SUMMARY
Herein we report that the VEGFR/PDGFR kinase inhibitor sunitinib/SU11248 can accelerate metastatic tumor growth and decrease overall survival in mice receiving short-term therapy in various metastasis assays, including after intravenous injection of tumor cells or after removal of primary orthotopically grown tumors. Acceleration of metastasis was also observed in mice receiving sunitinib prior to intravenous implantation of tumor cells, suggesting possible “metastatic conditioning” in multiple organs. Similar findings with additional VEGF receptor tyrosine kinase inhibitors implicate a class-specific effect for such agents. Importantly, these observations of metastatic acceleration were in contrast to the demonstrable antitumor benefits obtained when the same human breast cancer cells, as well as mouse or human melanoma cells, were grown orthotopically as primary tumors and subjected to identical sunitinib treatments.
INTRODUCTION
Multiple strategies for inhibiting the VEGF pathway have been shown in numerous preclinical studies to hinder tumor growth, and the recent approval of a VEGF-neutralizing antibody (bevacizumab) and VEGF receptor tyrosine kinase inhibitors (RTKIs) (sorafenib and sunitinib) has clinically validated targeting VEGF or its receptors (particularly VEGFR2) as an anticancer treatment (Folkman, 2007). The purpose of the present study was to test whether a VEGF RTKI, when administered daily for short periods, can influence the growth of experimental and spontaneous metastasis in mice. The rationale for this experimental design was based on a number of recent clinical and preclinical observations. First, while sorafenib and sunitinib have been approved for the treatment of renal cell carcinoma, as well as hepatocellular carcinoma (sorafenib only) and gastrointestinal stromal tumor (sunitinib only), enduring clinical responses are rare. Moreover, single-agent use of such drugs, including bevacizumab, has not led to meaningful beneficial activity in many cases. Second, when such agents are administered on a discontinuous schedule, such as with sunitinib (4 weeks on/2 weeks off), tumor regrowth has sometimes been observed during drug-free break periods (Burstein et al., 2008) or when treatment is discontinued (Johannsen et al., 2008). Third, rapid tumor revascularization has been reported when therapy is stopped in preclinical studies (Mancuso et al., 2006). Finally, we recently reported that a number of changes in proangiogenic plasma proteins observed in patients after sunitinib treatment could be recapitulated in non-tumor-bearing mice, and moreover, in a dose-dependent manner (Ebos et al., 2007). Together, these results suggest a systemic host response to VEGF inhibition that could play a role in regrowth of both tumor and vasculature in eventual evasion of response during continued antiangiogenic treatment (Bergers and Hanahan, 2008; Casanovas et al., 2005) as well as potentially influencing the progression of secondary (metastatic) disease—a possibility that could impact the outcome of neoadjuvant and adjuvant administration of such agents (von Mehren, 2008; Shuch et al., 2008). In this study, we examined the impact of sunitinib on metastasis, with the choice of sunitinib based not only on our aforementioned work but also on the discontinuous administration schedule of this agent currently used in clinical practice (Motzer et al., 2006).
RESULTS AND DISCUSSION
Our first experiments tested the effect of short-term sunitinib treatment in a model of experimental metastasis. Human metastatic breast cancer 231/LM2-4LUC+ cells expressing luciferase were injected into the tail vein of severe combined immunodeficiency (SCID) mice, which then received vehicle treatment or a short-term sunitinib therapy regimen (120 mg/kg/daily for 7 days) administered by gavage either before or after tumor cell inoculation (groups A, B, and C, respectively; Figure 1A). Short-term sunitinib treatment administered either before or after tumor cell injection resulted in accelerated experimental metastasis as measured by bioluminescence (Figure 1A) and significantly reduced median survival compared to vehicle-treated controls (Figure 1B). Representative images showing increased metastasis in sunitinib-treated mice are shown in Figure 1C. The choice of 120 mg/kg daily sunitinib for a 7-day period was based on prior preclinical studies that had demonstrated this short-term regimen to maximize the aforementioned multiple host-derived changes in proangiogenic proteins in mouse plasma. A sustained sunitinib therapy regimen of 60 mg/kg/day given continuously (described below) had previously been shown to result in optimal tumor inhibition with minimal toxicity after long-term therapy (Ebos et al., 2007). The treatment for group B was stopped 24 hr prior to injection of tumor cells in order to minimize any potential direct drug effect on tumor cells, as blood concentrations of sunitinib are significantly reduced 24 hr after treatment cessation, and to maximize the aforementioned sunitinib-induced host molecular changes, which were shown to be reversed within 2–5 days after stopping therapy (Ebos et al., 2007). Importantly, similar results were obtained when nu/nu mice were treated with other VEGF RTKIs including sorafenib (150 mg/kg/day) and SU10944 (225 mg/kg/day) 7 days prior to tumor cell inoculation (see Figures S1A–S1C available online). These results suggest that a host response to multitargeted angiogenic kinase inhibition can result in conditions that allow for increased tumor initiation even after drug has been removed.
