Abstract
OBJECTIVE
The study sought to examine the prevalence and outcomes of sports participation (both competitive and recreational) in our single-center LQTS genotype positive pediatric population.
BACKGROUND
The risks of sports participation in patients with long QT syndrome (LQTS) are not clearly elucidated.
METHODS
A retrospective review was performed on genotype positive patients referred for the evaluation and management of LQTS between 1998 and 2013 at the Children’s Hospital of Philadelphia. Pediatric patients participating in competitive or recreational sports were included in the analysis and their charts were reviewed for documented LQTS events during follow-up.
RESULTS
The cohort of genotype-positive LQTS patients included 212 patients, and 103 patients (49%, female n = 53, average follow-up 7.1 ± 4.0 years, average QTc 468 ± 42 ms) participated in sports. A total of 105 LQTS disease-causing mutations were identified: KCNQ1 n = 60 (58%), KCNH2 n = 36 (35%), SCN5A n = 6 (6%), KCNE1 n = 1 (1%), and KCNE2 n = 2 (2%). All patients were treated with beta-blockade, with noncompliance in 1 patient and intolerance in 1 patient. Twenty-six patients participated in competitive sports (26%, female n = 15, average follow-up 6.9 ± 4.1 years, average QTc 461 ± 35 ms). Seventy-seven patients (75%, female n = 35, average follow-up 7.3 ± 3.9 years, average QTc 470 ± 43 ms) participated in recreational sports. No patients had LQTS symptoms during sports participation. Five appropriate implantable cardioverter-defibrillator shocks occurred in 2 patients, though none were related to sports participation.
CONCLUSIONS
In this series no cardiac events and no deaths were observed in treatment-compliant LQTS children while participating in sports in 755 patient-years of follow-up.
Keywords: cardiac arrest, long QT syndrome, pediatrics, sports participation
Congenital long QT syndrome (LQTS) is a cardiac ion channelopathy characterized by syncope, ventricular arrhythmias (torsade de pointes) and sudden death (1). Children and adolescents are particularly at risk for life-threatening arrhythmias as this patient population is more often exposed to known triggers. As our understanding of the genotype-phenotype relationship has evolved, we now know that LQT triggers are genotype specific and risk assessment hinges on age, gender, mutation location, and QTc duration (2–5). Though several LQT subtypes exist, the majority (75%) involve the 3 most common forms, which are LQT1 (KCNQ1 gene), LQT2 (KCNH2 gene), and LQT3 (SCN5A gene).
Arrhythmia triggers, which are LQTS subtype specific, are often the result of the catecholaminergic surge seen with competitive exercise (6). For this reason, expert consensus statements have limited if not eliminated most competitive sports participation in children and young adults with LQTS. Specifically, the 36th Bethesda Conference guidelines restrict competition to billiards, bowling, cricket, curling, golf, and riflery, the well-known class IA sports (low static/low dynamic intensity) (7). Asymptomatic long QT patients with baseline QT prolongation (QTc duration of 470 ms or more in males, 480 ms or more in females) are also disqualified from commonly played competitive sports (Bethesda recommendation #2). Complicating these recommendations is the ill-defined distinction between LQTS cardiac events associated with competitive, recreational and school based physical education programs. Additionally, expert consensus statements do not allow for sports participation in asymptomatic LQTS patients who are adequately treated with beta-blockers despite accumulating evidence that beta-blockers are protective and confer great benefit among patients with LQT1, the most common form of LQTS. Contending with these complex issues, many parents of LQTS children have engaged in extensive dialogue with their pediatric electrophysiologist and have chosen sports participation for their children, despite published guidelines.
Recent data by Johnson and Ackerman (8,9) suggest that sports participation in LQTS may be safer than previously thought. Outside of this single study, no other data examining the prevalence of arrhythmic events in treated, genotype-positive, LQTS patients participating in sports exists in the literature. Similar to the tenets of self-determination and patient/family autonomy embraced by Johnson and Ackerman, our institution’s clinical practice has evolved over the years to allow sports liberalization in some LQTS patients. The aim of our series was to examine retrospectively the prevalence and outcomes of sports participation (both competitive and recreational) in our LQTS genotype positive pediatric population.
METHODS
STUDY DESIGN
After obtaining Institutional Review Board approval, a retrospective review was performed on patients referred to the Pediatric Arrhythmia Clinic at the Children’s Hospital of Philadelphia for the evaluation and management of LQTS between January 1, 1998 and May 15, 2013. Inclusion criteria for this series were: 1) LQTS patients (male or female) >4 and <21 years of age at the time of sports participation; 2) genotype-positive LQTS patients; and 3) patients actively participating in recreational or competitive sports. Patients excluded from the analysis were: 1) patients treated for LQTS without genotype confirmation; 2) patients found to have LQTS single-nucleotide polymorphisms not thought to be disease causing; 3) patients with incomplete medical records or inconsistent follow-up at the Children’s Hospital of Philadelphia; and 4) LQTS patients ≤4 years of age as they were deemed too young to engage in consistent athletic activity. The primary predetermined endpoint was a serious adverse event during or up to 2 h after sports defined as: 1) tachyarrhythmic death or externally resuscitated cardiac event; 2) syncope; or 3) severe injury, defined as requiring hospitalization, resulting from syncope or arrhythmia. The secondary endpoints included: 1) appropriate implantable cardioverter-defibrillator (ICD) shock; 2) inappropriate ICD shock; 3) automatic external defibrillator (AED) shock; and 4) ICD system damage. Genetic analysis of the susceptibility genes described previously was performed through 2 commercial laboratories (Familion, PGxHealth, New Haven, Connecticut; and Gene DX, Gaithersburg, Maryland). Medical records were reviewed for demographics, clinical history, symptoms, treatment regimen, documentation of sports participation, type of sports played, level of participation (competitive or recreational), and LQTS-related events at initial and each subsequent visit. A patient’s symptomatic status was defined as follows: 1) asymptomatic, if there were no symptoms referable to the cardiovascular system; 2) LQTS-related symptoms, in the presence of aborted cardiac arrest, exertional or atypical syncope; and 3) non–LQTS-related symptoms (e.g., vasodepressor syncope, chest pain, dizziness), if patients had complaints that were determined to be non-LQTS related by the evaluating electrophysiologist. Patients were considered genotype positive, phenotype negative if the QTc intervals were <470 males, <480 females and in the absence of rhythm-related syncope or sudden cardiac arrest. All patients in this series were evaluated and followed by a pediatric electrophysiologist and QT duration measurements on electrocardiogram (ECG), ambulatory Holter monitoring data, exercise stress test data, and medication compliance at each visit (initial and subsequent) were rereviewed from the medical records documentation.
