipital, entorhinal, and cingulate gyri, as well as basal ganglia, thalamus, hippocampus, amygdala, midbrain, pons, and medulla. Pathologic evaluations were done between 2003 and 2012, thus we used recommended procedures and criteria available during this time interval. The standard protocol included the use of the following stains: hematoxylin & eosin (H&E) stain, modified Bielschowsky silver stain, and immunostaining for tau, β-amyloid, ubiquitin, and α-synuclein. Pathologic evaluations were performed blinded to clinical diagnosis by 2 board-certified neuropathologists. Joshua Sonnen performed evaluation for microinfarcts with the same methodology he applied in other population-based cohorts.10,11 Ronald Kim performed evaluations for all other pathologies.
Braak & Braak tangle stage12 and CERAD (Consortium to Establish a Registry for Alzheimer's Disease) plaque score13 were combined using National Institute on Aging (NIA) Reagan Criteria14 to classify the neuropathologic findings as no, low, intermediate, or high likelihood of AD. Three different vascular pathologies were considered in the present study. Ischemic and hemorrhagic macroinfarcts identified grossly were evaluated for size, location, and number. Infarctions rated as proximal to death by the pathologist were excluded from analyses because they did not contribute to cognition before the end of life. The presence of microinfarcts was evaluated through H&E-stained sections of 6 predefined brain regions: middle frontal, inferior parietal, superior temporal, occipital, basal ganglia, and thalamus. Microinfarcts were defined as foci of pallor, loss of vulnerable cells, gliosis, and macrophage presence10 (figure e-1 on the Neurology® Web site at Neurology.org). White matter disease in the form of subcortical arteriolosclerotic leukoencephalopathy (SAE), defined by lipohyalinosis/arteriolar sclerosis, widening of perivascular spaces, white matter gliosis, and pallor of myelin staining,15 was assessed within periventricular white matter immediately adjoining the rostral cingulate gyrus and caudal cingulate gyrus (figure e-2). Lewy body disease (LBD) was identified using α-synuclein immunostaining according to established recommendations.16 Hippocampal sclerosis (HS) was characterized by the presence of severe nerve cell loss and astrocytosis within CA1 and subiculum17 (figure e-3). The presence of cerebral amyloid angiopathy (CAA) was assessed with β-amyloid immunostaining of cerebral blood vessels and classified as absent, mild, moderate, or severe. Corticobasal degeneration and other frontotemporal lobar degeneration were identified by finding achromatic neurons in H&E or Klüver-Barrera–stained sections as suggested in consensus criteria.18
Dementia determination.
Participants were examined every 6 months. The evaluations included a neurologic examination, physical examination, neuropsychological battery, including the Mini-Mental State Examination (MMSE),19 review of medical history, examination of medication containers, and interviews with informants. Dementia diagnosis (DSM-IV)20 was assigned postmortem during a consensus conference that used all available information from the longitudinal evaluations, brain imaging when clinically available, and medical records. If dementia was present, the year when the participant met dementia criteria was recorded. All clinical evaluations and cognitive diagnosis assignments were done blinded to the results of the pathologic evaluations.
Statistical analyses.
The 8 pathologies used in these analyses were dichotomized to represent substantial levels of each as follows: AD (NIA Reagan intermediate or high likelihood),7 microinfarcts (≥3),11 HS (present), macroinfarcts (≥2 or more large infarcts or lacunar infarcts),11 LBD (limbic or neocortical Lewy bodies), CAA (moderate or severe),11 white matter disease/SAE (present), and other pathologies (present). Dichotomies were selected to agree with standard criteria, previous similar studies, or to represent significant associations with dementia in our own cohort. A variable totaling the number of these pathologies was created as the sum of the 8 pathologies.
Logistic regression was used to first estimate the odds of dementia in relation to the presence of each individual pathology and then estimate the independent contribution of each pathology by including all pathologies simultaneously in a regression model. We also estimated the odds of dementia in relation to the number of pathologies using the group with no pathologies as the reference. Because no participant without dementia had more than 3 pathologies, all participants with 3 or more pathologies were combined into one group. We also classified people into mutually exclusive groups according to the types of pathologies present: no pathologies, intermediate/high AD pathology alone (AD alone), one non-AD pathology alone (non-AD alone), intermediate/high AD with another pathology (AD plus), and more than one non-AD pathology (non-AD plus). The odds of dementia were then compared in the 4 mutually exclusive groups using the no pathologies group as the reference. All analyses were adjusted for age at death and sex.