Figure 1. Accelerated Experimental Metastasis and Decreased Survival after Short-Term Sunitinib Treatment before and after Intravenous Tumor Inoculation.
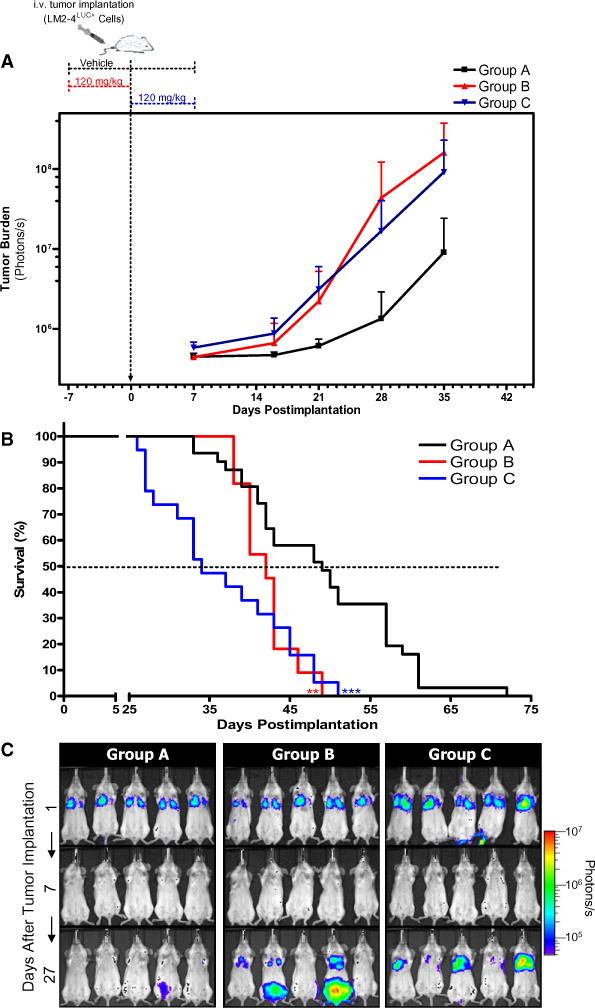
(A) 231/LM2-4LUC+ cells were injected into the tail vein of SCID mice that received vehicle (group A) or short-term sunitinib treatment daily for 7 days either before (group B) or after tumor inoculation (group C). Quantification of bioluminescence showed accelerated tumor growth in groups B and C compared with controls. A representative experiment is shown. Group A, n = 10; group B, n = 5; group C, n = 10. Data are presented as mean ± SD.
(B) Kaplan-Meier survival curve shows significantly decreased median survival of mice in group B (log-rank test, p = 0.0055) and group C (log-rank test, p < 0.0001) compared with group A. Data represent a summary of multiple experiments. Group A, n = 31; group B, n = 11; group C, n = 19. 0.001 < **p < 0.01; ***p < 0.001.
(C) Representative images for each group taken 1, 7, and 27 days following tumor implantation, with increased metastasis visible in sunitinib-treated mice. Sunitinib dose and treatment schedule were performed as illustrated in (A).
We next tested the effect of short-term sunitinib treatment on distant spontaneous metastasis generated after primary tumor removal using the protocol illustrated in Figure 2A. Mice receiving short-term adjuvant sunitinib therapy showed increased spontaneous metastatic tumor burden as measured by bioluminescence (Figures 2A and 2B), which corresponded with decreased overall survival (Figure 2C). To ensure equal tumor burden between groups prior to drug treatment, resected tumors were weighed prior to sorting into groups A and B (Figure 2D).
Figure 2. Short-Term Sunitinib Treatment Increases Spontaneous Metastasis and Decreases Survival after Removal of Primary Human Xenograft Tumors.