SPORTS PARTICIPATION
All patients underwent an initial and subsequent LQTS evaluation by a pediatric electrophysiologist. Follow-up evaluations were generally performed on an annual basis and included an ECG, ambulatory Holter monitor, and exercise stress test. During evaluations, as part of routine clinical care, the patient’s inclination to participate in sports was extensively discussed, including potential risks, data from published literature, our clinical experience, the Bethesda Conference Guidelines, and risk assessment as it related to the individual’s LQTS clinical presentation. This discussion was documented in the patient’s medical record and reviewed at each follow-up visit. Independent of the patient’s athletic status, all patients were treated with beta-blocker medication. In addition patients were advised to avoid QT prolonging drugs, electrolyte derangements, and overheating, and ensure proper hydration. ICD implantation was recommended when indicated: secondary prevention of sudden cardiac death, beta-blocker intolerance and/or noncompliance and those patients considered high risk as primary prevention. If the patient and his/her family chose sports participation, an AED was recommended at all sporting events if the patient did not already have an ICD. If the physician was asked to fill out a school sports participation form, the diagnosis, treatment regimen and AED recommendation were clearly stated on the form. Sports participation was categorized into: 1) recreational sports and 2) competitive sports. Individuals participating in a variety of informal sports and engaged in moderate to vigorous exercise levels, without systematic training or regular competition, were characterized as recreational athletes (10). A competitive athlete was defined as one who in addition to recreational sports also participated in an organized team or individual sport that required systematic training and regular competition against others. Sports were classified into static and dynamic components based on the Bethesda classification system for sports and were further categorized into low, moderate, and high intensity (7). In addition we also analyzed the prevalence and outcome of school-age LQTS patients engaged in school-based physical activity programs.
ELECTROCARDIOGRAPHIC QTc MEASUREMENT
The QTc was measured manually from a 15-lead standard pediatric ECG using lead II and V5 when possible and calculated using the standard Bazett’s formula (10). During exercise stress testing, QT intervals at rest (supine and standing), at peak exercise, and in recovery (1, 3, 5, 7, and 9 min) were measured and QTc was then calculated according to the Bazett formula according to our previously published protocol (5). The resting QTc intervals recorded on the initial ECG were used for analysis.
ADVERSE EVENTS AND SYMPTOMS
Adverse events and symptoms including pre-syncope, syncope, seizures, aborted cardiac arrest, or sudden death were determined from medical records and patient/family interview at each follow-up visit.
ICDs/PACEMAKERS/AEDs
In patients with a pacemaker or an ICD, all device interrogations including stored ICD electrograms, rhythm diagnosis, and data on system malfunction were reviewed by a pediatric electrophysiologist.
For patients without an ICD, an AED was always recommended during sports participation and this was documented in the sports/physical activity form. The availability of AED during sports was determined by family reports. For school and/or recreational venue based AEDs, the requirement for an AED at the site of the patient’s sporting/physical education event was stated in the school sports/physical activity form. For personal AEDs, we confirmed with families that the AED was transported (usually by a parent) to the site of the sporting event. In accordance to clinical practice at our institution, data from an activated AED is always analyzed by a pediatric electrophysiologist.
THERAPY AND COMPLIANCE
Documentation of the prescribed medical therapy was performed. Type of beta-blocker therapy as well as adjunctive medical therapies (antiarrhythmic drugs, presence of an ICD, sympathectomy) were also recorded. Assessment of medication compliance was routinely performed during the outpatient evaluation and was determined by patient/family report as well as blunted heart rate on exercise stress test during the initial visit or during follow-up.
STATISTICAL ANALYSIS
Descriptive statistics were used to summarize patient characteristics, sports participation, and ICD shock episodes. A subgroup analysis of patient characteristics was performed comparing participants, defined as competitive or recreational athletes. All analyses were performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). All continuous variables are reported as the mean ± SD. Means were analyzed using the independent group t test for means. A p value <0.05 was considered to be significant. Categorical variables were analyzed by use of chi-square analysis. As primary and secondary endpoints were not reached, further statistical analysis of event rates for each cohort (recreational sports, competitive sports, patients with and without ICDs) could not be performed.
RESULTS
PATIENT CHARACTERISTICS
During the 15 year period, 212 patients with genotype-positive LQTS were identified. Of these 212 patients, 103 patients (49%) participated in competitive or recreational sports and met the inclusion criteria (Table 1). The reasons for study exclusion were <4 years of age (n = 56), preference not to participate in sports (n = 38), follow-up at other institutions (n = 13), and irregular follow-up after initial evaluation (n = 2). The majority of study patients (n = 57, 55%) were asymptomatic at the time of diagnosis. Clinical presentations at LQTS diagnosis are shown in Figure 1. There were no statistically significant differences in the demographic variables between athletes in the competitive and the recreational groups (p > 0.05). A total of 105 LQT disease-causing mutations were identified (Table 1). Twenty-eight (27%) patients were identified to have LQTS through familial cascade genetic screening. Two patients had compound mutations that were included in this tabulation: KCNQ1/KNCH2 and KCNH2/KCNE1.
