Summary
One of the major recent clinical advances in cancer treatment is the use of antiangiogenic drugs such as bevacizumab, sorafenib, and sunitinib. Bevacizumab, the monoclonal anti-VEGF antibody, has been approved for the first line treatment of metastatic breast cancer (MBC) when combined with taxane. However, the clinical benefits are modest; despite a doubling of response rates and significant prolongation of progression free survival times, no increase in overall survival is attained. This review summarizes some of the possibilities to account for this discrepant result. These include rapid development of acquired drug resistance due to the redundancy of proangiogenic growth factors, acceleration of tumor growth after antiangiogenic drug treatments are stopped, and increases in tumor cell malignant aggressiveness driven by mechanisms such as increased tumor hypoxia. Some possible strategies to improve the benefits of antiangiogenic drug therapy are discussed such as prolonging the treatment beyond tumor progression, combination with other therapeutic modalities, e.g. long term (‘maintenance’) low-dose metronomic chemotherapy or additional targeted/biologic drugs, e.g. trastuzumab.
Keywords: Bevacizumab, VEGF, Drug resistance, Endothelial progenitor cells, Metronomic chemotherapy
Introduction and background
One of the major developments in medical oncology practice over the last five years has been the demonstrated success and approval of a number of antiangiogenic drugs for the treatment of a variety of malignancies, including breast cancer.1 The approvals followed from the results of several large randomized phase III clinical trials, and the antiangiogenic drugs tested in these trials included bevacizumab, the humanized anti-VEGF monoclonal antibody,2 sorafenib and sunitinib, both oral small molecule receptor tyrosine kinase inhibitors (RTKIs) which target multiple RTKs, including VEGF receptors and PDGF receptors, among others.1 In the case of bevacizumab, the successes thus far have involved its use in combination with various standard chemotherapy regimens for the treatment of first or second line colorectal cancer, first line non small cell lung cancer, and first line metastatic breast cancer (MBC).1,3,4 This combinatorial approach with bevacizumab was instigated primarily as a result of the minimal, if any, activity of bevacizumab as a monotherapy for the treatment of the aforementioned malignancies at an advanced (metastatic) stage of disease in prior phase I or II trials. Although the overall efficacy of the various chemotherapy/bevacizumab combinations is superior to the respective chemotherapy regimen, the overall clinical benefits gained are modest, e.g. several months prolongation of overall survival (OS) or progression-free survival (PFS) in the cases of colorectal cancer and non small cell lung cancer, but only a benefit of either 1–2 months in PFS with no OS benefit in the case of MBC when bevacizumab was combined with a weekly paclitaxel regimen (the E2100 trial)5 or with docetaxel given once every three weeks (the AVADO trial).6 The MBC trial successes came after the failure of a randomized phase III trial of bevacizumab and capecitabine used a second or third line treatment of refractory MBC patients.7 In addition, there has been a failed phase III clinical trial involving bevacizumab in combination with weekly gemcitabine for the treatment of pancreatic cancer despite earlier promising results in a randomized phase II trial.8
In the case of the small molecular antiangiogenic RTKIs, sorafenib and sunitinib have both been used successfully as monotherapies where they have shown clinical survival benefits in the treatment of renal cell carcinoma.1,9 In addition, sorafenib has shown a benefit for the treatment of hepatocellular carcinoma (HCC).9,10 However, thus far, no enhanced benefit has been observed in phase III trials when a small molecule antiangiogenic RTKI such as sorafenib or PTK-787 (vatalanib) is combined with chemotherapy, compared to the respective chemotherapy alone. In addition, monotherapy with drugs such as sunitinib seems to have minimal, if any, activity when used in the treatment of more common solid malignancies, including MBC.11 Another aspect that is beginning to emerge from both preclinical and clinical results is that exposure to antiangiogenic drugs can sometimes accelerate tumor growth when therapy is terminated.2,12,13 In addition, there have been some instances where the malignant phenotype, e.g. increased invasion, can be induced as a result of antiangiogenic drug therapy,2,14 as has been observed in the case of glioblastomas in numerous studies.15–17
Given the aforementioned information, a number of important questions and issues are raised. Discussion of these issues and questions is the focus of this review. They include the following: (i) how does bevacizumab function to enhance the efficacy of chemotherapy? (ii) why are the clinical benefits in PFS and OS caused by antiangiogenic drugs relatively transitory, and what is the basis of acquired resistance to such drugs?5,18 and (iii) what are some promising therapeutic strategies that might be used in conjunction with an antiangiogenic drug to improve overall clinical benefit for treating malignant disease, e.g. by delaying drug resistance, including for the treatment of breast cancer?
Discussion
How does bevacizumab function to enhance the efficacy of chemotherapy?