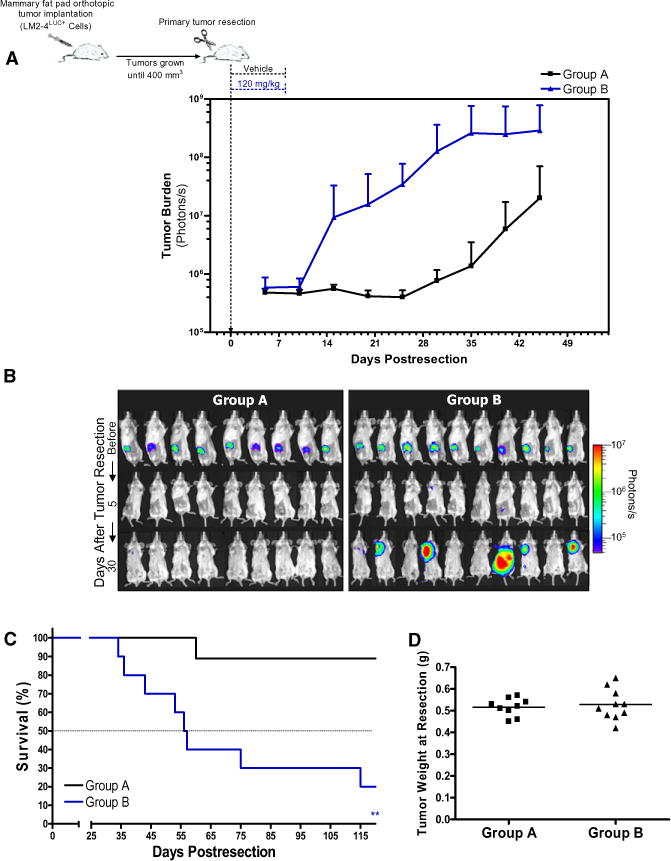
(A) Orthotopically grown 231/LM2-4LUC+ tumors were surgically removed, and SCID mice were treated daily with vehicle (group A) or short-term sunitinib therapy (group B). Biweekly quantification of bioluminescence showed accelerated tumor growth and increased spontaneous metastasis in group B compared with group A. Data are presented as mean ± SD.
(B) Representative bioluminescence images visualizing tumor cells before and after primary tumor resection (days 5 and 30 after resection).
(C) Kaplan-Meier survival curves of the corresponding mice show significantly decreased median survival in group B (log-rank test, p = 0.0024) compared with group A. 0.001 < **p < 0.01.
(D) Resected tumors were weighed prior to sorting into groups A and B to ensure equal tumor burden between groups. Sunitinib dose and treatment schedule were performed as illustrated in (A).
We have previously reported that surgical resection of highly metastatic orthotopically grown 231/LM2-4 tumors leads to spontaneous metastasis in the lungs, liver, and lymph nodes, with the primary determinant for euthanasia being extensive visceral and peripheral metastasis (Man et al., 2007). In the spontaneous and experimental metastasis studies described herein, we similarly considered visceral and peripheral disease as the primary reason for mouse sacrifice (data not shown). To test for differences in distribution of metastatic disease after sunitinib treatment, mice subjected to the same protocol as described in Figure 1 were sacrificed 27 days after tumor implantation to allow for assessment of 231/LM2-4LUC+ tumor burden using bioluminescence (Figure S2). Increased overall tumor burden was found in mice receiving sunitinib before or after tumor inoculation (groups B and C compared to group A; Figure 3A, inset), which corresponded to increased metastatic tumor burden in multiple organs, while no obvious differences in overall tumor distribution were observed (Figure 3A). Immunohistochemical staining for tumor tissue in mouse organs using an anti-human vimentin antibody confirmed increased micrometastasis in various organs in groups B and C compared with controls in group A (Figure 3B, upper panel). Importantly, increased metastasis in multiple organs was observed in an additional tumor model when human MeWo melanoma cells were injected intravenously (i.v.) into nu/nu mice pretreated with either vehicle or short-term sunitinib therapy (groups A and B, respectively; Figure 3B, lower panel). Examples of micrometastases detected using an antibody specific to human vimentin are shown in Figure 3C. Finally, increased visible lung surface nodules and lung weight were indicative of increased tumor burden in both tumor models, with representative hematoxylin and eosin (H&E) and anti-vimentin staining shown in Figure 3D.
Figure 3. Increased Multiorgan Metastasis in Mice after Short-Term Sunitinib Treatment.
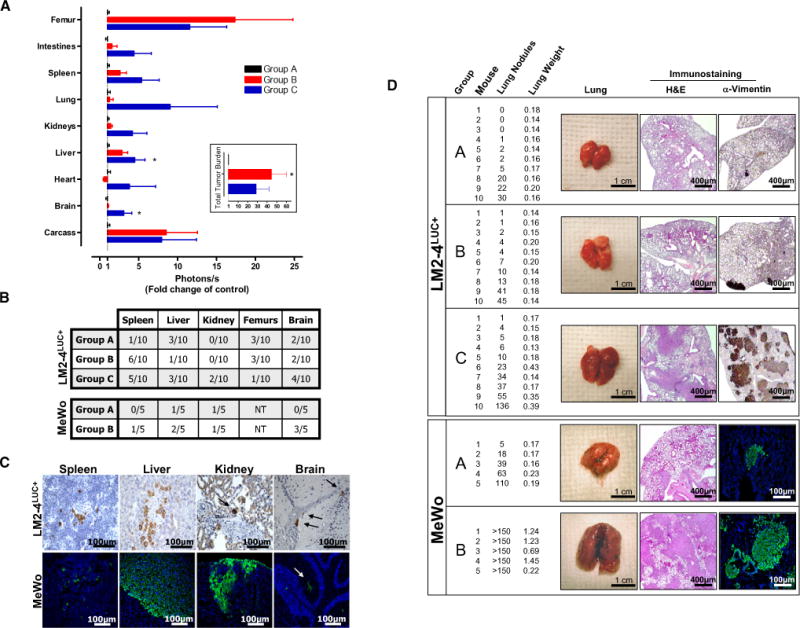
(A) Following the same experimental design as described in Figure 1A, SCID mice were sacrificed at day 27 to compare increases in overall tumor burden after short-term sunitinib treatment (inset) and corresponding increased bioluminescence in multiple organs (groups A, B, and C). Data are presented as mean ± SEM. Instances of highly divergent bioluminescence values did not permit statistical significance to be reached in all groups. 0.01 < *p < 0.05 by one-way ANOVA.
