Abstract
Objectives
We sought to investigate the prevalence and predictors of oral anticoagulation prescription among patients with atrial fibrillation (AF) at the lowest risk for thromboembolism, despite contemporary consensus guidelines that do not recommend anticoagulation therapy in this population.
Background
In young and healthy AF patients without significant thromboembolic risk factors, anticoagulant treatment carries bleeding risks that outweigh stroke prevention benefit.
Methods
Within a large contemporary registry of cardiology outpatients, we identified low-risk patients with AF meeting criteria for a contemporary consensus guideline class III indication against use of anticoagulation (age < 60 years, CHADS2 Score=0, and no structural heart disease) between 2008–2012, and a second cohort with the same criteria and a CHA2DS2-VASc Score of 0. Using hierarchical modified Poisson regression models adjusted for patient characteristics, we examined predictors of oral anticoagulation treatment in these low thromboembolic risk AF patients.
Results
Oral anticoagulation was prescribed in a total of 2,561 of 10,995 (23.2%) AF patients with a CHADS2 score of 0 and 1,787 of 6,730 (26.6%) AF patients with a CHA2DS2-VASc score of 0. In multivariable analysis, older age (RR 1.48 per 10 years; 95% CI, 1.41–1.56; p<0.0001), male sex (RR 1.34; 95% CI, 1.22–1.46; p<0.0001), higher body mass index (RR 1.18 per 5 kg/m2; 95% CI, 1.14–1.22; p<0.0001), and Medicare insurance (reference: private insurance; RR,1.32; 95% CI, 1.17–1.49; overall p<0.0001) were associated with a higher likelihood of oral anticoagulant prescription, whereas treatment in Southern states (reference: Northeast; RR 0.69; 95% CI, 0.49–0.98;overall p=0.1187) was associated with a lower likelihood of oral anticoagulant prescription.
Conclusions
In a large, real-world population of AF patients with the lowest thrombotic risk, approximately 1 in 4 were treated with oral anticoagulation against contemporary guideline recommendations.
Keywords: Atrial fibrillation, Anticoagulants, Stroke, Thromboembolism
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated 1 in 4 lifetime risk in those older than 40 years of age and a projected increase in prevalence to approximately 5.6 million affected individuals in the United States by the year 2050 (1,2). In patients with AF at higher risk for thromboembolism, anticoagulation with warfarin (a vitamin K antagonist) or the newer novel anticoagulants reduces morbidity and mortality (3–6). Because oral anticoagulant use carries a risk of bleeding, including potentially fatal intracranial hemorrhage, anticoagulant treatment is not recommended in AF patients at a particularly low risk for stroke. Specifically, previous AF guidelines recommend against the use of oral anticoagulation in patients under 60 years of age without heart disease or other known risk factors for thromboembolism (7), and more recently updated guidelines do not recommend oral anticoagulation in AF patients without any established risk factor for stroke (8). Although it is well known that appropriate oral anticoagulant prescription in AF patients at risk for stroke outside clinical trial settings falls short of guideline-based expectations (9–12), it is unknown whether young and healthy AF patients with the lowest stroke risk are being treated with oral anticoagulation that may increase bleeding risk without a commensurate reduction in stroke risk. To our knowledge, a real-world evaluation of guideline adhering practice patterns of anticoagulation prescription in AF patients with the lowest stroke risk has never been performed.
We evaluated the prevalence of oral anticoagulant prescription by cardiovascular specialists in a cohort of outpatients using data from the National Cardiovascular Data Registry (NCDR)’s Practice Innovation and Clinical Excellence (PINNACLE) Registry®. Use of this prospective national registry of cardiovascular care in the United States provides a unique opportunity to examine patterns of oral anticoagulant treatment in routine practice among outpatients as well as clinical predictors of this practice.
Methods
Data Source
The NCDR PINNACLE registry was created in 2008 by the American College of Cardiology as the first national, prospective, office-based cardiac quality improvement registry in the United States (13,14). Participating academic and private practices collect longitudinal, point-of-care data that includes patient demographics, symptoms, comorbidities, vital signs, medications, laboratory values, and recent hospitalizations with either paper forms, or modification of a practice’s electronic medical record using a standardized collection tool to comprehensively obtain and transmit uniform data. Quality checks and analyses of the data are performed at St. Luke’s Mid America Heart Institute (Kansas City, Missouri), the primary analytical center for the PINNACLE registry.
