Abstract
Amphetamine and stress can sensitize mesolimbic dopamine-related systems. In Pavlovian autoshaping, repeated exposure to uncertainty of reward prediction can enhance motivated sign-tracking or attraction to a discrete reward-predicting cue (lever CS+), as well as produce cross-sensitization to amphetamine. However, it remains unknown how amphetamine-sensitization or repeated restraint stress interact with uncertainty in controlling CS+ incentive salience attribution reflected in sign-tracking. Here rats were tested in three successive phases. First, different groups underwent either induction of amphetamine sensitization or repeated restraint stress, or else were not sensitized or stressed as control groups (either saline injections only, or no stress or injection at all). All next received Pavlovian autoshaping training under either certainty conditions (100% CS-UCS association) or uncertainty conditions (50% CS-UCS association and uncertain reward magnitude). During training, rats were assessed for sign-tracking to the lever CS+ versus goal-tracking to the sucrose dish. Finally, all groups were tested for psychomotor sensitization of locomotion revealed by an amphetamine challenge. Our results confirm that reward uncertainty enhanced sign-tracking attraction toward the predictive CS+ lever, at the expense of goal-tracking. We also report that amphetamine sensitization promoted sign-tracking even in rats trained under CS-UCS certainty conditions, raising them to sign-tracking levels equivalent to the uncertainty group. Combining amphetamine sensitization and uncertainty conditions together did not add together to elevate sign-tracking further above the relatively high levels induced by either manipulation alone. In contrast, repeated restraint stress enhanced subsequent amphetamine-elicited locomotion, but did not enhance CS+ attraction.
Keywords: Motivation and Reward, Uncertainty, Incentive Salience, Sensitization, Amphetamine and Stress
1. Introduction
Rewards (unconditioned stimuli or UCSs) and the Pavlovian conditioned stimuli (CSs+) or cues that predict them can take on motivational magnet properties that make them ‘wanted’ and approached. The incentive salience hypothesis suggests that mesolimbic dopamine, released in the nucleus accumbens and other brain limbic structures in response to encountering a cue for reward, is part of the mechanism that triggers surges of cue-triggered ‘wanting’ for rewards, and produces motivated attraction toward their associated cues that become ‘wanted’ CSs+ (Berridge & Robinson, 1998). Repeated exposure to drugs of abuse such as amphetamine are known to sensitize mesolimbic dopamine-related systems and to enhance incentive salience (Bradberry, Barrett-Larimore, Jatlow, & Rubino, 2000; Giorgetti, Hotsenpiller, Ward, Teppen, & Wolf, 2001; Vezina, 2004; Wyvell & Berridge, 2001). Incentive-sensitization has been suggested to be an important mechanism of addiction (M. J. F. Robinson, Robinson, & Berridge, 2014b; T. E. Robinson & Berridge, 1993).
Similarly, recent evidence suggests that uncertainty of reward delivery in CS-UCS relations, creating an approach toward a gambling-like scenario, can also raise dopamine levels and similarly contribute to the motivational attraction to cues for uncertain rewards, which could be potentially relevant to addiction-type pursuit of gambling by some individuals (Boileau et al., 2013; Hart, Clark, & Phillips, 2015; Linnet et al., 2012; M. J. F. Robinson, Anselme, Fischer, & Berridge, 2014a; Zack, Featherstone, Mathewson, & Fletcher, 2014). We have previously demonstrated the incentive salience amplification as enhancement of sign-tracking, or higher motivated attraction toward a reward-predictive CS+ when the CS-UCS relationship is uncertain (e.g., a 50% probability that sucrose UCS will follow the CS+) than when it is certain (i.e., 100%). A sign-tracking response occurs when an animal approaches, sniffs and nibbles its CS+ (insertion of a metal lever accompanied by an auditory label) that predicts subsequent sucrose reward, whereas a goal-tracking response involves Pavlovian approach instead toward the goal or sucrose dish where the UCS will appear, also triggered by the CS+ presentation (Boakes, 1977; Hearst & Jenkins, 1974). Higher attraction to an uncertain cue is paradoxical in one sense, in that it contradicts the idea that the motivation value of a reward cue should always be linearly proportional to the predictive value of that CS+. But the dissociation between motivational attraction and predictive certainty is consistent with the incentive salience hypothesis that cue ‘wanting’ (CS attraction) is separable from cue learning (CS-UCS association). Enhanced attraction to an uncertain reward CS+ is also compatible with the idea that gambling-related cues become potent triggers of incentive motivation in part because of the uncertainty of their reward association.
Potential interaction between sensitization and uncertainty is suggested by findings that exposure to uncertainty in reward conditions can promote cross-sensitization to subsequent amphetamine (Berridge & Robinson, 1998; Boileau et al., 2013; Linnet et al., 2012; Singer, Scott-Railton, & Vezina, 2012; Zack et al., 2014). Also, the excitatory effects of reward uncertainty on behavior are relatively persistent in time, similar to sensitization (Bradberry et al., 2000; Giorgetti et al., 2001; Vezina, 2004; Wyvell & Berridge, 2001), even after the uncertainty conditions are reduced (M. J. F. Robinson et al., 2014a). However, it is not yet known how reward uncertainty and amphetamine sensitization would compare or interact regarding the motivational attraction to reward CSs+. Finally, stress interacts with drug use, cue-induced craving and reinstatement of drug-taking, and exhibits cross-sensitization to amphetamine (Berridge & Robinson, 1998; Buffalari & See, 2009; Logrip & Zorrilla, 2012; Stewart, 2003; Zorrilla, Logrip, & Koob, 2014). It is therefore of interest to know how amphetamine sensitization or repeated restraint stress might interact subsequently with reward uncertainty and sign-tracking.
Here rats were initially sensitized to amphetamine or exposed to repeated restraint stress, or else were not sensitized or stressed as controls. Then all rats received Pavlovian conditioning (autoshaping) with a lever insertion as CS+ and sucrose pellet delivery as UCS, to assess sign-tracking and goal-tracking responses in an undrugged state. Locomotor activity and sensitization was then assessed by administering a single amphetamine challenge. Based on previous work, it was predicted that (i) reward uncertainty would enhance sign-tracking, and that (ii) previous amphetamine exposure or stress exposure would similarly enhance sign-tracking toward a reward-predictive CS+.