(B) Micrometastases were confirmed by immunostaining for human vimentin in organs of the 231/LM2-4LUC+ tumor model in (A) (upper panel) or in nu/nu mice receiving vehicle (group A) or short-term sunitinib therapy (group B) prior to intravenous (i.v.) inoculation with human MeWo melanoma cells (lower panel). Tissue sections were scored as positive or negative based on the presence or absence of detectable micrometastases. NT = not tested.
(C) Representative examples of micrometastases in spleen, liver, kidney, and brain shown using human-specific vimentin antibodies.
(D) Excised lungs from 231/LM2-4LUC+ and MeWo tumor models were scored visually for surface tumor nodules, with confirmation of macrometastasis by hematoxylin and eosin (H&E) and anti-vimentin immunostaining (representative images shown). For groups A, B, and C in the 231/LM2-4LUC+ tumor model, n = 10 per group. For groups A and B in the MeWo tumor model, n = 5 per group.
Since previously published studies had demonstrated potent tumor growth inhibition of established primary tumors in mice after sunitinib treatment (Christensen, 2007), we compared short-term and sustained sunitinib therapies in both orthotopic primary tumor and experimental metastasis models. 231/LM2-4LUC+ cells were implanted into the mammary fat pad of nu/nu mice and treated with vehicle or sustained sunitinib therapy (60 mg/kg/daily) when tumors reached 200 mm3 in size (groups A and B; Figure 4A). A third group of mice received short-term sunitinib treatment (120 mg/kg/day for 7 days) starting the day after tumor implantation (group C; Figure 4A). Significant tumor growth delay was observed after either short-term or sustained treatment compared to controls, with sustained sunitinib therapy having more potent tumor-inhibitory effects. In contrast, accelerated tumor growth was observed when the same cells were injected i.v. into nu/nu mice receiving short-term sunitinib therapy either before or immediately after tumor inoculation (groups A, B, and C; Figure 4B). Short-term treatment followed by sustained sunitinib therapy did not abrogate this acceleration of tumor growth, and mice receiving only sustained treatment, initiated 7 days following tumor inoculation, did not exhibit reduced tumor burden compared to controls (groups D and E; Figure 4B). Corresponding survival curves show significantly decreased median survival in groups B, C, and D when compared to controls, with group E showing no overall prolongation of survival. Importantly, as an additional example of opposing efficacies of sunitinib therapy in both orthotopic primary tumor and experimental metastasis models, we observed potent tumor growth inhibition in nu/nu mice bearing orthotopically growing human MeWo melanoma tumors that were treated with sustained sunitinib therapy (Figure S3). This was in contrast to the increased metastasis observed in the lungs of mice 58 days after i.v. inoculation of tumor cells immediately following short-term sunitinib therapy (Figure 3D).
Figure 4. Differentiating Opposing Efficacies of Short-Term and Sustained Sunitinib Treatment in Primary and Metastatic Disease.