Study Population
Of 1,711,326 patients enrolled into the PINNACLE registry between January 1, 2008 and December 30, 2012, 359,315 (21.0%) had a diagnosis of AF. We based our inclusion criteria on the American College of Cardiology/American Heart Association/European Society of Cardiology guidelines in place during the study timeframe, which defined as a class III indication (i.e., recommended against) the use of oral anticoagulation for the primary prevention of stroke in patients below the age of 60 years without structural heart disease or any risk factors for thromboembolism (7). In order to identify these young and healthy patients, our primary cohort (referred to as the “CHADS2 score = 0 cohort”) was restricted to patients <60 years old [n=296,014 patients excluded], patients with non-valvular AF [n=824 patients excluded], and patients without structural heart disease (by excluding patients with coronary artery disease, prior myocardial infarction, or left ventricular ejection fraction< 50%); patients with a CHADS2 Score >0 were excluded by omitting patients with congestive heart failure, hypertension, age ≥75 years, diabetes, or previous stroke/transient ischemic attack (15) [n=51,176 patients excluded]. We also excluded patients from cardiology practices with ≥10 eligible patients [n=88 patients excluded] and patients without known contraindications to oral anticoagulation [n=218 patients excluded]. Therefore, our final study cohort was comprised of 10,995 young and healthy patients with AF and no structural heart disease at low risk for thromboembolism from __ practices (Figure 1). We also conducted a secondary analysis, using the CHA2DS1-VASc score, since it has been shown to be a more sensitive tool to risk-stratify AF patients who may be at risk for stroke and benefit from anticoagulant therapy (16), use of this risk score may have influenced cardiovascular specialist prescription of oral anticoagulation during the study timeframe, as reflected in subsequently published updated guidelines after the study (8). These updated guidelines advise the use of CHA2DS2-VASc Score for the assessment of stroke risk and state that it is reasonable to omit antithrombotic therapy for patients with nonvalvular AF and a CHA2DS2-VASc Score =0 (8). As a result, we also compared anticoagulant treatment patterns in a mirrored analysis of a more strictly defined cohort (deemed the “CHA2DS2-VASc Score =0 Cohort”) of the AF study population. In addition to carrying over all of the same exclusion criteria above, this cohort was restricted to those young and healthy patients with a CHA2DS2-VASc Score =0, with 1 point for congestive heart failure, hypertension, age ≥65 years [2 points if age ≥75 years], diabetes, female sex, and coronary or peripheral arterial disease, and 2 points for stroke/transient ischemic attack. Selection of this CHA2DS2-VASc Score =0 Cohort led to exclusion of an additional 4,265 patients, for a total cohort of 6,730 patients. To minimize over-representation by patients with multiple visits, only data from the index visit of each patient during the study period were used.
Figure 1. Flowchart of Practice Innovation and Clinical Excellence (PINNACLE) Registry Patients Included in the Analyses.
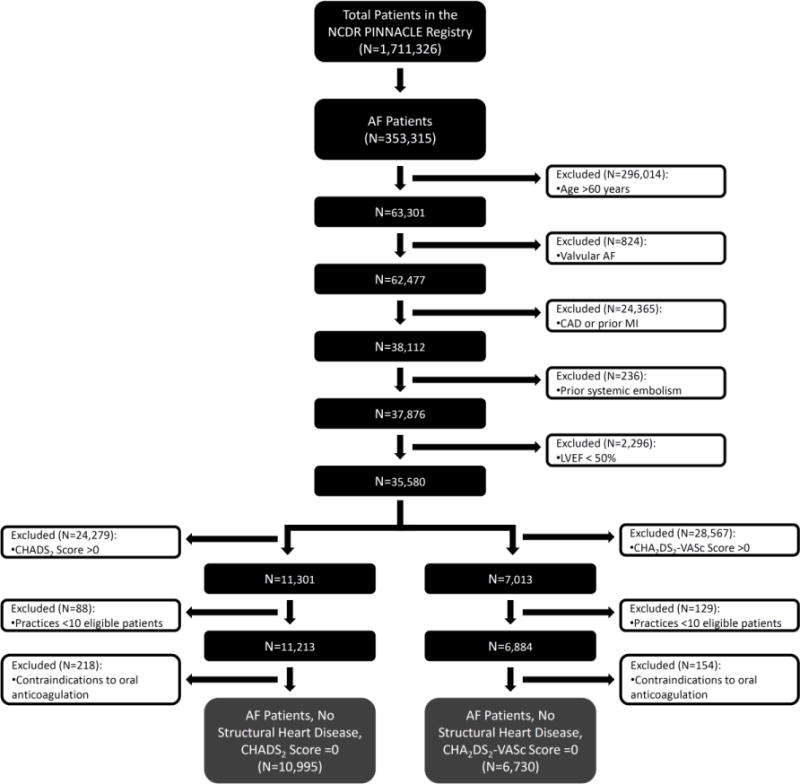
The flowchart depicts the total cohort of patients in the PINNACLE Registry from which exclusion criteria were administered to arrive at the final cohorts.
