Abstract
An enduring problem in cancer research is the failure to reproduce highly encouraging preclinical therapeutic findings using transplanted or spontaneous primary tumours in mice in clinical trials of patients with advanced metastatic disease. There are several reasons for this, including the failure to model established, visceral metastatic disease. We therefore developed various models of aggressive multi-organ spontaneous metastasis after surgical resection of orthotopically transplanted human tumour xenografts. In this Opinion article we provide a personal perspective summarizing the prospect of their increased clinical relevance. This includes the reduced efficacy of certain targeted anticancer drugs, the late emergence of spontaneous brain metastases and the clinical trial results evaluating a highly effective therapeutic strategy previously tested using such models.
Limited value of mouse therapy models
Preclinical tumour models are a fundamental component of the study and design of new regimens for cancer treatment. Nonetheless, there are considerable shortcomings in the models used, both past and present. To cite just a few examples, tumour cell lines implanted subcutaneously in mice generally tend to grow rapidly and thus do not mimic the much slower doubling times of most human cancers. This may render them, for example, much more sensitive to most chemotherapy drugs that target dividing cells. It is also unclear whether ectopic (out of the normal place) subcutaneously implanted tumours — still a standard methodology — will respond to a therapy in the same way if grown in an orthotopic site1 (in their organ or tissue of origin, such as breast cancers in mammary fat pads). In addition, tumour-bearing mice are often treated with drugs at levels, or with pharmacokinetics, that are not relevant to humans2–4. Furthermore, almost all the preclinical models that have been studied have not involved tumours that were pre-exposed to another therapy, whereas many Phase I and Phase II clinical trials involve patients who have already undergone and progressed under first, second, or even more therapies and to which their tumours have become refractory. In addition, these models fail to reflect Phase I, Phase II and most Phase III clinical trials of patients with advanced metastatic disease in multiple organ sites, which presents a much more formidable therapeutic challenge than treating localized primary tumours, single metastases or minimal residual microscopic disease.
Possible alternatives to human cancer xenografts include genetically engineered mouse models (GEMMs), which have been developed and used to study many aspects of tumour biology5. Such models are generated through alterations in the level of expression (overexpression or deletion) of genes, especially those that are relevant to the human tumorigenic process in the respective tumour type6,7, and offer several advantages, including generating orthotopic tumours in immune competent hosts that often reflect their respective human tumour histotypes and which contain a stroma and a vasculature of the same species. In this respect, they have been especially effective for studying the early events in tumorigenesis. However, they have not replaced xenograft models as reliable clinically predictive tools for examining the efficacy of therapeutic approaches to treat metastatic disease. This may be due, at least in part, to the fact that such models generally show a low incidence of distant metastatic disease8,9 (for example, <10% in Brca1+/−- and Pten+/−-mutant mice, compared with much higher incidences of metastasis in patients with germline BRCA1 and BRCA2 mutations10,11, or to the reported 59% of patients with prostate cancer that lack PTEN who have lymph node metastases12). Moreover, even when metastatic disease occurs, it often develops only after long periods of latency, and in an asynchronous manner, thus making it difficult to monitor in vivo7,13. Despite these handicaps some promising advances are being made14,15. For example, mouse models in which the oncogenes Erbb2 (also known as Neu and Her2) and polyoma middle T antigen (PyMT) have been expressed under the control of mouse mammary tumour virus (MMTV) — the MMTV–PyMT and MMTV–Erbb2 models of breast cancer16 — do develop metastatic disease (for example, to the lungs and other organs), as does the model of pancreatic islet carcinoma in which SV40 T antigen (Tag) is expressed under the control of the rat insulin promoter (RIP–Tag)17. These models have been used to test the efficacy of experimental drugs; thus, the RIP–Tag model has been used to test smallmolecule kinase inhibitors, as well as inhibitors of angiogenesis and inhibitors of matrix metalloproteinases14,18. In addition, Singh et al. used different mutant Kras GEMMs to study, in a retrospective manner, therapies that had been used in various prior successful or unsuccessful randomized Phase III trials. Excellent correlation was found when clinically relevant end points were used, such as progression-free survival based on imaging19,20. Similarly, a GEMM model of pancreatic cancer was recently used to show increased tumour perfusion, with concomitant improved delivery of chemotherapy to the tumours, following the administration of a Hedgehog pathway (Smoothened) inhibitor21,22. However, routine surgical resection of the multiple asynchromously arising primary tumours in such mice is, by definition, difficult or not practical. As such, this severely limits duplicating preoperative neoadjuvant therapy and postoperative adjuvant therapy of either early-stage microscopic metastatic disease or late-stage metastatic disease — all of which are standard clinical procedures.