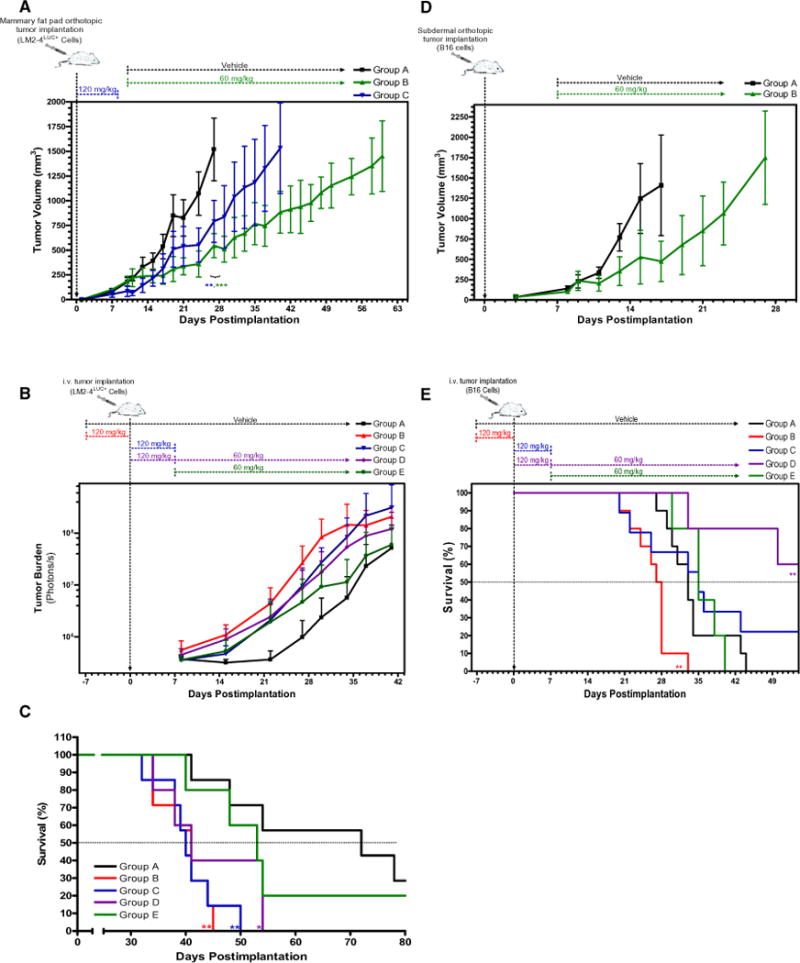
(A) nu/nu mice bearing orthotopically grown 231/LM2-4LUC+ tumors received either vehicle (group A) or sustained sunitinib therapy (group B) when tumors reached an average volume of 200 mm3. A third group received short-term sunitinib therapy (group C) starting the day after tumor implantation. Group A reached tumor volume endpoint (1500 mm3) at 27 days, with comparative tumor volume significantly reduced in group B (p = 0.0002 by Student’s t test) and group C (p = 0.0027 by Student’s t test) at the same time point. Mice receiving sustained sunitinib therapy showed greater tumor growth inhibition compared to mice receiving short-term therapy (41 to 62 days to endpoint, respectively). n = 5 for all groups.
(B) nu/nu mice injected i.v. with231/LM2-4LUC+cells were treated daily with vehicle (group A) or received short-term sunitinib therapy for 7 days either before (group B) or after tumor inoculation (group C). Quantification of bioluminescence showed accelerated metastatic tumor growth in groups B and C after short-term sunitinib therapy. Mice in groups D and E received sustained sunitinib therapy starting on day 8, with group D also receiving short-term sunitinib therapy similar to group C.
(C) Corresponding Kaplan-Meier survival curves show that median survival was significantly decreased in groups B, C, and D (p=0.0011, p=0.0022, and p=0.0365 by log-rank test, respectively) and was not significantly different in group E (p=0.4485 by log-rank test) compared to control mice in group A. For (B) and (C): group A, n = 7; group B, n = 7; group C, n = 7; group D, n = 5; group E, n = 5.
(D) In a syngeneic tumor model, C57BL/6 mice bearing mouse B16 melanoma tumors grown orthotopically showed delayed primary tumor growth after sustained sunitinib therapy compared with control mice (groups A and B, with time to endpoint 17 and 28 days, respectively). Group A, n = 4; group B, n = 4.
(E) C57BL/6 mice receiving short-term sunitinib therapy prior to i.v. inoculation with the same mouse B16 melanoma tumor cells showed accelerated experimental metastasis and decreased survival compared to controls (group B; p = 0.0014 by log-rank test). Delayed metastasis and increased survival were observed in mice receiving short-term sunitinib treatment followed by sustained sunitinib treatment (group D; p = 0.0047 by log-rank test). Mice receiving sustained sunitinib therapy 7 days after tumor implantation showed no difference in survival compared to vehicle-treated control mice (group E; p=0.6368 by log-ranktest). Mice receiving short-term sunitinib therapy following tumor implantation exhibited a bimodal response that included either accelerated metastasis and reduced survival or extended survival (group C; p= 0.3391 by log-rank test). Group A, n = 10; group B, n=10; group C, n=9; group D, n=5; group E, n= 5. Sunitinib dose and treatment schedule were performed as illustrated.
Data are presented as mean ± SD. 0.01 < *p < 0.05; 0.001 < **p < 0.01; ***p < 0.001.
We next tested whether similar results would be observed in a syngeneic mouse tumor model following the same treatment protocol as in Figure 4A. Mouse B16 melanoma cells were implanted subdermally into the flanks of C57BL/6 mice and, as in the aforementioned human xenograft experiments, primary tumor growth was found to be delayed in mice receiving sustained sunitinib treatment compared to controls (groups A and B; Figure 4D). In contrast, and similar to Figure 4C, C57BL/6 mice receiving short-term sunitinib treatment for 7 days prior to i.v. tumor inoculation showed decreased survival compared to controls, whereas sustained sunitinib therapy, initiated 8 days after inoculation, showed no survival advantage (groups A, B, and E; Figure 4E). Interestingly, mice receiving short-term sunitinib therapy immediately following tumor inoculation, which was then either stopped after 7 days (group C) or followed by sustained sunitinib therapy (group D), exhibited a survival advantage in some instances. Group D mice showed a significant prolongation of survival compared to control mice, while mice in group C had biphasic effects, with about half of the mice progressing with accelerated metastasis and the remainder showing a prolongation in survival, even after cessation of treatment (Figure 4E). One potential explanation for these results could stem from possible direct antitumor activity of sunitinib therapy against B16 cells—something that has been demonstrated previously with other VEGF RTKIs (Beaudry et al., 2008).