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction
Study Outcomes
Our main study outcome was treatment with any U.S. Food and Drug Administration (FDA)-approved oral anticoagulant for stroke prevention in patients with AF, which would include warfarin, dabigatran or rivaroxaban (apixaban had not yet been approved by the U.S. FDA during the study timeframe). Among patients not treated with anticoagulant therapy, we also examined whether these patients were treated with an antiplatelet agent or were not receiving either oral anticoagulant or antiplatelet therapy. Treatment with an antiplatelet agent was defined as prescription of aspirin, clopidogrel, ticlopidine, prasugrel and/or dipyridamole.
Statistical Analysis
Normally distributed continuous variables are expressed as means and standard deviations, whereas categorical variables are expressed as proportions. Unadjusted differences were compared using the χ2 test for categorical variables and t-tests for continuous variables.
To investigate the independent associations of various characteristics with the outcome of oral anticoagulant prescription, we constructed hierarchical modified Poisson regression models adjusted for patient demographic and clinical characteristics. These models included site as a random effect to account for patient clustering within sites. Covariates considered to be potential predictors were entered as fixed effects in the multivariable model and included age, sex, U.S. geographical region, health insurance, body mass index (BMI), AF classification, peripheral arterial disease, dyslipidemia, and tobacco use. Covariates selected for the multivariate analyses were chosen based on the plausibility that they could be associated with differential prescription of anticoagulation. Because sex is inherent to the CHA2DS2-VASc Score, it was not included as a predictor in related models.
Race was not included in the multivariable model due to a high rate of missing data (42.3%). The highest missing rate for other variables included BMI (32.0%), tobacco use (35.7%), and insurance payer (14.2%), therefore, a missing indicator was included in models that contained these variables. Additionally, missing data were assumed to be missing at random and were imputed with 10 imputation data sets (17), in which all patient variables were used to inform the imputation model (18).
Since the rate of oral anticoagulant prescription exceeded 10%, we used modified Poisson regression models at all steps to estimate relative risks (RRs) with 95% confidence intervals (CI) directly (instead of odds ratios obtained from logistic regression, which may overestimate effect differences) (19,20). Statistical tests were 2-sided and considered significant if they yielded a p value <0.05. Analyses were performed using the SAS statistical package version 9.3 (SAS Institute, Cary, NC), R version 2.15.3 (Foundation for Statistical Computing, Vienna, Austria), and IVEWare (Institute for Social Research, University of Michigan, Ann Arbor).
Results
A total of 10,995 patients without structural heart disease and a CHADS2 score of 0 (CHADS2 cohort), and 6,730 with a CHA2DS2-VASc Score of 0 (CHA2DS2-VASc cohort) were identified. In CHADS2 cohort, a total of 2,561 (23.2%) AF patients were prescribed an oral anticoagulant. Warfarin was the most commonly used therapy (n=____ [__%]), followed by dabigatran (n=_____ [___%]) and rivaroxaban (n=___ [___%]). In the CHA2DS2-VASc cohort, a total of 1,787 (26.6%) AF patients were prescribed an oral anticoagulant. Similarly, warfarin was the most commonly used therapy (n=____ [__%]), followed by dabigatran (n=_____ [___%]) and rivaroxaban (n=___ [___%]).
Demographic and clinical characteristics among patients in each cohort stratified by prescription of oral anticoagulation are shown in Table 1. In both the CHADS2 Score =0 Cohort and CHA2DS2-VASc Score =0 Cohort, AF patients without structural heart disease who were prescribed oral anticoagulation were older and more frequently insured by Medicare or were uninsured. Compared with those AF patients who were not prescribed oral anticoagulation, patients in both cohorts who were prescribed oral anticoagulation had a higher BMI and were more likely to reside in the Northeast and West. Patients prescribed oral anticoagulation were less likely to have paroxysmal AF or to be current smokers. In the CHADS2 Score =0 Cohort only, patients who were prescribed oral anticoagulation were more likely to be male and have dyslipidemia.
