Abstract
OBJECTIVE
All national organizations now recommend that women be screened for cervical cancer beginning at age 21 years, regardless of age of sexual initiation; however, studies have shown that providers continue to screen much earlier than recommended. Two federal cancer surveillance systems were used to quantify the burden of invasive cervical carcinoma among women younger than 40 years of age.
METHODS
We examined combined data from the National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program covering 92% of the U.S. population. We calculated the age-adjusted incidence of cervical carcinoma among women younger than age 40 years by age, race, ethnicity, and histology for the time period of 1999–2008.
RESULTS
For women younger than age 40 years, 78% of the cervical cancer cases were diagnosed in women aged 30–39, 21% were diagnosed in women 20–29 years of age, and 1% was diagnosed in women younger than age 20 years. There was an average of 3,063 cases of invasive cervical carcinomas annually from 1999 through 2008, with an average of 14 carcinomas per year (rate of 0.15 per 100,000 females) among those aged 15–19 years, and 125 carcinomas per year (rate of 1.4 per 100,000 females) among those aged 20–24 years.
CONCLUSION
Cervical cancer is very rare in young women. Widespread implementation of Pap testing over the past four decades has detected very few cases of cervical cancer in women younger than 25 while potentially causing harm with unnecessary follow-up interventions.
LEVEL OF EVIDENCE
III
Invasive cervical cancer is generally avoidable with appropriate screening and follow-up, which allow for detection and treatment of most lesions before invasion. Cervical cancer screening in women younger than 21 years is thought to be less effective than for older age groups; thus, all national organizations have increased the age to begin screening regardless of onset of sexual activity.1–4 Cancers that do arise in young women have been suggested to be less detectable by traditional screening, or more aggressive and likely to arise during screening intervals.5
Cervical cancer is rare in young women, yet cytologic abnormalities found with Pap tests are very common because of the high prevalence of human papillomavirus, a sexually transmitted infection, in these women. An abnormal Pap test result can lead to being labeled with a sexually transmitted disease, anxiety, extended surveillance, and invasive procedures.6 When precancerous lesions are found, they often are treated with excisional procedures, which are associated with adverse pregnancy outcomes, including preterm delivery and low birth weight.7,8 Also, precancerous lesions diagnosed in young women usually spontaneously regress without treatment.9,10 However, studies have shown that providers are continuing to overscreen and overtreat this young population.11,12
Previous studies have analyzed national surveillance data for a majority of women in the United States with invasive cervical cancer without focusing on this younger population.13–17 With the recent recommendations from all national organizations to start screening at 21 years of age, more recent nationwide data could help reassure both clinicians and patients about safety in complying with these recommendations. The purpose of this study is to use nationwide surveillance data to examine invasive cervical cancer among women younger than 40 years of age in the United States, focusing on age, race or ethnicity, and histology.
MATERIALS AND METHODS
We used data from two federal cancer surveillance programs, the Centers for Disease Control and Prevention’s National Program of Cancer Registries and the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program to examine microscopically confirmed cervical carcinoma cases diagnosed from 1999 through 2008. These data cover 42 registries, which included 92% of the U.S. population for our years of study.18 The Centers for Disease Control and Prevention Institutional Review Board approved this study.
Rates were calculated in SEER*Stat, standardized to the 2000 United States Standard Population and expressed per 100,000 females. Age-adjusted rates of cervical carcinoma incidence are reported for 5-year age group (15–19, 20–24, 25–29, 30–34, and 35–39), combined race or Hispanic ethnicity, and histology. Patients classified as white and black are non-Hispanic; Hispanic patients include females from all race categories identified as Hispanic in the medical record or by use of an algorithm.19 The “all cases” category includes patients from all races and ethnicities. Histology was reported for carcinomas only, including squamous cell, glandular, and unspecified.
