Abstract
Recent experimental and clinical studies have shown that the epicardial autonomic ganglia play an important role in the initiation and maintenance of atrial fibrillation (AF). In this review, we present the current data on the role of the autonomic ganglia in the pathogenesis of AF and discuss potential therapeutic implications. Experimental studies have demonstrated that acute autonomic remodeling may play a crucial role in AF maintenance in the very early stages. The benefit of adding ablation of the autonomic ganglia to the standard pulmonary vein (PV) isolation procedure for patients with paroxysmal AF is supported by both experimental and clinical data. The interruption of axons from these hyperactive autonomic ganglia to the PV myocardial sleeves may be an important factor in the success of PV isolation procedures. The vagus nerve exerts an inhibitory control over the autonomic ganglia and attenuation or loss of this control may allow these ganglia to become hyperactive. Autonomic neuromodulation using low-level vagus nerve stimulation inhibits the activity of the autonomic ganglia and reverses acute electrical atrial remodeling during rapid atrial pacing and may provide an alternative non-ablative approach for the treatment of AF, especially in the early stages. This notion is supported by a preliminary human study. Further studies are warranted to confirm these findings.
Keywords: atrial fibrillation, autonomic nervous system, neuromodulation
Recent experimental and clinical studies have shown that the intrinsic cardiac autonomic nervous system (CANS), which is formed by interconnected clusters of autonomic ganglia, known as ganglionated plexi (GP), plays an important role in the initiation and maintenance of atrial fibrillation (AF) (1). Variations in autonomic tone in humans (2) and hyperactivity of the GP in ambulatory dogs (3) often precede episodes of paroxysmal AF. Four of the left atrial GP heavily innervate each of the myocardial sleeves of the four pulmonary veins (PVs) (4, 5). Recent experimental evidence suggests that it is the GP activity to the PVs that is important in the pathogenesis of AF, and that the interruption of axons from these hyperactive GP to the PV myocardial sleeves may be an important factor in the success of PV isolation procedures (6, 7). In this review, we will present the current data on the role of the autonomic ganglia in the pathogenesis of AF and discuss potential therapeutic implications.
Anatomy of the autonomic ganglia
The heart receives innervation from both the extrinsic (central) and the intrinsic CANS. The extrinsic CANS includes the ganglia in the brain or along the spinal cord, where the cell bodies reside, as well as their axons (e.g., the vagosympathetic trunk) en route to the heart. The intrinsic CANS is essentially an extensive, highly interconnected epicardial neural network which consists of multiple GP, nerve axons and interconnecting neurons (Figure 1). These GP, except the ligament of Marshall, are embedded within epicardial fat pads and vary in size, from those that contain just a few neurons, to those that contain over 400 neurons (4, 5). It should be noted that although the epicardial surface of both atria is covered by a dense neural plexus, the highest density of neurons is found at the posterior wall of the left atrium (5). Several studies have demonstrated that the GP contain both sympathetic and parasympathetic elements, in addition to a variety of neuropeptides and neuromodulators (8, 9). Four of the left atrial GP each innervate one of the four PVs, as well as the surrounding atrial myocardium (4, 5). These four GP are located within areas of fractionated atrial potentials during AF and can be identified during electrophysiological study by applying high frequency stimulation (20 Hz) at the respective anatomical locations (10, 11). The high frequency stimulation activates the GP, leading through interconnecting neurons to activation of the inferior right GP. The latter depresses AV nodal conduction, increasing the R-R interval by >50% during AF (Figure 2). Although the AV block is not mediated by the vagus nerve, this positive response is often referred to as “vagal response.” This response is specific, but not completely sensitive.
Figure 1.
Anatomical location of the major left atrial ganglionated plexi (GP). A posteroanterior projection of the left atrium is shown. Hatched areas represent anterior surface and solid areas represent posterior left atrial surface. The GP innervate the adjacent pulmonary vein and surrounding atrium. Interconnecting neurons connect within and between GP.
Figure 2.
Identification of GP by high frequency stimulation. High frequency stimulation of the endocardium close to the inferior left GP elicits a profound “vagal response”, resulting in marked prolongation of the R-R interval during atrial fibrillation (AV nodal block).
Based on the response to high frequency stimulation, the anterior right GP is located immediately anterior to the right superior PV and often extends inferiorly, to the region anterior to the right inferior PV (Figure 1). This is the only GP directly adjacent to a PV. The superior left GP is located at the roof of the left atrium, 1–2 cm medial to the left superior PV (Figure 1). The right and left inferior GP are located at the inferior aspect of the posterior wall of the left atrium, 2–3 cm below the right and left PV, respectively (Figure 1) (11).
Autonomic ganglia and AF
The mechanism of AF initiation has been a matter of debate during the last 50 years. It was not until 1998, when a seminal observation by Haissaguerre and co-workers established the role of the PVs in the pathogenesis of AF. These investigators found that, in most patients with paroxysmal AF, AF was initiated by rapid focal firing arising from the myocardial sleeves of the PVs (12, 13). This recurring, rapid, non-sustained focal firing was often responsible for maintaining episodes of AF. This breakthrough observation laid the foundation for the present methodology for catheter ablation of AF, which includes wide, circumferential PV isolation by applying radiofrequency current or a cryo-application around the PV ostia. The premise of PV isolation is to prevent the ectopic activity arising from the PVs from reaching the atria and thereby inducing AF (14, 15). However, this approach failed to answer at least 4 fundamental questions: 1. How does the PV myocardium initiate premature beats? 2. How do bursts of premature beats from the PVs initiate AF rather than an atrial tachycardia? 3. Why do the PVs (with a very small mass of myocardium) rather than other atrial regions become the sites of focal firing in patients with AF? 4. How does PV isolation affect the substrate for AF maintenance? Addressing these questions may help explain the less than optimal long-term results of PV isolation procedures (16, 17) and provide the basis for a novel, mechanistic-based approach to treating AF.