Table 1.
Baseline Characteristics of Atrial Fibrillation Patients at Low Stroke Risk with No Structural Heart Disease Categorized by CHADS2 Score =0 and CHA2DS2-VASc Score =0, Stratified by Prescription of Oral Anticoagulation
| Characteristic | CHADS2 Score =0 Cohort (N=10,995) |
P Value | CHA2DS2-VASc Score =0 Cohort (N=6,730) |
P Value | ||
|---|---|---|---|---|---|---|
| Prescribed Oral Anticoagulant (n=2,561) |
No Oral Anticoagulant (n=8,434) |
Prescribed Oral Anticoagulant (N=1,787) |
No Oral Anticoagulant (N=4,943) |
|||
| Patient demographic characteristics | ||||||
| Age, years | 50.9 ± 7.6 | 46.3 ± 10.4 | <0.001 | 50.7 ± 7.7 | 46.6 ± 10.1 | <0.001 |
| Male sex | 70.8% | 59.6% | <0.001 | — | — | — |
| Race | 0.105 | 0.558 | ||||
| White | 92.4% | 92.6% | 93.3% | 94.0% | ||
| Black | 5.1% | 5.8% | 4.5% | 4.4% | ||
| Other | 2.4% | 1.6% | 2.2% | 1.6% | ||
| Hispanic ethnicity | 2.3% | 2.4% | 0.866 | 1.8% | 2.1% | 0.597 |
| Insurance | 0.002 | 0.002 | ||||
| Private | 81.3% | 82.4% | 83.1% | 84.8% | ||
| Medicare | 7.0% | 5.2% | 6.4% | 4.2% | ||
| Medicaid | 2.4% | 3.3% | 1.5% | 2.1% | ||
| Other | 2.6% | 3.1% | 2.7% | 3.4% | ||
| Uninsured | 6.7% | 6.0% | 6.3% | 5.5% | ||
| Region | <0.001 | <0.001 | ||||
| Northeast | 14.1% | 10.4% | 14.9% | 10.9% | ||
| Midwest | 25.8% | 25.2% | 25.3% | 27.4% | ||
| South | 35.4% | 45.3% | 34.4% | 41.8% | ||
| West | 24.7% | 19.1% | 25.4% | 19.9% | ||
| Body mass index | 31.6 ± 7.3 | 29.0 ± 6.3 | <0.001 | 31.5 ± 6.7 | 29.3 ± 5.7 | <0.001 |
| Atrial fibrillation classification | 0.003 | 0.002 | ||||
| First episode detected | 46.4% | 39.8% | 45.8% | 42.8% | ||
| Paroxysmal | 43.2% | 48.4% | 42.8% | 48.8% | ||
| Persistent | 10.4% | 11.7% | 11.4% | 8.4% | ||
| Comorbidities | ||||||
| Peripheral arterial disease | 0.5% | 0.5% | 0.795 | — | — | — |
| Dyslipidemia | 19.6% | 17.8% | 0.039 | 19.5% | 18.6% | 0.375 |
| Tobacco use | <0.001 | <0.001 | ||||
| Never | 52.7% | 53.0% | 49.6% | 52.5% | ||
| Current | 17.9% | 22.5% | 19.6% | 23.2% | ||
| Quit within 12 months | 4.9% | 3.5% | 4.8% | 3.4% | ||
| Quit more than 12 months ago | 24.4% | 21.0% | 26.1% | 20.8% | ||
Categorical data are reported as percentages. Continuous data are reported as mean ± SD.
Abbreviations: CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
In the CHADS2 Score =0 Cohort, of all 2,561 patients who were prescribed an oral anticoagulant, 31.2% of patients (n=799) were also taking an antiplatelet agent. The same was true in the CHA2DS2-VASc Score =0 Cohort of 1,787 patients who were prescribed an oral anticoagulant, with 30.0% of patients (n=589) also taking an antiplatelet agent. In both the CHADS2 Score =0 Cohort and the CHA2DS2-VASc Score =0 Cohort, more than one-third of patients were prescribed antiplatelet therapy only (34.8% and 38.1%, respectively) or no antithrombotic agent at all (41.9% and 35.3%, respectively) (Table 2).