Age-specific incidence rates per 100,000 females are reported for 1999–2008 using the combined National Program of Cancer Registries and SEER data with 5-year age groups (15–19, 20–24, 25–29, 30–34, and 35–39). In addition, incidence rates are reported for 1973–2008 using SEER-only data to include a longer time of follow-up and age 20 years in the younger age category (0–20, 21–24, 25–29, 30–34, and 35–39) to reflect the guideline to start screening at age 21 years. Linear trends were examined using the weighted least-squares method to determine annual percentage change among the time periods for each age category.
We also provide the number of Pap tests conducted per age group using data from the National Survey of Family Growth, which gathers information on female reproductive health as young as age 15 through 44 years.20 The survey asks information about whether a female had a Pap test in the past year. National Survey of Family Growth survey data from 2006–2010 were weighted to the U.S. Census to provide national estimates of the number of annual Pap tests per 5-year age group. These estimates of Pap tests were then divided by the number of cancers per age group to allow for a proxy of the number of Pap tests that are conducted each year in relationship to the number of cancers diagnosed each year.
RESULTS
There was an average of 3,063 cases of invasive cervical carcinomas annually in our dataset from 1999 through 2008, with an incidence rate of 4.30 per 100,000 females (Table 1). For women younger than 40 years of age, 78% of the cervical cancer cases were diagnosed in women aged 30–39, 21% were diagnosed in women 20–29 years of age, and 1% was diagnosed in women younger than 20 years of age. Up to age 14 years, incidence rates were close to zero and were suppressed from the table because of low case counts. Rates increased steadily with age from 0.15 among those 15–19 years of age to 14.16 among women 35–39 years of age. Most cancers were squamous cell carcinomas; Hispanic females had the highest rate of squamous cell carcinomas of all groups studied. Black females had a significantly lower rate of glandular carcinomas than did other females.
Table 1.
Incidence Rates* and Counts of Cervical Carcinoma by Age, Race or Ethnicity, and Histology, United States 1999–2008
| Squamous Cell
|
Glandular
|
Unspecified
|
All Carcinomas
|
|||||
|---|---|---|---|---|---|---|---|---|
| Average Annual Count | Rate (95% CI) | Average Annual Count | Rate (95% CI) | Average Annual Count | Rate (95% CI) | Average Annual Count | Rate (95% CI) | |
| All cases | 2,120 | 2.98 (2.94–3.02) | 793 | 1.12 (1.09–1.14) | 150 | 0.21 (0.20–0.22) | 3,063 | 4.30 (4.26–4.35) |
| Age (y) | ||||||||
| 15–19 | 6 | 0.07 (0.05–0.09) | 5 | 0.06 (0.04–0.07) | 3 | 0.03 (0.02–0.04) | 14 | 0.15 (0.13–0.18) |
| 20–24 | 88 | 0.98 (0.92–1.05) | 25 | 0.28 (0.25–0.32) | 13 | 0.14 (0.12–0.17) | 125 | 1.40 (1.33–1.48) |
| 25–29 | 363 | 4.15 (4.02–4.29) | 122 | 1.40 (1.32–1.48) | 33 | 0.38 (0.34–0.43) | 519 | 5.94 (5.78–6.10) |
| 30–34 | 719 | 8.02 (7.83–8.21) | 270 | 3.01 (2.90–3.13) | 46 | 0.51 (0.46–0.56) | 1,035 | 11.54 (11.32–11.76) |
| 35–39 | 944 | 9.76 (9.57–9.96) | 370 | 3.82 (3.70–3.95) | 55 | 0.57 (0.53–0.62) | 1,369 | 14.16 (13.92–14.40) |
| Race or ethnicity | ||||||||
| White | 1,263 | 2.78 (2.73–2.82) | 574 | 1.26 (1.23–1.30) | 94 | 0.21 (0.19–0.22) | 1,931 | 4.24 (4.18–4.30) |
| Black | 299 | 3.31 (3.19–3.43) | 44 | 0.49 (0.45–0.54) | 20 | 0.21 (0.18–0.25) | 362 | 4.02 (3.89–4.15) |
| Hispanic | 444 | 3.80 (3.69–3.92) | 131 | 1.14 (1.08–1.20) | 27 | 0.23 (0.20–0.26) | 603 | 5.17 (5.04–5.30) |
Rates are per 100,000 females.