TABLE 1.
| Total Participants | Competitive Sports | Recreational Sports | Physical Education | |
|---|---|---|---|---|
| Number of patients | 103 | 26 | 77 | 84 |
| Age at diagnosis, yrs | 8.4 ± 5.6 | 9.3 ± 4.5 | 8.0 ± 5.6 | 7.4 ± 4.8 |
| Age at follow-up, yrs | 15.4 ± 5.1 | 16.2 ± 3.0 | 15.4 ± 5.5 | 14.0 ± 3.7 |
| Percent female | 51 | 58 | 45 | 50 |
| Average QTc, ms | 468 ± 42 | 461 ± 35 | 470 ± 43 | 466 ± 42 |
| QTc 25th percentile, ms | 442 | 436 | 449 | 440 |
| QTc 75th percentile, ms | 484 | 481 | 484 | 483 |
| Genotype positive, phenotype negative | 43 (42) | 13 (50) | 46 (45) | 36 (44) |
| Gene mutations | 105 | 26 | 79 | 84 |
| KCNQ1 | 60 (58) | 15 (58) | 46 (58) | 48 (57) |
| KCNH2 | 36 (35) | 8 (31) | 25 (32) | 31 (37) |
| SCN5A | 6 (6) | 1 (4) | 5 (6) | 3 (4) |
| KCNE1 | 1 (1) | 1 (4) | 1 (1) | 0 (0) |
| KCNE2 | 2 (2) | 1 (4) | 2 (3) | 2 (2) |
| >1 Genotype (included above) | 2 (2) | 0 | 2 (3) | 2 (2) |
| Beta-blockers | 101 (98) | 26 (100) | 75 (97) | 82 (100) |
| Personal AED | 36 (36) | 15 (58) | 21 (27) | 30 (36) |
| ICD | 6 (6) | 2 (8) | 5 (6) | 6 (7) |
| Noncompliance | 3 (3) | 2 (8) | 2 (3) | 2 (2) |
| Known follow-up, yrs | 7.1 ± 4.0 | 6.9 ± 4.1 | 7.3 ± 3.9 | 6.6 ± 4.0 |
| LQTS cardiac events during participation | 0 (0)* | 0 (0) | 0 (0) | 0 (0) |
Values are mean ± SD or n (%).
There were no long QT syndrome (LQTS) cardiac events during sports participation. However 1 patient had an appropriate implantable cardioverter-defibrillator (ICD) shock while running casually in the backyard when she was noncompliant with beta-blockers.
AED = automated external defibrillator.
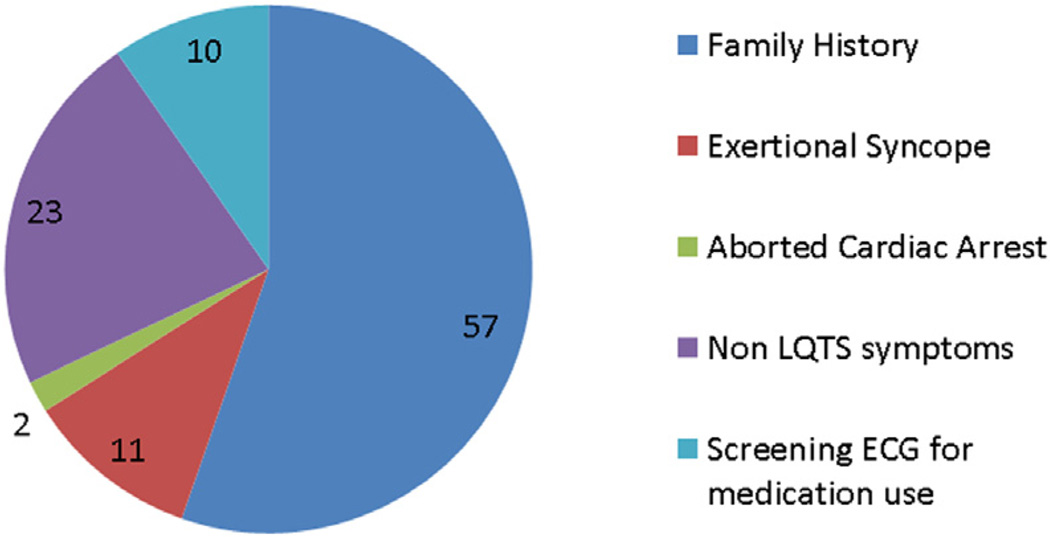
FIGURE 1. Athlete Presentation.
Athlete presentation is categorized for all sports participants. The majority of patients were identified through familial electrocardiogram (ECG) screening. LQTS = long QT syndrome.
Beta-blockade was prescribed for all patients in this series: nadolol (n = 97, 93%), propranolol (n = 5, 5%), or atenolol (n = 1, 1%). The maximum heart rate achieved during exercise stress testing after treatment with beta-blockers was 71 ± 2.3% predicted for age (151 ± 7 beats/min, range 85 to 184 beats/min). In addition to beta-blockade, 3 patients (3%) were on mexiletine, 2 of which had SCN5A mutations. Six patients (6%) had ICDs. The indications for ICD implantation were syncope despite therapy (n = 1), documented self-limited torsade de pointes on therapy (n = 1), aborted cardiac arrest prior to treatment (n = 2), noncompliance with beta-blockers (n = 1), and beta-blocker intolerance (n = 1). None of the 6 ICD patients with LQTS symptoms had events during sports or exertion. Two patients had dual chamber pacemakers, 1 of whom was upgraded to an ICD secondary to documented torsade de pointes on the pacemaker during the analysis period and this patient was included in the ICD group.