Table 2.
Prevalence of Antithrombotic Prescription in Atrial Fibrillation Patients at Low Stroke Risk with No Structural Heart Disease in Both the CHADS2 Score =0 and CHA2DS2-VASc Score =0 Cohorts
| Therapy | CHADS2 Score =0 Cohort (N=10,995) |
CHA2DS2-VASc Score =0 Cohort (N=6,730) |
|---|---|---|
| Antithrombotic therapy | ||
| Any oral anticoagulant therapy only | 16.0% | 17.8 % |
| Any oral anticoagulant therapy and any antiplatelet therapy | 7.3% | 8.8% |
| Any antiplatelet therapy only | 34.8% | 38.1% |
| No therapy | 41.9% | 35.3% |
Categorical data are reported as percentages.
Oral anticoagulant therapy was defined as prescription of either warfarin, dabigatran, or rivaroxaban. Antiplatelet therapy was defined as prescription of either individual or combination of aspirin, clopidogrel, ticlopidine, prasugrel, and/or dipyridamole.
In multivariable analysis of the CHADS2 Score =0 Cohort assessing clinical predictors of oral anticoagulant prescription adjusted for clustering of patients within sites, older age (adjusted RR, 1.48 per 10 years; 95% CI, 1.41–1.56; p<0.0001), male sex (adjusted RR, 1.34; 95% CI, 1.22–1.46; p<0.0001), higher BMI (adjusted RR, 1.18 per 5 kg/m2; 95% CI, 1.14–1.22; p<0.0001), and Medicare compared to private insurance (adjusted RR, 1.32; 95% CI, 1.17–1.49; overall p<0.0001) were associated with a higher likelihood of being prescribed oral anticoagulation, whereas treatment in the South compared to the Northeast of the United States was associated with a lower likelihood of being prescribed oral anticoagulation (adjusted RR, 0.69; 95% CI, 0.49–0.98; overall p=0.1187) (Figure 2a). In multivariable analysis of the CHA2DS2-VASc Score =0 Cohort, older age (adjusted RR, 1.44 per 10 years; 95% CI, 1.36–1.54; p<0.0001), higher BMI (adjusted RR, 1.19 per 5 kg/m2; 95% CI, 1.15–1.23; p<0.0001), Medicare compared to private insurance (adjusted RR, 1.29; 95% CI, 1.13–1.47; overall p<0.0001), and no insurance compared to private insurance (adjusted RR, 1.19; 95% CI, 1.03–1.37; overall p<0.0001) were associated with a higher likelihood of being prescribed oral anticoagulation, whereas treatment in the South compared to the Northeast of the United States was associated with a lower likelihood of being prescribed oral anticoagulation (adjusted RR, 0.67; 95% CI, 0.47–0.96; overall p=0.0745) (Figure 2a).
Figure 2. Predictors of Oral Anticoagulant Prescription in Atrial Fibrillation Patients at Low Stroke Risk Among Both CHADS2 Score =0 and CHA2DS2-VASc Score =0 Cohorts.