Resolving the discrepancy: an approach
Because of the shortcomings of commonly used human tumour xenograft models, particularly subcutaneously transplants, and the limited numbers of mouse models that readily develop extensive visceral metastatic disease, we decided to try and improve the clinical predictive power of preclinical models based on the biology of metastasis (FIG. 1). Our approach was to initially develop new models that could develop advanced, established and spontaneous visceral metastatic disease by using transplanted cell line-based human tumour xenografts (FIG. 2). We began our studies using human breast and melanoma cell lines, as orthotopic transplantation of such tumours is relatively straightforward in comparison to other cancer types, such as lung or prostate cancer. Orthotopic transplantation1,23–27 of tumours enhances the possibility of distant metastatic spread, compared with ectopic (subcutaneous) transplants28, and advanced multiple metastases can be obtained29–33, especially if the primary tumours are surgically resected34. This prolongs survival and allows sufficient time for disseminated cells from the primary tumour to develop into established metastases, thus recreating the multiple sequential steps that are associated with the metastatic cascade34–38. The metastatic process can be further monitored if the cells are tagged with molecular markers39–41 such as luciferase41, which was a key factor in our decision to use tumour cell lines, rather than tumour tissue, to establish the xenografts.
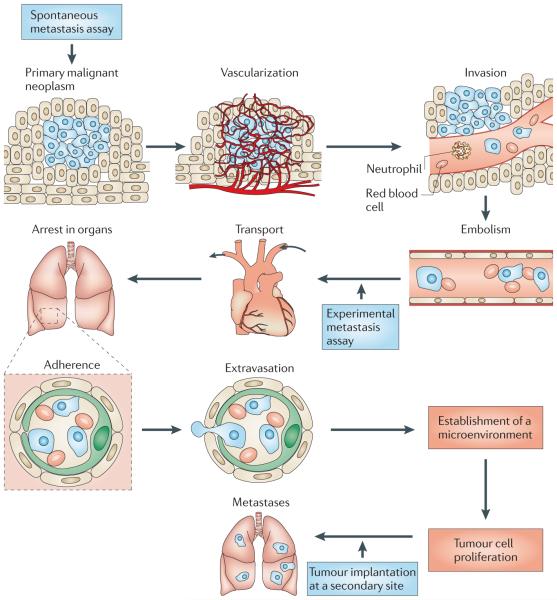
Figure 1. The metastatic cascade.
Metastatically competent primary tumours grow, invade the local host tissue and eventually shed tumour cells into the circulation. These cells travel to and colonize distant organs, and their subsequent growth at the secondary sites constitutes metastatic disease37,73. Spontaneous metastasis assays involve establishing a primary tumour that is allowed to grow and spread in the host, whereas experimental metastasis assays circumvent the initial growth and invasion stages as a result of directly injecting tumour cells into the circulation. Other assays mimic aspects of metastatic growth by seeding tumour cells at the secondary site (for example, by intrasplenic injection of colorectal cancer cells, which directly targets the cells to the liver55 where they grow as metastases). Figure is modified, with permission, from REF. 37 © (1982) Amerian Association for the Advancement of Science.
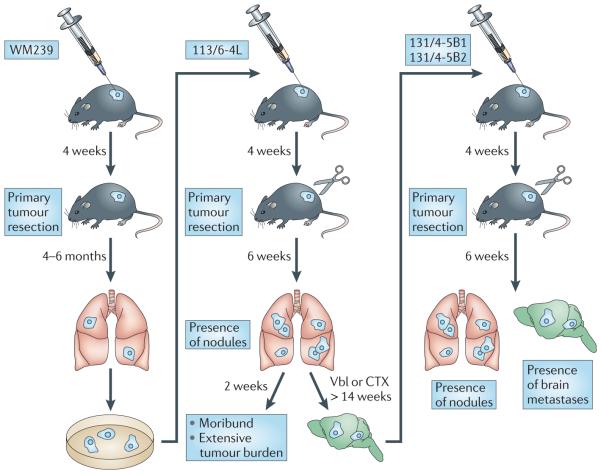
Figure 2. The selection of metastatically aggressive subpopulations of tumour cells often requires rounds of in vivo selection.
For example, a primary tumour of the human melanoma cell line WM239 was established by the injection of the cells subdermally (orthotopically) into severe combined immunodeficient (SCID) mice, allowed to grow and establish itself for 3 weeks, and was then surgically removed (to prevent the rapidly growing primary tumour from causing end point termination of the experiment). Several months later the mice showed evidence of lung metastases — from which the subline 113/6-4L was derived35. In some studies, these cells were re-implanted into a new host for an additional round of selection. This type of in vivo enrichment procedure was also used to derive, for example, the highly metastatic 231/LM2-4 variant of the human breast cancer cell line MDA-MB-231 (REF. 34). By using a similar selection procedure, the H2N/met2.hCG metastatic variant was derived from MDA-MB-231 cells that previously expressed ERBB2 by gene transduction and which were then transfected to express and secrete the β-subunit of human choriogonadotropin (β-hCG) protein, which can be used as a surrogate molecular marker of disease burden and response to therapy36,39. The treatment of mice with established 113/6-4L spontaneous metastases using a metronomic doublet vinblastine (Vbl) and CTX regimen led to prolonged survival and to the eventual emergence of brain metastases. Isolation of a brain metastases resulted in the derivation of a variant subpopulation that spontaneously metastasizes to the brain without any therapeutic intervention to prolong the survival times of mice. Figure is modified, with permission, from REF. 35 © (2008) American Association for Cancer Research.