PRIMARY AND SECONDARY ENDPOINTS
There were no occurrences of the primary endpoint (tachyarrhythmic death or externally resuscitated tachyarrhythmia, syncope, or severe injury resulting from arrhythmia-related syncope during or after sports participation) or the secondary endpoints (appropriate shock, inappropriate shock, AED shock during or after sports, or sports-related ICD system damage).
ATHLETIC SCREENING QTc CUTOFF VALUES
In athletic screening QTc values <480 ms for females and <470 ms for males have been recommended (7). In this study, there were 36 females (36 of 53 females, 68%) with a QTc <480 ms and there were 29 males (29 of 50 males, 58%) with a QTc <470 ms. There were 11 patients with a QTc >500 ms (4 females, 7 males). Given that no patients had cardiac events during sports participation, a substratification based on QTc intervals was not performed.
COMPETITIVE SPORTS PARTICIPANTS
Twenty-six patients participated in competitive sports (Table 1). Three patients had exertional syncope, and the remainder of patients were diagnosed with LQTS after evaluation for non-LQTS symptoms (n = 6) and screening ECGs for medication use (n = 4) (Figure 1).
Beta-blockade was prescribed for all patients in this group: nadolol (n =24, 92%), propranolol (n =1,4%), or atenolol (n =1, 4%). Self-reported noncompliance with prescribed medical therapy occurred in 2 patients (8%). Two (8%) had ICDs (intolerance to beta-blockers in 1, aborted cardiac arrest prior to treatment in 1).
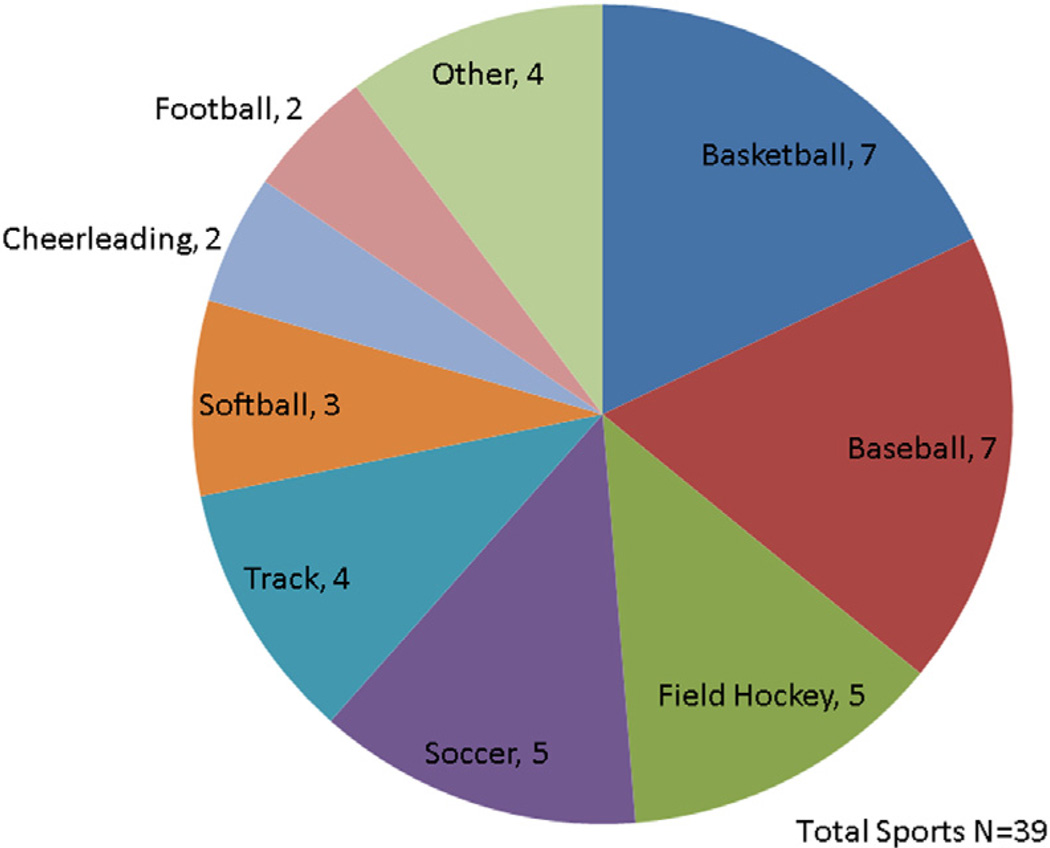
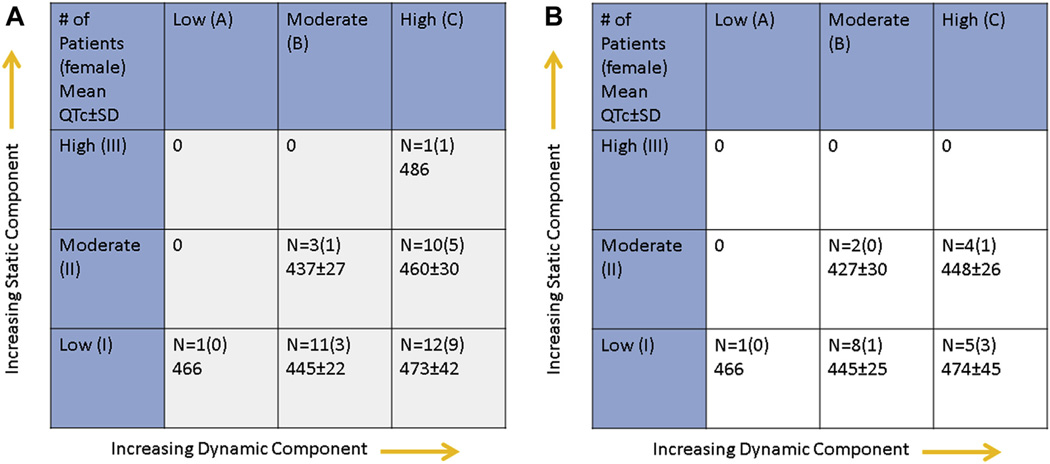
A total of 39 competitive sports were played among the 26 athletes (Figure 2). Using the static and dynamic designations in the Bethesda guidelines (7), sports were further categorized into low, moderate and high stratification as shown in Figure 3. Eighteen (69%) competed in 1 sport, 3 (12%) competed in 2 sports, and 5 (19%) competed in 3 sports. The majority of patients (12 of 26, 46%) participated in class IC sports. Figure 3B shows a proportionate distribution of LQT1 competitive athletes. No patients participating in competitive sports had syncope, documented arrhythmia, or aborted cardiac arrest during sports-related activity.