A series of recent experimental studies provides several lines of evidence linking the intrinsic CANS with focal firing from the PVs. Scherlag et al. demonstrated that stimuli applied to PVs would not induce AF unless there was simultaneous stimulation of the adjacent GP (20 Hz, 0.1 ms pulse width) that did not excite the atrial myocardium (18). In another series of experiments, Po et al. showed that injection of acetylcholine into the GP induced, within minutes, focal firing originating from the adjacent PV and sustained AF (19). Patterson et al. demonstrated that PV myocytes have cellular electrophysiological properties which differ from the adjacent left atrial myocardium, specifically, a shorter action potential duration, which plays an important role in the initiation of AF (20, 21). In isolated canine PV preparations (with a surrounding left atrial myocardial cuff), local autonomic (both parasympathetic and sympathetic) nerve electrical stimulation (21), or simultaneous administration of acetylcholine plus norepinephrine (or isoproterenol) (20), induced early afterdepolarizations (EADs) and short bursts of triggered firing from the PVs (Figure 3). This triggered firing was similar to the pattern recorded from the PVs in patients with paroxysmal AF. Importantly, local autonomic nerve stimulation had a much greater effect on the PV myocardium compared to the adjacent left atrial myocardium in terms of shortening of action potential duration, EAD formation and triggered firing, indicating that the PV myocardium is more sensitive to autonomic stimulation than left atrial myocardium (21). These observations may explain why the first beat of AF often arises from the PVs.
Figure 3.
The role of autonomic ganglia in AF initiation at the cellular level. A. Microelectrode recordings from the left atrium (LA) and left superior pulmonary vein (LSPV) at baseline and during high frequency stimulation (HFS) at the LA myocardium, 3mm from the PV ostium. At baseline, LSPV has a shorter action potential duration (APD) than adjacent LA myocardium. With HFS, early afterdepolarizations (EADs) and triggered firing are induced in the LSPV, preceding the LA activation. B. In PV myocytes, cells with an abbreviated action potential, the duration of the calcium transient exceeds action potential duration, leading to activation of the forward mode of the sodium-calcium exchanger, which in turn generates an EAD. With increased GP activity, parasympathetic activation which causes action potential shortening, and sympathetic activation which enhances calcium loading, result in EAD formation and triggered firing.
The ionic mechanisms underlying PV firing have been recently elucidated (20, 21). Under increased GP activity, the increase in parasympathetic tone strikingly shortens action potential duration in the PV myocardium and the increase in sympathetic tone increases calcium loading. The high intracellular calcium concentration during repolarization activates the sodium-calcium exchanger to extrude calcium. Each calcium ion is exchanged for three sodium ions, leading to a net inward (depolarizing) current, and EAD as well as triggered firing from the PV myocardium (Figure 3) (20). Triggered firing from the PVs can be suppressed by muscarinic cholinergic receptor blockade (atropine), beta-adrenoceptor antagonists, inhibition of calcium release from the sarcoplasmic reticulum (ryanodine), or sodium-calcium exchange blockade (21), corroborating that both sympathetic and parasympathetic activity, intracellular Ca2+ load and sodium-calcium exchanger are all critical elements responsible for rapid PV firing. This mechanism of EAD and triggered firing needs to be contrasted with late phase 3 EADs, which are associated with a prolonged, rather than an abbreviated, action potential duration. Action potential prolongation, caused by loss of depolarizing K currents or excessive late Na currents allows for L-type calcium current to recover from inactivation, resulting in an inward depolarizing current, leading to an EAD (22, 23). Late phase 3 atrial EADs and triggered firing caused by action potential prolongation have been implicated in the pathogenesis of AF in congenital long QT syndrome patients (23).
Autonomic ganglia and atrial remodeling
AF causes electrophysiological changes in the atrial myocardium (atrial electrical remodeling) and structural remodeling (e.g. fibrosis), providing the substrate for AF maintenance (24). Although the chronic rapid atrial pacing model developed by Wijffels et al. (25) provided strong evidence to explain how “AF begets AF” in a chronic state, how AF maintains itself in the first few hours remained poorly understood. We have recently shown that rapid atrial pacing for 6 hours induced acute atrial remodeling (shortening of ERP and increased inducibility of AF) mediated through the GP, evidenced by reversal of these effects by GP ablation (26). More recently, we reported that the autonomic neural activity recorded from the anterior right GP increased hour by hour during 6 hours of rapid atrial pacing (27). We proposed that acute atrial electrical remodeling induced by rapid atrial pacing-simulated AF and autonomic remodeling form a vicious cycle, so that one perpetuates the other to maintain AF (Central illustration). These results may help explain how AF sustains itself in the very early stage (first few hours). Importantly, these data suggest that the autonomic ganglia may provide not only the trigger for AF initiation, but also the substrate for AF maintenance.
Central illustration.

The vicious cycle among AF, autonomic remodeling, atrial electrical remodeling and inflammation. AF induces atrial electrical remodeling and promotes autonomic remodeling, as well as inflammation. These 3 remodeling processes form a vicious cycle, each perpetuating the other, thereby maintaining AF. Autonomic neuromodulation (low level vagus nerve stimulation or low level tragus stimulation) may reverse acute autonomic remodeling and inflammation, and suppress AF.