Finally, to explore whether the number of pathologies was related to dementia severity, we compared the mean MMSE score closest to death in people with different numbers of pathologies. We used linear regression with MMSE score as the outcome, number of pathologies as a categorical variable, and adjusted for age at death, sex, and interval between last MMSE and death. Because MMSE scores are likely related to the duration of dementia, we also included the number of years between dementia diagnosis and death as a covariate in the analyses. This last set of analyses excluded 4 participants without an MMSE score.
RESULTS
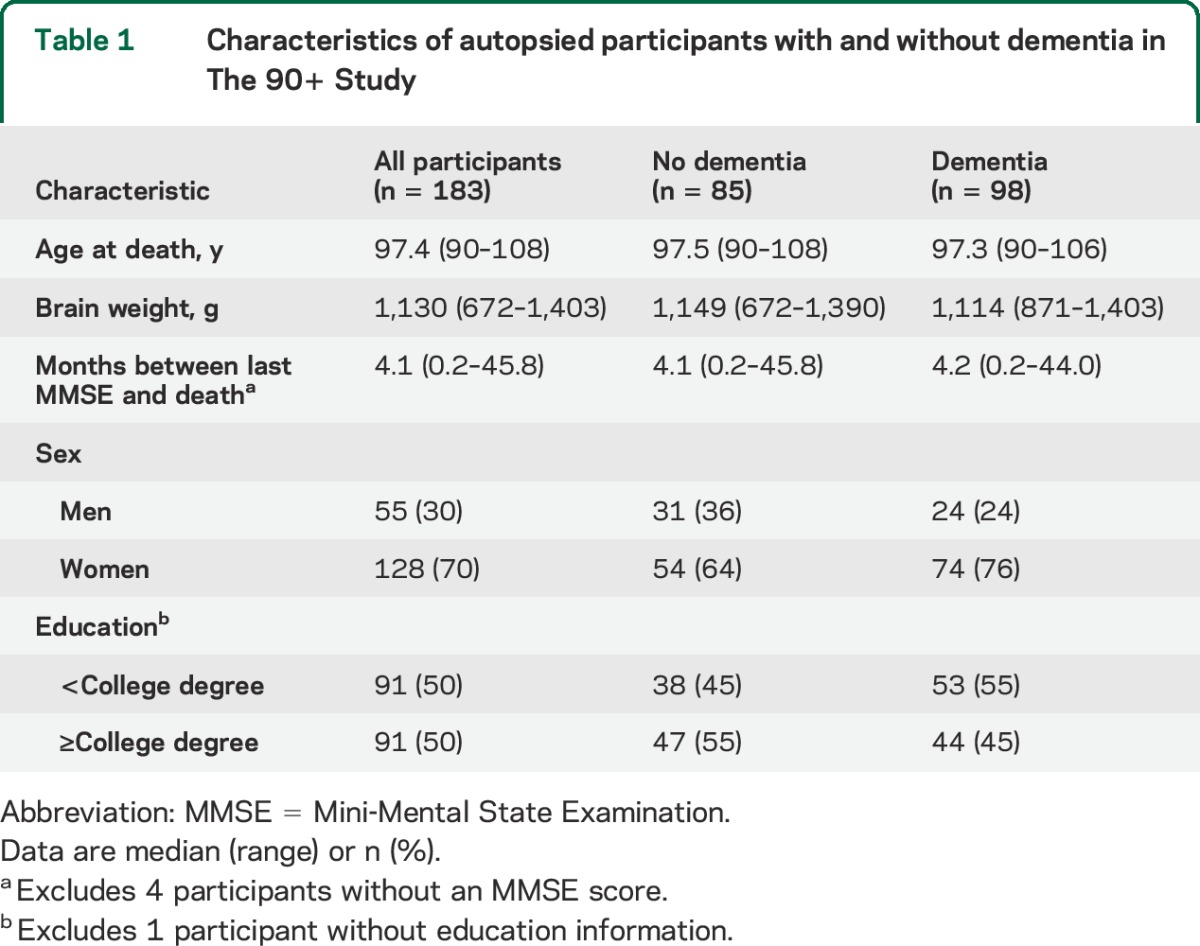
At the time of death, participants' median age was 97 years (range: 90–108), 70% were women, 50% had a college education or higher, and 54% had dementia (table 1). The median time between the last in-person evaluation and death was 4 months (range: 0.2–45.8).
Table 1.