FIGURE 2. Competitive Sports Participation.
A total of 39 sports are depicted by type in this pie chart. The most frequently participated sports were basketball, baseball, soccer, and field hockey.
FIGURE 3. Bethesda Classification (Competitive Athletes as Well as LQT1 Subtype).
(A) Sports classified in accordance with the Bethesda Guidelines. Increasing static component (I–III) is depicted on the y-axis and increasing dynamic component (A–C) is depicted on the x-axis. (B) Sports classified for long QT1 (LQT1) patients in accordance with Bethesda guidelines. This figure shows a proportionate distribution of LQT1 athletes compared to Figure 2.
BETHESDA GUIDELINES COMPLIANCE
Twelve (46%) LQTS patients engaged in competitive sports contrary to the Bethesda guidelines for LQTS (Tables 1 and 2). Two (17%) of these patients had ICDs (Bethesda guideline #4), 7 (58%) patients were asymptomatic but had QTc intervals exceeding 470 ms for males and 480 ms for females (Bethesda guideline #2), and 3 patients had suspected LQTS-precipitated syncopal episodes (Bethesda guideline #1). A total of 14 sports were played among the 12 competitive athletes, including basketball (n = 3), field hockey (n = 3), baseball (n = 2), soccer (n = 2), lacrosse (n = 1), softball (n = 1), biking (n = 1), and running (n = 1).
TABLE 2.
Summary of Most Recent Bethesda Recommendations for Sports Restriction in LQTS
| Summary of Recommendation |
|---|
| Recommendation #1 Patients with a prior cardiac arrest or syncopal event related to LQTS should be restricted to class IA sports |
| Recommendation #2 Asymptomatic patients with borderline QTc prolongation (<470 in males, <480 in females) are restricted to class IA sports. |
| Recommendation #3 Patients with phenotype-negative, genotype-positive LQTS are not restricted. LQT1 patients should refrain from swimming. |
| Recommendation #4 LQTS patients with an ICD should be restricted from all sports. |
ICD = implantable cardioverter-defibrillator; LQTS = long QT syndrome.
RECREATIONAL SPORTS PARTICIPANTS
Seventy-seven (75%) patients participated in recreational sports (Table 1). Similar to competitive sports participants, the majority of patients (n = 42, 55%) were diagnosed with LQTS through familial cascade genetic screening (Figure 1).
Beta-blockade was prescribed for all patients in this group: nadolol (n = 72, 94%), propranolol (n = 4, 5%), or atenolol (n = 1, 1%). In addition to betablockade 3 patients (4%) received mexiletine, 2 of which had SCN5A mutations. The indications for ICD implantation in this group were syncope despite therapy (n = 1), documented torsade de pointes on therapy (n = 1), beta-blocker noncompliance (n = 1), and aborted cardiac arrest (n = 1).
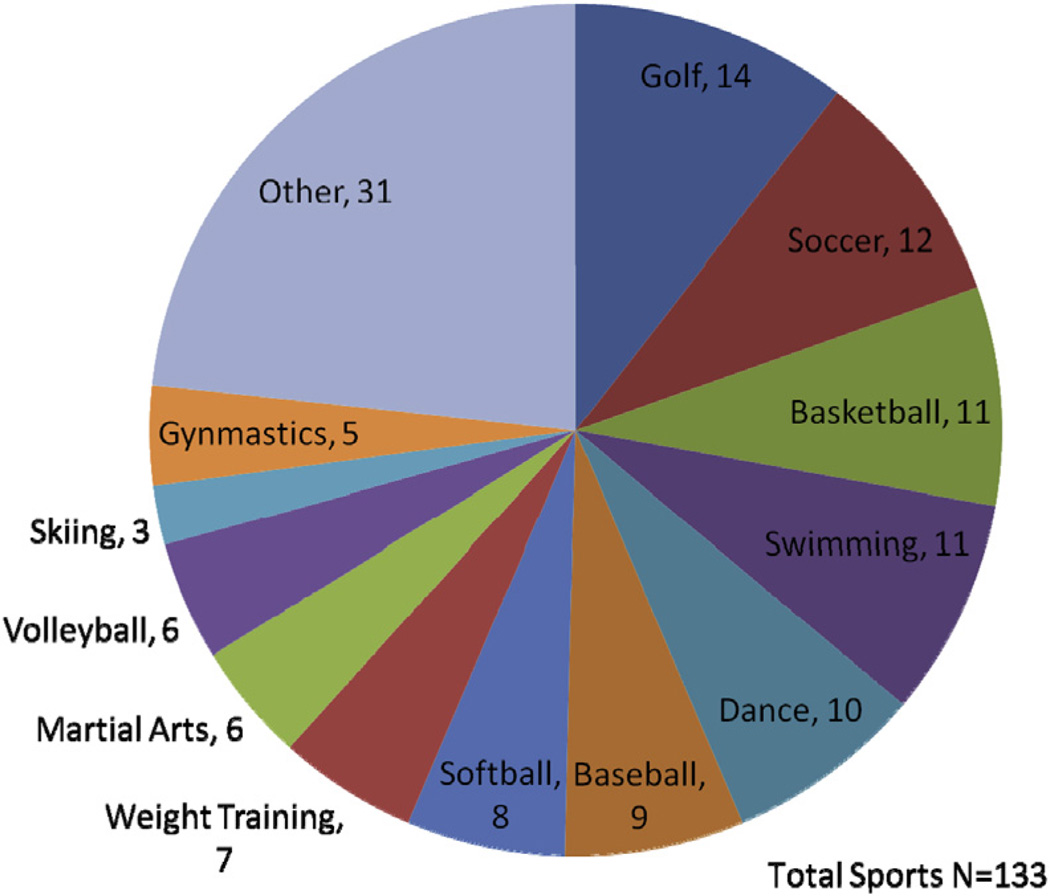
A total of 133 recreational sports were played among the 84 participants (Figure 4). The QTc of the recreational swimmers was 459 ± 29 ms. Two swimmers were equipped with an ICD and 6 swimmers owned personal AEDs.