More than 25 years ago we and other groups reported the development of metastatic melanoma models42–44. We described the derivation of variants of the human MeWo melanoma cell line that were capable of spontaneous metastatic spread44. More recently, we also derived highly metastatic variants, such as the 113/6-4l subline derived from the WM-239 human melanoma35 (TABLE 1). These highly metastatic variants were generated through two rounds of in vivo selection involving orthotopic primary tumour cell implantation, subsequent tumour resection and isolation of metastatic cells from visceral (lung) metastasis that emerged several months later (FIG. 2). For example, whereas the parental WM239 cells require 4–6 months for the formation of visible metastatic nodules after tumour resection, the 113/6-4l variant requires only 4–6 weeks for metastases to develop in the lungs and elsewhere, such as in the pleural cavity35. Variants of the human MDA-MB-231 breast cancer cell line that were capable of spontaneous metastatic spread (TABLE 1) were also isolated following a similar in vivo selection procedure45. One such variant, called 231/LM2-4, aggressively and spontaneously metastasizes to various host organs such as the liver and lungs34. Additionally, we developed the first ERBB2-positive breast cancer model of advanced visceral spontaneous metastasis36,46. MDA-MB-231 triple-negative cells (that is, breast cancer cells that do not express oestrogen receptor (ER), progesterone receptor (PR) or ERBB2) were first genetically manipulated to express high levels of ERBB2 by gene transduction47, and the resulting cell line, H2N/met2, was used to select sublines that had competence for spontaneous metastatic spread by the aforementioned strategy36,46 (FIG. 2).
Table 1.
Examples of preclinical models of spontaneous human metastases*
| Model | Common sites of metastasis | Refs |
|---|---|---|
| Breast (231/LM2-4) | Lung | 34 |
| Breast (H2N/met2) | Lung and lymph nodes | 36 |
| Colon (KM12) | Lymph nodes and liver | 23 |
| Colon (Co-3, Col-3-JCK and Col-5-JCK) | Liver | 24 |
| Colon (LSLiM6) | Lymph nodes and liver | 30 |
| Gastric (St-4, St-40, H-111 and Sc-1NU) | Lymph nodes and liver | 24 |
| Melanoma (113/6-4L) | Lung | 35 |
| Melanoma (113/4-5B1 and 113/4-5B2) | Lung and central nervous system | 35 |
| Pancreatic (PANC-4) | Liver and peritoneum | 25 |
| Ovarian (RMG-1) | Peritoneum, lymph nodes and diaphragm | 26 |
Some clinically relevant outcomes in mice
As noted above, conventional primary tumour xenograft models are often poorly predictive of whether a new drug (evaluated as a monotherapy) will be effective, and to what extent it will be effective, in a more advanced metastatic disease treatment setting. An example of this kind of discrepancy was observed when we compared the response of primary tumours with macroscopic metastases to various antibody-based drugs. Thus, using the metastatic ERBB2-positive breast cancer cell line36,46 (H2N/met2) we noted that treatment with the monoclonal antibody trastuzumab alone did not affect the growth of established visceral metastases, whereas it potently inhibited the corresponding cells that were grown as established orthotopic primary tumours. A similar observation was noted when the parental MDA-MB-231 breast cancer was implanted orthotopically; this resulted in the development of both primary tumours and distant metastases48. Monotherapy with DC101, an anti-angiogenic anti-mouse vascular endothelial growth factor receptor 2 (VEGFR2) monoclonal antibody, inhibited primary tumour growth but had no effect on metastatic disease48. By contrast, when mice were then treated with daily oral cyclophosphamide (CTX) administered through the drinking water at low minimally toxic doses over prolonged periods and with no long drug-free break periods (metronomic chemotherapy) and with DC101, the combination inhibited both primary tumour growth, as well as the appearance of metastases. These results would seem to reflect the clinical experiences of using the single agent trastuzumab or the monoclonal antibody bevacizumab against VEGF when treating patients with advanced metastatic breast cancer, as rather limited, if any, benefit is generally the outcome49,50. Instead, they must usually be combined beforehand with another drug or therapy, often chemotherapy, to provide a clinical benefit in the metastatic setting49–51, after which maintenance therapy with the antibody alone may sometimes be effective52. Thus, although primary orthotopic and perhaps even subcutaneous tumour models may be useful for the initial screening and the identification of potentially active antitumour agents, ideally they should probably be coupled with additional preclinical models involving advanced metastatic disease that confirm the therapeutic activity of the agents with respect to the appropriate tumour type and in relevant sites of distant metastasis53. If such activity is not detected, further combination treatments should be evaluated in the metastatic setting. In addition, antitumour agents should also be confirmed in GEMM models; for example metronomic CTX was also shown to have antitumour activity in the RIP–Tag model48.