Characteristics of autopsied participants with and without dementia in The 90+ Study
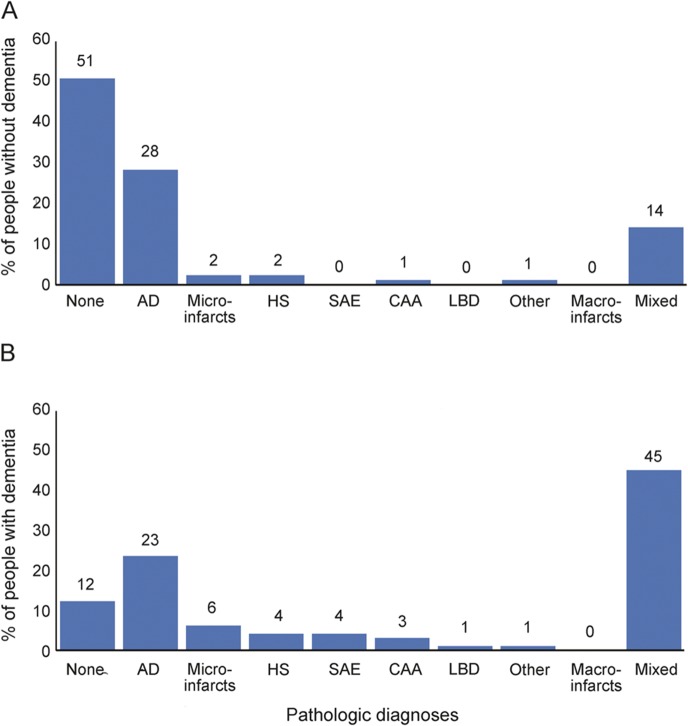
The distribution of the individual pathologies is shown in table 2. A pathologic diagnosis of AD was the most common with half of the participants in the intermediate/high level category, followed by ≥3 microinfarcts in 17% of participants, and HS in an equal proportion of participants (17%). Moderate or severe levels of CAA pathology were detected in 13% of participants whereas white matter disease/SAE was seen in 8%. Macroinfarcts were less frequent with only 4% of participants showing evidence of ≥2 lacunar or large infarcts. Similarly, Lewy body pathology (limbic or neocortical) was detected in only 4% of participants. Finally, 2 participants had other pathologies, one with corticobasal degeneration and another with glioblastoma. Note that some pathologies, such as HS, white matter disease/SAE, ≥2 macroinfarcts, and LBD were present almost exclusively in people with dementia. However, other than HS, these pathologies were relatively infrequent, occurring in fewer than 10% of participants. Some pathologies commonly associated with dementia in younger elderly, such as Parkinson disease and most types of frontotemporal lobar degeneration, were not identified in this cohort.
Table 2.
Association between pathologies and dementia in the oldest-old
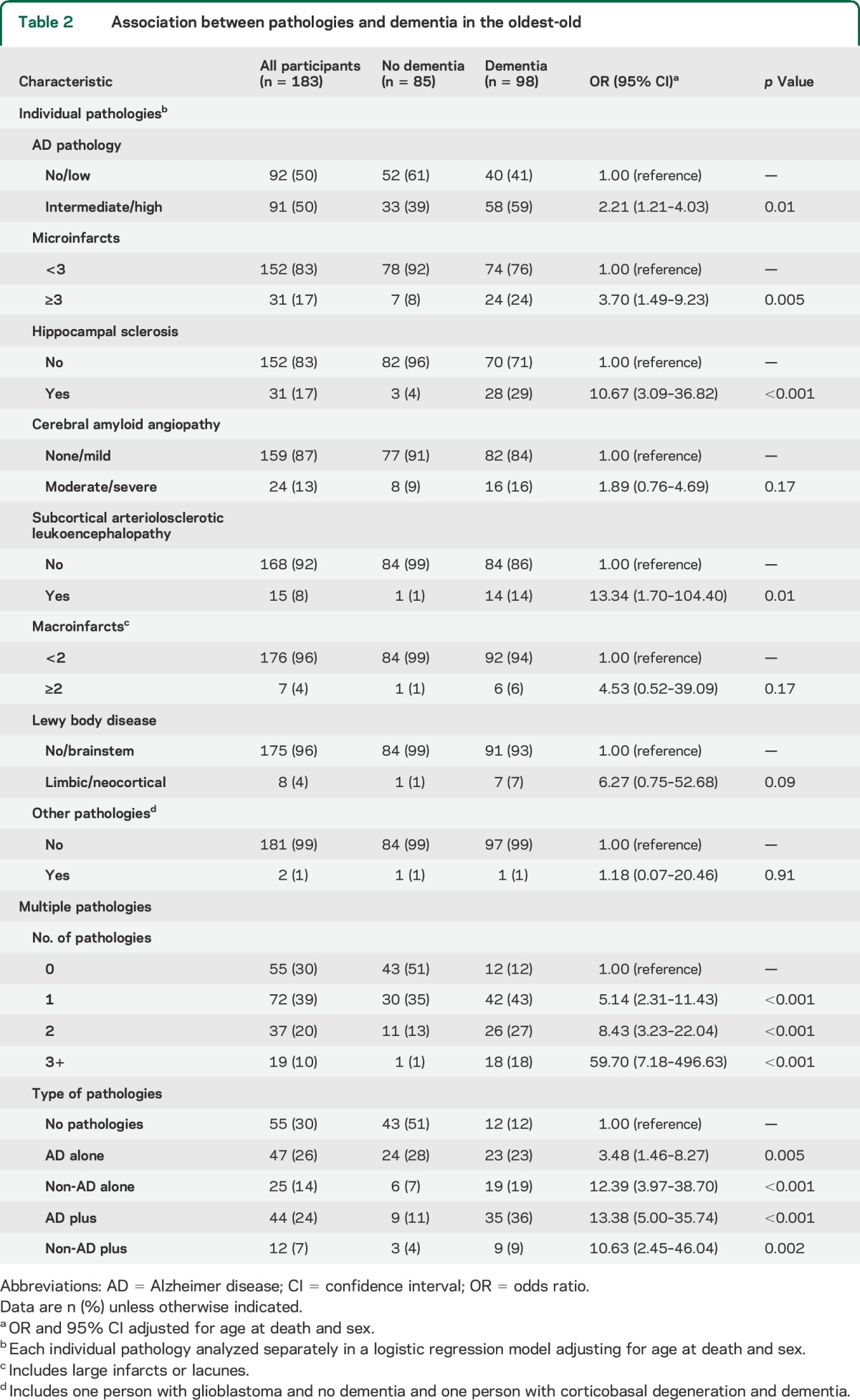