FIGURE 4. Recreational Sports Participation.
A total of 133 sports are depicted by type in this pie chart.
No patients competing in recreational sports had syncope, documented arrhythmia, or aborted cardiac arrest during sports-related activity.
PHYSICAL EDUCATION PROGRAM
Eighty-four subjects (81%) were ≤18 years of age during follow-up and participated in a school physical education program in addition to playing either competitive or recreational sports (Table 1). All school gyms had access to an AED.
No patients participating in a school structured physical education program had syncope, documented arrhythmia or aborted cardiac arrest during program activities.
ICD DISCHARGES
During the analysis period, there were 5 appropriate ICD discharges that occurred in 2 patients. One patient with a KCNH2 mutation and a QTc duration of 510 ms had 4 appropriate ICD discharges for polymorphic ventricular tachycardia (average cycle length 210 ms) in a short span of time during a febrile illness associated with influenza A infection unrelated to exertional activity. The second patient with a KCNQ1 mutation and QTc duration of 470 ms had 1 appropriate ICD discharge for torsade de pointes that occurred while she ran a few feet in her backyard and by self-admission had stopped taking her beta-blocker for a few weeks prior. This patient also had experienced an inappropriate ICD shock for T-wave oversensing. No patients with ICDs experienced inappropriate ICD shocks for sinus tachycardia or lead fracture.
AUTOMATIC EXTERNAL DEFIBRILLATOR USE
In accordance with our recommendation of having an AED at all sporting events for LQTS children participating in any type of sports, many patients had access to a personal AED as described in Table 1. During a follow-up of 755 patient-years, there were no events that triggered AED use.
DISCUSSION
Due to an association of sudden death during exercise (11,12), limiting or avoiding significant physical activity has been recommended in individuals with certain genetic cardiovascular disorders (7). Sports eligibility guidelines for patients with LQTS have been predicated on the concept that intense physical exertion and increased catecholamine surges may create greater susceptibility to sudden death in the setting of an unstable cardiac repolarization substrate (13). Consequently, sports disqualification for patients with LQTS, in accordance with current guidelines, is commonly adopted by physicians (7). More recent literature has demonstrated a significant risk reduction in patients compliant with beta-blocker therapy, further fueling the debate (13).
Apart from the current series in which we focus on sports participation in school-age children with LQTS, there is only 1 other study describing the outcome of athletes with LQTS engaging in competitive sports. Johnson and Ackerman (9) reported no deaths and observed only 1 athlete experiencing 2 LQTS-related cardiac events during athletics in 650 patient-years of follow-up. Our results mirror those of the aforementioned study, however, there are several differences. First, our analysis was aimed primarily at school-age LQTS children. The average age at diagnosis of our LQTS cohort was 8.4 ± 5.6 years of age compared to 17 ± 11 years of age in the study by Johnson and Ackerman. Second, our analysis was not restricted to competitive athletes. While competitive athletes distinguish themselves with systematic training and participate in regular competition, young people participating in recreational, non-organized sports and physical education classes are also capable of intense physical exertion and some may place an equally high premium on excellence and achievement. In fact, the majority of sudden death events have been reported to occur in young persons who are not competitive athletes (11,14). We therefore felt it important to also analyze the outcomes of LQTS children engaged in recreational sports and school-based physical education programs in addition to competitive sports. The majority of our patients (n = 90, 88%) were previously participating in some type of sports at the time of initial evaluation and continued to pursue sports despite the diagnosis of LQTS and discussion of Bethesda guidelines. Finally, beta-blocker treatment was prescribed to 100% of our patients irrespective of genotype.
Our series represents a LQTS population skewed towards asymptomatic patients. By comparison, in the multi-institutional study by Garson et al (15), 45% of their patients presented with “serious” symptoms (sudden cardiac arrest, syncope, seizures). With a mortality of 9%, the Garson et al. (15) study clearly describes a higher risk cohort than the LQTS athletes in this series. We suspect that the substrate and severity of disease in our study population reflects that of the current era, where a number of asymptomatic patients are diagnosed with LQTS as a result of liberal use of ECG testing and genotype positive-phenotype negative individuals are identified secondary to readily available familial cascade genetic screening. Given the changing diagnostic trends, it may be clinically beneficial to re-examine sports recommendations that were made based on data from an earlier era.