Taken together, our results show that VEGF receptor tyrosine kinase inhibition by the same drug, administered in different schedules and doses using three different tumor cell models, can have opposing effects on tumor growth. Sustained sunitinib treatment of preestablished tumors led to significant growth inhibition in both orthotopically grown primary human xenograft and syngeneic tumors. In contrast, short-term treatment prior to i.v. inoculation with the same cells produced an acceleration of metastasis and corresponding significant reduction in median survival. Furthermore, sustained treatment in both 231/LM2-4LUC+ and B16 experimental metastasis models, initiated 7 days after tumor cell inoculation, did not produce a survival advantage compared to controls. Conversely, short-term sunitinib treatment immediately following tumor inoculation was shown to accelerate metastatic disease (in the 231/LM2-4LUC+ model) or produce a biphasic response (in the B16 model).
Our results complement those of Pàez-Ribes et al. (2009) in this issue of Cancer Cell. Importantly, in both studies, antiangiogenic drug treatment was shown to have potent inhibitory effects in localized tumors. Furthermore, Pàez-Ribes et al. show that treatment of tumor-bearing mice with antiangiogenic drugs, including the VEGFR2-blocking monoclonal antibody DC101 and VEGF RTKIs such as sunitinib and SU10944, leads to increased local tumor cell invasion and enhanced distant metastasis after prolonged treatment or, in the case of DC101, after only short-term treatment. As Pàez-Ribes et al. demonstrate, these effects appear to be an adaptive/evasive response by the tumor cells themselves triggered by a disruption of the tumor vasculature. Our results present a second possibility independent of adaptations by an established tumor that involves microenvironmental changes in mouse organs that are “conditioned” to be more permissive to tumor extravasation. But how might such a metastatic “conditioning” effect occur? A number of potential mechanisms alone or in combination could play a role. One is the aforementioned induced upregulation of multiple circulating proangiogenic cytokines and growth factors in response to treatment, including osteopontin, G-CSF, and SDF1α (Ebos et al., 2007)—all of which have been implicated in angiogenesis and/or metastasis (McAllister et al., 2008; Ben Baruch, 2008; Wai and Kuo, 2008; Natori et al., 2002; Zhang et al., 2000). Second, and likely related to such molecular changes, the mobilization of bone marrow-derived cells may facilitate an enhanced “pre-metastatic niche,” including circulating endothelial (Okazaki et al., 2006) and myeloid progenitors (Shojaei et al., 2008), CXCR4+ recruited bone marrow circulating cells (Grunewald et al., 2006), and circulating VEGFR1+ bone marrow cells (Kaplan et al., 2005). Finally, the well-recognized target promiscuity of RTKIs such as sunitinib and sorafenib (Karaman et al., 2008) may produce a plethora of broad host microenvironmental responses to cellular inhibition/injury. These responses in turn may promote tumor extravasation, similar in principal to the enhanced metastasis observed after treatment with radiation and numerous chemotherapeutic drugs (van Putten et al., 1975; Vollmer and Conley, 1984; de Ruiter et al., 1982)—all of which may involve various proinflammatory responses (Noonan et al., 2008) or alterations in the endothelial microenvironment. Collectively, such effects could create a more favorable metastatic niche (Bidard et al., 2008). Importantly, however, unlike chemotherapy and radiation treatments, which act in large part via direct tumor cytotoxicity by nonspecifically targeting proliferating cells and are administered for defined periods, antiangiogenic agents act in large part against host tumor support processes, thus indirectly prohibiting tumor growth, and are meant (at least theoretically) to be administered indefinitely. Regardless of the actual mechanisms involved, our results may be pertinent to the consideration of several prominent issues in cancer therapeutics, including the relative benefits of discontinuous versus continuous treatment schedules of antiangiogenic drugs (such as sunitinib), duration of treatment, use of such drugs in the neoadjuvant and adjuvant setting, and the prospect that drugs targeting metastatic mechanisms might be considered in the future for combination with antiangiogenic agents, aiming to improve their overall antitumor efficacy.