Figure 1 shows the frequency of each pathology alone and the frequency of multiple pathologies in people with or without dementia. No pathologies were found in 51% of people without dementia and 12% of people with dementia. Of note, intermediate/high AD pathology alone was present in approximately the same proportion of people without dementia (28%) and with dementia (23%). Mixed pathologies were present in 45% of participants with dementia and 14% of participants without dementia.
Figure 1. Distribution of single and multiple pathologies in oldest-old participants without (A) and with (B) dementia.
AD = intermediate/high likelihood of Alzheimer disease; CAA = moderate/severe cerebral amyloid angiopathy; HS = hippocampal sclerosis present; LBD = limbic/neocortical Lewy bodies; macroinfarcts = 2 or more lacunar or large infarcts; microinfarcts = 3 or more microinfarcts; Other includes one participant with glioblastoma and no dementia and one participant with corticobasal degeneration and dementia; SAE = white matter disease/subcortical arteriolosclerotic leukoencephalopathy.
Figure 2 shows Venn diagrams of various combinations of pathologies in people with and without dementia. All possible combinations of pathologies were evident in people with dementia but not in participants without dementia. In people with dementia, AD + other and AD + vascular were the most frequent combinations. In participants with no dementia, the most common combination was AD + CAA. The number of people with AD pathology alone was similar in individuals with and without dementia, but vascular, other, and mixed pathologies were much more frequent in individuals with dementia.
Figure 2. Venn diagram showing the number of oldest-old participants with no pathologies or combinations of 4 different pathologies.