CI, confidence interval.
Data are from population-based cancer registries that participate in the National Program of Cancer Registries, the Surveillance, Epidemiology, and End Results Program, or both, and meet high-quality data criteria (www.cdc.gov/uscs).
The 0- to 14-year data are suppressed because of small numbers.
Limits of CIs were 95% and were based on the Gamma method using the Tiwari modification (http://seer.cancer.gov/seerstat/).
Age-specific incidence rates of cervical carcinomas using National Program of Cancer Registries and SEER for 1999–2008 are shown in Figure 1. Of note is that over the course of the 10-year time period for those 0–19 years of age, the line could not be graphed because of small rates ranging from 0 to 0.06 and statistically significant (P<.05) decrease in incidence rates for all other age groups, with exception of those 34–39 years of age. Figure 2 provides the incidence rates using SEER data for 1973–2008. Similar findings are noted with the additional time and different age categories, again for the longer 36-year time span for females 0–20 years of age the rate was too small to graph and ranged from 0 to 0.15. A significant decrease (P<.05) in incidence is noted for all other age categories.
Fig. 1.
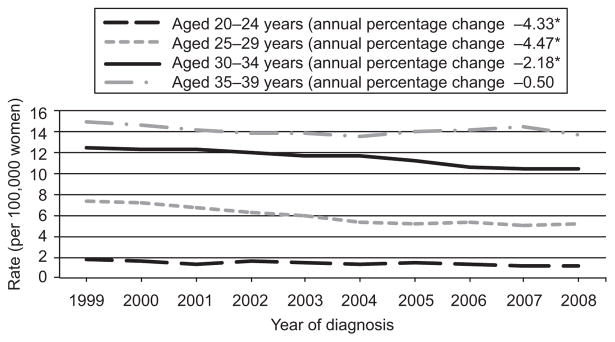
Recent trends in cervical carcinoma among females younger than 40 years of age from the National Program of Cancer Registries and Surveillance, Epidemiology, and End Results (SEER) program, 1999–2008. The estimated rates for ages 0–19 years are too close to zero to graph (rates range from 0 to 0.06). *The annual percent change is significantly different from zero (P<.05). Data from the Centers for Disease Control and Prevention’s National Program of Cancer Registries and National Cancer Institutes’ SEER Program covering 92% of the United States population for 1999–2008. Rates are per 100,000 and age-adjusted to the 2000 United States Standard Population (single ages to 84, Census P25–1130 standard).
Benard. Cervical Carcinoma Among Young Females. Obstet Gynecol 2012.
Fig. 2.
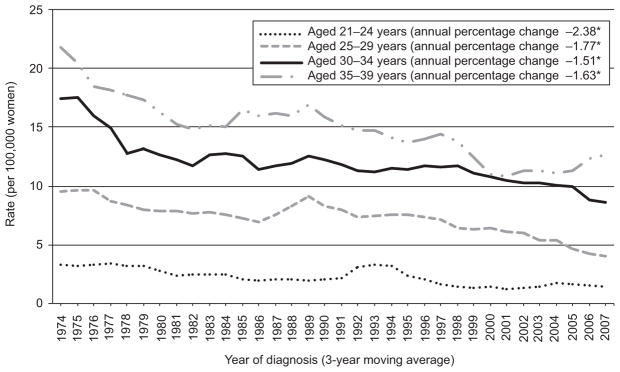
Trends in cervical carcinoma among females younger than 40 years of age from the National Program of Cancer Registries (National Program of Cancer Registries) and Surveillance, Epidemiology, and End Results (SEER) program, 1973–2008. The estimated rates for ages 0–20 years are too close to zero to graph (rates range from 0 to 0.15). *The annual percent change is significantly different from zero (P<.05). Data from the Centers for Disease Control and Prevention’s National Program of Cancer Registries and National Cancer Institutes’ SEER Program covering 92% of the United States population for 1999–2008. Rates are per 100,000 and age-adjusted to the 2000 United States Standard Population (single ages to 84, Census P25–1130 standard).