2. Materials and methods
2.1. Animals and housing conditions
Female Sprague-Dawley rats (N = 48; 200–280 g) were bred and reared by the research group from animals purchased from Harlan, similarly to our previous studies. Rats were weaned at 21 days of age and housed (41 cm x 25.4 cm x 20.3 cm) in groups of 2–3 animals with possible litter effects controlled for during group assignment. Animals had ad-lib access to water and chow, and were food restricted to 90% of free feeding body weight during autoshaping procedures. All rats were handled for a minimum of four days prior to the start of any of the procedures. For additional details, see Robinson et al. (2014). All experimental procedures were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
2.2. Apparatus
Locomotor activity chambers
Sixteen locomotion chambers (actometers) controlled by Crossbreak software were used to measure the activity level of the rats. Locomotor activity was monitored in Plexiglas chambers (41 cm x 25.4 cm x 20.3 cm) that contained a clear plastic insert in the cage center (23 × 6.3 × 20.3 cm). Locomotor activity was measured by the total number of photocell beam breaks in a photobeam array.
Sensory stimulation and restraint stress chambers
In clear Plexiglas cages (41 cm x 25.4 cm x 20.3 cm), rats received restraint-stress by being placed in clear cylindrical restraints equipped with air holes, a tail slot, and Velcro straps, and being exposed to a light source (1000–1300 lx) and loud sounds supplied by a continuous hard rock album (by Iggy Pop & The Stooges; 80–86 dB).
Autoshaping
Each rat was trained and tested in one of eight Med Associates autoshaping chambers (30.5 cm x 24.1 cm x 21.0 cm) with Plexiglass floor, ceiling, and walls. Each chamber was equipped with a magazine dish (3 cm x 2 cm x 1 cm) and with two levers (one acting as a CS and the other as a control stimulus) on either side of the magazine, and two video cameras. For more details, see Robinson et al. (2014).
2.3. Procedure
2.3.1. Phase 1: Sensitization induction
Amphetamine sensitization
On day 0, all rats (amphetamine sensitization and saline control groups) received an intraperitoneal injection of saline (1 ml/kg) and were allowed to habituate in the novel locomotion chambers for 45 minutes to measure their baseline locomotor activity. Starting on day 1, depending on group assignment, rats received either amphetamine injections (N = 16; 2 mg/kg, i.p.) or saline (N = 16) each day for 7 days, and each time were immediately afterwards placed in the locomotion chamber. To assess locomotion, photobeam breaks were measured in 5-minute bins for the span of 45 minutes. Following the 7 days of injections, rats were returned to their home cage to allow sensitization to incubate for an additional period of 7 days.
Repeated restraint stress
Stress rats were daily exposed to restraint and sensory-stress stimulation (N = 16; exposure to bright light and loud discordant soundtrack), for a period of 45 minutes each day for 7 days. Restraint has previously been shown to produce a robust activation of the hypothalamic-pituitary-adrenal axis (Evanson & Herman, 2015; Maghsoudi et al., 2014), while sensory-stress stimulation potentiates mesolimbic elicitation of defensive treading and is avoided when given a choice (Reynolds & Berridge, 2008). No Stress control animals were singly placed in a separate cage for 45 minutes each day for 7 days and allowed to freely explore. All animals were then returned to their home cage and allowed to incubate for 7 days.
Prior to Pavlovian training, rats were pre-exposed to sucrose pellets in their home cage, and received one day of magazine training (25 pellets were dropped one-by-one into the magazine dish in the absence of CS lever presentation, 30–90 s ITI) 7 days after the last day of sensitization treatments.
2.3.2. Phase 2: Pavlovian autoshaping
Rats in the amphetamine, saline, stress, and no stress groups were further subdivided into either CS-UCS certainty (C, 100%-1) or uncertainty (U, 50%-1-2-3) autoshaping conditions. In the certainty condition, 100% of CS lever presentations were immediately followed by the delivery of a single sucrose pellet. In the uncertainty condition, CS lever presentations were either followed by no sucrose pellet 50% of the time or by either 1, 2, or 3 pellets with an equal probability (16.7%) for the remaining 50% of CS+ presentations – rewarded and non-rewarded trials were intermixed on a random basis. We showed elsewhere that, in itself, the variability in food amounts used here generates a similar performance to that obtained with fixed food amounts (100%-1), but that it contributes to enhance performance when associated with a 50% probability of reward (Anselme, Robinson, & Berridge, 2013). All groups underwent 8 autoshaping sessions over 8 days. In every condition each rat received a total of 36 CS+ presentations per session for a total of 36 UCS sucrose pellets delivered. Each CS+ presentation consisted of the insertion of a lever into the chamber for 8 seconds, accompanied by a 2.9 kHz tone and a light at the base of the lever. Sucrose pellet delivery to the magazine dish occurred immediately after retraction of the lever. Seven groups of eight individuals were obtained: amphetamine + certainty (Amph+C), amphetamine + uncertainty (Amph+U), saline + certainty (Saline+C), saline + uncertainty (Saline+U), stress + certainty (Stress+C), stress + uncertainty (Stress+U), and no stress + uncertainty (NoStress+U). The saline groups were used as controls for comparison to both the amphetamine and stress groups. Indeed, based on the results of group NoStress+U, we will see that the injection of saline neither impacted performance in autoshaping or performance during the test of locomotor sensitization.
Sign-tracking versus goal-tracking assessment
An animal was classified as a sign-tracker if it was to sniff, nibble, bite and grasp the CS+ lever resulting in lever presses three times more frequently than it did the sucrose dish during CS+ presentations on the final training day. The criterion for classification as goal-tracker was to sniff, nibble, bite and grasp the dish resulting in magazine entries at least three times more frequently than the lever during CS+ presentations on the last day of training. An individual could be classified as a mixed responder if it directed between 33% and 66% of its total number of responses to lever CS+, and the remaining responses to the goal dish (Flagel, Watson, Robinson, & Akil, 2007). All animals developed some form of conditioned response across testing, whether sign-tracking, goal-tracking or some mixture of both.