EXPERIMENTAL PROCEDURES
Drug
SU11248/sunitinib malate (Sutent, Pfizer) and SU10944 (Pfizer) were suspended in vehicle 1, which contained carboxymethylcellulose sodium (USP, 0.5% w/v), NaCl (USP, 1.8% w/v), Tween 80 (NF, 0.4% w/v), benzyl alcohol (NF, 0.9% w/v), and reverse osmosis deionized water (added to final volume) adjusted to pH 6.0. Sorafenib (Nexavar, Bayer) was suspended in vehicle 2, which contained Cremophor (Sigma), 95% ethanol, and reverse osmosis deionized water in a ratio of 1:1:6. Drug aliquots were prepared once weekly and kept in the dark at 4°C.
Cell Lines
The human 231/LM2-4LUC+ cells were 231/LM2-4 cells (Munoz et al., 2006) co-transfected with plasmids expressing the firefly luciferase gene (pGL3-control, Promega Corporation) and neomycin resistance gene as described previously (Ebos et al., 2008). 231/LM2-4LUC+ cells, human MeWo melanoma cells, and mouse B16 melanoma cells (obtained from the American Type Culture Collection) were maintained in Dulbecco’s modified Eagle’s medium with 5% heat-inactivated fetal bovine serum (GIBCO Invitrogen Corp.). All cells were incubated at 37°C in 5% CO2 in a humidified incubator.
Mouse Tumor Models
All animal studies, including maintenance and determination of experimental endpoints, were performed according to the Sunnybrook Health Sciences Centre Animal Care Committee and the Canadian Council on Animal Care.
Experimental Metastasis Assays
231/LM2-4LUC+(1 × 106 cells) or human MeWo melanoma cells (1 × 106 cells) were injected directly into the tail vein of 6-to 8-week-old female CB-17 SCID mice or BALB/c allogeneic athymic nude (nu/nu) mice (Charles River Canada). B16 mouse melanoma cells (2 × 104 cells) were injected directly into the tail vein of 4-to 6-week-old female C57BL/6 mice (Charles River Canada). Metastatic disease progression in 231/LM2-4LUC+ tumor-bearing animals was monitored biweekly (see imaging details below). For 231/LM2-4LUC+ and human MeWo melanoma models presented in Figure 3, mice were sacrificed for examination at 27 and 58 days, respectively, after tumor inoculation.
Spontaneous Human Xenograft Metastasis
231/LM2-4LUC+cells (2 × 106 cells) were orthotopically implanted into the right inguinal mammary fat pads of 6-to 8-week-old female CB-17 SCID mice as described previously (Munoz et al., 2006). Tumors reaching approximately 400 mm3 2–3 weeks later were surgically removed and weighed, and 24 hr later, mice received either vehicle or short-term sunitinib treatment.
Syngeneic and Human Xenograft Orthotopic Tumors
Orthotopic tumor implantation of 231/LM2-4LUC+ cells was performed as described above, while human MeWo (2 × 106 cells) and mouse B16 cells (1 × 106 cells) were implanted into the dermis of 4-to 6-week-old female nu/nu or C57BL/6 mice, respectively. Treatment with vehicle or sustained sunitinib therapy was initiated when tumors reached an average volume of 600 mm3 (MeWo) or 200 mm3 (B16); experimental endpoint was reached when 50% of the group averaged tumor volumes of >1500 mm3.
Statistical Analysis
Results were subjected to statistical analysis using GraphPad Prism v4.0 software. Survival curves were analyzed using the Kaplan-Meier method, with groups compared by respective median survival or number of days taken to reach 50% morbidity. Two-tailed p values were calculated using the Mantel-Haenszel log-rank test. One-way ANOVA was followed by Dunnett’s multiple-comparison test. Student’s t tests were two-tailed and unpaired.
Supplementary Material
SIGNIFICANCE.
The use of VEGF pathway inhibitors to impair angiogenesis now represents a clinically validated anticancer treatment strategy. However, the benefits of VEGF-targeted agents in the treatment of late-stage cancers can be transitory, resulting in eventual drug resistance, tumor growth and/or regrowth, and rapid vascular recovery when therapy is stopped. Our findings here demonstrate that angiogenesis inhibition in mice can lead to opposing effects on tumor growth and metastasis depending on tumor stage and treatment duration. These observations could have clinical implications with respect to optimal dose, treatment schedule, and therapy in the adjuvant/neoadjuvant setting and highlight the importance of testing additional drugs in combination as a possible approach to abrogate this effect.
Acknowledgments
We thank S. Wilhelm (Bayer) for sorafenib and G. Francia for critical analysis and experimental assistance. The Terry Fox Foundation supports J.M.L.E. through an award from the National Cancer Institute of Canada (NCIC), and R.S.K. is a Canada Research Chair. This work was supported by grants from the Ontario Institute for Cancer Research, the Canadian Institutes of Health Research, and the NCIC to R.S.K.
J.G.C. is an employee and shareholder of Pfizer Inc.
Footnotes
SUPPLEMENTAL DATA
The Supplemental Data include Supplemental Experimental Procedures, Supplemental References, and three figures and can be found with this article online at http://www.cancercell.org/supplemental/S1535-6108(09)00029-4.