To date, several studies have used advanced disease models to test new therapeutic regimens54 (TABLE 2). For example, Napoleone Ferrara’s group tested bevacizumab monotherapy on experimental liver metastases of human colon cancer cells, as well as on subcutaneously implanted tumours55. Rakesh Jain and colleagues reported the effect of anti-VEGFR1 therapy on spontaneous metastases of the B16 melanoma murine cell line, and Pratima Nangia-Makker et al. described the effect of modified citrus pectin therapy on LSLiM6 human colon carcinoma metastases. In addition, Beverly Teicher and colleagues reported the effect of alkylating agent-based therapies on EMT-6 mouse breast cancer spontaneous metastases32, as well as the effects of TNP-470-based therapies on experimental lung metastases in a fibrosarcoma model31.
Table 2.
Experimental versus spontaneous metastasis assays in cancer therapeutics*
| Experimental metastasis | Spontaneous metastasis | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Examples of use in experimental therapeutics |
|
|
CTX, cyclophosphamide; HIF1α, hypoxia-inducible factor 1\g=a\; i.c., intracardiac injection; i.s., intrasplenic injection; i.v., intravenous injection; TGFβ, transforming growth factor-β; VEGF, vascular endothelial growth factor; VEFGR, VEGF receptor.
Additional examples of spontaneous and experimental metastasis assays in cancer therapeutics were recently reviewed by Steeg and Theodorescu (REF. 68).
Effective anti-metastatic therapies
One surprising discovery to arise from the use of models of advanced multi-organ spontaneous metastasis is the responsiveness of metastases to therapies that had initially been found to be ineffective in the treatment of the primary tumours developed by those cells. For example, Ann Chambers and colleagues56 found that when the MDA-MB-435 tumour cell line was grown in the mammary fat pads of nude mice, which were subsequently placed on a diet supplemented with the antioxidant genistein (750 μg per g) or a control diet, no difference in primary tumour growth rate between the two diets was observed. By contrast, spontaneous metastases of MDA-MB-435 (in mice still harbouring the primary implants) were inhibited by the genistein diet. The selective anti-metastatic effect of genistein was confirmed by growing primary tumours and then surgically removing them — at which point the mice were placed on a genistein diet56.
We have observed similar findings when studying metastases of the MDA-MB-435 model that responded well to a metronomic chemotherapy protocol of the antimitotic agent vinblastine. This result would not have been predicted from the minimal response observed from primary tumours in control experiments3 — a discrepancy that has also been noted in other tumour models57. Similarly, we found that orthotopic 231/LM2-4 primary tumours showed a limited growth delay when mice were treated with a doublet metronomic CTX and UFT (tegafur plus uracil, an oral 5-Fluorouracil (5-FU) prodrug) chemotherapy regimen34. By contrast, the therapy was remarkably effective against established spontaneous metastases (FIG. 3). This result was important in the decision to initiate a Phase II clinical trial in patients with metastatic breast cancer of metronomic capecitabine (another oral 5-FU prodrug), with metronomic CTX, plus bevacizumab, which yielded very promising results58 that are now being evaluated in a randomized Phase III trial (ClinicalTrails.gov Identifier NCT01131195; see Further information). Such preclinical results clearly raise the question of whether many potentially promising therapeutic regimens in the past were discarded because of unimpressive effects observed on primary tumours.
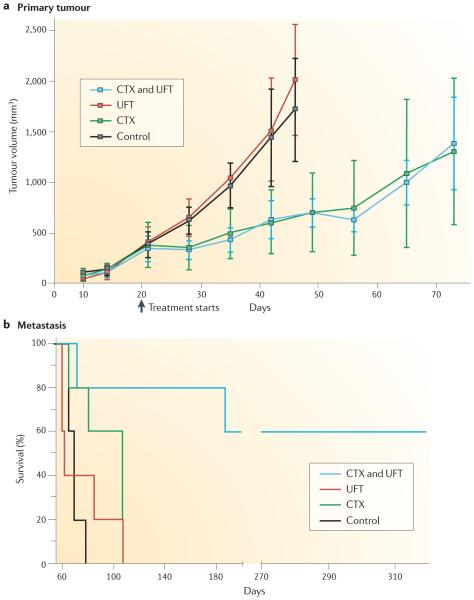
Figure 3. Differential response of metastases and primary tumours to therapy.