Autonomic ganglia and PV isolation
Some degree of autonomic denervation is common following PV isolation (28, 29) and has been associated with decreased risk of AF recurrence (30, 31). In a recent elegant study, Lemola et al. (6) investigated the role of PVs versus the GP in maintenance of experimental vagal AF. These investigators performed PV isolation in dogs while preserving the GP and in others ablated the GP while leaving the PVs intact. They demonstrated that intact PVs are not needed to maintain experimental vagal AF, whereas ablation of GP prevented AF (6). The same group of investigators expanded these results in a different experimental model of AF showing that PVs play a minor role in AF induced by chronic rapid atrial pacing, whereas intact GP play an important role in AF maintenance in the presence of rapid atrial pacing-induced remodeling (7). The degree to which the specific neural elements within the GP (efferent neurons, afferent neurons or interconnecting neurons) are responsible for the beneficial results of GP ablation remains unclear. Clinical studies showing that complete electrical isolation of the PVs is not necessary for the maintenance of sinus rhythm support these experimental data (32, 33). It should be pointed out that conventional wide-area PV isolation transects both axons transiting from the GP to the PV myocardium, and much of the anterior right GP and some of the superior left GP, but has little impact on the inferior left or right GP. The above studies suggest that injury to some GP tissue and interruption of their axons to PVs may contribute to the success of PV isolation procedures, and may explain the increase in success found by extending PV isolation from the ostia further into the antrum (34, 35). Interruption of the autonomic axons may explain the usual finding of elimination of firing within the PVs by PV isolation procedures (36). This phenomenon was systematically examined in a recent study, where PV firing induced by intravenous infusion of isoproterenol and adenosine triphosphate was markedly suppressed by PV isolation (36). In a minority of patients who exhibited PV firing after PV isolation, this was eliminated by GP ablation outside the PV isolation line (36). If the PVs themselves were the source of firing, PV firing should have continued after PV isolation, since there was no injury to the tissues inside the isolation lines. These data raise the possibility that PV firing may be an epiphenomenon and that elimination of substrate outside the PV isolation lines (GP and axons) may be responsible for the clinical ablation results. Targeting the cell bodies within the GP may offer the advantage of permanent denervation, as axons interrupted by PV isolation may regenerate (37). Finally, in light of the recent promising findings of focal source (possibly rotor) ablation (38–40), it is possible that areas of heavy GP innervation overlap with focal AF sources. Although the exact mechanism by which GP innervation may interact with focal AF sources remains to be determined, experimental studies suggest that rotor formation in some forms of AF requires intense autonomic stimulation (41, 42), supporting the notion that elimination of GP activity (by ablation) may limit rotor formation. In isolated sheep hearts, elevated acetylcholine concentrations increase rotor frequency in the left atrium, thus promoting AF (41), while combined acetylcholine and isoproterenol infusion leads to high-frequency focal discharges which perpetuate AF (42). Elevated local acetylcholine and adrenergic levels is also the hallmark of GP hyperactivity (20, 21), and may represent the common link between GP and localized AF sources.
It has been suggested that PV isolation affects the substrate for AF maintenance. However, the mechanisms remain elusive. Fractionated atrial potentials, also referred to as complex fractionated atrial electrograms (CFAE) represent one of the most specific substrate features of AF (43). Recent studies have provided evidence that CFAE may be related to GP hyperactivity (44). For example, in a canine model, the distribution of the CFAE correlated well with the anatomical locations of the GP, consistent with the original clinical report by Nademanee et al. (43), and the CFAE could be eliminated by ablating the GP at a distance (44). In addition, CFAE were correlated with increased GP activity in ambulatory dogs, and preceded the onset of paroxysmal AF, suggesting that GP may serve as both the source for CFAE and the trigger for AF (3). In patients with AF, Katritsis et al. showed that CFAE are usually found surrounding the GP areas (45, 46). Recent studies have shown that the prevalence of CFAE was reduced after combined autonomic blockade with atropine and a beta blocker (47, 48). We have also shown that the GP (identified by high frequency stimulation) are always located within an area of fractionated atrial potentials, and the area of fractionated atrial potentials is much larger than the GP, suggesting that although GP ablation consistently produces CFAE (or fractionated atrial potential) ablation, CFAE ablation is not equivalent to GP ablation (49). In a series of 33 patients with either paroxysmal or persistent AF, after GP ablation (before PV isolation), AF was no longer inducible in 14/33 patients. After GP ablation, if AF remained inducible, the area of fractionated atrial potentials was markedly reduced (median 27.9 cm2 to 1.8 cm2, p<0.01) (Figure 4) (49).
Figure 4.
Fractionated atrial potential (FAP) maps of the left atrium and PVs before (upper panel) and after (lower panel) left atrial GP ablation (without PV isolation or other ablation). An automated algorithm was programmed to identify segments between electrogram peaks of 15–80 ms duration and define FAP as those demonstrating more than 40 such segments per 2.5 seconds. After GP ablation, there was a marked decrease in the area of FAP represented by the red area.