2.3.3. Phase 3: Test of locomotor sensitization
Following the last day of autoshaping, all animals received an amphetamine injection (0.75 mg/kg, i.p.) and were placed in locomotion chambers (the same as in Phase 1) for 45 minutes. Activity levels were measured in 5 minutes bins. Animals in the stress and no-stress conditions first received a saline injection and were placed in locomotion chambers for 45 minutes (to control for habituation and provide a baseline recording of general activity) prior to receiving an amphetamine injection.
2.3.4 Statistical analysis
Mixed ANOVAs were used for most between- and within-subject comparisons. Planned comparisons allowed us to assess one data set relative to another. One-way ANOVAs were used as appropriate. Two-tailed tests were used, and the null hypothesis was rejected at p < 0.05. All measurements are indicated as mean ± SE. Due to recording malfunction, data for one animal in the Saline+C group was removed from the analysis on day 5 of autoshaping.
3. Results
3.1. Phase 1: Sensitization induction
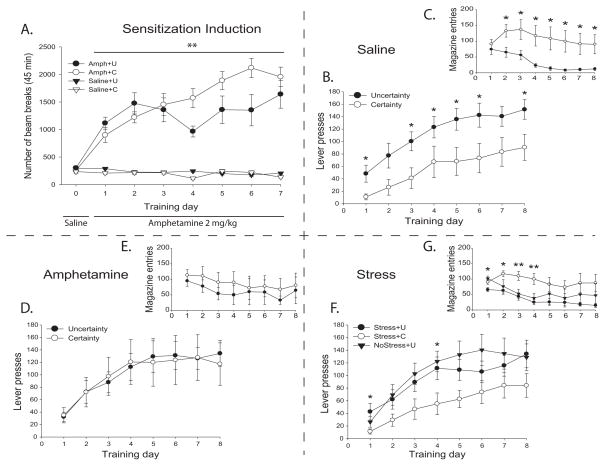
Saline and amphetamine groups did not differ initially in locomotion following a saline injection on day 0 (F(1,28) = 0.109, p = 0.744). During initial induction of sensitization (Figure 1A), amphetamine administration elevated locomotor responses (beam breaks) compared to all rats receiving saline injections (Group: F(1,28) = 114.077, p = 0.000). Further, amphetamine-exposed rats showed signs of an incrementally sensitized locomotor response with increased activity across the 7 days of sensitization induction (Day: F(7,196) = 22.574, p = 0.000; Group × Day interaction: F(7,196) = 27.491, p = 0.000), which became increasingly pronounced (d1–7: F(1,28) = 43.675, p = 0.000). By contrast, locomotion levels remained unchanged across the 7 days in the saline group (d1–7: F(1,28) = 0.446, p = 0.510) (Figure 1A). Overall, these results suggest that repeated amphetamine administration produced detectable sensitization over the 7-day period, reflected as increased locomotion over initial basal activity level. The amphetamine sensitization regimen used here did not significantly reduce body weight below saline group levels, which might have otherwise influenced subsequent sign-tracking and goal-tracking behavior (F(1,30) = 1.719, p = 0.200).
Fig. 1. Amphetamine sensitization and performance in autoshaping under reward certainty or reward uncertainty.
(A) Greater locomotor activity and sensitization in response to repeated amphetamine (but not saline) injections. Autoshaping: Saline treated animals showed greater cue attraction in the form of sign-tracking (B) under conditions of reward uncertainty, and lower levels of goal-tracking (C). Amphetamine groups did not differ in terms of lever presses (D) or magazine entries (E) on any day. Chronically stressed animals exposed to uncertain reward conditions showed an increased attraction to the cue (F) and decreased attraction to the goal (G) on certain days. Legend: * p < 0.05; ** p < 0.01; data is Mean +/− SEM.
3.2. Phase 2: Pavlovian sign-tracking (uncertainty, prior sensitization and prior stress effects)
Uncertainty versus Certainty of CS-UCS Prediction
Following an additional week to allow for incubation of any sensitization treatment, autoshaping training was conducted for all rats, and individuals were assessed for sign-tracking versus goal-tracking phenotypes. Exposure to reward uncertainty during Pavlovian autoshaping in saline-treated rats (Saline+U group) led to significantly greater sign-tracking attraction toward the CS+ lever over 8 days, as measured by grasps and/or consummatory nibble and bite responses on the metal object, resulting in lever presses, compared to similar saline-treated rats that were trained under certainty conditions (Saline+C group) (Figure 1B). There was an effect of Group (F(1,14) = 6.373, p = 0.024), and of Day (F(7,98) = 22.548, p = 0.000), but no Interaction (F(7,98) = 0.544, p = 0.799). Rats in group Saline+U pressed the CS lever 167% more than rats in group Saline+C on day 8. As noted in Figure 1B, a significant difference between groups Saline+C and Saline+U was shown for six of the training days (F(1,14)’s ≥ 4.683, p’s ≤ 0.048). Both saline-treated groups gradually increased response rates across 8 days of training (d1–8: C: F(1,14) = 24.226, p = 0.000; U: F(1,14) = 40.637, p = 0.000).