A disparity between primary tumours (part a) and metastasis (part b) in terms of response to therapy was observed with the human breast cancer model 231/LM2-4 treated with the combination of metronomic daily cyclophosphamide (CTX) and the 5-Fluorouracil (5-FU) oral prodrug UFT (tegafur plus uracil)34. Thus, primary tumour growth assays involving treatment showed no antitumour effect by combining UFT with CTX, and did not foreshadow the UFT and CTX doublet metronomic therapy34 (part a). Figure is modified, with permission, from REF. 34 © (2006) American Association for Cancer Research.
The aforementioned results are not specific for the MDA-MB-231 or MDA-MB-435 tumour models. For example, spontaneous metastases of the WM-239 melanoma metastatic variant 113/6-4L were found to respond to metronomic vinblastine and CTX doublet chemotherapy, whereas primary tumours responded poorly59 (FIG. 4). This model of spontaneous melanoma metastasis also mirrored the minimal clinical efficacy that was noted for a maximum tolerated dose of dacarbazine (which is standard therapy and the only uS Food and Drug Administration (FDA)-approved chemotherapy agent for malignant melanoma60) even when combined with other agents59. However, we found that a doublet metronomic dacarbazine plus CTX protocol, with vinblastine, caused an increase in the survival of mice with extensive visceral metastases59. Similar survival benefits were observed with DC101 plus metronomic vinblastine59. This result seems to confirm our earlier observations from treating mice with MDA-MB-435 metastases with metronomic vinblastine plus DC101 (REF. 3).
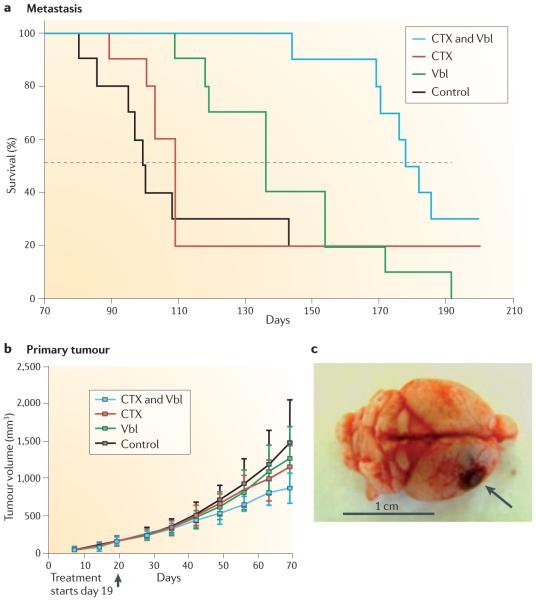
Figure 4. Treatment of visceral metastatic disease can result in the appearance of metastases to the brain.
a | Mice with established spontaneous metastases from the highly metastatic variant 113/6-4L derived from the WM239 melanoma were treated using a metronomic doublet vinblastine (Vbl) and cyclophosphamide (CTX) regimen (FIG. 2), and this led to prolonged survival and to the eventual emergence of the brain metastases. b | A therapeutic benefit was not observed when the Vbl and CTX regimen was administered to 113/6-4L grown as primary tumours. c | Image of brain metastasis from this experiment35. Figure is modified, with permission, from REF. 35 © (2008) American Association for Cancer Research.
The disparity of response to therapy between primary tumours and advanced metastases has also been reported in the rat. Thus, McCulloch and George57 found that spontaneous metastases that were derived from MTln3 rat mammary carcinoma were inhibited by warfarin treatment. The same therapy, however, did not have an effect on the growth of MTln3 subcutaneously implanted primary tumours.
Late emergence of CnS metastases
The successful control of visceral metastatic disease that was achieved in the aforementioned breast and melanoma spontaneous metastasis models resulted in the manifestation of a finding that is assuming increasing clinical importance: the emergence of central nervous system (CNS) metastases. We have noted this in studies of the 113/6-4l spontaneous melanoma model35 and also more recently in the 231/lM2-4 breast cancer model34. Ironically, this phenomenon is probably the result of the more prolonged control of systemic metastatic disease, which is also observed in certain cancer patients receiving various therapies. For example, the therapy of ERBB2-positive breast cancer with trastuzumab-based chemotherapy regimens has led to a marked increase in the incidence of brain metastases at relapse61–63. This is probably due to the increase in survival times caused by temporarily controlling visceral metastatic disease, which allows seeded microscopic metastases in the CNS sufficient time to develop into established lesions61–63. As such, this bolsters the rationale for developing preclinical models of spontaneous CNS metastases to study the biology and treatment of such lesions. In this respect, one group has reported that spontaneous CNS metastases can also develop from orthotopically implanted 4T1 mouse mammary tumour cells64.