Recent clinical evidence from small randomized and cohort studies, where GP ablation was performed either in addition to PV isolation (11, 50), or as a stand-alone procedure (51–53) supports the experimental data. In these studies, the overall success in eliminating AF was increased with the addition of GP ablation by approximately 25% (11, 50), whereas GP ablation alone in patients with either paroxysmal or persistent AF was successful in 71% to 86% of the patients (51–53). To provide a more definitive answer to the question of whether the addition of GP ablation to PV isolation improves the success of AF ablation in patients with paroxysmal AF and whether GP ablation alone is as effective as PV isolation, Katritsis et al. (54) designed a large clinical study, which randomized a total of 242 patients with paroxysmal AF to conventional PV isolation, PV isolation plus GP ablation and GP ablation alone. The patients were followed for at least 2 years. Freedom from atrial tachyarrhythmias was achieved in a similar number of patients in the PV isolation and GP ablation groups (56% and 48%, respectively), and in a significantly greater number of patients in the PV isolation plus GP ablation group (74%; p=0.004 by log-rank test), supporting the important role of autonomic nervous system in the pathophysiology of AF (Figure 5). In another randomized study including 264 patients with persistent or long standing persistent AF, GP ablation as an adjunct to PV isolation resulted in higher rates of sinus rhythm maintenance at 3 years (49%) compared to PV isolation plus left atrial linear lesions (34%) (55) (Figure 5). In addition, left atrial tachycardias were less common with PV isolation plus GP ablation than with PV isolation plus linear lesions. GP ablation alone was also tested in patients with drug-refractory long-standing persistent AF, albeit with less optimal results (38% sinus rhythm maintenance at 2 years) (56). These studies lend credence to the autonomic hypothesis for AF, which states that GP activity is most important in the early stages of AF, while its importance may diminish with progression of the disease to more advance stages and the development of atrial remodeling and fibrosis.
Figure 5.

Randomized clinical trials including GP ablation for paroxysmal (A) or persistent (B) AF. In patients with paroxysmal AF, pulmonary vein isolation (PVI) plus GP ablation improved AF/atrial tachycarrhythmia (AT) free survival compared to GP ablation alone or PVI alone. In patients with persistent AF, PVI plus GP ablation resulted in better AF/AT free survival compared to PVI plus left atrial linear lesions (LL).
Further insight into the relative importance of PV isolation in maintaining sinus rhythm comes from studies investigating the PV reconnection rate in patients with or without AF recurrence after AF ablation. A high prevalence of PV reconnection has been reported in patients with AF recurrence (57–59), but studies including patients without recurrence are limited, mainly because there is no compelling clinical indication to perform a repeat electrophysiological study in patients without AF recurrence. In such a study, Jiang et al. systematically examined the prevalence of PV reconnection during a repeat procedure among patients with and without clinical AF recurrence (60). Reconnection in at least one PV at repeat electrophysiological study was found in 29 of 32 (91%) patients without clinical AF recurrence and in 40 of 43 (93%) with AF recurrence, supporting the notion that sustained PV isolation may not be required for freedom from clinical recurrence of AF, consistent with prior experimental studies (6, 7). In other words, the success of AF ablation procedures may also depend on elimination of substrate outside the PV isolation lines, which probably represents the GP.
Interactions between extrinsic and intrinsic CANS
The interactions between the extrinsic cardiac autonomic nervous system and the intrinsic CANS were studied systematically by Hou et al. (61). They found that the GP function as “integration centers”, which modulate autonomic innervation. GP ablation attenuated the effects of vagus nerve stimulation on sinus rate, AV nodal conduction and AF inducibility. Multiple interconnections exist among the GP and there is a common final pathway to the sinus node and AV node through the anterior right GP and inferior right GP, respectively (61). Similar neural pathways connecting the GP with the PVs, AV node, and sinus node exist in humans, as shown recently in an elegant study by Malcolme-Lawes et al. (62). Further studies have shown that ablation of the “head station” GP, located between the extrinsic and intrinsic CANS at the junction of the superior vena cava, aorta, and right pulmonary artery, resulted in progressive electrical remodeling with progressive increase in AF burden, suggesting that there is a tonic inhibitory input to the GP which is important in preserving sinus rhythm (63). Moreover, we have recently provided evidence that modulating the extrinsic neural input to the GP by applying vagus nerve stimulation (both at the suprathreshold level which slows the sinus rate, as well as at the subthreshold level which does not alter the sinus rate) suppresses GP activity (64–66). These studies collectively suggest that the extrinsic autonomic input to the heart, i.e., from the brain and spinal cord, exerts an inhibitory control over the GP and that attenuation or loss of this control would allow the GP to become hyperactive. GP hyperactivity due to the loss of tonic vagal inhibition with advancing age may explain the increased prevalence of AF in the elderly. This hypothesis is consistent with previous data indicating that baroreflex function and vagal efferent control of the heart is attenuated with aging (67). Importantly, there is evidence of marked degeneration of vagal efferent axons and terminals in cardiac autonomic ganglia, which in turn may provide the substrate for reduced vagal control of the heart rate, attenuated baroreflex function, and increased propensity for AF with aging (68). It should be noted that the interaction between the extrinsic and intrinsic CANS is characterized by a constant coordination between the central and peripheral neurons in respond to afferent information, to control regional electrophysiological, vascular and contractile function. This interaction can be both excitatory and inhibitory, depending on afferent neuronal transduction of the cardiovascular milieu (69).