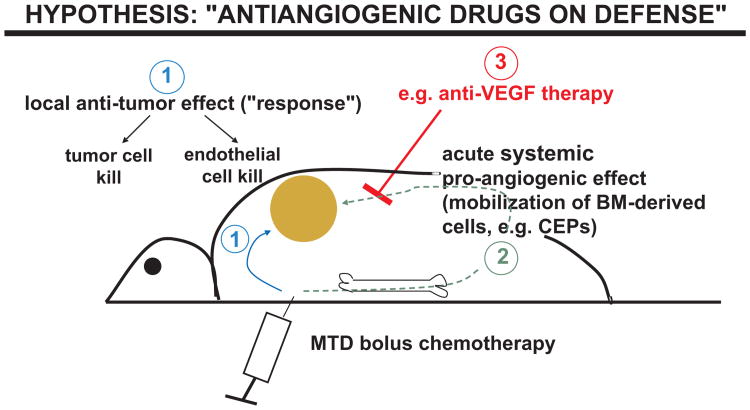
A number of hypotheses have been put forward to account for the preclinical and clinical phenomenon of enhancement of chemotherapy efficacy as a result of co-treatment with an antiangiogenic drug.3 These include the ‘vessel normalization’ hypothesis in which it has been proposed that an antiangiogenic drug such as bevacizumab may actually transiently improve tumor vascular function by decreasing vessel leakiness (caused in part by the potent vascular permeability function of VEGF) and ‘normalizing’ some of the chaotic dysfunctional tumor blood vessels thus decreasing the high interstitial fluid pressures within tumors, and in so doing, actually improve the delivery and distribution of chemotherapy into and within tumors provided the chemotherapy is given during this ‘normalization window’.19,20 Another theory, one which we have been actively studying, involves a concept that we have termed “antiangiogenic drugs on defense”.3,21 In brief, the basis of this hypothesis is that certain chemotherapy drugs can induce a transient but very rapid pro-vasculogenic/angiogenic systemic response which can contribute to tumor repopulation/regrowth during the break periods between successive cycles of conventional chemotherapy, as shown in Fig. 1.22,23 The basis for this host effect is that some chemotherapy drugs, e.g. paclitaxel, 5-FU, and cyclophosphamide, can induce a rapid and marked mobilization of circulating bone marrow-derived cells (BMDCs), including endothelial progenitor cells (CEPs), which then enter the peripheral blood circulation and migrate to sites of drug-treated tumor masses where they subsequently invade and colonize the drug-treated tumors.22,23 These BMDCs can then amplify the intrinsic ability of the drug-treated tumors to repopulate, at least in part, by stimulating tumor angiogenesis. This host response may also reverse some of the potential local antiangiogenic effects caused by chemotherapy within the tumor neovasculature. By this is meant the finding that dividing, activated endothelial cells present in the tumor neovasculature may be sensitive to the cytotoxic effects of a number of chemotherapy drugs, similar to the sensitivity of dividing cells present in other tissues or organs such as the bone marrow, gut, or hair follicle cells.24–28 Put in another way, chemotherapy can have opposite effects on tumor angiogenesis, i.e., inhibiting it within the tumor but promoting it by the systemic BMDC response such that the latter may cancel or blunt the former effect. But the latter effect can be blocked by certain antiangiogenic drugs.
Fig. 1.
Diagrammatic representation of one proposed mechanism to explain how an antiangiogenic drug may enhance the efficacy of maximum tolerated dose (MTD) chemotherapy. An injection of chemotherapy, e.g. MTD paclitaxel leads to a local tumor response by direct cell kill, and possibly a local (tumor) antiangiogenic effect, as a result of death of dividing endothelial cells in the tumor-associated angiogenic neovasculature (1). However, a systemic host response (2) is also induced comprised in part of a rapid mobilization of various bone marrow-derived cell populations, including circulating endothelial progenitor cells (CEPs), which subsequently migrate to and invade the chemotherapy-treated tumors. These bone marrow colonizing cells accelerate the recovery of the drug treated tumors thus reducing the duration and extent of tumor responses induced by the chemotherapy. However, this systemic CEP response can be blunted by co-treatment with an antiangiogenic drug, e.g. anti-VEGF or anti-VEGFR-2 antibodies, thus optimizing the effect of the chemotherapy treatment. The chemotherapy-induced mobilization of bone marrow-derived cells, including CEPs, is caused, in part, by a rapid induction of growth factors such as SDF-1.
The basis for the rapid BMDC responses induced by certain chemotherapy drugs is under investigation but recent results have implicated at least one mechanism, namely, rapid systemic induction of circulating stromal derived factor-1 (SDF-1).23 Furthermore, mobilization of a number of BMDCs, including CEPs, appears to be VEGF-dependent so that co-treatment with an antiangiogenic drug such as an antibody to VEGF or VEGF receptor-2 (the main signalling receptor for VEGF-mediated angiogenesis) largely blunts the systemic BMDC response and subsequent tumor invasion by these cells – including CEPs.22,23 As a result, the ability of tumors to repopulate is compromised and the extent or duration of the tumor response thus achieved is enhanced.22,23 Of some interest in this regard is the finding that gemcitabine chemotherapy appears unable to induce the aforementioned rapid BMDC/CEP response, at least in mice.23 Perhaps this could be a factor in explaining why addition of bevacizumab to weekly gemcitabine did not enhance the efficacy of the latter drug for the treatment of pancreatic cancer in a randomized phase III clinical trial. In addition it is not yet known whether small molecule antiangiogenic RTKIs can block the chemotherapy-induced BMDC/CEP response similar to other drugs such as anti-VEGF or anti-VEGFR-2 antibodies.