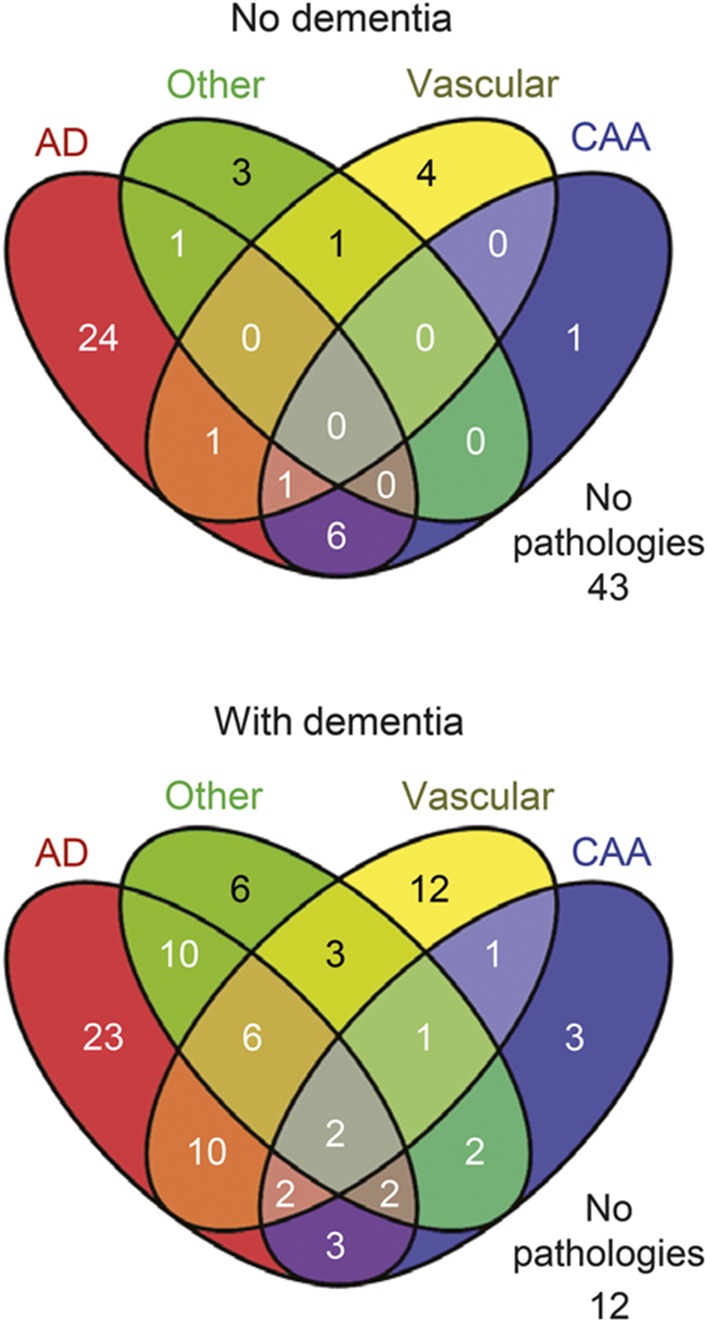
AD = intermediate/high likelihood of Alzheimer disease; CAA = cerebral amyloid angiopathy; Other = hippocampal sclerosis, Lewy body disease, one participant with corticobasal degeneration but no other pathologies, and one participant with glioblastoma but no other pathologies; Vascular = 3+ microinfarcts, 2+ macroinfarcts, and subcortical arteriolosclerotic leukoencephalopathy.
We first analyzed the contribution of each individual pathology to dementia using the absence of the individual pathology as the reference (table 2). The odds of dementia were significantly related to the presence of several individual pathologies including intermediate/high AD (odds ratio [OR] = 2.21), ≥3 microinfarcts (OR = 3.70), HS (OR = 10.67), and white matter disease/SAE (OR = 13.34). Although the ORs were high in people with LBD (OR = 6.27) or ≥2 macroinfarcts (OR = 4.53), they were not significant most likely because of the relatively few people with these pathologies. When we analyzed all pathologies simultaneously in one model, the same 4 pathologies were estimated to contribute significantly and independently to dementia (intermediate/high AD: OR = 2.56; ≥3 microinfarcts: OR = 3.20; HS: OR = 9.70; and white matter disease/SAE: OR = 14.85).
We then analyzed the contribution of multiple pathologies to dementia using no pathologies as the reference group (table 2). Prevalence of dementia increased with number of pathologies from 22% in people with no pathologies to virtually all individuals (95%) with 3 or more pathologies. The odds of dementia increased significantly with increasing number of pathologies. The OR was 5.14 for 1 pathology, 8.43 for 2 pathologies, and 59.7 for 3 or more pathologies. We also analyzed the contribution of specific combinations of pathologies. People with intermediate/high AD pathology alone had more than 3 times the odds of dementia (OR = 3.48) compared with people who had no pathologies. The odds of dementia increased considerably among people with intermediate/high AD plus other pathologies (OR = 13.48), people with 1 non-AD pathology alone (OR = 12.39), and non-AD pathology in combination with other pathologies (OR = 10.63) suggesting that the presence of pathologies other than AD are more likely to result in dementia than AD alone. We repeated these analyses reclassifying AD pathology as high likelihood (vs no/low/intermediate) and the results were similar. The OR for people with high AD pathology alone was still the lowest (OR = 4.13) and the OR for people in the AD plus group was the highest (OR = 18.47).