The incidence of sudden cardiac death is estimated to be in the range of 1:40,000 to 1:80,000 in high school athletes (12). The exact prevalence of sudden death in LQTS patients participating in sports is unknown. Even less information exists regarding the prevalence of sudden death in LQTS pediatric athletes who are adequately treated and compliant with medications and/or protected with an ICD. Currently, outside of our current report and that of Johnson and Ackerman, there are no data regarding sports-related LQTS cardiac events in patients who have undergone a full diagnostic evaluation, demonstrated compliance with treatment and are protected with a safety net of an ICD/AED. Vincent et al. (13) found a high heart rate to be a risk factor for cardiac events in a cohort of LQT1 patients (mean age of 35 years), but beta-blocker therapy was not uniformly used in all patients. Earlier reports of sports-related deaths in LQTS focused on swimming as a trigger, especially in patients with the KCNQ1 mutations. While the prevalence of swimming-related deaths is undoubtedly high as shown in the report by Moss et al. (16), it should be noted only 14 of 118 (12%) were taking betablockers at the time of the cardiac event. In our series, 11 (11%) patients participated in recreational swimming, and 6 (54%) had a KCNQ1 mutation, but none of the patients had a LQTS event during the follow-up period.
Patients with ICDs are conventionally advised against sports participation (17,18), though recent prospective data suggests that participation is safer than previously recognized (19). No serious arrhythmic events or ICD system damage related to sports was observed in the 6 patients with ICDs in our series. There was 1 appropriate ICD shock during mild physical activity in a patient with beta-blocker noncompliance.
Although we routinely recommend that LQTS patients opting to participate in competitive or recreational sports have access to an AED and preferably purchase a personal AED, there were no instances of AED activations in this cohort during the follow-up period.
Decisions regarding whether children with LQTS should engage in sports participation, both competitive and recreational, are difficult. The physiologic benefits of exercise at all ages have been emphasized repeatedly and promoted as a national public health agenda (20). Equally important are the enhanced self-confidence; sense of psychological, physical, and social well-being; and the improved overall quality of life that sports participation brings to children and adolescents (21,22). On the other hand are the potentially adverse consequences of exercise and increased susceptibility to sudden death in certain types of genetic cardiovascular disease. Given the lack of past and present data and questionable feasibility of future randomized studies, a timely resolution of this debate is unlikely. As reflected in this series, our approach to this issue evolved overtime and our results describe a single institutional experience.
STUDY LIMITATIONS
Our retrospective review was limited to a single institution; whether the results are representative of all LQTS patients participating in sports is unknown. The majority of our patients engaged in recreational sports and since the level of exercise may vary considerably in nonorganized sports, the lack of LQTS events, ICD shocks, and ICD system damage may simply reflect a lower level of exertion. Similarly, competitive athletes engaged mostly in class IB, IC, and IIC sports that may have a limited degree of catecholamine surge than othermore physically demanding activities listed in the class IIIC category, such as rowing, triathlon, and speed skating. Nevertheless, the data from this retrospective case series are encouraging that patients with LQTS may be able to engage in some levels of sports. We only included patients who were actively participating in sports and we cannot exclude the possibility of survival or selection bias (i.e., that those who had been participating uneventfully before the initiation of this analysis continued, whereas those who had suffered adverse events or were deemed high risk by their physician and therefore were disqualified did not). Two of 212 patients were excluded from the study due to inconsistent follow-up and it is possible that their death was responsible for the lack of follow-up but to date our institution has not received the information of such an event. There were no primary endpoints in any patients, and this may be a result of almost uniform treatment compliance. However, treatment compliance may fluctuate and may not be accurately reported by patients. Therefore, this data should be considered relevant only to patients who are definitively compliant with beta-blockers while participating in sports. Majority of patients had a KCNQ1 mutation that is exquisitely sensitive to beta-blocker treatment and the absence of LQTS events may be due to a genotype-specific treatment response. Additional risk stratification based on serial QTc intervals was not performed, as no cardiac events occurred during formal sports participation in this study (23). The determination of AED availability was based on information provided by families and other means of confirmation were not available. This series involved patients <21 years of age and therefore no intercollegiate athletes or professional sports participants were involved in the analysis. Our younger population might have added a selection bias in that the level of intensity is presumably less than that of competitive, professional athletes. Therefore, this data strictly applies to the pediatric athlete. Finally, this is a retrospective review reflecting our clinical practice and a prospective study was not conducted. Longer-term follow-up and a multi-institutional registry are needed.
CONCLUSIONS
In this series, no cardiac events and no deaths were observed in treatment-compliant LQTS children during sports participation in 755 patient years of follow-up. One patient with beta-blocker noncompliance received 1 appropriate ICD shock during mild physical activity. A prospective registry of LQTS patients participating in sports is necessary to quantify risks associated with sports participation to assist physicians and patients in making informed decisions regarding sports participation.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE
The decision to allow sports participation in patients with long QT syndrome is controversial and should be deferred to expert clinicians.
TRANSLATIONAL OUTLOOK
Prospective registries are required to examine the risk of arrhythmia and sudden cardiac death in treated patients with long QT syndrome.
The objective of this analysis was to examine the outcomes of sports participation in pediatric long QT syndrome (LQTS) patients. A retrospective review was performed on LQTS at the Children’s Hospital of Philadelphia. One hundred and three patients (49%, female n = 53, average follow-up 7.1 ± 4.0 years, average QTc 468 ± 42 ms) participated in sports. Twenty-six patients participated in competitive sports (26%, female n = 15, average follow-up 6.9 ± 4.1 years, average QTc 461 ± 35 ms). Seventy-seven patients (75%, female n = 35, average follow-up 7.3 ± 3.9 years, average QTc 470 ± 43 ms) participated in recreational sports. In this series no cardiac events/deaths were observed in 755 patient years of follow-up.