Benard. Cervical Carcinoma Among Young Females. Obstet Gynecol 2012.
Table 2 provides information on the estimated number of Pap tests per cancer case as a crude proxy for the number of Pap tests needed to prevent a single cancer or screening efficiency. The number of cervical carcinomas in each age group was taken from Table 1. National Survey of Family Growth survey data were used to provide estimates of the number of Pap tests conducted annually for each age group. For females aged 15–19 years, an estimated 2,737,000 Pap tests were conducted and an average of 14 cases of cervical cancer were diagnosed annually, or nearly 200,000 screenings per cervical cancer. For women aged 20–24 years, an estimated 6,866,000 Pap tests were conducted and an average of 125 cases of cervical cancer were diagnosed annually, or nearly 55,000 screenings per cervical cancer. As the number and rate of cancer cases increased with older age groups, the number of screenings per cancer detected declined, to a low of 4,921 Pap tests to detect one invasive cancer case among women aged 35–39 years.
Table 2.
Comparison of Annual Cervical Carcinomas and the Estimated Number of Pap Tests by Age Group
| Age (y) | Average Annual Count of Cervical Carcinomas* | Estimated No. of Pap Tests in the United States† | No. of Pap Tests Conducted for Every Cancer Diagnosed‡ |
|---|---|---|---|
| 15–19 | 14 | 2,737,000 | 194,113 |
| 20–24 | 125 | 6,866,000 | 54,753 |
| 25–29 | 519 | 7,492,000 | 14,444 |
| 30–34 | 1,035 | 6,496,000 | 6,275 |
| 35–39 | 1,369 | 6,736,000 | 4,921 |
Average annual count from National Program of Cancer Registries and the Surveillance, Epidemiology, and End Results Program, 1999–2008.
Estimated number of Pap tests in the United States from the National Survey of Family Growth.
Number of Pap tests per cancer case indicates estimated number of Pap tests yearly divided by average annual count (numbers may differ because of rounding).
DISCUSSION
Cervical cancer is not a very common cancer in the developed world and is even rarer in younger populations, with an average of only 14 carcinomas per year among those aged 15–19 years, and 125 carcinomas per year among those aged 20–24 years. Precancerous lesions are frequently found among these age groups and are more likely to regress than at older ages.9,10 Based on the rarity of invasive disease and the potential harms of overscreening and treating this young population, national organizations have all moved toward a later starting age to screen for cervical cancer regardless of onset of sexual activity. However, studies have shown that providers are continuing to overscreen and overtreat this young population.11,12 With the introduction of the human papillomavirus vaccine, the prevalence of true cancer precursors is expected to decrease, thereby increasing the rate of false-positive results and unnecessary treatment.21
Studies have shown that cervical cancers arising among young females are often less detectable by traditional screening or are more aggressive and thus more likely to arise during screening intervals.5 In our study, histology varied according to age, with a larger proportion of noncarcinomas (defined as all histologies not included in International Classification of Disease codes 8010–8671 and 8940–8941) among the youngest age groups (data not shown). These non-carcinomas were often childhood cancers and would not be detected through screening. For the youngest age group, the most common tumor diagnosis was embryonal rhabdomyosarcoma.