Finally, we tested whether the number of pathologies was related to dementia severity (as measured by MMSE score) independent of duration of disease. Lower MMSE scores were associated with a higher number of pathologies (F4,85 = 3.71, p = 0.008). The average adjusted MMSE score decreased significantly from 17.7 in people with no pathologies to 5.7 in people with 4 pathologies. Duration of disease (years between dementia diagnosis and death) was not significantly different between the groups (figure 3).
Figure 3. Adjusted MMSE score by number of pathologies in participants with dementia.
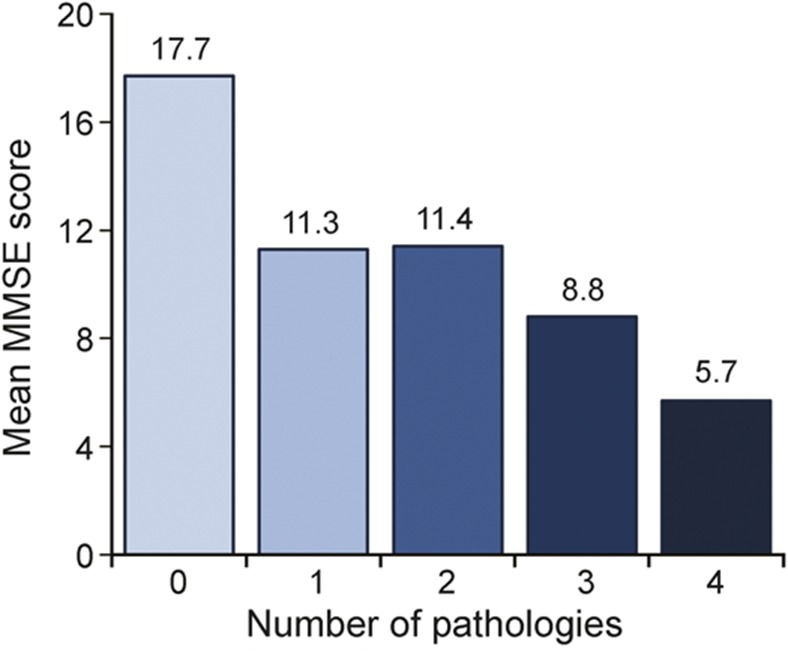
From a linear regression with MMSE score as the outcome, number of pathologies as the main predictor, and adjusted for age at death, sex, interval between last MMSE score and death, and duration of dementia (years between dementia diagnosis and death). Duration of dementia was 4.9 years in people with no pathologies, 4.1 years in people with 1 pathology, 5.0 years in people with 2 pathologies, 6.6 years in people with 3 pathologies, and 6.3 years in people with 4 pathologies. MMSE = Mini-Mental State Examination.
DISCUSSION
This clinical pathologic study of dementia showed that the presence of multiple cerebral pathologies is frequent in the oldest-old and closely related to dementia. Two or more pathologic diagnoses were present in approximately a third of all 90+ Study individuals, and in almost half of those with dementia. Prevalence of dementia increased in relation to number of pathologies from approximately half of the individuals (58%) with a single pathology to virtually all individuals (95%) with 3 or more pathologies. Moreover, in individuals with dementia, higher numbers of pathologies were associated with greater severity of dementia despite similar disease duration.
This study in the oldest-old extends the results of investigations performed in younger community-dwelling elderly that show dementia is generally associated with mixed rather than single brain pathologies.7,21,22 Even in the carefully characterized patients with AD of the NIA Alzheimer Disease Centers, HS, small vessel disease, and CAA were frequent copathologies and contributed independently to cognitive loss with a magnitude similar to AD.23
Only a handful of pathologic studies address multiple pathologies specifically in the oldest-old. Consistent with our investigation, these studies found that mixed rather than single pathologic diagnoses were most frequently associated with dementia in this age group.3,5,24,25 AD with various forms of vascular disease was the most frequent of the mixed diagnoses in these studies and increased the likelihood of dementia compared with AD alone.5,25 However, many of these studies did not include HS,5,25 microinfarcts,24 or other pathologic diagnoses included in the present investigation. Moreover, none of the investigations examined the relationship between number of pathologic diagnoses and severity of dementia. Thus, this investigation adds to the growing evidence that mixed pathologies contribute to dementia in the majority of elderly and may be particularly relevant to the oldest-old, who have the highest rates of mixed pathologies and dementia.5,22
Higher-grade AD pathology was the most prevalent dementia-related pathology in this oldest-old sample (50%). Compared with younger elderly, some non-AD pathologies, including HS (17%), ≥3 microinfarcts (17%), amyloid angiopathy (13%), and white matter disease/SAE (8%), were found more frequently, whereas Lewy bodies (4%) and ≥2 macroinfarcts (4%), frontotemporal subtypes (<1%), and Parkinson disease (0%) were detected less frequently or not at all compared with younger elderly. The relative differences in frequency of these pathologies have been reported in other epidemiologic studies that included the oldest-old.26–28
When alone, non-AD pathologies appeared more likely to result in dementia than intermediate/high AD pathology. Individuals with a single non-AD pathology (such as HS, white matter disease/SAE, or ≥3 microinfarcts) were 12 times more likely to have dementia than individuals with no pathology, and the likelihood of dementia did not change with the addition of a second pathology. In contrast, individuals with intermediate/high AD pathology alone were only 3 times more likely to have dementia than individuals with no pathologies. However, when AD pathology was accompanied by a second pathology, the likelihood of dementia increased to the level of non-AD pathologies. These results suggest that pathologies may interact in different ways, an important area for future research that will require larger samples to adequately study combinations and interactions of pathologies.