Spontaneous brain metastatic variants from resected primary tumour transplants have recently been derived using the 113/6-4l and 231/lM2-4 metastatic models34,35. Such tumour variants with enhanced metastatic affinity for CNS35, termed 131-4-5B1 and 131-4-5B2 (melanoma) and 161/8-1B (breast cancer), can be used to investigate aspects of tropism and mechanisms of spontaneous metastasis65,66, as well as to test therapeutic strategies for the treatment of brain metastases.
Metastasis models: pros and cons
One advantage of using preclinical models of spontaneous metastasis is that they allow researchers to test therapies in laboratory conditions that mimic disease present in most clinical trials more faithfully than do the simpler subcutaneous xenograft models, for example. Nonetheless, there are several challenges associated with the setting up of spontaneous metastasis experiments7,67 (FIG. 2). For instance, once the primary tumours have grown to a considerable size (for example, 400–500 mm3) they have to be surgically resected, and at that stage several complications can arise. Tumours may grow asynchronously making it difficult to decide when surgery is to be carried out, and they can also locally invade the surrounding host tissues. Such invasion may become evident only at the time of surgery, which then becomes either impractical or more laborious, and this may reduce the number of mice that remain available for the experiment. It is also imperative that the incidence and the extent of primary tumour invasion at the time of surgery be noted, as mice with locally invasive tumours are more likely to develop metastases, and they also tend to have a worse prognosis. These factors make randomization of the mice, to ensure equal disease burden in the different treatment groups, more difficult.
One additional complication is the uneven appearance of spontaneous metastases following the surgical removal of the primary tumours; thus, in some mice detectable disease may develop 1 week after surgery, whereas in others it may take up to 1 month. This, in turn, requires further careful planning of how to randomize the mice into treatment groups, and can increase both the overall cost as well as the time it takes to complete an experiment. Such difficulties can nonetheless be offset by starting the experiment with more mice per group. Furthermore, one advantage of such complexity is that the observed difference in metastatic patterns from animal to animal is more realistic and reflective of the clinical presentation of metastasis.
TABLE 2 summarizes the major advantages and disadvantages of spontaneous metastasis assays and provides a comparison with the more commonly used experimental metastasis assay. In this assay, disseminated disease (typically to the lungs) develops following intravenous injection of tumour cells (FIG. 1). Therapy studies using experimental metastasis, in retrospect, often involve the initiation of treatment when only low volume (microscopic) disease is present68. This is actually more reflective of postoperative adjuvant therapies for occult (early-stage) disease. Patients with such disease can sometimes be cured, in part because of its minimal nature and lack of exposure to previous therapies. Nonetheless, there are also studies in which the initiation of treatment is delayed until the experimental metastases have become evident; thus, effectively treating late-stage disease69. Furthermore, the use of experimental metastasis assays has also been highly successful for investigating the tissue specificity of metastases (for example, for experimental breast cancer metastases65,66,70, using models that are also capable of spontaneous metastasis71,72), as well as their response to targeted therapies55 (TABLE 2) and the identification of possible relevant molecular drivers of metastatic disease.
Overall, given the benefits and limitations of these assays, it is reasonable to assume that a combination of both experimental and spontaneous metastasis models constitutes an effective and robust strategy to preclinically evaluate the efficacy of new therapeutic regimens.
Conclusions
On the basis of the results of the studies that have been summarized above, we suggest that the incorporation of the preclinical evaluation of anticancer drug activity against established visceral metastases constitutes an important, and perhaps even essential, step in the overall sequential development and assessment of new therapeutic regimens. As the spontaneous metastasis models outlined here recapitulate all the events involved in the multistep process of the metastatic cascade, they should also help to improve our understanding of the mechanisms that regulate metastatic spread and growth. The development of similar models involving other types of cancer, for example, colorectal, lung, renal, liver and prostate cancers, should be considered (along with strategies designed to enhance visceral metastasis in GEMMs). As illustrated by the aforementioned results, the use of these preclinical metastasis models can also lead to the development of interesting drug-resistant variants such as those that escape therapy by preferentially metastasising to the brain. Therefore, although our proposal represents a considerable leap of faith for preclinical cancer therapeutics in that spontaneous metastasis assays are more expensive and technically challenging, we would argue that this is a leap worth taking.
Acknowledgements
The authors would like to thank C. Cheng for her excellent secretarial assistance; L. Ellis, I. Hart, I. J. Fidler, C. Hackl and U. Emmenegger for their comments, past and present. R.S.K.’s metastasis therapy studies are supported by grants from the US National Institutes of Health (NIH)(CA-41233), Canadian Cancer Society Research Institute (CCSRI), Canadian Institutes of Health Research (CIHR) and the Ontario Institute for Cancer Research (OICR), as well as past or present sponsored research agreements with Taiho Pharmaceuticals, Tokyo, Japan, ImClone Systems, New York, GSK, Philadelphia and Pfizer, La Jolla, USA. R.S.K. holds a Tier I Canada Research Chair in Tumour Biology, Angiogenesis and Antiangiogenic Therapy.