Additionally, cervical cancer incidence varied by race or ethnicity and age. Overall, Hispanic females had the highest rates, although the magnitude of difference by race or ethnicity was lower among younger females than in previous studies examining all ages.14 We found that black females had lower rates overall than white females, contrary to analyses including all ages. Black females also had the lowest rate of glandular carcinomas, consistent with other studies.13,14 These differences in race and ethnicity may be attributable to a combination of screening and follow-up rates as well as hormonal-based risk factors such as obesity, parity, and use of oral contraceptives.22,23
Although screening occurs less often in the youngest age group (15–19 years), an alarming 2.7 million Pap tests still are conducted annually among this age group to detect an average of 14 cases of cancer that occur annually, or just less than 200,000 Pap tests per cervical cancer diagnosed (Table 2). Assuming a screening cost of approximately $60 (Pap test with office visit),24 the total cost of screening in this age group is estimated to be approximately $164,220,000, or approximately $11,646,800 per cancer case. For women aged 20–24, the cost is $3,285,200 per cancer case diagnosed. As age increases, the rate and number of cancers per year increase, and Pap testing becomes more effective, with women in their 30s averaging approximately 5,000–6,000 Pap tests to detect one cancer case and an estimated cost per cancer case of approximately $300,000–$375,000.
We acknowledge that abnormal Pap test findings and subsequent follow-up ideally result in detecting precancerous lesions before they become cancer; thus, our calculation does not take into account cancers that may have been avoided. However, analyses examining the effect of screening on future cancer risk have found cervical cancer screening to be less effective for younger women.25,26 As found in our data, there was no change in the incidence of invasive cervical cancer across the 36-year and 10-year time periods for this younger population of females. The updated screening guidelines were based on a systematic evidence review that also provided rationale of the rarity of cervical cancer in young women.1 Additionally, a recent article by Habbema et al provides a cross-national case study of cervical cancer prevention efforts in both the United States and the Netherlands describing cancer-related outcomes to screening intensity.27 The authors found that even though women in the United States undergo more than three-times as much Pap testing as do women in the Netherlands, the decrease in cervical cancer mortality over the past five decades has been nearly identical and the rates of cancer comparable, noting that in the Netherlands women are not screened until age 30. A commentary to this article cites that the implication is clear: women in the United States typically undergo far too much screening (as well as too many false-positive results, colposcopies, and other downstream consequences).28
The goal of cervical cancer screening is to detect and treat preinvasive lesions that begin to peak in women in their late 20s.3 Colposcopy with biopsy is performed when evaluation of an abnormal Pap test result is needed.30 Recent data suggest that the risk for adverse effects from this procedure is not trivial, including pain, bleeding, and discharge.31 In addition, once precancers are identified they must be treated, and this includes potential harms both in the short-term (pain, discharge, bleeding)30 and in the long-term (increased risk of preterm delivery).7,8 Given these short-term and long-term risks and the high likelihood of regression among young women, available data suggest that screening women younger than 21 years would result in more harms than benefits;3 therefore, all screening organizations now recommend against screening before age 21 years. Therefore, comprehensive vaccination in young women may be a better and more efficacious solution for preventing cancers in young women, because we can expect that it will be 70%–80% effective and without the harms of screening.
Data from two federal cancer surveillance programs were used in this analysis. The SEER data, which only cover 9% of the U.S. population, was used to give a longer time span (36 years) to observe changes in cervical cancer rates. However, the combined data covering 92% of the population were presented to give a more complete picture of the burden of the disease. Although population-based registries covering a majority of the U.S. population provide an excellent system to measure invasive cancers, they do not capture patient-level risk factors, including tobacco use, oral contraceptive use, or screening history, which could provide additional information for reasons for cervical cancer incidence by age or race or ethnicity.31 Additionally, race and ethnicity data come from medical records that may not be accurate for a small number of cases.32 However, our findings with regard to race and ethnicity were similar to other studies.13,14 A final limitation to the collected data may be in the reproducibility of histologic classification of these cervical cancers and may affect the observed case distribution.33
Self-reported data (National Survey of Family Growth) were used to estimate the annual number of Pap tests because the United States does not currently have a national cervical cancer screening surveillance system. Additionally, the time period for current Pap tests (2006–2010) was not the same for the incidence data (1999–2008). However, we did examine the 2002 National Survey of Family Growth data and found a slight increase in the number of Pap tests, because this was before organizations updated their guidelines to suggest later initiation and longer screening intervals. Therefore, by using the current estimates of Pap test use, we are presenting a more conservative estimate.