Why are the clinical benefits in PFS and OS caused by antiangiogenic drugs relatively transitory, and what is the basis of acquired resistance to such drugs?
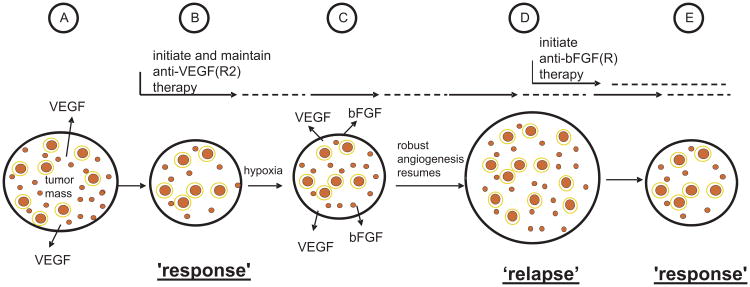
One of the more obvious explanations for the modest benefits obtained thus far using antiangiogenic drugs is that acquired resistance develops fairly rapidly, e.g. over several months, in patients whose tumors initially respond to the drug treatments. There was early speculation (and hope) that resistance to antiangiogenic therapies might not be as serious a problem as it is with virtually all other therapeutic modalities based on the notion that antiangiogenic drugs ultimately target normal host cells such as vascular endothelial cells rather than genetically unstable tumor cell populations as it is well known such genetic instabilities can be a major driving force for the selection and overgrowth of drug resistant subpopulations with respect to other therapies.24,29 However, clinical experience has shown that similar to other drugs, patients with advanced cancers whose tumors initially respond to bevacizumab, sorafenib, or sunitinib, almost always relapse and become drug resistant.1 Thus there is currently considerable interest in exploring the mechanisms of resistance to antiangiogenic therapies and in this regard several relevant hypotheses have been advanced.18 One that has attracted considerable attention was actually presented more than a decade ago on the basis of an analysis of human breast cancer tissue specimens.30 A large number of breast cancer clinical specimens obtained from various stages of breast cancer progression were analyzed for the expression of six different pro-angiogenic growth factors, including VEGF. In general, tumors from the earliest stage lesions expressed mainly or only VEGF.30 However, successive stages of tumor development were associated with expression of increasing numbers of factors, e.g. bFGF and TGFβ, among others. On the basis of these results, it was predicted that targeting a single pathway, e.g. the VEGF pathway of angiogenesis, would likely result in resistance, i.e., loss of response, due to selection of subpopulations expressing alternate proangiogenic mediators.30 There is now experimental support for this hypothesis. Thus treatment of islet cell pancreatic tumors spontaneously arising in mice with a drug such as DC101 – the antibody that specifically targets mouse VEGFR-2 function – leads to an initial tumor response rapidly followed by relapse/resistance within one month of therapy.31 This was found to be caused by upregulation of bFGF in the drug-treated tumors which likely occurred as a result of induction of elevated sustained levels of tumor hypoxia by the DC101 treatment, given every 3 days,31 as outlined schematically in Fig. 2. Thus salvage treatment with a drug that blocks bFGF receptors re-induced an antiangiogenic effect accompanied by an anti-tumor effect.31
Fig. 2.
Schematic representation of one of the ways resistance can develop, in principle, to a targeted antiangiogenic drug in tumors which initially respond to the drug, e.g. anti-VEGF or anti-VEGFR-2 antibody, as exemplified by the results of Casanovas et al.31 Angiogenesis in untreated tumors (A) is driven mainly, for example, by VEGF; upon treatment with an agent such as an anti-VEGFR-2 antibody (B), some regression of newly formed immature tumor neovasculature (small red circles) occurs, and further angiogenesis is blunted along with reduced perfusion/flow in some remaining vessels, many of which are more mature, pericyte covered vessels (larger red circles with a yellow border to symbolize pericyte coverage) leading to a tumor ‘response’, e.g. a reduction in tumor mass or no new growth (“stable disease”); however, these effects on the tumor vasculature lead to an overall increase in the levels of tumor hypoxia which in turn leads to induction of expression of new hypoxia regulated proangiogenic growth factors, such as bFGF (C); the induction of bFGF induces angiogenesis despite ongoing anti-VEGFR-2 therapy, leading to tumor ‘relapse’, i.e., resumption of angiogenesis and robust expansion of tumor mass (D). Initiation of bFGF(R) directed antiangiogenic therapy at this point could lead to angiogenesis inhibition once again and a tumor response (E). Taken and adapted from Kerbel RS; Therapeutic implications of intrinsic or induced angiogenic growth factor redundancy in tumors revealed.