Autonomic neuromodulation
Autonomic neuromodulation is a novel therapeutic approach that takes advantage of the plasticity of the neural tissue to provide therapeutic advantage without damage to nerves or myocardium. This approach has been successfully used in some diseases, such as drug-refractory epilepsy, where vagus nerve stimulation can be delivered through an implantable device (70, 71). We (27, 64, 65, 72, 73) and others (74) have recently shown that low level vagus nerve stimulation (LLVNS), at voltages substantially below the threshold for slowing the sinus rate or AV conduction, significantly increased the ERP in the atria as well as the PV myocardium, suppressed AF inducibility and decreased the duration of acetylcholine-induced AF episodes (Figure 6). In those experiments, LLVNS applied to both vagal trunks dissected in the neck (64, 72, 73), to the right vagal trunk alone (65), or to the vagal preganglionic fibers at the posterior wall of the superior vena cava (27), exerted equally strong anti-arrhythmic effects. We have also provided evidence that LLVNS resulted in marked inhibition of GP activity, as indicated by a decrease in the frequency and amplitude of direct neural recordings (27, 73) (Figure 6). The anti-arrhythmic effects of LLVNS were also observed in ambulatory dogs. In this experimental model, left sided LLVNS suppressed left stellate ganglion neural activity, especially in the morning and decreased tyrosine-hydroxylase positive cells in the left stellate ganglion 1 week after cessation of LLVNS (74). In the same study, LLVNS prevented paroxysmal AF induced by rapid atrial pacing (74). Although the exact downstream targets of LLVNS remain to be determined, there is preliminary evidence that these may involve the anti-adrenergic neuropeptide vasostatin-1 (75), the nitric oxide signaling pathway (76) and upregulation of the small conductance calcium activated potassium channel 2 in the left stellate ganglion (77).
Figure 6.
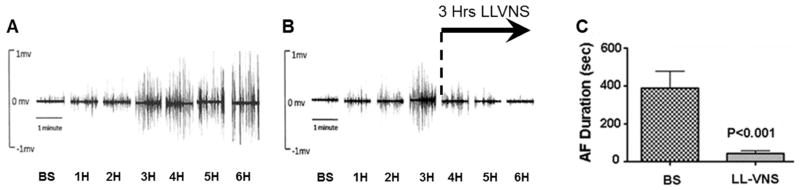
Acute autonomic remodeling induced by 6 hours of rapid atrial pacing in a canine model, and reversal by low level vagus nerve stimulation (LLVNS). A. Neural activity recorded by a tungsten electrode in the anterior right GP increased steadily over 6 hours of rapid atrial pacing. B. Three hours (4H, 5H, and 6H) of LLVNS progressively suppressed GP neural activity to baseline value (6H) despite persistence of rapid atrial pacing. C. LLVNS for 3 hours significantly reduced acetylcholine-induced AF duration.
Based on the observation that transcutaneous electrical stimulation of the tragus, the anterior protuberance of the outer ear, where the auricular branch of the vagus nerve is located, elicits evoked potentials in the brainstem in humans (78), we recently examined the effects of low-level tragus stimulation (LLTS) for inhibiting AF in a canine model of rapid atrial pacing (79). The anti-arrhythmic effects of LLTS were similar to those of LLVNS delivered to the cervical vagus nerve trunk(s). However, LLTS after transection of both vagus nerves failed to show any antiarrhythmic effect, indicating that the efferent vagus nerves are essential for its antiarrhythmic effects (79). LLTS reversed the autonomic remodeling process which was induced by rapid atrial pacing, as indicated by a return of the neural activity of the anterior right GP towards baseline values (79). More recently, we translated these data in humans with paroxysmal AF. In a randomized study, we examined the antiarrhythmic and anti-inflammatory effects of 1 hour of LLTS (n=20) compared to sham (n=20) in patients referred for AF ablation (80). We demonstrated for the first time in humans that LLTS compared to control decreased rapid atrial pacing-AF duration, increased AF cycle length, increased atrial ERP and suppressed inflammatory cytokines (Figure 7). The importance of the latter finding is highlighted by accumulating evidence that inflammation promotes the persistence of AF (81). The results of this proof-of-concept and first-in-man study support the notion that non-invasive autonomic neuromodulation may emerge as a novel, non-pharmacologic, non-ablative therapy for the treatment of paroxysmal AF (Central illustration). Further large-scale, double-blind clinical trials measuring long-term outcome endpoints are warranted to evaluate the clinical importance of these findings.
Figure 7.

Antiarrhythmic and anti-inflammatory effect of low level tragus stimulation (LLTS) in humans. AF was induced by rapid atrial pacing at baseline and again after 1 hour of LLTS (n=20) or sham (control, n=20). There was a significant decrease in AF duration (A) and serum tumor necrosis factor (TNF)-α after 1 hour of LLTS, but not after 1 hour of sham (control).
Conclusions
The intrinsic CANS plays an important role in the initiation and maintenance of AF and may provide both the substrate and trigger for AF. Acute autonomic remodeling may play a crucial role in AF maintenance in the very early stages. The benefit of adding GP ablation to the standard PV isolation procedure for patients with paroxysmal AF is supported by both experimental and clinical data. In addition, given the close association between GP activity and CFAE, GP ablation may replace CFAE ablation as part of the ablation procedure for persistent AF. The beneficial effects of this approach may also include decreasing the autonomic activity required for rotor formation. Autonomic neuromodulation inhibits GP activity and reverses acute electrical atrial remodeling during rapid atrial pacing and may provide an alternative non-ablative approach for the treatment of AF, especially in the early stages. This notion is supported by a preliminary human study. Further studies are warranted to confirm these findings in ambulatory patients with AF and identify the effects of this novel approach on the molecular, cellular and tissue level.