As noted, invasive cervical cancer is extremely rare in females younger than 25 years of age. However, this age group has higher rates of transient human papillomavirus infection and regressive cervical abnormalities, the treatment of which possibly having harmful effects on future reproduction. Screening organizations have weighed the balance between potential harms associated with diagnosis and treatment with any potential benefits, and all agree that screening should not occur before age 21 years.
Acknowledgments
The authors thank Gladys Martinez, PhD, Centers for Disease Control and Prevention, National Center for Health Statistics, for providing National Survey of Family Growth data in Table 2, and Donatus Ekwueme PhD, Centers for Disease Control and Prevention, National Center for Chronic Disease Health Promotion, for consultation on costs in the Discussion section.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.U. S. Preventive Services Task Force. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. 2012 Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/cervcancer/cervcancerrs.htm. Retrieved April 25, 2012.
- 2.Cervical cytology screening. ACOG Practice Bulletin No. 109. American College of Obstetricians and Gynecologist. Obstet Gynecol. 2009;114:1409–20. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 3.Vesco KK, Whitlock EP, Eder M, Burda BU, Senger CA, Lutz K. Risk factors and other epidemiologic considerations for cervical cancer screening: a narrative review for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:698–705. doi: 10.7326/0003-4819-155-10-201111150-00377. [DOI] [PubMed] [Google Scholar]
- 4.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;137:516–42. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 5.Hildesheim A, Hadjimichael O, Schwartz PE, Wheeler CM, Barnes W, Lowell DM, et al. Risk factors for rapid-onset cervical cancer. Am J Obstet Gynecol. 1999;180(3 Pt 1):571–7. doi: 10.1016/s0002-9378(99)70256-5. [DOI] [PubMed] [Google Scholar]
- 6.Gray NM, Sharp L, Cotton SC, et al. Psychological effects of a low-grade abnormal cervical smear test result: anxiety and associated factors. Br J Cancer. 2006;94:1253–62. doi: 10.1038/sj.bjc.6603086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 8.Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscicki AB, Ma Y, Wibbelsman C, Darragh TM, Powers A, Farhat S, et al. Rate of and risks for regression of cervical intra-epithelial neoplasia 2 in adolescents and young women. Obstet Gynecol. 2010;116:1373–80. doi: 10.1097/AOG.0b013e3181fe777f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAllum B, Sykes PH, Sadler L, Macnab H, Simcock BJ, Mekhail AK. Is the treatment of CIN 2 always necessary in women under 25 years old? Am J Obstet Gynecol. 2011;205:478, e471–7. doi: 10.1016/j.ajog.2011.06.069. [DOI] [PubMed] [Google Scholar]
- 11.Yabroff KR, Saraiya M, Meissner HI, Haggstrom DA, Wideroff L, Yuan G, et al. Specialty differences in primary care physician reports of papnicolaou test screening practices: A national survey, 2006 to 2007. Ann Intern Med. 2009;151:602–11. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Saraiya M, Berkowitz Z, Yabroff KR, Wideroff L, Kobrin S, Benard V. Cervical cancer screening with both human papillomavirus and Papanicolaou testing vs Papanicolaou testing alone: what screening intervals are physicians recommending? Arch Intern Med. 2010;170:977–85. doi: 10.1001/archinternmed.2010.134. [DOI] [PubMed] [Google Scholar]
- 13.Saraiya M, Ahmed F, Krishnan S, Richards TB, Unger ER, Lawson HW. Cervical cancer incidence in a prevaccine era in the United States, 1998–2002. Obstet Gynecol. 2007;109:360–70. doi: 10.1097/01.AOG.0000254165.92653.e8. [DOI] [PubMed] [Google Scholar]