In addition to the aforementioned mechanism of acquired resistance, i.e., pro-angiogenic growth factor redundancy, there are a number of other possible mechanisms.18 These include rapid vascular remodelling or maturation during or after antiangiogenic therapy, thus resulting in vessels with a more mature phenotype; such vessels tend to be less or non-responsive to antiangiogenic drugs compared to immature, growing vessel capillaries.32 Selection and overgrowth of tumor cell mutant or variant subpopulation that can survive under more hypoxic conditions, as a result of various genetic mutations, may be another mechanism of acquired resistance.33 Yet another is the ability of tumor cells aggressively “co-opt” the normal and abundant vasculature in certain organs such as the lungs, liver, and brain. In the case of glioblastomas, this can result in increased tumor cell invasion/infiltration through the brain at tumor relapse.15,16
The ability of potent antiangiogenic drugs, when given continuously or sequentially to cause increases in tumor hypoxia, may constitute not only a mechanism by which several proangiogenic growth factors can be induced or increased, but may also provide a means by which tumors become more aggressive over time.14,34 Tumor hypoxia has long been associated with a more aggressive malignant phenotype in a number of cancers. One way this could occur, in theory, is by elevated levels of the HIF-1 transcription factor, which in turn is known to induce a number of genes that are not only involved in angiogenesis, such as VEGF and PlGF, but also in tumor cell motility and invasion, e.g. c-met34,35 and twist – an inducer of metastasis.36 As a result, although antiangiogenic drug-treated tumors may initially respond and have their growth slowed by such treatments, it appears that a change in their biology can sometimes take place over time such that the surviving tumor cell populations express a more aggressive invasive and/or metastatic phenotype.14 This phenomenon could represent one explanation, for example, why treatment of metastatic breast cancer patients with bevacizumab (and taxane chemotherapy) results in a PFS advantage but not an OS advantage. Simply put, the degree of the initial benefit obtained, e.g. slowing down of tumor growth and increased tumor responses (shrinkage) could be partially reversed by the onset of more aggressive tumor growth later on, that is in some way caused by the initial (and successful) antiangiogenic drug treatment effects.
Also currently considerable recent attention is the possibility that temporary or permanent termination of antiangiogenic therapy, including the regular drug-free breaks when using drugs such as sunitinib (which is generally administered in a 4 week on/2 week off schedule over a 6 week cycle) can sometimes result in acceleration, i.e., “rebound” of tumor growth.13 For example, preclinical studies have shown that treatment of tumors in mice with a small molecule antiangiogenic RTKI can result in rapid reduction of tumor vascularity. However, if the drug treatment is stopped, there is a very rapid rebound in tumor revascularization that occurs within one week.37 This observation may help explain certain clinical observations of a similar nature. For example, it has been noted that cutaneous tumor nodules in some breast cancer patients treated with sunitinib show evidence of rapid tumor (re)growth during the 2 week drug-free break periods in between successive 4 week cycles of daily therapy with this drug.13 Similarly, cessation of bevacizumab therapy might result in subsequent acceleration in the growth rate of tumors such as liver metastases in colorectal cancer patients.12 Such observations are having an impact on the debate over the duration of antiangiogenic treatments, and whether such treatments should be continued beyond tumor progression, for which evidence of increased survival benefit is beginning to emerge.38–40
Such clinical observations are prompting ‘bedside-to-bench’ experimental analysis of the various possible mechanisms involved. To cite one example of such translational research, it is well known that cancer patients, including breast cancer patients, receiving small molecule antiangiogenic RTKIs such as sunitinib almost invariably show reversible increases in the level of VEGF and another member of the VEGF family, placental growth factor (PlGF).13,41–43 One hypothetical mechanism proposed to account for such findings is that the drug induces elevated levels of tumor hypoxia and hypoxia then serves as a trigger for increased expression of tumor cell associated VEGF and PlGF, i.e., the phenomenon is essentially tumor-dependent. However, recent results have cast some doubt on the validity or overall contribution of this mechanism. Thus, treatment of healthy normal non-tumor bearing mice with sunitinib can result in similar increases in VEGF and PlGF, and they occur in a dose-dependent and reversible fashion.43 Furthermore, there are a number of other growth factors, cytokines, and chemokines that are induced by the drug treatment, in addition to VEGF and PlGF. Some of these are not known to bind to receptors that are targets of the drug, e.g. SDF-1 and granulocyte colony-stimulating factor (G-CSF),43 among others, including osteopontin, a mediator of metastasis.44 Thus systemic induction in host tissues throughout the body of such multiple (and potentially tumor growth promoting and pro-angiogenic growth factors) could conceivably contribute to tumor regrowth during the breaks in between cycles of sunitinib therapies. In addition, these kinds of molecular changes could conceivably also contribute to acceleration of metastatic disease – which we reported recently, especially when sunitinib is administered over short periods of time.2 For example, daily treatment of non-tumor bearing mice for one week with sunitinib followed by intravenous injection of breast cancer cells a day later can actually result in acceleration of the rate of subsequent tumor growth and metastasis compared to control mice.2 Similarly, implantation of the same human breast cancer line (a metastatic variant of the MDA-MB-231 cell line) into the mammary fat pads followed by surgical resection was used as another model to evaluate the effects of briefly treating the tumor bearing mice, either before tumor resection (a form of neoadjuvant therapy) or immediately after tumor resection (a form of adjuvant therapy). In both cases acceleration of subsequent metastatic disease was observed.2 These results clearly have possible implications for the use of antiangiogenic drugs in the neoadjuvant and adjuvant settings, including for the treatment of breast cancer. Ongoing neoadjuvant and adjuvant clinical trials using antiangiogenic drugs should shortly reveal whether the aforementioned preclinical results have any clinical relevance.