Acknowledgments
Funded in part by NIH/NIGMS #8P20GM103447 to Stavros Stavrakis
Abbreviations
- CANS
cardiac autonomic nervous system
- GP
ganglionated plexi
- AF
atrial fibrillation
- PV
pulmonary vein
- AV
atrioventricular
- EAD
early afterdepolarization
- CFAE
complex fractionated atrial electrogram
- LLVNS
low level vagus nerve stimulation
- LLTS
low level tragus stimulation
Footnotes
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol. 2014;64:823–31. doi: 10.1016/j.jacc.2014.06.1172. [DOI] [PubMed] [Google Scholar]
- 2.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 3.Choi EK, Shen MJ, Han S, et al. Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs. Circulation. 2010;121:2615–23. doi: 10.1161/CIRCULATIONAHA.109.919829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armour JA, Murphy DA, Yuan BX, Macdonald S, Hopkins DA. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec. 1997;247:289–98. doi: 10.1002/(SICI)1097-0185(199702)247:2<289::AID-AR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. 2000;259:353–82. doi: 10.1002/1097-0185(20000801)259:4<353::AID-AR10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Lemola K, Chartier D, Yeh YH, et al. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation. 2008;117:470–7. doi: 10.1161/CIRCULATIONAHA.107.737023. [DOI] [PubMed] [Google Scholar]
- 7.Nishida K, Maguy A, Sakabe M, et al. The role of pulmonary veins vs. autonomic ganglia in different experimental substrates of canine atrial fibrillation. Cardiovasc Res. 2011;89:825–33. doi: 10.1093/cvr/cvq332. [DOI] [PubMed] [Google Scholar]
- 8.Hoover DB, Isaacs ER, Jacques F, et al. Localization of multiple neurotransmitters in surgically derived specimens of human atrial ganglia. Neuroscience. 2009;164:1170–9. doi: 10.1016/j.neuroscience.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Steele PA, Gibbins IL, Morris JL, Mayer B. Multiple populations of neuropeptide-containing intrinsic neurons in the guinea-pig heart. Neuroscience. 1994;62:241–50. doi: 10.1016/0306-4522(94)90327-1. [DOI] [PubMed] [Google Scholar]
- 10.Lemery R, Birnie D, Tang AS, Green M, Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3:387–96. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1186–9. doi: 10.1111/j.1540-8167.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 12.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 13.Jais P, Haissaguerre M, Shah DC, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. 1997;95:572–6. doi: 10.1161/01.cir.95.3.572. [DOI] [PubMed] [Google Scholar]
- 14.Oral H, Knight BP, Tada H, et al. Pulmonary vein isolation for paroxysmal and persistent atrial fibrillation. Circulation. 2002;105:1077–81. doi: 10.1161/hc0902.104712. [DOI] [PubMed] [Google Scholar]
- 15.Pappone C, Oreto G, Rosanio S, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–44. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang F, Tilz R, Chun J, et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation. 2010;122:2368–77. doi: 10.1161/CIRCULATIONAHA.110.946806. [DOI] [PubMed] [Google Scholar]
- 17.Weerasooriya R, Khairy P, Litalien J, et al. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–6. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 18.Scherlag BJ, Yamanashi W, Patel U, Lazzara R, Jackman WM. Autonomically induced conversion of pulmonary vein focal firing into atrial fibrillation. J Am Coll Cardiol. 2005;45:1878–86. doi: 10.1016/j.jacc.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 19.Po SS, Scherlag BJ, Yamanashi WS, et al. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3:201–8. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Patterson E, Lazzara R, Szabo B, et al. Sodium-calcium exchange initiated by the Ca2+ transient: an arrhythmia trigger within pulmonary veins. J Am Coll Cardiol. 2006;47:1196–206. doi: 10.1016/j.jacc.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–99. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–74. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 24.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 25.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997;96:3710–20. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 26.Lu Z, Scherlag BJ, Lin J, et al. Atrial fibrillation begets atrial fibrillation: autonomic mechanism for atrial electrical remodeling induced by short-term rapid atrial pacing. Circ Arrhythm Electrophysiol. 2008;1:184–92. doi: 10.1161/CIRCEP.108.784272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu L, Scherlag BJ, Sha Y, et al. Interactions between atrial electrical remodeling and autonomic remodeling: how to break the vicious cycle. Heart Rhythm. 2012;9:804–9. doi: 10.1016/j.hrthm.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Tan AY, Li H, Wachsmann-Hogiu S, et al. Autonomic innervation and segmental muscular disconnections at the human pulmonary vein-atrial junction: implications for catheter ablation of atrial-pulmonary vein junction. J Am Coll Cardiol. 2006;48:132–43. doi: 10.1016/j.jacc.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 29.Verma A, Saliba WI, Lakkireddy D, et al. Vagal responses induced by endocardial left atrial autonomic ganglion stimulation before and after pulmonary vein antrum isolation for atrial fibrillation. Heart Rhythm. 2007;4:1177–82. doi: 10.1016/j.hrthm.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Scanavacca M, Pisani CF, Hachul D, et al. Selective atrial vagal denervation guided by evoked vagal reflex to treat patients with paroxysmal atrial fibrillation. Circulation. 2006;114:876–85. doi: 10.1161/CIRCULATIONAHA.106.633560. [DOI] [PubMed] [Google Scholar]