Footnotes
Competing interests statement
The authors declare competing financial interests. See Web version for details.
References
- 1.Wilmanns C, Fan D, O’Brian CA, Bucana CD, Fidler IJ. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int. J. Cancer. 1992;52:98–104. doi: 10.1002/ijc.2910520118. [DOI] [PubMed] [Google Scholar]
- 2.Kerbel RS. What is the optimal rodent model for anti-tumor drug testing? Cancer Metastasis Rev. 1998;17:301–304. doi: 10.1023/a:1006152915959. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived - but they can. be improved. Cancer Biol. Ther. 2003;2:108–113. [PubMed] [Google Scholar]
- 4.Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur. J. Cancer. 2004;40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 6.Ottewell PD, Coleman RE, Holen I. From genetic abnormality to metastases: murine models of breast cancer and their use in the development of anticancer therapies. Breast Cancer Res. Treat. 2006;96:101–113. doi: 10.1007/s10549-005-9067-x. [DOI] [PubMed] [Google Scholar]
- 7.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 2007;170:793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie SG, et al. Multiple genetic changes are associated with mammary tumorigenesis in Brca1 conditional knockout mice. Oncogene. 2001;20:7514–7523. doi: 10.1038/sj.onc.1204929. [DOI] [PubMed] [Google Scholar]
- 9.Stambolic V, et al. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten± mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 10.Gourley C, et al. Increased incidence of visceral metastases in scottish patients with BRCA1/2-defective ovarian cancer: an extension of the ovarian BRCAness phenotype. J. Clin. Oncol. 2010;28:2505–2511. doi: 10.1200/JCO.2009.25.1082. [DOI] [PubMed] [Google Scholar]
- 11.Albiges L, et al. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann. Oncol. 2005;16:1846–1847. doi: 10.1093/annonc/mdi351. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz M, et al. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int. J. Cancer. 2007;120:1284–1292. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 13.Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26:513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- 14.Sharpless NE, DePinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature Rev. Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 15.Heyer J, Kwong LN, Lowe SW, Chin L. Non-germline genetically engineered mouse models for translational cancer research. Nature Rev. Cancer. 2010;10:470–480. doi: 10.1038/nrc2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim IS, Baek SH. Mouse models for breast cancer metastasis. Biochem. Biophys. Res. Commun. 2010;394:443–447. doi: 10.1016/j.bbrc.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 17.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J. Clin. Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh M, et al. Assessing therapeutic responses in Kras mutant cancers using genetically engineered mouse models. Nature Biotech. 2010;28:585–593. doi: 10.1038/nbt.1640. [DOI] [PubMed] [Google Scholar]
- 20.Francia G, Kerbel RS. Raising the bar for cancer therapy models. Nature Biotech. 2010;28:561–562. doi: 10.1038/nbt0610-561. [DOI] [PubMed] [Google Scholar]
- 21.Van DT. Approximating a human cancer. Nature Med. 2010;16:976–977. doi: 10.1038/nm0910-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 24.Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J. Cell. Biochem. 1994;56:4–8. doi: 10.1002/jcb.240560103. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53:3070–3072. [PubMed] [Google Scholar]
- 26.Kiguchi K, et al. A patient-like orthotopic implantation nude mouse model of highly metastatic human ovarian cancer. Clin. Exp. Metastasis. 1998;16:751–756. doi: 10.1023/a:1006537013317. [DOI] [PubMed] [Google Scholar]
- 27.Price JE, Zhang RD. Studies of human breast cancer metastasis using nude mice. Cancer Metastasis Rev. 1990;8:285–297. doi: 10.1007/BF00052605. [DOI] [PubMed] [Google Scholar]
- 28.Fidler IJ. Models for spontaneous metastasis. Cancer Res. 2006;66:9787. doi: 10.1158/0008-5472.CAN-06-2396. [DOI] [PubMed] [Google Scholar]
- 29.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:e4. doi: 10.1038/nature08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nangia-Makker P, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J. Natl Cancer Inst. 2002;94:1854–1862. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 31.Teicher BA, et al. Potentiation of cytotoxic cancer therapies by TNP-470 alone and with other anti-angiogenic agents. Int. J. Cancer. 1994;57:920–925. doi: 10.1002/ijc.2910570624. [DOI] [PubMed] [Google Scholar]
- 32.Holden SA, Emi Y, Kakeji Y, Northey D, Teicher BA. Host distribution and response to antitumor alkylating agents of EMT-6 tumor cells from subcutaneous tumor implants. Cancer Chemother. Pharmacol. 1997;40:87–93. doi: 10.1007/s002800050631. [DOI] [PubMed] [Google Scholar]
- 33.Barnett SC, Eccles SA. Studies of mammary carcinoma metastasis in a mouse model system. II: lectin binding properties of cells in relation to the incidence and organ distribution of metastases. Clin. Exp. Metastasis. 1984;2:297–310. doi: 10.1007/BF00135169. [DOI] [PubMed] [Google Scholar]