What are some promising therapeutic strategies that might be used in conjunction with an antiangiogenic drug to improve overall clinical benefit for treating malignant disease, including breast cancer?
A recurring concept or theme from the aforementioned discussion is the possible negative impact of reactive host responses induced by either chemotherapy drugs when used at maximum tolerated doses or antiangiogenic agents administered at near full (supposedly optimal) doses. These host responses have the potential to diminish the overall anti-tumor effectiveness of the aforementioned therapies, whether used alone or together. One possible strategy to avoid or minimize such reactive host responses could be to use lower doses of the drugs administered, at least in the case of chemotherapy. This leads to the concept of low-dose ‘metronomic’ chemotherapy, a therapeutic strategy which we have been studying actively for almost a decade, including in the context of MBC.27,45–48
Metronomic chemotherapy refers to the frequent, i.e., close, regular administration of relatively low, minimally or non-toxic doses of a chemotherapy drug over long periods of time with no prolonged drug-free interruptions.27,28,45,49 One mechanism of action to account for the anti-tumor effects of metronomic chemotherapy is through antiangiogenesis. This can occur in at least two ways. First, by targeting the dividing/activated endothelial cells in newly forming tumor associated blood vessel,27,28 and/or by targeting circulating endothelial progenitor cells.50–53
Of considerable interest with respect to metronomic chemotherapy are many preclinical studies which have shown striking anti-tumor activity of low-dose metronomic chemotherapy regimens, which often equal or exceed the anti-tumor efficacy of the same chemotherapy drug tested in a more conventional pulsatile MTD fashion.28,47,54 In the case where equivalent anti-tumor efficacy has been observed, the metronomic regimen is almost always less toxic.47 Given the frequency of drug administration, oral chemotherapy drugs are the most commonly used for metronomic chemotherapy clinical trials, e.g. cyclophosphamide, UFT, capecitabine, methotrexate, among others, which are also more convenient for patients.55–57 Given the reduced or minimal toxicity associated with most metronomic chemotherapy regimens,56,58–60 there is no need for the use of hemopoietic supportive care drugs such as recombinant G-CSF, unlike the necessity to use such an agent for conventional ‘dose-dense’ but more intensive and toxic chemotherapy regimens.61 This is important given the possibility that G-CSF may be able to promote tumor growth, e.g. by mobilizing either CEPs62 or other types of BMDCs that are pro-angiogenic such as certain types of myeloid/dendritic cells.63 Such effects could serve to reduce some of the efficacy of dose-dense chemotherapy regimens, including for the treatment of early stage breast cancer, that would otherwise be expected in the absence of the G-CSF therapy. If this is correct then addition of an antiangiogenic drug such as bevacizumab to G-CSF supported dose-dense chemotherapy regimens should significantly improve clinical (survival) benefits-– but without affecting recovery from myelosuppression.
Prior preclinical metronomic chemotherapy studies by several groups have shown that the combination of an antiangiogenic drug such as DC101, sorafenib, or TNP470 can considerably increase the anti-tumor efficacy compared to either agent alone, and moreover do so with minimal associated host toxicity.27,28,45,54 In addition, certain ‘doublet’ metronomic chemotherapy combinations, even without an added antiangiogenic drug, can sometimes cause striking anti-tumor benefits, even when used to treat advanced high volume, established visceral metastatic disease-– including that of human breast cancer in immune deficient mice.46 An example of the latter shown in Fig. 3 in which daily oral administration of cyclophosphamide through the drinking water along with daily gavage of UFT (i.e., tegafur plus uracil, a 5-FU prodrug), was able to cause striking survival effects in SCID mice which had extensive visceral metastatic disease at the time therapy was initiated; moreover this was observed in the absence of any overt toxicity.46 Partly as a result of this preclinical study, and others showing the benefit of adding an antiangiogenic drug to metronomic chemotherapy, as discussed above, a phase II clinical trial was initiated at the European Institute of Oncology in MBC patients using daily metronomic cyclophosphamide and capecitabine along with bevacizumab administered every two weeks.56 The results of this non-randomized trial look extremely promising both in terms of efficacy and minimal host associated toxicity and reflect the results of some other metronomic chemotherapy trials in other indications, e.g. the use of daily oral cyclophosphamide with bevacizumab for the treatment of recurrent ovarian epithelial carcinoma.55
One of the salient points regarding metronomic chemotherapy in the context of the concepts presented herein is the possibility that the continuous or frequent administration of a chemotherapy drug can sometimes have surprisingly efficacious anti-tumor activity, something which may appear counterintuitive. However, in retrospect, one possible explanation for the robust anti-tumor activity observed is that the lower doses of drug would be less potent in terms of inducing reactive host responses that can act to promote tumor growth and angiogenesis such as elevated levels of multiple growth factors, cytokines, and chemokines, as well as bone marrow-derived CEPs, which, as discussed above, are often observed when using MTDs of agents such as a taxane,23 or vascular disrupting agents,22 or even certain antiangiogenic drugs (in the case of induced growth factor responses), as also discussed above. In this regard, one of the interesting preclinical findings regarding mobilization of CEPs induced by certain conventional MTD chemotherapy regimens, e.g. MTD paclitaxel, is the finding that not only are such CEP spikes avoided by giving chemotherapy at lower metronomic doses, but the levels of such cells, compared to controls, can actually be significantly reduced.46,53,64 This may occur, at least in part, through mechanisms such as the induced upregulation of endogenous inhibitors of angiogenesis such as thrombospondin-1 by the metronomic chemotherapy protocols.65,66 The mechanisms for this are currently unknown.