- 31.Scherlag BJ, Nakagawa H, Jackman WM, et al. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;13 (Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 32.Lemola K, Oral H, Chugh A, et al. Pulmonary vein isolation as an end point for left atrial circumferential ablation of atrial fibrillation. J Am Coll Cardiol. 2005;46:1060–6. doi: 10.1016/j.jacc.2005.05.069. [DOI] [PubMed] [Google Scholar]
- 33.Stabile G, Turco P, La Rocca V, et al. Is pulmonary vein isolation necessary for curing atrial fibrillation? Circulation. 2003;108:657–60. doi: 10.1161/01.CIR.0000086980.42626.34. [DOI] [PubMed] [Google Scholar]
- 34.Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation. 2003;108:2355–60. doi: 10.1161/01.CIR.0000095796.45180.88. [DOI] [PubMed] [Google Scholar]
- 35.Arentz T, Weber R, Burkle G, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. 2007;115:3057–63. doi: 10.1161/CIRCULATIONAHA.107.690578. [DOI] [PubMed] [Google Scholar]
- 36.Jiang RH, Jiang CY, Sheng X, et al. Marked Suppression of Pulmonary Vein Firing After Circumferential Pulmonary Vein Isolation in Patients with Paroxysmal Atrial Fibrillation: Is Pulmonary Vein Firing an Epiphenomenon? J Cardiovasc Electrophysiol. 2014;25:111–8. doi: 10.1111/jce.12288. [DOI] [PubMed] [Google Scholar]
- 37.Kangavari S, Oh YS, Zhou S, et al. Radiofrequency catheter ablation and nerve growth factor concentration in humans. Heart Rhythm. 2006;3:1150–5. doi: 10.1016/j.hrthm.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct or coincidental elimination of stable rotors or focal sources may explain successful atrial fibrillation ablation: on-treatment analysis of the CONFIRM trial (Conventional ablation for AF with or without focal impulse and rotor modulation) J Am Coll Cardiol. 2013;62:138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shivkumar K, Ellenbogen KA, Hummel JD, Miller JM, Steinberg JS. Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: first multicenter experience of focal impulse and rotor modulation (FIRM) ablation. J Cardiovasc Electrophysiol. 2012;23:1277–85. doi: 10.1111/jce.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130:530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 41.Sarmast F, Kolli A, Zaitsev A, et al. Cholinergic atrial fibrillation: I(K,ACh) gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res. 2003;59:863–73. doi: 10.1016/s0008-6363(03)00540-6. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki M, Vaquero LM, Hou L, et al. Mechanisms of stretch-induced atrial fibrillation in the presence and the absence of adrenocholinergic stimulation: interplay between rotors and focal discharges. Heart Rhythm. 2009;6:1009–17. doi: 10.1016/j.hrthm.2009.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Scherlag BJ, Zhou J, et al. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18:1197–205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 45.Katritsis D, Giazitzoglou E, Sougiannis D, Voridis E, Po SS. Complex fractionated atrial electrograms at anatomic sites of ganglionated plexi in atrial fibrillation. Europace. 2009;11:308–15. doi: 10.1093/europace/eup036. [DOI] [PubMed] [Google Scholar]
- 46.Katritsis D, Sougiannis D, Batsikas K, et al. Autonomic modulation of complex fractionated atrial electrograms in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol. 2011;31:217–23. doi: 10.1007/s10840-011-9558-0. [DOI] [PubMed] [Google Scholar]
- 47.Chaldoupi SM, Linnenbank AC, Wittkampf FH, et al. Complex fractionated electrograms in the right atrial free wall and the superior/posterior wall of the left atrium are affected by activity of the autonomic nervous system. J Cardiovasc Electrophysiol. 2012;23:26–33. doi: 10.1111/j.1540-8167.2011.02145.x. [DOI] [PubMed] [Google Scholar]
- 48.Knecht S, Wright M, Matsuo S, et al. Impact of pharmacological autonomic blockade on complex fractionated atrial electrograms. J Cardiovasc Electrophysiol. 2010;21:766–72. doi: 10.1111/j.1540-8167.2009.01712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakagawa H, Scherlag BJ, Patterson E, et al. Pathophysiologic basis of autonomic ganglionated plexus ablation in patients with atrial fibrillation. Heart Rhythm. 2009;6:S26–34. doi: 10.1016/j.hrthm.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 50.Katritsis DG, Giazitzoglou E, Zografos T, et al. Rapid pulmonary vein isolation combined with autonomic ganglia modification: A randomized study. Heart Rhythm. 2011;8:672–8. doi: 10.1016/j.hrthm.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 51.Pokushalov E, Romanov A, Artyomenko S, et al. Left atrial ablation at the anatomic areas of ganglionated plexi for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2010;33:1231–8. doi: 10.1111/j.1540-8159.2010.02800.x. [DOI] [PubMed] [Google Scholar]
- 52.Pokushalov E, Romanov A, Shugayev P, et al. Selective ganglionated plexi ablation for paroxysmal atrial fibrillation. Heart Rhythm. 2009;6:1257–64. doi: 10.1016/j.hrthm.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Pokushalov E, Turov A, Shugayev P, et al. Catheter ablation of left atrial ganglionated plexi for atrial fibrillation. Asian Cardiovasc Thorac Ann. 2008;16:194–201. doi: 10.1177/021849230801600304. [DOI] [PubMed] [Google Scholar]
- 54.Katritsis DG, Pokushalov E, Romanov A, et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–25. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 55.Pokushalov E, Romanov A, Katritsis DG, et al. Ganglionated plexus ablation vs linear ablation in patients undergoing pulmonary vein isolation for persistent/long-standing persistent atrial fibrillation: a randomized comparison. Heart Rhythm. 2013;10:1280–6. doi: 10.1016/j.hrthm.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 56.Pokushalov E, Romanov A, Artyomenko S, et al. Ganglionated plexi ablation for longstanding persistent atrial fibrillation. Europace. 2010;12:342–6. doi: 10.1093/europace/euq014. [DOI] [PubMed] [Google Scholar]