Conversely, the number of goal-tracking responses (Figure 1C), reflected by magazine beam-breaks caused by nose entries into the sucrose dish during CS+ presentations, declined under uncertainty conditions from on average 75 on the first day to approximately 10 by the final few days (F(1,14) = 8.136, p = 0.013). By contrast, goal-tracking responses for rats in the certainty conditions remained relatively stable over the week, and did not change significantly from day 1 to day 8 (F(1,14) = 0.001, p = 0.973). Consequently, the uncertainty group of saline-treated rats emitted markedly fewer goal-tracking responses than the certainty group by day 8 (F(1,14) = 6.608, p = 0.022). Among the sign-trackers of the two saline groups, 100% of individuals emitted at least some goal-tracking responses but the amount of those responses remained limited (≤ 41 on day 8). Figure 1C indicates an overall goal-tracking difference between groups Saline+U and Saline+C (F(1,14) = 7.519, p = 0.016) as well as an effect of Day (F(7,98) = 5.576, p = 0.000), and a Group x Day interaction (F(7,98) = 2.313, p = 0.032). Consistent with the evidence shown above that uncertainty enhanced sign-tracking responses, uncertainty also lowered goal-tracking responses (probably due to response competition as rats can only perform one behavior at a time). As noted, a significant difference was shown on all but the first day (F(1,14)’s ≥ 5.396, p’s ≤ 0.036). Here also, 100% of goal-trackers (18.7% of saline-treated individuals) emitted a small number of sign-tracking responses (≤ 75 on day 8).
Incidence of goal-tracker versus sign-tracker phenotypes
A direct comparison between certainty and uncertainty groups indicated that uncertainty produced more sign-trackers (Saline+U = 100%; Saline+C = 50%), and fewer goal-trackers (Saline+U = 0%; Saline+C = 37.5%). For these groups, uncertainty actually abolished the existence of any individuals who met criterion for classification as a ‘goal-tracker’ phenotype (i.e., 3 times more approaches to goal dish than to lever during CS+ lever presentations; although we cannot rule out the possibility that the uncertainty group contained more exclusive sign-trackers by chance).
Prior amphetamine-sensitization effects
In contrast to saline-treated rats described above, all rats that had previously been exposed to amphetamine-sensitization (Amph+C and Amph+U) displayed relatively higher sign-tracking approaches to the CS+ lever than saline-exposed rats even when trained under certainty conditions (Figure 1D). In this respect, rats trained with CS-UCS certainty that had earlier received amphetamine exposure (Amph+C) pressed their CS+ lever more than saline-treated rats trained under certainty as described above (Saline+C) (t126 = 3.219, p = 0.002). Adding uncertainty to amphetamine-sensitization as a combination (Amph+U) did not further elevate sign-tracking responses detectably above the already elevated sensitized level (Amph+C) indicating the two forms of sign-tracking elevation were not additive here or that the elevation had reached a measurement ceiling (Group: F(1,14) = 0.002, p = 0.962; Interaction: F(7,98) = 0.236, p = 0.975) (Figure 1D). Both groups showed a steady increase in lever responses across the 8 daily sessions (Day: F(7,98) = 13.325, p = 0.000), a clear indication that both groups correctly learned the task (d1–8: C: F(1,14) = 11.943, p = 0.004; U: F(1,14) = 18.322, p = 0.001), but the two groups did not significantly differ on any day.
However, despite higher sign-tracking responses, amphetamine-sensitization rats did not necessarily make fewer goal-tracking entries into the sucrose magazine than saline-treated rats (Amph+U vs. Saline+U: F(1,14) = 1.386, p = 0.259; Amph+C and Saline+C: F(1,14) = 0.248, p = 0.626). It should be noted also that goal-tracking performance did not significantly change between day 1 and day 8 for the rats sensitized to amphetamine (U: F(1,14) = 0.653, p = 0.432; C: F(1,14) = 0.753, p = 0.400), unlike the decline over days seen in the saline-treated uncertainty group above. Also, amphetamine-sensitization rats all showed similar numbers of goal-tracking responses regardless of whether trained under uncertainty or certainty conditions: although there seemed to be a slight trend for uncertainty rats to goal-track less (Figure 1E), that difference did not reach statistical significance (F(1,14) = 0.451, p = 0.513). Overall, it appears that amphetamine-sensitization enhanced sign-tracking without reducing goal-tracking, and possibly even protected goal-tracking from being significantly reduced by concomitant uncertainty training (only a slight and non-significant trend toward reduction of goal-tracking was observed). That sensitization pattern of sign-tracking enhancement was different from the uncertainty pattern described above, which enhanced sign-tracking at the expense of reduced goal-tracking.
Incidence of goal-tracker versus sign-tracker phenotypes
How is it possible that amphetamine maintained goal-tracking responses at normal levels while enhancing sign-tracking? In terms of individual classification as phenotypes, amphetamine-sensitized rats were primarily sign-trackers (Amph+U = 75%; Amph+C = 62.5%), with only few goal-trackers (Amph+U = 12.5%; Amph+C = 37.5%), and only one animal in the uncertain group displaying mixed behavior. Of the animals exposed to certainty following sensitization, sign-tracking responses ranged from 230 to 5, while goal-tracking ranged from 281 to 0 (3 goal-trackers = 281–171, all others ≤ 8). Among animals sensitized and exposed to uncertainty, sign-tracking responses ranged from 205 to 34, while goal-tracking responses ranged from 354 to 0 on day 8 (single goal-tracker = 354, all others ≤ 84). Consequently, the lack of a significant decrease in goal-tracking in sensitized animals under uncertainty may have been largely driven by a single goal-tracker and explained by a high standard error (Amph+U: SE = 42.8). In fact, 354 magazine entries is the largest number of goal-tracking responses done by any animal across all groups and days, which may suggest that in the few cases where goal-tracking behavior prevails under conditions of uncertainty, the addition of amphetamine sensitization produces extreme levels of attraction to the goal.
Prior-stress effects
Previous stress exposure did not elevate sign-tracking under certainty conditions compared to non-stressed rats, unlike amphetamine sensitization. For example, the no stress/no-injection control group (NoStress+U) did not differ from the stressed group (Stress+U) under uncertainty conditions (Group: F(1,21) = 0.272, p = 0.608) (Figure 1F), nor did the saline-injected control groups differ from stressed groups under certainty conditions (Stress+C versus Saline+C: F(1,14) = 0.006, p = 0.937) or under uncertainty conditions (Stress+U versus Saline+U: F(1,14) = 0.833, p = 0.377). However, adding uncertainty to prior over-stimulation restraint stress did appear to marginally raise sign-tracking in Stress+U rats compared to Stress+C rats, indicating that uncertainty can elevate sign-tracking in animals previously exposed to chronic stress (Group: F(1,14) = 4.274, p = 0.058).