- 14.Watson M, Saraiya M, Benard V, Coughlin SS, Flowers L, Cokkinides V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer. 2008;113(Suppl 10):2855–64. doi: 10.1002/cncr.23756. [DOI] [PubMed] [Google Scholar]
- 15.Benard VB, Coughlin SS, Thompson T, Richardson LC. Cervical cancer incidence in the United States by area of residence, 1998–2001. Obstet Gynecol. 2007;110:681–6. doi: 10.1097/01.AOG.0000279449.74780.81. [DOI] [PubMed] [Google Scholar]
- 16.Carter JJ, Madeleine MM, Shera KA, Schwartz SM, et al. Human papillomavirus 16 and 18 L1 serology compared across anogenital cancer sites. Cancer Res. 2001;61:1934–40. [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan J, Patel N, Rollison D, Kortepeter K, MacKinnon J, Giuliano A. Incidence trends of invasive cervical cancer in the United States by combined race and ethnicity. Cancer Causes Control. 2009;20:1129–38. doi: 10.1007/s10552-009-9317-z. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. United States Cancer Statistics (USCS) 1999–2008 Cancer Incidence and Mortality Data. 2012 Available at: http://apps.nccd.cdc.gov/uscs/. Retrieved May 22, 2012.
- 19.Naaccr Latino Research Work Group. NAACCR guideline for enhancing Hispanic-Latino identification: revised NAACCR Hispanic/Latino identification algorithm (NHIA v2) Spring-field (IL): North American Association of Central Cancer Registries; 2005. [Google Scholar]
- 20.Centers for Disease Control and Prevention. National Survey of Family Growth. 2012 Available at: http://www.cdc.gov/nchs/nsfg.htm. Retrieved April 25, 2012.
- 21.Massad LS, Einstein M, Myers E, Wheeler CM, Wentzensen N, Solomon D. The impact of human papillomavirus vaccination on cervical cancer prevention efforts. Gynecol Oncol. 2009;114:360–4. doi: 10.1016/j.ygyno.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benard VB, Lawson HW, Eheman CR, Anderson C, Helsel W. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet Gynecol. 2005;105:1323–8. doi: 10.1097/01.AOG.0000159549.56601.75. [DOI] [PubMed] [Google Scholar]
- 23.Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. JNCI Cancer Spectrum. 2006;98:303–15. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 24.Ekwueme DU, Gardner JG, Subramanian S, Tangka FK, Bapat B, Richardson LC. Cost analysis of the National Breast and Cervical Cancer Early Detection Program: selected states, 2003 to 2004. Cancer. 2008;112:626–35. doi: 10.1002/cncr.23207. [DOI] [PubMed] [Google Scholar]
- 25.Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ. 2009;339:b2968. doi: 10.1136/bmj.b2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tota J, Franco EL. Effectiveness of cervical cancer screening at different ages. Womens Health. 2009;5:613–6. doi: 10.2217/whe.09.57. [DOI] [PubMed] [Google Scholar]
- 27.Habbema D, De Kok IM, Brown ML. Cervical cancer screening in the United States and the Netherlands: a tale of two countries. Milbank Q. 2012;90:5–37. doi: 10.1111/j.1468-0009.2011.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirovich BE. Too much of a good thing? Milbank Q. 2012;90:42–6. doi: 10.1111/j.1468-0009.2011.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 30.Tombola Group. Cytological surveillance compared with immediate referral for colposcopy in management of women with low grade cervical abnormalities: multicentre randomised controlled trial. BMJ. 2009;339:b2546. doi: 10.1136/bmj.b2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.North American Association of Central Cancer Registries. Data standards and data dictionary. Springfield (IL): NAACCR; 2012. [Google Scholar]
- 32.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 33.Thoburn KK, German RR, Lewis M, Nichols PJ, Ahmed F, Jackson-Thompson J. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer. 2007;109:1607–16. doi: 10.1002/cncr.22566. [DOI] [PubMed] [Google Scholar]