- 57.Verma A, Kilicaslan F, Pisano E, et al. Response of atrial fibrillation to pulmonary vein antrum isolation is directly related to resumption and delay of pulmonary vein conduction. Circulation. 2005;112:627–35. doi: 10.1161/CIRCULATIONAHA.104.533190. [DOI] [PubMed] [Google Scholar]
- 58.Gerstenfeld EP, Callans DJ, Dixit S, Zado E, Marchlinski FE. Incidence and location of focal atrial fibrillation triggers in patients undergoing repeat pulmonary vein isolation: implications for ablation strategies. J Cardiovasc Electrophysiol. 2003;14:685–90. doi: 10.1046/j.1540-8167.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 59.Lemola K, Hall B, Cheung P, et al. Mechanisms of recurrent atrial fibrillation after pulmonary vein isolation by segmental ostial ablation. Heart Rhythm. 2004;1:197–202. doi: 10.1016/j.hrthm.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 60.Jiang RH, Po SS, Tung R, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11:969–76. doi: 10.1016/j.hrthm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Hou Y, Scherlag BJ, Lin J, et al. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–8. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 62.Malcolme-Lawes LC, Lim PB, Wright I, et al. Characterization of the left atrial neural network and its impact on autonomic modification procedures. Circ Arrhythm Electrophysiol. 2013;6:632–40. doi: 10.1161/CIRCEP.113.000193. [DOI] [PubMed] [Google Scholar]
- 63.Lo LW, Scherlag BJ, Chang HY, et al. Paradoxical long-term proarrhythmic effects after ablating the “head station” ganglionated plexi of the vagal innervation to the heart. Heart Rhythm. 2013;10:751–7. doi: 10.1016/j.hrthm.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 64.Sheng X, Scherlag BJ, Yu L, et al. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 2011;57:563–71. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 65.Sha Y, Scherlag BJ, Yu L, et al. Low-level right vagal stimulation: anticholinergic and antiadrenergic effects. J Cardiovasc Electrophysiol. 2011;22:1147–53. doi: 10.1111/j.1540-8167.2011.02070.x. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Scherlag BJ, Lu Z, et al. Comparison of atrial fibrillation inducibility by electrical stimulation of either the extrinsic or the intrinsic autonomic nervous systems. J Interv Card Electrophysiol. 2009;24:5–10. doi: 10.1007/s10840-008-9297-z. [DOI] [PubMed] [Google Scholar]
- 67.Matsukawa T, Sugiyama Y, Mano T. Age-related changes in baroreflex control of heart rate and sympathetic nerve activity in healthy humans. J Auton Nerv Syst. 1996;60:209–12. doi: 10.1016/0165-1838(96)00057-4. [DOI] [PubMed] [Google Scholar]
- 68.Ai J, Gozal D, Li L, et al. Degeneration of vagal efferent axons and terminals in cardiac ganglia of aged rats. J Comp Neurol. 2007;504:74–88. doi: 10.1002/cne.21431. [DOI] [PubMed] [Google Scholar]
- 69.Armour JA. Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol. 2004;287:R262–71. doi: 10.1152/ajpregu.00183.2004. [DOI] [PubMed] [Google Scholar]
- 70.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–82. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 71.Uthman BM, Reichl AM, Dean JC, et al. Effectiveness of vagus nerve stimulation in epilepsy patients: a 12-year observation. Neurology. 2004;63:1124–6. doi: 10.1212/01.wnl.0000138499.87068.c0. [DOI] [PubMed] [Google Scholar]
- 72.Li S, Scherlag BJ, Yu L, et al. Low-level vagosympathetic stimulation: a paradox and potential new modality for the treatment of focal atrial fibrillation. Circ Arrhythm Electrophysiol. 2009;2:645–51. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 73.Yu L, Scherlag BJ, Li S, et al. Low-level vagosympathetic nerve stimulation inhibits atrial fibrillation inducibility: direct evidence by neural recordings from intrinsic cardiac ganglia. J Cardiovasc Electrophysiol. 2011;22:455–63. doi: 10.1111/j.1540-8167.2010.01908.x. [DOI] [PubMed] [Google Scholar]
- 74.Shen MJ, Shinohara T, Park HW, et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation. 2011;123:2204–12. doi: 10.1161/CIRCULATIONAHA.111.018028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stavrakis S, Scherlag BJ, Fan Y, et al. Antiarrhythmic effects of vasostatin-1 in a canine model of atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:771–7. doi: 10.1111/j.1540-8167.2012.02317.x. [DOI] [PubMed] [Google Scholar]
- 76.Stavrakis S, Scherlag BJ, Fan Y, et al. Inhibition of atrial fibrillation by low-level vagus nerve stimulation: the role of the nitric oxide signaling pathway. J Interv Card Electrophysiol. 2013;36:199–208. doi: 10.1007/s10840-012-9752-8. [DOI] [PubMed] [Google Scholar]
- 77.Shen MJ, Hao-Che C, Park HW, et al. Low-level vagus nerve stimulation upregulates small conductance calcium-activated potassium channels in the stellate ganglion. Heart Rhythm. 2013;10:910–5. doi: 10.1016/j.hrthm.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fallgatter AJ, Neuhauser B, Herrmann MJ, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm. 2003;110:1437–43. doi: 10.1007/s00702-003-0087-6. [DOI] [PubMed] [Google Scholar]
- 79.Yu L, Scherlag BJ, Li S, et al. Low-level transcutaneous electrical stimulation of the auricular branch of the vagus nerve: a noninvasive approach to treat the initial phase of atrial fibrillation. Heart Rhythm. 2013;10:428–35. doi: 10.1016/j.hrthm.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 80.Stavrakis S, Humphrey MB, Scherlag BJ, et al. Low level transcutaneous electrical vagus nerve stimulation suppresses atrial fibrillation. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.12.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]