Figure 1G shows that, here also, goal-tracking responses for groups Stress+U and NoStress+U were very similar (F(1,21)’s ≤ 0.669, p’s ≥ 0.422), except on day 1 (F(1,21) = 12.691, p = 0.002). Although there was a trend, no overall difference was shown between the two uncertainty conditions (Stress+U and NoStress+U) and Stress+C rats (F(2,21) = 3.226, p = 0.060). The Stress+U rats alone did however show lower levels of magazine entries when compared to Stress+C (F(1,14) = 13.031, p = 0.003), particularly during the first four days of training (F(1,21)’s ≥ 5.935, p’s ≤ 0.024).
Incidence of goal-tracker versus sign-tracker phenotypes
Again, uncertainty groups had a higher proportion of sign-trackers than certainty groups, regardless of whether they were previously stressed: Stress+U (ST = 87.5%; GT = 0%), NoStress+U (ST = 87.5%; GT = 12.5%), and Stress+C (ST = 50%; GT = 25%). As with all groups across all conditions, there was a general trend for more sign-trackers than goal-trackers.
The main result emerging from this analysis is that current CS-UCS uncertainty for both non-sensitized rats and prior amphetamine sensitization produces similar increases in sign-tracking performance, but reduces goal-tracking performance only in saline-treated rats. Current uncertainty and prior amphetamine sensitization groups become similar in sign-tracking under these conditions, and show higher sign-tracking than their certainty control or unsensitized control conditions. However, uncertainty and drug sensitization did not combine additively to produce a super-elevation in a combined Amph+U group above those separately elevated levels (compared to Amph+C or Saline+U groups). Finally, repeated sensory and restraint stress exposure alone, by comparison, does not produce as robust an elevation in sign-tracking, which allows the effects of uncertainty to still be detected, at least on some training days.
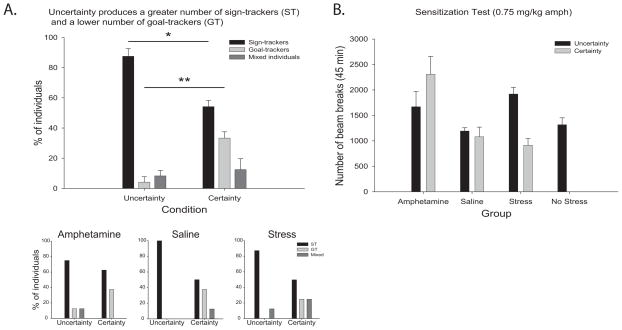
Comparing sign-tracker versus goal-tracker phenotypes across all training, sensitization and stress conditions, uncertainty conditions generally produced a higher proportion of sign-tracker individuals (87.50%; F(1,5) = 22.857, p = 0.005) and fewer goal-trackers (4.17%; F(1,5) = 24.143, p = 0.004) than certainty (ST = 54.17%; GT = 33.33%). Very few individuals showed a mixed phenotype, and the low proportion was equivalent in both uncertainty and certainty groups (F(1,5) = 0.714, p = 0.436). The propensity of uncertainty to generate a larger proportion of sign-trackers (and hence a lesser proportion of goal-trackers) had previously been suspected (Anselme et al., 2013; M. J. F. Robinson et al., 2014a) but not confirmed until now (Figure 2A).
Fig. 2. Distribution of sign-trackers and goal-trackers, and test of locomotor (cross-) sensitization.
(A) Percentage of sign-trackers (ST), goal-trackers (GT), and mixed individuals in uncertainty groups as opposed to certainty groups on day 8, showing a greater proportion of sign-trackers and lower proportion of goal-trackers following reward uncertainty. Inserts show the percentage of individuals for each autoshaping phenotype across Amphetamine, Saline and Stress conditions. (B) Following an amphetamine challenge (0.75 mg/kg), rats sensitized to amphetamine and exposed to either certain or uncertain reward conditions showed increased locomotor activity in comparison to their saline treated counterparts. Of the chronically stressed rats only those exposed to uncertain reward conditions showed cross-sensitization and enhanced locomotor activity. Rats that received saline injections or No Stress conditions showed similar performance whether trained under uncertainty or certainty. Legends: * p < 0.05; ** p < 0.01; data is Mean +/− SEM.
3.3. Phase 3: Confirmation of psychomotor sensitization
Following the final day of Pavlovian autoshaping testing, all rats received an amphetamine challenge (0.75 mg/kg, i.p.) and were placed in locomotion chambers for 45 min to confirm whether psychomotor sensitization had been previously induced by either earlier amphetamine treatment or earlier stress exposure (Figure 2B). One-way ANOVA revealed an overall sensitization effect of increased locomotor activity for both the amphetamine-treatment group (regardless of certainty/uncertainty) and the stress-treatment group (at least in uncertainty condition) compared to saline and no stress groups (Group: F(6,49) = 5.746, p = 0.000). That is, group Stress+U displayed higher or sensitized locomotion in comparison with group Saline+U (F(1,49) = 6.059, p = 0.017), and Stress+U was similarly higher than NoStress+U (F(1,49) = 4.164, p = 0.047), although Stress+C and Stress+U groups did differ (F(1,49) = 11.611, p = 0.001). Similarly, prior amphetamine sensitization elevated locomotion in rats trained under certainty (Amph+C vs. Saline+C: F(1,49) = 17.254, p = 0.000), but not under uncertainty (Amph+U vs. Saline+U: F(1,49) = 2.614, p = 0.112). However, a comparison of the two amphetamine groups (C and U) with the two saline groups (C and U) indicated amphetamine-induced sensitization (F(1,30) = 11.026, p = 0.002). The saline groups should be relevant controls for the stress groups, as control groups did not differ from each other: neither between Saline+U and NoStress+U (F(1,49) = 0.177, p = 0.676) nor between Saline+U and Saline+C (F(1,49) = 0.144, p = 0.706).