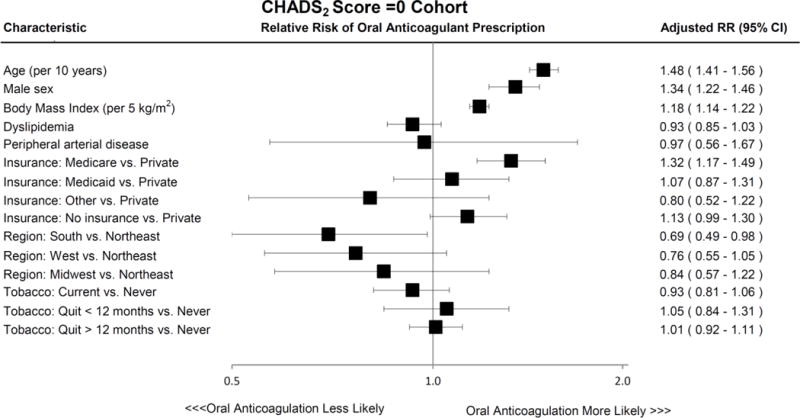
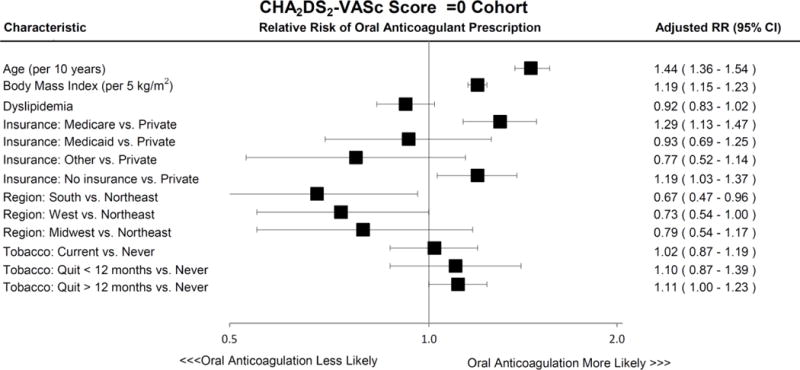
Characteristics associated with oral anticoagulant treatment after multivariable adjustment in AF patients with low thromboembolic risk, as defined by CHADS2 Score =0 (Panel A, and CHA2DS2-VASc Score =0 (Panel B). Error bars denote 95% confidence intervals.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; RR, relative risk
Discussion
In a large, nationally representative sample of young (<60 years of age) and healthy outpatients with AF at the lowest risk of stroke (both CHADS2 Score =0 and CHA2DS2-VASc Score =0) treated by cardiovascular specialists, approximately 25% of patients were prescribed oral anticoagulant therapy contrary to contemporary guideline recommendations. Specific patient characteristics predicted an increased likelihood of oral anticoagulant prescription. These findings may have important public health implications, since young and healthy AF patients at the lowest risk of stroke are felt to have an unfavorable risk/benefit profile when prescribed oral anticoagulation.
Despite a well-established association of AF with stroke, significant failure of guideline-adherence in the prescription of oral anticoagulation to reduce thromboembolism in at-risk candidates has been demonstrated in several large-scale studies (10,21,22). While improved educational campaigns and awareness of this important issue may help to reduce the risk of cardioembolic stroke and systemic thromboembolism, the “spill over” effects that might lead to inappropriate prescription in those who need oral anticoagulation the least has not previously been examined. Because oral anticoagulants bring the potential for both substantial benefit and harm, practitioner decision-making in regards to stroke prophylaxis in AF patients presents a unique clinical challenge. Clinical risk scores have been developed to elucidate and quantify stroke risk in AF patients to aid in the decision to prescribe antithrombotic therapies (15,16), realizing that the ultimate decision for oral anticoagulation involves a shared-decision making process supported by formal guidelines (7,8). Prescription of oral anticoagulation by cardiovascular specialists in a significant proportion of patients at the lowest thrombotic risk suggests that these providers may not be fully aware of the potential risks associated with oral anticoagulation or the particularly low risk of stroke in this population.
Previous studies evaluating oral anticoagulation (predominantly vitamin K antagonists such as warfarin) in AF patients at risk for thromboembolic stroke have shown that significant adverse events related to drug administration including the combination of intracranial hemorrhage and other major bleeding to occur in at least 1 to 3% of drug recipients per year (3). In more recent clinical trials of novel oral anticoagulants including dabigatran and rivaroxaban, the rate of significant bleeding was incrementally less, but overall similar to warfarin at approximately 2 to 3% per year (5,6). In a population-based study of Olmsted County, Minnesota patients with “lone” AF (defined as patients <60 years with no clinical history of echocardiographic evidence of concomitant overt cardiopulmonary disease) who were similar to the two low risk cohorts in our study, the overall thromboembolic stroke risk was much less than 1% at approximately 0.35% per year, with a 15-year cumulative stroke risk of 1.3% (23). In comparison, the intracranial hemorrhage risk was higher in several contemporary studies during treatment with warfarin, approaching 0.6–0.7% per year (5,6,24). Use of the novel oral anticoagulants, which are generally regarded as safer than warfarin regarding intracranial hemorrhage, still resulted in an absolute intracranial hemorrhage risk approaching 0.3–0.5% per year (5,6). Taken together, these studies suggest that the risk of intracranial hemorrhage itself from administration of oral anticoagulation may outweigh any benefit of thromboembolic stroke reduction in these low thromboembolic risk patients. In fact, using a recent cohort in an analysis of an administrative dataset, Friberg et al. evaluated 182,678 patients with AF and found that those with a CHA2DS2-VASc Score =0 did not derive a net clinical benefit from anticoagulation (25), suggesting that the patients identified in our current study may indeed be placed at unnecessary risk when exposed to full oral anticoagulation.