- 34.Munoz R, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma CNS metastasis. Cancer Res. 2008;68:4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 36.Francia G, et al. Long term progression and therapeutic response of visceral metastatic disease non-invasively monitored in mouse urine using β-hCG choriogonadotropin secreting tumor cell lines. Mol. Cancer Ther. 2008;7:3452–3459. doi: 10.1158/1535-7163.MCT-08-0200. [DOI] [PubMed] [Google Scholar]
- 37.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 38.Barnett SC, Eccles SA. Studies of mammary carcinoma metastasis in a mouse model system. I: derivation and characterization of cells with different metastatic properties during tumour progression in vivo. Clin. Exp. Metastasis. 1984;2:15–36. doi: 10.1007/BF00132304. [DOI] [PubMed] [Google Scholar]
- 39.Shih IM, et al. Assessing tumors in living animals through measurement of urinary β-human chorionic gonadotropin. Nature Med. 2000;6:711–714. doi: 10.1038/76299. [DOI] [PubMed] [Google Scholar]
- 40.Hoffman RM. Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin. Exp. Metastasis. 2009;26:345–355. doi: 10.1007/s10585-008-9205-z. [DOI] [PubMed] [Google Scholar]
- 41.Sahai E. Illuminating the metastatic process. Nature Rev. Cancer. 2007;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 42.Kozlowski JM, et al. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44:3522–3529. [PubMed] [Google Scholar]
- 43.Poste G, Doll J, Hart IR, Fidler IJ. In vitro selection of murine B16 melanoma variants with enhanced tissue-invasive properties. Cancer Res. 1980;40:1636–1644. [PubMed] [Google Scholar]
- 44.Kerbel RS, Man MS, Dexter D. A model of human cancer metastasis: extensive spontaneous and artificial metastasis of a human pigmented and derived variant sublines in nude mice. J. Natl Cancer Inst. 1984;72:93–108. doi: 10.1093/jnci/72.1.93. [DOI] [PubMed] [Google Scholar]
- 45.Price JE, Polyzos A, Zhang RD, Daniels LM. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 46.Francia G, et al. Comparative impact of trastuzumab and cyclophosphamide on HER-2 positive human breast cancer xenografts. Clin. Cancer Res. 2009;15:6358–6366. doi: 10.1158/1078-0432.CCR-09-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.du Manoir JM, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin. Cancer Res. 2006;12:904–916. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 48.Man S, et al. Antitumor and anti-angiogenic effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 49.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 50.Hudis CA. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 51.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 52.Burger RA, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group study. J. Clin. Oncol. Abst. 2010;28 LBA1. [Google Scholar]
- 53.Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–747. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- 54.Gril B, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J. Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warren RS, Yuan H, Mati MR, Gillett NA, Ferrara N. Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J. Clin. Invest. 1995;95:1789–1797. doi: 10.1172/JCI117857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vantyghem SA, et al. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65:3396–3403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 57.McCulloch P, George WD. Warfarin inhibits metastasis of Mtln3 rat mammary carcinoma without affecting primary tumour growth. Br. J. Cancer. 1989;59:179–183. doi: 10.1038/bjc.1989.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dellapasqua S, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer: clinical and biological activity. J. Clin. Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 59.Cruz-Munoz W, Man S, Kerbel RS. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin. Cancer Res. 2009;15:4867–4874. doi: 10.1158/1078-0432.CCR-08-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lawson DH. Choices in adjuvant therapy of melanoma. Cancer Control. 2005;12:236–241. doi: 10.1177/107327480501200405. [DOI] [PubMed] [Google Scholar]
- 61.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin. Cancer Res. 2007;13:1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 62.Steeg PS, et al. Preclinical drug development must consider the impact on metastasis. Clin. Cancer Res. 2009;15:4529–4530. doi: 10.1158/1078-0432.CCR-09-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palmieri D, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin. Cancer Res. 2009;15:6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carbonell WS, Ansorge O, Sibson N, Muschel R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE. 2009;4:e5857. doi: 10.1371/journal.pone.0005857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minn. AJ, et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 2005;115:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang XH, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welch DR. Technical considerations for studying cancer metastasis in vivo. Clin. Exp. Metastasis. 1997;15:272–306. doi: 10.1023/a:1018477516367. [DOI] [PubMed] [Google Scholar]
- 68.Steeg PS, Theodorescu D. Metastasis: a therapeutic target for cancer. Nature Clin. Pract. Oncol. 2008;5:206–219. doi: 10.1038/ncponc1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dunn LK, et al. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 71.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Price JE. Analyzing the metastatic phenotype. J. Cell. Biochem. 1994;56:16–22. doi: 10.1002/jcb.240560105. [DOI] [PubMed] [Google Scholar]