4. Discussion
Here we confirm that uncertainty about the prediction triggered by a CS+ for sucrose reward as UCS enhances sign-tracking behavior (approaches and grasping, nibbling and biting of metal lever) leading to greater attraction toward that CS+ lever in Pavlovian autoshaping, compared to certainty conditions. The sign-tracking enhancement by uncertainty occurred at the reciprocal expense of goal-tracking. Uncertainty enhancement of sign-tracking was observed for unsensitized (no previous amphetamine), and repeated restraint stress rats. Consequently, there was a greater proportion of sign-tracker phenotypes, and lower proportion of goal-tracker phenotypes in these groups, as well as a higher number of overall sign-tracking responses in individuals when trained and tested under uncertainty conditions, compared to under CS-UCS 100% certainty conditions.
By comparison, we report that prior sensitization to amphetamine induced an equivalent elevation of sign-tracking here, even under current certainty conditions, but did not reciprocally decrease in goal-tracking under any training condition. We also report that, once sign-tracking was elevated by either current uncertainty conditions or previous amphetamine sensitization, no further elevation was produced by combining both manipulations together, perhaps indicating a ceiling effect for sign-tracking enhancement. Our results suggest that prior amphetamine sensitization and current CS-UCS uncertainty are equivalent potentiators of incentive salience attributed to a discrete CS+ cue that predicts reward.
Amphetamine sensitization
Our finding of increased sign-tracking in female rats after amphetamine sensitization is similar to the report of Doremus-Fitzwater and Spear (Doremus-Fitzwater & Spear, 2011). However, we note that opposite sensitization enhancement of goal-tracking has been reported by others (Simon, Mendez, & Setlow, 2009). Although the reason for different results in our study and Simon and colleagues’ remains unknown, we note that their study was done in male rather than female rats and involved 5 rather than 7 daily injections of amphetamine. Also, their sensitization was conducted in the home cage whereas ours was conducted in a novel context, and home administration of amphetamine has been reported to diminish or modify sensitization induction compared to administration in a novel context (Crombag et al., 2001).
Here we found, rather unusually, that when amphetamine-sensitization enhanced sign-tracking, goal-tracking still persisted at moderate levels and did not decline in amphetamine-sensitized rats, not even when uncertainty during training was added to prior amphetamine-sensitization. The persistence of sensitized goal-tracking responses at control levels seems at least a step toward Simon et al.’s (2009) findings, in that goal-tracking defected from its usual reciprocal relationship to sign-tracking, suggesting that amphetamine-sensitization may have contributed to goal-tracking maintenance as a competitive alternative response. It may also reveal a potential incentive-salience component of goal-tracking, as a motivated attraction to the sucrose dish, particularly in the few animals displaying a goal-tracking phenotype. We acknowledge that sign-tracking is generally taken to more purely reflect Pavlovian incentive salience (Flagel et al., 2011; Saunders & Robinson, 2012), whereas goal-tracking can be mediated separately either by a cognitive expectation of impending sucrose delivery (i.e., an act-outcome representation) or by a simple stimulus-response motor habit (i.e., traditional conception of Pavlovian conditioned approach). However, enhanced goal-tracking can occur as a motivated response in goal-tracking individuals, together with faster approach and more intense consummatory sniffs, licks and nibbles of the metal sucrose dish, after the same brain limbic stimulations that enhance motivated sign-tracking in sign-tracking individuals, such as mu opioid-stimulation of the central amygdala by DAMGO microinjections (DiFeliceantonio & Berridge, 2012; Mahler & Berridge, 2009). Enhanced goal-tracking as a motivated response was interpreted as indicating that a third psychological component, namely, incentive salience targeted toward the dish, can potentially contribute to goal-tracking in some individuals, especially in states of mesocorticolimbic activation. Similarly, acute amphetamine administration has been reported to increase goal-tracking while drug is on board (Holden & Peoples, 2010). Finally, either amphetamine or opioid stimulation of nucleus accumbens can increase the relative incentive salience of a reward-proximal CS (analogous to the sucrose dish here) at the expense of a more predictive but reward-distal CS (analogous to the CS+ lever here) (Smith, Berridge, & Aldridge, 2011; Tindell, Berridge, Zhang, Peciña, & Aldridge, 2005). These considerations highlight that mesolimbic activation can produce complicated patterns of effects on incentive salience of lever versus dish as potential CSs, with consequences for sign-tracking versus goal-tracking. That complexity needs further research and explanation to better understand the conditions under which sign-tracking versus goal-tracking becomes most enhanced, but in general our results do fit a hypothesis that sensitization can enhance ‘wanting’ for a reward-predictive cue.
Uncertainty
The excitatory effect of reward uncertainty in CS+ autoshaping has long been noted (Anselme et al., 2013; Boakes, 1977; Collins, Young, Davies, & Pearce, 1983; Gibbon, Farrell, Locurto, Duncan, & Terrace, 1980; Gottlieb, 2004; Kaye & Pearce, 1984; M. J. F. Robinson et al., 2014a) although its explanation also remains controversial. Some authors have suggested that increased behavioral vigor in uncertainty reflects the animal’s aversive frustration caused by intermittent non-reward (Amsel, 1958; Papini, 2003). However, we hypothesized that it was caused by increased appetitive ‘wanting’ directed to the CS+ (Anselme, 2015; Anselme et al., 2013) because the increased approach occurs under Pavlovian autoshaping conditions even when no lever-pressing response has ever been instrumentally reinforced, and because evidence suggests that uncertainty in rewards may stimulate the midbrain-mesolimbic dopamine system both in animals and humans (Anselme & Robinson, 2013; Boileau et al., 2013; L. Clark, Lawrence, Astley-Jones, & Gray, 2009; Dreher, Kohn, & Berman, 2006; Fiorillo, Tobler, & Schultz, 2003; Joutsa et al., 2012; Linnet et al., 2012; Singer et al., 2012; Zack et al., 2014). An appetitive interpretation may be consistent with our observation here of overlap in effect between uncertainty and prior amphetamine sensitization. Previous research has shown that amphetamine sensitization promotes mesolimbic reactivity in the form of increased dopaminergic release in areas such as the nucleus accumbens, ventral pallidum and amygdala, and greater motivation for drug rewards (Vezina, 2004). Behaviorally, sensitization enhances incentive salience by increasing excitatory associations (Harmer & Phillips, 1999a; 1999b) and ‘wanting’ (Tindell et al., 2005) for reward associated cues, all of which can be expected to affect levels of sign-tracking which has been suggested to heavily rely on dopaminergic activity (Flagel et al., 2011; Saunders & Robinson, 2012). Our conclusion is simply that uncertainty and sensitization produced similar effects, namely with the procedures used here, to enhance sign-tracking toward the reward-predictive CS+ lever.