Because dabigatran and rivaroxaban were approved towards the end of our study timeframe, the majority of patients prescribed oral anticoagulation in our study were taking warfarin. Although three novel anticoagulants are now currently available in clinical practice, warfarin remains the most common drug prescription for anticoagulation in AF (26). Pooled analyses have found an overall lower major bleeding risk with the novel oral anticoagulants compared to warfarin, with significantly reduced risks of intracranial hemorrhage but higher risk of gastrointestinal hemorrhage with two of the three newer agents (27). Notably, the bleeding risk may be lower with apixaban, and the overall risk versus benefit of that drug in low risk AF patients remains unknown. However, there remains a 2.1% per year risk of major bleeding and 0.3% per year risk of intracranial hemorrhage with apixaban (28), and it is not unreasonable to suggest that this may not adequately balance protection against a 0.35% per year risk of stroke in those AF patients at particularly low risk of thromboembolism (23). Indeed, the most recent 2014 AF guidelines clearly state that it is reasonable to omit oral anticoagulation in AF patients without any established risk factor for stroke (8).
We discovered several clinical predictors of oral anticoagulant prescription in our cohort of young and healthy AF patients at the lowest risk of stroke. Interestingly, males with AF at the lowest risk of stroke were more likely to be prescribed oral anticoagulation than females despite previous studies that suggest an increased stroke risk in females (16,29). This is a clear indication that the important enhanced stroke risk in females remains largely unappreciated, even among cardiovascular specialists. Older AF patients (albeit within those less than 60 years of age) were also more likely to be prescribed oral anticoagulant therapy, which may reflect practitioner perception of age as a risk factor that has a more continuous spectrum rather than strict cutoff values. Higher BMI patients without stroke risk factors were also more often prescribed oral anticoagulation, which may suggest that physicians generally perceive overweight patients as less healthy or at higher risk for adverse events from AF. Although higher BMI has not been incorporated into any major clinical risk tool evaluating stroke risk in AF, there has been previously published evidence that suggests a potential link (30). More frequent prescription of oral anticoagulation in Medicare insured patients and differences in likelihood of oral anticoagulation prescription across regions of the United States in these low stroke risk AF patients may reflect social, economic, or cultural effects that should be the focus of future investigations.
Study limitations
Our study has several limitations. First, the PINNACLE Registry did not capture data on certain diagnoses such as previous pulmonary embolism or deep vein thrombosis, which may have warranted use of oral anticoagulation independent of AF. However, as such misclassification (if present) would have represented a small proportion of patients in our study, we do not believe it could be responsible for a meaningful contribution to the substantial number receiving anticoagulation. Using all of the data available whenever possible, we did take care to exclude patients from the analysis that would warrant anticoagulation independent of AF, such as patients with mechanical heart valves. Second, the PINNACLE Registry did not capture any procedural data on electrical cardioversion or catheter ablation for AF. Since oral anticoagulation is often administered in the post-procedural time period for 1–2 months, patients who recently underwent these procedures may have been categorized as taking oral anticoagulation despite the actual intention to treat only transiently. Once again, although this miscategorization would potentially inflate the overall number of lowest risk AF patients prescribed oral anticoagulation, we do not believe this would realistically comprise approximately 1 in 4 patients of the study cohort. Third, the PINNACLE program enrolled patients from motivated cardiology practices dedicated to quality improvement. Therefore, antithrombotic therapy prescription patterns in other U.S. practices may differ from those reported in this study, potentially reducing the generalizability of our results. Such bias may in fact lead to an underestimate of inappropriate anticoagulation in these low risk patients. Fourth, some may argue that the PINACCLE data collection form may not reflect actual prescription of the drug much less what patients actual receive or consume—however, that distinction is arguably minimally relevant for the purposes of this study as the data recorded on the form likely more purely mirrors the intent or perceived “correct” prescription of medications. Finally, there was limited registry data available regarding the cardiovascular specialists providing care for these AF patients, including the type of cardiologist (e.g. general, interventional, or cardiac electrophysiologist), board certification of treating specialist, or whether treated by cardiologists in a training program. This last limitation does not undermine our primary findings, but rather may limit our ability to identify accurate predictors of anticoagulation prescription in these patients.