References
- Beaudry P, Nilsson M, Rioth M, Prox D, Poon D, Xu L, Zweidler-Mckay P, Ryan A, Folkman J, Ryeom S, Heymach J. Potent antitumor effects of ZD6474 on neuroblastoma via dual targeting of tumor cells and tumor endothelium. Mol Cancer Ther. 2008;7:418–424. doi: 10.1158/1535-7163.MCT-07-0568. [DOI] [PubMed] [Google Scholar]
- Ben Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–356. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidard FC, Pierga JY, Vincent-Salomon A, Poupon MF. A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer Metastasis Rev. 2008;27:5–10. doi: 10.1007/s10555-007-9103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, Deprimo SE, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- Casanovas O, Hicklin D, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Christensen JG. A preclinical review of sunitinib, a multitargeted receptor tyrosine kinase inhibitor with anti-angiogenic and antitumour activities. Ann Oncol. 2007;18(Suppl 10):x3–x10. doi: 10.1093/annonc/mdm408. [DOI] [PubMed] [Google Scholar]
- de Ruiter J, Cramer SJ, Lelieveld P, van Putten LM. Comparison of metastatic disease after local tumour treatment with radiotherapy or surgery in various tumour models. Eur J Cancer Clin Oncol. 1982;18:281–289. doi: 10.1016/0277-5379(82)90047-5. [DOI] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, Xu P, Mutsaers AJ, Dumont DJ, Kerbel RS. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–529. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, Chimenti S, Landsman L, Abramovitch R, Keshet E. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Johannsen M, Florcken A, Bex A, Roigas J, Cosentino M, Ficarra V, Kloeters C, Rief M, Rogalla P, Miller K, Grunwald V. Can tyrosine kinase inhibitors be discontinued in patients with metastatic renal cell carcinoma and a complete response to treatment? A multicentre, retrospective analysis. Eur Urol. 2008 doi: 10.1016/j.eururo.2008.10.021. Published online October 18, 2008. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Man S, Munoz R, Kerbel RS. On the development of models in mice of advanced visceral metastatic disease for anti-cancer drug testing. Cancer Metastasis Rev. 2007;26:737–747. doi: 10.1007/s10555-007-9087-6. [DOI] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Hoosen S, Bello CL, Christensen JG. Sunitinib malate for the treatment of solid tumours: a review of current clinical data. Expert Opin Investig Drugs. 2006;15:553–561. doi: 10.1517/13543784.15.5.553. [DOI] [PubMed] [Google Scholar]
- Munoz R, Man S, Shaked Y, Lee C, Wong J, Francia G, Kerbel RS. Highly efficacious non-toxic treatment for advanced metastatic breast cancer using combination UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–3391. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002;297:1058–1061. doi: 10.1016/s0006-291x(02)02335-5. [DOI] [PubMed] [Google Scholar]
- Noonan DM, De Lerma BA, Vannini N, Mortara L, Albini A. Inflammation, inflammatory cells and angiogenesis: decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ebihara S, Asada M, Kanda A, Sasaki H, Yamaya M. Granulocyte colony-stimulating factor promotes tumor angiogenesis via increasing circulating endothelial progenitor cells and Gr1+CD11b+ cells in cancer animal models. Int Immunol. 2006;18:1–9. doi: 10.1093/intimm/dxh334. [DOI] [PubMed] [Google Scholar]
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Anti-angiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuch B, Riggs SB, Larochelle JC, Kabbinavar FF, Avakian R, Pantuck AJ, Patard JJ, Belldegrun AS. Neoadjuvant targeted therapy and advanced kidney cancer: observations and implications for a new treatment paradigm. BJU Int. 2008;102:692–696. doi: 10.1111/j.1464-410X.2008.07660.x. [DOI] [PubMed] [Google Scholar]
- van Putten LM, Kram LK, van Dierendonck HH, Smink T, Fuzy M. Enhancement by drugs of metastatic lung nodule formation after intravenous tumour cell injection. Int J Cancer. 1975;15:588–595. doi: 10.1002/ijc.2910150408. [DOI] [PubMed] [Google Scholar]
- Vollmer TL, Conley FK. Effect of cyclophosphamide on survival of mice and incidence of metastatic tumor following intravenous and intracardial inoculation of tumor cells. Cancer Res. 1984;44:3902–3906. [PubMed] [Google Scholar]
- von Mehren M. The role of adjuvant and neoadjuvant therapy in gastrointestinal stromal tumors. Curr Opin Oncol. 2008;20:428–432. doi: 10.1097/CCO.0b013e328302ed82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev. 2008;27:103–118. doi: 10.1007/s10555-007-9104-9. [DOI] [PubMed] [Google Scholar]
- Zhang W, Stoica G, Tasca SI, Kelly KA, Meininger CJ. Modulation of tumor angiogenesis by stem cell factor. Cancer Res. 2000;60:6757–6762. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