Ceiling effects for Sensitization + Uncertainty combination?
It might have been expected that prior amphetamine sensitization combined with current uncertainty together (Amph+U) would produce a greater sign-tracking attraction to the reward CS+ lever than either manipulation alone (Saline+U or Amph+C). But it did not: the combination produced an elevation over control levels that was merely equivalent to elevations produced either by sensitization alone or by uncertainty alone. That the whole was less than the sum of its parts suggests that some sort of measurement ceiling existed which precluded further enhancement. A ceiling effect at either the behavioral level or in mesocorticolimbic circuitry may have occurred because rats pressed up to 6 times on average per CS+ presentation (almost one press per second) on day 8. It is possible that a ceiling effect occurred centrally in mesolimbic circuitry, such as when dopamine released upon amphetamine administration occupies 50% of receptors and cannot occupy receptors any further (Kortekaas et al., 2004). Alternatively, perhaps the combination did indeed boost incentive salience further in a way that might be detected by other measurement procedures, but simply not detected here.
Sensory stress sensitizes and interacts with uncertainty for psychomotor locomotion but not motivation CS+ attraction?
Prior amphetamine pretreatment produced robust sensitization of locomotion (psychomotor sensitization) as well as of sign-tracking (incentive sensitization). Stress pre-treatment did produce psychomotor sensitization, reflected in higher drug-induced locomotion here at least for rats trained under uncertainty conditions, but did not produce significant incentive sensitization. Even the psychomotor sensitization by stress that appeared only was significant in some stressed rats (i.e. stressed and then uncertainty trained) but not others (i.e., stressed and then trained under certainty), suggesting a potential interaction between the conditions. Certainty conditions following the stress regimen could have had normalizing effects on behavior and contributed to preventing the expression of locomotor cross-sensitization. The psychomotor sensitization by stress did not transfer into incentive sensitization of CS+ motivation, suggesting either that the degree of stress sensitization was relatively weak here, or that there are differences in neural circuitry underlying psychomotor sensitization versus incentive sensitization, or both (T. E. Robinson & Berridge, 2008). Also, we note that the relationships between stress and the reward circuit are complex and may lead to apparent contradictions, such as decreased reactivity of striatal neurons (Cabib & Puglisi-Allegra, 2012). Finally, we did not find a potentiation of locomotion by mere uncertainty exposure here, though we did find a potentiation of stress psychomotor sensitization by uncertainty, even though others have reported that uncertainty training by itself can induce psychomotor sensitization to amphetamine (Singer et al., 2012). However, our procedures were quite different from Singer et al. (2012), who used a variable-ratio schedule operant task to induce uncertainty (not Pavlovian autoshaping) and a much longer training period (55 days), making it difficult to directly compare across studies.
5. Conclusion
Our results suggest commonalities in the pathways involved in the enhancement of cue attraction by reward uncertainty and amphetamine sensitization. This further ties the behavioral effects of reward uncertainty to changes in brain dopaminergic pathways. It suggests that unpredictable reward environments may result in an increased motivation towards reward related cues similarly to psychostimulant sensitization, which may be of particular importance to the development of problem gambling.
Acknowledgments
We thank Terry Robinson for use of his locomotion chambers and Huda Akil for use of her animal restraints. Funding for this research was provided by NIH grants (MH63649 and DA015188) to KCB.
Footnotes
The authors report no conflict of interest.
References
- Amsel A. The role of frustrative nonreward in noncontinuous reward situations. Psychological Bulletin. 1958;55(2):102. doi: 10.1037/h0043125. [DOI] [PubMed] [Google Scholar]
- Anselme P. Incentive salience attribution under reward uncertainty: A Pavlovian model. Behavioural Processes. 2015;111:6–18. doi: 10.1016/j.beproc.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Anselme P, Robinson MJF. What motivates gambling behavior? Insight into dopamine’s role. Frontiers in Behavioral Neuroscience. 2013;7:182. doi: 10.3389/fnbeh.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselme P, Robinson MJF, Berridge KC. Reward uncertainty enhances incentive salience attribution as sign-tracking. Behavioural Brain Research. 2013;238:53–61. doi: 10.1016/j.bbr.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Boakes RA. Performance on Learning to Associate a Stimulus with Positive Reinforcement. In: Davis H, Hurvitz HMB, editors. Operant Pavlovian Interactions. Hillsdale, N.J: Erlbaum Associates; 1977. pp. 67–97. Vol. Book Chapter. [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo DSS, Houle S, Wilson AA, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [11C]-( + )-PHNO. Molecular Psychiatry. 2013:1–9. doi: 10.1038/mp.2013.163. [DOI] [PubMed] [Google Scholar]
- Bradberry CW, Barrett-Larimore RL, Jatlow P, Rubino SR. Impact of self-administered cocaine and cocaine cues on extracellular dopamine in mesolimbic and sensorimotor striatum in rhesus monkeys. Journal of Neuroscience. 2000;20(10):3874–3883. doi: 10.1523/JNEUROSCI.20-10-03874.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE. Footshock stress potentiates cue-induced cocaine-seeking in an animal model of relapse. Physiology & Behavior. 2009;98(5):614–617. doi: 10.1016/j.physbeh.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neuroscience and Biobehavioral Reviews. 2012;36(1):79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling Near-Misses Enhance Motivation to Gamble and Recruit Win-Related Brain Circuitry. Neuron. 2009;61(3):481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Young DB, Davies K, Pearce JM. The influence of partial reinforcement on serial autoshaping with pigeons. The Quarterly Journal of Experimental Psychology B, Comparative and Physiological Psychology. 1983;35(4):275–290. doi: 10.1080/14640748308400893. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2001;24(6):680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- DiFeliceantonio AG, Berridge KC. Which cue to “want?” Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behavioural Brain Research. 2012;230(2):399–408. doi: 10.1016/j.bbr.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Amphetamine-induced incentive sensitization of sign-tracking behavior in adolescent and adult female rats. Behavioral Neuroscience. 2011 doi: 10.1037/a0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher J-C, Kohn P, Berman KF. Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex (New York, NY : 1991) 2006;16(4):561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Evanson NK, Herman JP. Metabotropic glutamate receptor-mediated signaling dampens the HPA axis response to restraint stress. Physiology & Behavior. 2015 doi: 10.1016/j.physbeh.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science (New York, NY) 2003;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469(7328):53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology. 2007;191(3):599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Farrell L, Locurto CM, Duncan HJ, Terrace HS. Partial reinforcement in autoshaping with pigeons. Animal Learning & Behavior. 1980;8(1):45–59. [Google Scholar]
- Giorgetti M, Hotsenpiller G, Ward P, Teppen T, Wolf ME. Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats. Journal of Neuroscience. 2001;21(16):6362–6369. doi: 10.1523/JNEUROSCI.21-16-06362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DA. Acquisition with partial and continuous reinforcement in pigeon autoshaping. Learning & Behavior. 2004;32(3):321–334. doi: 10.3758/bf03196031. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced conditioned inhibition following repeated pretreatment with d-amphetamine. Psychopharmacology. 1999a;142(2):120–131. doi: 10.1007/s002130050870. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999b;90(1):119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Hart AS, Clark JJ, Phillips PEM. Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiology of Learning and Memory. 2015;117:84–92. doi: 10.1016/j.nlm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst ES, Jenkins HM. Sign Tracking: The Stimulus-reinforcer Relation and Directed Action. Austin, TX: Psychonmic Society; 1974. [Google Scholar]
- Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behavioural Brain Research. 2010;208(1):270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. NeuroImage. 2012;60(4):1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology Animal Behavior Processes. 1984;10(1):90–109. [PubMed] [Google Scholar]
- Kortekaas R, Maguire RP, Cremers TI, Dijkstra D, van Waarde A, Leenders KL. In vivo Binding Behavior of Dopamine Receptor Agonist (+)-PD 128907 and Implications for the “Ceiling Effect” in Endogenous Competition Studies with [11C]raclopride - a Positron Emission Tomography Study in Macaca mulatta. Journal of Cerebral Blood Flow & Metabolism. 2004;24(5):531–535. doi: 10.1097/00004647-200405000-00007. [DOI] [PubMed] [Google Scholar]
- Linnet J, Mouridsen K, Peterson E, Møller A, Doudet DJ, Gjedde A. Striatal dopamine release codes uncertainty in pathological gambling. Psychiatry Research. 2012;204(1):55–60. doi: 10.1016/j.pscychresns.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addiction Biology. 2012;17(5):920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani AM, Nooshinfar E, Tahzibi A. Effect of Chronic Restraint Stress on HPA Axis Activity and Expression of BDNF and Trkb in the Hippocampus of Pregnant Rats: Possible Contribution in Depression during Pregnancy and Postpartum Period. Basic and Clinical Neuroscience. 2014;5(2):131–137. [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Berridge KC. Which cue to “want?” Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. The Journal of Neuroscience. 2009;29(20):6500–6513. doi: 10.1523/JNEUROSCI.3875-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini MR. Comparative psychology of surprising nonreward. Brain, Behavior and Evolution. 2003;62(2):83–95. doi: 10.1159/000072439. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nature Neuroscience. 2008;11(4):423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Anselme P, Fischer AM, Berridge KC. Initial uncertainty in Pavlovian reward prediction persistently elevates incentive salience and extends sign-tracking to normally unattractive cues. Behavioural Brain Research. 2014a;(266):119–130. doi: 10.1016/j.bbr.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Robinson TE, Berridge KC. Incentive Salience in Addiction and Over-Consumption. In: Preston S, kringelbach ML, Knutson B, whybrow PC, editors. The Interdisciplinary Science of Consumption. MIT Press; 2014b. pp. 185–197. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences. 2008;363(1507):3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. European Journal of Neuroscience. 2012;36(4):2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Effects of prior amphetamine exposure on approach strategy in appetitive Pavlovian conditioning in rats. Psychopharmacology. 2009;202(4):699–709. doi: 10.1007/s00213-008-1353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Scott-Railton J, Vezina P. Unpredictable saccharin reinforcement enhances locomotor responding to amphetamine. Behavioural Brain Research. 2012;226(1):340–344. doi: 10.1016/j.bbr.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(27):E255–E264. doi: 10.1073/pnas.1101920108/-/DCSupplemental. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. Stress and Relapse to Drug Seeking: Studies in Laboratory Animals Shed Light on Mechanisms and Sources of Long-Term Vulnerability. The American Journal on Addictions. 2003;12(1):1–17. doi: 10.1111/j.1521-0391.2003.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Peciña S, Aldridge JW. Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. The European Journal of Neuroscience. 2005;22(10):2617–2634. doi: 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews. 2004;27(8):827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. The Journal of Neuroscience. 2001;21(19):7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zack M, Featherstone RE, Mathewson S, Fletcher PJ. Chronic exposure to a gambling-like schedule of reward predictive stimuli can promote sensitization to amphetamine in rats. Frontiers in Behavioral Neuroscience. 2014;8:36. doi: 10.3389/fnbeh.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: a key role in the neurobiology of addiction. Frontiers in Neuroendocrinology. 2014;35(2):234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]