Acknowledgments
Dr. Shah has received research grant support from Medtronic.
ABBREVIAT IONS AND ACRONYMS
- AED
automatic external defibrillator
- ECG
electrocardiogram
- ICD
implantable cardioverter-defibrillator
- LQTS
long QT syndrome
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Ackerman MJ, Clapham DE. Ion channels-basic science and clinical disease. N Engl J Med. 1997;336:1575–1586. doi: 10.1056/NEJM199705293362207. [DOI] [PubMed] [Google Scholar]
- 2.Liu JF, Jons C, Moss AJ, et al. Risk factors for recurrent syncope and subsequent fatal or near-fatal events in children and adolescents with long qt syndrome. J Am Coll Cardiol. 2011;57:941–950. doi: 10.1016/j.jacc.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldenberg I, Horr S, Moss AJ, et al. Risk for life-threatening cardiac events in patients with genotype-confirmed long-qt syndrome and normal-range corrected qt intervals. J Am Coll Cardiol. 2011;57:51–59. doi: 10.1016/j.jacc.2010.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldenberg I, Moss AJ, Peterson DR, et al. Risk factors for aborted cardiac arrest and sudden cardiac death in children with the congenital long-QT syndrome. Circulation. 2008;117:2184–2191. doi: 10.1161/CIRCULATIONAHA.107.701243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz PF, Wieand TS, Ganley J, et al. Genotype-and mutation site-specific qt adaptation during exercise, recovery, and postural changes in children with long-qt syndrome. Circ Arrhythm Electrophysiol. 2011;4:867–873. doi: 10.1161/CIRCEP.111.963330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Ackerman MJ, George AL, Jr, Wilde AA. Impact of genetics on the clinical management of channelopathies. J Am Coll Cardiol. 2013;62:169–180. doi: 10.1016/j.jacc.2013.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zipes DP, Ackerman MJ, Estes NA, 3rd, Grant AO, Myerburg RJ, Van Hare G. Task force 7: Arrhythmias. J Am Coll Cardiol. 2005;45:1354–1363. doi: 10.1016/j.jacc.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JN, Ackerman MJ. Competitive sports participation in athletes with congenital long qt syndrome. JAMA. 2012;308:764–765. doi: 10.1001/jama.2012.9334. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JN, Ackerman MJ. Return to play? Athletes with congenital long qt syndrome. Br J Sports Med. 2013;47:28–33. doi: 10.1136/bjsports-2012-091751. [DOI] [PubMed] [Google Scholar]
- 10.Bazett HC. An analysis of time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 11.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42:1959–1963. doi: 10.1016/j.jacc.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: A state-of-the-art review. Heart. 2014;100:1227–1234. doi: 10.1136/heartjnl-2014-093872.rep. [DOI] [PubMed] [Google Scholar]
- 13.Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of beta-blockers in long-qt syndrome type 1: Contribution of noncompliance and qt-prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation. 2009;119:215–221. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 14.Maron BJ, Chaitman BR, Ackerman MJ, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. 2004;109:2807–2816. doi: 10.1161/01.CIR.0000128363.85581.E1. [DOI] [PubMed] [Google Scholar]
- 15.Garson A, Jr, Dick M, 2nd, Fournier A, et al. The long qt syndrome in children. An international study of 287 patients. Circulation. 1993;87:1866–1872. doi: 10.1161/01.cir.87.6.1866. [DOI] [PubMed] [Google Scholar]
- 16.Moss AJ, Robinson JL, Gessman L, et al. Comparison of clinical and genetic variables of cardiac events associated with loud noise versus swimming among subjects with the long QT syndrome. Am J Cardiol. 1999;84:876–879. doi: 10.1016/s0002-9149(99)00458-0. [DOI] [PubMed] [Google Scholar]
- 17.Heidbuchel H, Corrado D, Biffi A, et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part II: ventricular arrhythmias, channelopathies and implantable defibrillators. Eur J Cardiovasc Prev Rehabil. 2006;13:676–686. doi: 10.1097/01.hjr.0000239465.26132.29. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, Zipes DP. Introduction: Eligibility recommendations for competitive athletes with cardiovascular abnormalities—general considerations. J Am Coll Cardiol. 2005;45:1318–1321. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Lampert R, Olshansky B, Heidbuchel H, et al. Safety of sports for athletes with implantable cardioverter-defibrillators: results of a prospective, multinational registry. Circulation. 2013;127:2021–2030. doi: 10.1161/CIRCULATIONAHA.112.000447. [DOI] [PubMed] [Google Scholar]
- 20.Physical fitness and activity in schools. American Academy of Pediatrics. Pediatrics. 2000;105:1156–1157. doi: 10.1542/peds.105.5.1156. [DOI] [PubMed] [Google Scholar]
- 21.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241–246. [PubMed] [Google Scholar]
- 22.Fletcher GF, Blair SN, Blumenthal J, et al. Statement on exercise. Benefits and recommendations for physical activity programs for all americans. A statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1992;86:340–344. doi: 10.1161/01.cir.86.1.340. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg I, Mathew J, Moss AJ, et al. Corrected QT variability in seral electrocardiograms in long QT syndrome: the importance of maximum corrected QT for risk stratification. J Am Coll Cardiol. 2006;48:1047–1052. doi: 10.1016/j.jacc.2006.06.033. [DOI] [PubMed] [Google Scholar]