Given the convenience, lesser toxicity, and sometimes highly significant anti-tumor efficacy of metronomic chemotherapy, even when treating advanced stage cancer,46 there is the possibility that chemotherapy administered in this fashion might be a particularly promising therapeutic approach for the adjuvant treatment of early stage cancer after surgery, including breast cancer. Indeed, this possibility is currently under investigation in a large randomized phase III clinical trial being run by the International Breast Cancer Study Group (IBCSG). This is the IBCSG-00-22 clinical trial involving daily long term oral low-dose cyclophosphamide combined with low-dose methotrexate administered two days a week as a maintenance treatment in early stage breast cancer patients after they have undergone surgery followed by conventional adjuvant chemotherapy (http://www.ibcsg.org/Public/Health_Professionals/Open_Trials/IBCSG_22-00/Pages/IBCSG22-00.aspx). In this trial the one year long maintenance metronomic chemotherapy regimen is not accompanied by concurrent combination of a biologic agent such as bevacizumab. However, depending on the results obtained, future clinical trials could evaluate such a combination treatment regimen. One rationale for using such a combination would be to avoid the possibility that the antiangiogenic agent may eventually cause accelerated tumor growth or increases in invasion and metastasis (as discussed above); this possibility could be blocked by co-treatment with an effective metronomic chemotherapy regimen such that the benefits of both types of regimens are mutually enhanced, but with minimal associated host toxicity.
Also noteworthy with respect to the idea of combining metronomic chemotherapy with a targeted biologic agent for the treatment of breast cancer is that this concept has been extended to evaluating trastuzumab plus metronomic chemotherapy for the treatment of Her-2/erbB-2 positive breast cancer. Preclinical results have shown the superiority of the metronomic regimen compared to an MTD regimen of the same metronomic chemotherapy (using cyclophosphamide) as a result of equivalent anti-tumor efficacy but lesser host toxicity.47 There are now clinical investigations designed to evaluate trastuzumab plus metronomic chemotherapy for the treatment of women with MBC and preliminary results look encouraging, but clearly have to be extended to larger randomized clinical trials.67
There are of course many other possible combinatorial strategies involving antiangiogenic drugs for the treatment of breast cancer. One that is attracting considerable interest is the combination of trastuzumab and bevacizumab, i.e., two biologic agents (http://clinicaltrials.gov/ – “A study of Avastin (Bevacizumab) plus Herceptin (trastuzumab) in patients with primary inflammatory HER2-positive breast cancer”). But in this case one problem could be the financial costs associated with the use of two very expensive biologic agents.68 This reality is one of the factors that provides an additional rationale for the need to continue investigations into the possible benefits of combining drugs such as bevacizumab or trastuzumab, and others such as aromatase inhibitors, e.g. letrozole, with extended low-dose metronomic chemotherapy regimens.59 The rationale would certainly appear to be strong for treatment of metastatic disease and in some respects also for early stage disease. In this regard, one argument against the use of metronomic chemotherapy for early stage disease might be the possibility that long term therapy with a drug such as low-dose cyclophosphamide could promote the eventual emergence of secondary cancers, including in patients who were initially cancer-free, i.e., cured at the time that adjuvant therapy was initiated. Perhaps using other drugs such as methotrexate, UFT, or capecitabine for longer term metronomic chemotherapy regimens may avoid this possibility. For example, daily low-dose oral UFT for 2 years has been used as an adjuvant therapy in non small cell lung cancer.57 In addition, the very low doses of the agents used such as 50 mg/day cyclophosphamide would hopefully be associated with minimal risk of inducing secondary neoplasms that might even be lesser in magnitude than when early stage patients are treated with conventional shorter term but higher dose chemotherapy regimens.