Conclusions
In a large, real-world national registry of cardiology outpatients with AF and no structural heart disease who were at the lowest risk of stroke, cardiovascular disease specialists prescribed oral anticoagulation in approximately 1 out of 4 patients against contemporary evidence-based guideline recommendations. These findings draw attention to potential inappropriate prescription of oral anticoagulation in young and healthy patients with AF in whom bleeding risks may outweigh an antithrombotic benefit and highlight opportunities to reduce the risks of severe bleeding complications through better physician education. In short, contrary to previous reports documenting failure of physicians to take a recommended action, this study reveals an area where physicians should potentially withhold prescription in select patients in order to “do no harm” to those at particularly low risk.
Acknowledgments
None
Funding Sources:
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. PINNACLE Registry® is an initiative of the American College of Cardiology Foundation. Bristol-Myers Squibb and Pfizer Inc. are Founding Sponsors of the PINNACLE Registry.
Abbreviations List
- AF
Atrial fibrillation
- CI
Confidence interval
- NCDR
National Cardiovascular Data Registry
- PINNACLE
Practice Innovation and Clinical Excellence
- RR
Risk ratio
Footnotes
Disclosures:
Dr. Paul S. Chan is supported by a Career Development Grant Award (K23HL102224) from the National Heart Lung and Blood Institute.
Dr. Thomas M. Maddox is supported by a Health Services Research and Development career development award from the U.S. Department of Veterans Affairs.
Dr. Gregory M. Marcus receives research support from Medtronic, Baylis Medical, Gilead, and SentreHeart Inc.
References
- 1.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–6. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. Jama. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.van Walraven C, Hart RG, Singer DE, et al. Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta-analysis. JAMA. 2002;288:2441–8. doi: 10.1001/jama.288.19.2441. [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 7.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Alpert JS, et al. AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014. 2014 doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program) Am J Cardiol. 2011;108:1136–40. doi: 10.1016/j.amjcard.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds MR, Shah J, Essebag V, et al. Patterns and predictors of warfarin use in patients with new-onset atrial fibrillation from the FRACTAL Registry. Am J Cardiol. 2006;97:538–43. doi: 10.1016/j.amjcard.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher AM, Rietbrock S, Plumb J, van Staa TP. Initiation and persistence of warfarin or aspirin in patients with chronic atrial fibrillation in general practice: do the appropriate patients receive stroke prophylaxis? J Thromb Haemost. 2008;6:1500–6. doi: 10.1111/j.1538-7836.2008.03059.x. [DOI] [PubMed] [Google Scholar]
- 13.Chan PS, Oetgen WJ, Buchanan D, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry’s PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan PS, Oetgen WJ, Spertus JA. The Improving Continuous Cardiac Care (IC(3)) program and outpatient quality improvement. Am J Med. 2010;123:217–9. doi: 10.1016/j.amjmed.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE. Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 18.Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: imputation and variance estimation software. Ann Arbor, MI: Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan; 2002. [Google Scholar]
- 19.Greenland S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am J Epidemiol. 2004;160:301–5. doi: 10.1093/aje/kwh221. [DOI] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Glazer NL, Dublin S, Smith NL, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167:246–52. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
- 22.Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–7. doi: 10.1161/01.str.31.4.822. [DOI] [PubMed] [Google Scholar]
- 23.Kopecky SL, Gersh BJ, McGoon MD, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–74. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Tonarelli SB, Pearce LA. Avoiding central nervous system bleeding during antithrombotic therapy: recent data and ideas. Stroke. 2005;36:1588–93. doi: 10.1161/01.STR.0000170642.39876.f2. [DOI] [PubMed] [Google Scholar]
- 25.Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–307. doi: 10.1161/CIRCULATIONAHA.111.055079. [DOI] [PubMed] [Google Scholar]
- 26.Lip GY, Laroche C, Dan GA, et al. ‘Real-World’ Antithrombotic Treatment in Atrial Fibrillation: The EORP-AF Pilot Survey. Am J Med. 2014;127:519–529 e1. doi: 10.1016/j.amjmed.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 27.Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 28.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–56. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 30.Overvad TF, Rasmussen LH, Skjoth F, Overvad K, Lip GY, Larsen TB. Body mass index and adverse events in patients with incident atrial fibrillation. Am J Med. 2013;126:640 e9–17. doi: 10.1016/j.amjmed.2012.11.024. [DOI] [PubMed] [Google Scholar]