In The 90+ Study, we also found that multiple pathologies were associated with increasing severity of dementia. These results are consistent with findings from other investigations. For example, patients with AD who have cerebral infarcts show faster rates of cognitive decline and lower Clinical Dementia Rating scores compared with those who have AD pathology alone.29 Similarly, individuals with mixed AD and Lewy body pathologies decline more rapidly30 and have more severe dementia proximal to death compared with AD alone.31 Moreover, in individuals with the same level of cognitive impairment, studies show that those with mixed pathologies have lower levels of AD pathology compared with individuals who have AD pathology alone.32–34 Together, these investigations add further support to the notion that the effect of multiple pathologies may be additive or perhaps may interact in some way.
Finally, it is worth noting that 12% of individuals with dementia in The 90+ Study did not have substantial levels of neuropathology. Earlier research, including our own, has suggested that as many as half of individuals aged 90+ years have insufficient pathology to explain their dementia.9,35 These studies, however, frequently did not consider microinfarcts and other pathologies included in the current investigation. Moreover, many 90+ individuals with dementia but insufficient pathology do, in fact, have low levels of pathologies including AD, mild amyloid angiopathy, or 1 or 2 microinfarcts. Additional research is necessary to determine whether combinations of these low levels of pathology or other undetected pathologies contributed to their dementia.
Strengths of this clinical pathologic investigation include the variety of pathologic diagnoses assessed and the large number of well-characterized, oldest-old participants with comprehensive follow-up every 6 months. The frequent follow-up allowed us to detect changes in cognitive and physical status, which can occur rapidly at these ages. The evaluations were multidisciplinary including neuropsychological assessments, neurologic examinations, informant questionnaires, medical records, and other sources. Furthermore, the clinical and pathologic measures were done using standardized protocols blinded to other information about the participant.
Some limitations also deserve attention. First, our investigation of the oldest-old limits our ability to comment on younger elderly, but it is worth noting that most literature of multiple pathologies has included younger elderly with similar findings. Participants of The 90+ Study are also predominantly Caucasian and well-educated, limiting our ability to generalize our findings to other ethnic and racial groups. However, a Census Bureau report on the oldest-old suggests that characteristics of the participants in The 90+ Study are similar to those of the oldest-old presently in the United States36 particularly regarding sex and ethnicity.
Our investigation highlights that multiple neuropathologies are frequently present and contribute to the presence and severity of dementia in the oldest-old. The presence of mixed pathologies is common at these extreme ages and may account for the persistent increase in risk of dementia among the oldest-old. The relationships between different pathologies are not well understood and more research is necessary to determine whether pathologies are additive or interrelate in other ways.
At present, dementia is one of the most frequent and most devastating obstacles to successful aging. The presence of multiple dementia-related pathologies suggests that new research paradigms should include the targeting of non-AD pathologies, more quantitative assessments of neuropathologic lesions (particularly non-AD), and multimodal approaches to treatment. If most of the dementia in the population is attributable to multiple pathologies, we will have to broaden our translational approach to successfully prevent and treat dementia, one of our greatest public health challenges.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participants and their relatives, testers, and examiners of The 90+ Study, and the staff of the UCI brain repository.