Other promising developments for improving the clinical benefit of antiangiogenic drugs for breast cancer
There has been considerable interest in finding surrogate markers, especially those which are reliably predictive that a patient is likely to benefit from receiving an antiangiogenic drug such as bevacizumab. An obvious candidate would be elevated VEGF expression, detected either within tumors, or systemically in the circulation. However, elevated VEGF expression does not seem to correlate with response/clinical benefit as reported, for example, in colorectal cancer studies.69 Recently, however, Schneider et al. reported pharmaco/genomic molecular markers, namely, various single nucleotide polymorphisms (SNPs) in the VEGF gene that may have utility for predicting PFS or even OS benefit, and for toxicity as well, namely, highly grade hypertension. This was based on a retrospective analysis of tumor DNA of breast cancer specimens obtained from the randomized E2100 phase III MBC trial of weekly paclitaxel plus bevacizumab.70
Summary and conclusions
Antiangiogenic therapy has begun to make therapeutic inroads in the first line treatment of metastatic breast cancer, but the gains are quite incremental: one to two months benefit in PFS, but no OS benefit when a taxane is combined with bevacizumab. No such benefit has yet been obtained when treating refractory breast cancer patients with bevacizumab and chemotherapy, e.g. capecitabine. Whether small molecule antiangiogenic RTKIs will have a benefit when used to treat MBC is not yet known. Resistance to antiangiogenic drugs can develop quickly through multiple mechanisms including angiogenic growth factor redundancy. In addition antiangiogenic drugs can sometimes alter the natural history of tumors in undesirable ways such as accelerating tumor growth when therapy is stopped or promoting invasion/metastasis, especially short term therapies. Such effects may act to diminish the overall positive impact of antiangiogenic therapies and provide an explanation for why a benefit in PFS but not OS is obtained when MBC patients are treated with bevacizumab plus a taxane. Several strategies to improve the clinical benefits of antiangiogenic drugs are being actively investigated. Some promising developments include discovery of potentially useful predictive markers of clinical benefit and toxicity such as VEGF gene polymorphisms and combining antiangiogenic drugs with other biologic agents or treatments such as trastuzumab or metronomic chemotherapy. The latter may also be useful as a maintenance adjuvant therapy treatment of early stage breast cancer.
Acknowledgments
I thank Cassandra Cheng for her excellent secretarial assistance.
Funding: Grants from the National Cancer Institute of Canada (NCIC), the Canadian Institutes of Health Research (CIHR), the Ontario Institute for Cancer Research (OICR), and National Institutes of Health (NIH), USA.
Footnotes
Competing interests: Consultancy: Taiho Pharmaceuticals, Glaxo-SmithKline. Honoraria: Pfizer, Genentech.
References
- 1.Kerbel RS. Tumor Angiogenesis. N Engl J Med. 2008;358:2039–49. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ebos JML, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009 doi: 10.1016/j.ccr.2009.01.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–5. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 4.Chan A. Antiangiogenic therapy for metastatic breast cancer: current status and future directions. Drugs. 2009;69:167–81. doi: 10.2165/00003495-200969020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Miles D, Chan A, Romieu G, et al. Randomized, double-blind placebo-controlled, phase III study of bevacizumab (BV) with docetaxel (D) or D with placebo (PL) as 1st-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. Proc Am Soc Clin Oncol. 2008;26:LBA1011. [Google Scholar]
- 7.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–9. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 8.Kindler HL, Friberg G, Singh DA, Locker G, Nattam S, Kozloff M, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–40. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 9.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–40. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Aspitia A, Morton RF, Hillman DW, Lingle WL, Rowland KM, Jr, Wiesenfeld M, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–5. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cacheux W, Boisserie T, Staudacher L, Vignaux O, Dousset B, Soubrane O, et al. Reversible tumor growth acceleration following bevacizumab interruption in metastatic colorectal cancer patients scheduled for surgery. Ann Oncol. 2008;19:1659–61. doi: 10.1093/annonc/mdn540. [DOI] [PubMed] [Google Scholar]
- 13.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–6. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 14.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Anti-angiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009 doi: 10.1016/j.ccr.2009.01.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–87. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 18.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 21.Shaked Y, Kerbel RS. Antiangiogenic strategies on defense: blocking rebound by the tumor vasculature after chemotherapy. Cancer Res. 2007;67:7055–8. doi: 10.1158/0008-5472.CAN-07-0905. [DOI] [PubMed] [Google Scholar]
- 22.Shaked Y, Ciarrocchi A, Franco M, Lee CR, Man S, Cheung AM, et al. Therapy-induced acute recruitment of circulating endothelial progenitor cells to tumors. Science. 2006;313:1785–7. doi: 10.1126/science.1127592. [DOI] [PubMed] [Google Scholar]
- 23.Shaked Y, Henke E, Roodhart J, Mancuso P, Langenberg M, Colleoni M, et al. Rapid chemotherapy-induced surge in endothelial progenitor cells: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–73. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti-cancer therapeutic agents. BioEssays. 1991;13:31–6. doi: 10.1002/bies.950130106. [DOI] [PubMed] [Google Scholar]
- 25.Miller KD, Sweeney CJ, Sledge GW., Jr Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001;19:1195–206. doi: 10.1200/JCO.2001.19.4.1195. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–72. [PubMed] [Google Scholar]
- 27.Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin D, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Browder T, Butterfield CE, Kraling BM, Marshall B, O'Reilly MS, Folkman J. Antian-giogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86. [PubMed] [Google Scholar]