GLOSSARY
- AD
Alzheimer disease
- CAA
cerebral amyloid angiopathy
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- H&E
hematoxylin & eosin
- HS
hippocampal sclerosis
- LBD
Lewy body disease
- MMSE
Mini-Mental State Examination
- NIA
National Institute on Aging
- OR
odds ratio
- SAE
subcortical arteriolosclerotic leukoencephalopathy
- UCI
University of California, Irvine
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
C.H.K. conceived the study, interpreted the data, and drafted the manuscript. R.C.K. performed pathologic analyses and critically revised the manuscript. J.A.S. performed pathologic analyses and critically revised the manuscript. S.S.B. helped with the conception of the study and critically revised the manuscript. T.T. contributed to the displaying of data and critically revised the manuscript. M.M.C. conceived the study, performed the statistical analyses, interpreted the data, and drafted the manuscript.
STUDY FUNDING
This research was funded by grants from the NIH (R01AG21055, P50AG16573, and R01AG042444).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: results from The 90+ Study. Neurology 2008;71:337–343. [DOI] [PubMed] [Google Scholar]
- 2.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest-old: The 90+ Study. Ann Neurol 2010;67:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 2010;119:421–433. [DOI] [PubMed] [Google Scholar]
- 4.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–2309. [DOI] [PubMed] [Google Scholar]
- 5.James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 2012;307:1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol 2005;18:224–227. [DOI] [PubMed] [Google Scholar]
- 7.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. [DOI] [PubMed] [Google Scholar]
- 8.Kawas CH. The oldest old and The 90+ Study. Alzheimers Dement 2008;4:S56–S59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: The 90+ Study. Curr Alzheimer Res 2012;9:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White L, Petrovitch H, Hardman J, et al. Cerebrovascular pathology and dementia in autopsied Honolulu-Asia Aging Study participants. Ann NY Acad Sci 2002;977:9–23. [DOI] [PubMed] [Google Scholar]
- 11.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 2007;62:406–413. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 13.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486. [DOI] [PubMed] [Google Scholar]
- 14.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol 1997;56:1095–1097. [DOI] [PubMed] [Google Scholar]
- 15.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry 1997;63:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leverenz JB, Hamilton R, Tsuang DW, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 2008;18:220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amador-Ortiz C, Dickson DW. Neuropathology of hippocampal sclerosis. Handb Clin Neurol 2008;89:569–572. [DOI] [PubMed] [Google Scholar]
- 18.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 21.Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS). Lancet 2001;357:169–175. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs GG, Alafuzoff I, Al-Sarraj S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord 2008;26:343–350. [DOI] [PubMed] [Google Scholar]
- 23.Serrano-Pozo A, Qian J, Monsell SE, Frosch MP, Betensky RA, Hyman BT. Examination of the clinicopathologic continuum of Alzheimer disease in the autopsy cohort of the National Alzheimer Coordinating Center. J Neuropathol Exp Neurol 2013;72:1182–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imhof A, Kovari E, von Gunten A, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? J Neurol Sci 2007;257:72–79. [DOI] [PubMed] [Google Scholar]
- 25.Sinka L, Kovari E, Gold G, et al. Small vascular and Alzheimer disease-related pathologic determinants of dementia in the oldest-old. J Neuropathol Exp Neurol 2010;69:1247–1255. [DOI] [PubMed] [Google Scholar]
- 26.Ubhi K, Peng K, Lessig S, et al. Neuropathology of dementia with Lewy bodies in advanced age: a comparison with Alzheimer disease. Neurosci Lett 2010;485:222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson PT, Head E, Schmitt FA, et al. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol 2011;121:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jellinger KA, Attems J. Prevalence and pathology of dementia with Lewy bodies in the oldest old: a comparison with other dementing disorders. Dement Geriatr Cogn Disord 2011;31:309–316. [DOI] [PubMed] [Google Scholar]
- 29.Heyman A, Fillenbaum GG, Welsh-Bohmer KA, et al. Cerebral infarcts in patients with autopsy-proven Alzheimer's disease: CERAD, part XVIII. Consortium to Establish a Registry for Alzheimer's Disease. Neurology 1998;51:159–162. [DOI] [PubMed] [Google Scholar]
- 30.Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA. Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci 2013;5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 2010;20:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toledo JB, Arnold SE, Raible K, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer's Coordinating Centre. Brain 2013;136:2697–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy Z, Esiri MM, Jobst KA, et al. The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 1997;56:165–170. [DOI] [PubMed] [Google Scholar]
- 34.Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol 2002;103:481–487. [DOI] [PubMed] [Google Scholar]
- 35.Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 2000;57:713–719. [DOI] [PubMed] [Google Scholar]
- 36.He W, Muenchrath MN; US Census Bureau. American Community Survey Reports, ACS-17, 90+ in the United States: 2006–2008. Washington, DC: U.S. Government Printing Office; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.