- 29.Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404–7. doi: 10.1038/37126. [DOI] [PubMed] [Google Scholar]
- 30.Relf M, LeJeune S, Scott PA, Fox S, Smith K, Leek R, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–9. [PubMed] [Google Scholar]
- 31.Casanovas O, Hicklin D, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Glade Bender J, Cooney EM, Kandel JJ, Yamashiro DJ. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–8. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 34.Steeg PS. Angiogenesis inhibitors: motivators of metastasis? Nat Med. 2003;9:822–3. doi: 10.1038/nm0703-822. [DOI] [PubMed] [Google Scholar]
- 35.Bottaro DP, Liotta LA. Cancer: Out of air is not out of action. Nature. 2003;423:593–5. doi: 10.1038/423593a. [DOI] [PubMed] [Google Scholar]
- 36.Peinado H, Cano A. A hypoxic twist in metastasis. Nat Cell Biol. 2008;10:253–4. doi: 10.1038/ncb0308-253. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso MR, Davis R, Norberg SM, O'Brien S, Sennino B, Nakahara T, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 39.Grothey A, Sugrue MM, Purdie DM, Dong W, Sargent D, Hedrick E, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE) J Clin Oncol. 2008;26:5326–34. doi: 10.1200/JCO.2008.16.3212. [DOI] [PubMed] [Google Scholar]
- 40.Ellis LM, Haller DG. Bevacizumab beyond progression: does this make sense? J Clin Oncol. 2008;26:5313–5. doi: 10.1200/JCO.2008.17.4540. [DOI] [PubMed] [Google Scholar]
- 41.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 42.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–50. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 43.Ebos JML, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with anti-tumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki M, Mose E, Galloy C, Tarin D. Osteopontin gene expression determines spontaneous metastatic performance of orthotopic human breast cancer xenografts. Am J Pathol. 2007;171:682–92. doi: 10.2353/ajpath.2007.070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerbel RS, Kamen BA. Antiangiogenic basis of low-dose metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 46.Munoz R, Man S, Shaked Y, Lee C, Wong J, Francia G, et al. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT–cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–91. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 47.du Manoir JM, Francia G, Man S, Mossoba M, Medin JA, Viloria-Petit A, et al. Strategies for delaying or treating in vivo acquired resistance to trastuzumab (Herceptin®) in human breast cancer xenografts. Clin Cancer Res. 2006;12:904–16. doi: 10.1158/1078-0432.CCR-05-1109. [DOI] [PubMed] [Google Scholar]
- 48.Francia G, Emmenegger U, Lee CR, Shaked Y, Folkins C, Mossoba M, et al. Long term progression and therapeutic response of visceral metastatic disease non-invasively monitored in mouse urine using beta-hCG choriogonadotropin secreting tumor cell lines. Mol Cancer Ther. 2008;7:3452–9. doi: 10.1158/1535-7163.MCT-08-0200. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000;105:1045–7. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bertolini F, Paul S, Mancuso P, Monestiroli S, Gobbi A, Shaked Y, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res. 2003;63:4342–6. [PubMed] [Google Scholar]
- 51.Fürstenberger G, von Moos R, Lucas R, Thürlimann B, Senn HJ, Hamacher J, et al. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy or primary breast cancer. Br J Cancer. 2006;94:524–31. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, et al. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis: implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–11. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Shaked Y, Emmengger U, Man S, Cervi D, Bertolini F, Ben-David Y, et al. The optimal biological dose of metronomic chemotherapy regimens is associated with maximum antiangiogenic activity. Blood. 2005;106:3058–61. doi: 10.1182/blood-2005-04-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–52. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 55.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low dose metronomic oral cyclophosphamide in recurrent ovarian cancer. A trial of the California, Chicago and Princess Margaret Hospital Phase II Consortia. J Clin Oncol. 2007;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 56.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer: clinical and biological activity. J Clin Oncol. 2008;26:4899–905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 57.Kato H, Ichinose Y, Ohta M, Hata E, Tsubota N, Tada H, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med. 2004;350:1713–21. doi: 10.1056/NEJMoa032792. [DOI] [PubMed] [Google Scholar]
- 58.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, et al. Low dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 59.Bottini A, Generali D, Brizzi MP, Fox SB, Bersiga A, Bonardi S, et al. Randomized phase II trial of letrozole and letrozole plus low-dose metronomic oral cyclophosphamide as primary systemic treatment in elderly breast cancer patients. J Clin Oncol. 2006;24:3623–8. doi: 10.1200/JCO.2005.04.5773. [DOI] [PubMed] [Google Scholar]
- 60.Emmenegger U, Man S, Shaked Y, Francia G, Wong JW, Hicklin DJ, et al. A comparative analysis of low dose metronomic cyclophosphamide reveals absent or low grade toxicity on tissues highly sensitive to the toxic effects of maximum tolerated dose regimens. Cancer Res. 2004;64:3994–4000. doi: 10.1158/0008-5472.CAN-04-0580. [DOI] [PubMed] [Google Scholar]
- 61.Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–9. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 62.Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–61. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- 63.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 64.Ng SSW, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006;12:4331–8. doi: 10.1158/1078-0432.CCR-05-2762. [DOI] [PubMed] [Google Scholar]
- 65.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin-1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–22. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, et al. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–4. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 67.Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kolata G, Pollack A. Costly cancer drug offers hope, but also a dilemma. New York Times. 2008 Jul 6; [Google Scholar]
- 69.Jubb AM, Oates AJ, Holden S, Koeppen H. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–35. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 70.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]