Abstract
Objectives
To test agreement and define differences in periodic limb movements in sleep (PLMS) measured by polysomnography and an ankle activity monitor, and to describe PLMS variability across nights, feasibility of home monitoring, and correlates of PLMS in children with sickle cell disease (SCD).
Methods
Twenty children with SCD and restless legs syndrome (RLS) symptoms or polysomnography-documented PLMS underwent concurrent attended polysomnography and ankle activity monitoring over 1–2 nights and home activity monitoring for 3 nights. Serum iron and ferritin were measured pre- and post-polysomnography.
Results
Adequate sensitivity (1.00), specificity (0.69), and mean bias (5.0±7.4 PLMS/h) for identifying elevated PLMS by activity monitor were obtained when scoring the period from sleep onset to offset rather than time in bed per manufacturer recommendation, and using a cut-point of 10 PLMS/h. Compared to activity monitor, only polysomnographic PLMS demonstrated periodicity, at inter-movement intervals (IMI) 20–35 s; the activity monitor overscored PLMS at the beginning and end of sleep and at shorter IMI (5–15 s; p≤0.003), suggesting misclassification of nonperiodic leg movements as PLMS by activity monitor. PLMS varied across 4 nights by 16.1±13.4 PLMS/h. Post-polysomnography ferritin was associated (positively) with PLMS (p=0.034); RLS symptoms were not.
Conclusions
Ankle activity monitoring is a valid screening measure for PLMS in children with SCD and can readily be performed at home. Interpretation should incorporate a threshold for elevated PLMS of 10/h and scoring from sleep onset to offset which could be identified with concurrent wrist actigraphy, to better account for true PLMS.
Keywords: periodic limb movements in sleep, anemia, sickle cell, child, adolescent, actigraphy, validation studies, inter-movement interval
BACKGROUND
Periodic limb movements in sleep (PLMS) are stereotypic limb movements that occur during sleep and are associated with hypertension, cardiovascular disease, stroke, attention deficit-hyperactivity disorder and restless legs syndrome (RLS) [1–3]. A temporal relationship between PLMS and morbidity, however, has not been documented, perhaps in part due to the difficulty of measuring PLMS longitudinally using the “gold standard” measure, polysomnography (PSG), which is lengthy, inconvenient and labor-intensive. Additionally, PLMS vary substantially from night to night [4,5] and the likelihood of identifying PLMS exceeding the threshold for abnormal PLMS of 5/h increases with multiple nights of measurement [6]. Consequently, while providing diagnostic validity for PLMS, PSG is impractical for screening or longitudinal investigation. To determine the long-term consequences of PLMS and identify susceptible individuals and groups who might benefit from treatment, screening large numbers of individuals and serial measurement are required.
PLMS have been identified at an unusually high rate in children with sickle cell disease (SCD) [7,8]. Reasons for this phenomenon are largely unexplored. SCD is a disorder in which vaso-occlusion and hemolysis contribute to significant cardiovascular, cerebrovascular and neurocognitive deficits [9–12] and a severely curtailed life expectancy [13]. Given the well-established sequelae of SCD, and the potential additional consequences of PLMS, a reliable and valid method of measuring PLMS longitudinally to study their effects in children with SCD is needed. The main aim of this study was to test the agreement and define differences between PSG and ankle-worn activity monitors on the measurement of PLMS in children with SCD. Secondary aims were to test the feasibility of measuring PLMS in the home using activity monitoring, to describe night-to-night variability of PLMS and to test their association with serum iron and ferritin and symptoms of RLS.
METHODS
Participants
A convenience sample of 20 children with SCD was prospectively recruited through the Sickle Cell Center of a children’s hospital from October, 2010 to June, 2011. Children qualified for participation if they were age 2–18 years; had a confirmed SCD diagnosis of one of the three major SCD subtypes, SCD-SS, SCD-SC or SCD-S-Beta Thalassemia; and had symptoms suggestive of RLS [14] or PLMS [15] based on parent report or a PLMS index ≥ 5/h by overnight PSG within the preceding 2 years. Craniofacial or neuromuscular disorders, congenital heart disease, chronic lung condition unrelated to SCD (except asthma), acute illness, SCD-related exacerbation (e.g. painful crisis or acute chest syndrome) in the preceding 2 months and non-English speaking parent or child were criteria for exclusion.
Procedure
Polysomnography
All children underwent overnight, attended PSG in a pediatric sleep laboratory. According to our protocol, five children had a second, consecutive night PSG. Recruitment for the second night was targeted at those with elevated PLMS on a previous PSG. A Rembrandt PSG system (Embla, Broomfield, CO) recorded the following parameters: electroencephalogram (F3-M2, F4-M1, C3-M2, C4-M1, O1-M2, O2-M1), left and right electrooculogram, submental and tibial electromyogram (EMG), electrocardiogram, oro-nasal airflow via a 3-pronged thermistor (Pro-Tech Services, Inc., Mukilteo, WA), nasal pressure with a pressure transducer (Pro-Tech Services, Inc.), chest and abdominal wall motion with respiratory inductance plethysmography (Viasys Healthcare, Yorba Linda, CA), arterial oxygen saturation with oximeter pulse waveform (Masimo, Irvine, CA) and end-tidal carbon dioxide level (Novametrix Medical Systems, Inc., Wallingford, CT). Respiratory events, sleep architecture and arousals were scored using pediatric scoring criteria [16]. Obstructive sleep apnea was an obstructive apnea-hypopnea index ≥ 1.5 [17–19].
PLMS were recorded with two surface EMG electrodes positioned over the tibialis anterior (TA) muscle, using separate channels for each leg. Scoring of PLMS required a series of at least 4 consecutive leg movements each lasting 0.5–10 s (PLMS duration), having an interval between onset of leg movements of 5–90 s (inter-movement interval; IMI) and an amplitude increase in EMG voltage of ≥ 8 μ-V above resting baseline. Limb movements were not scored if they occurred within 0.5 s preceding or following an apnea or hypopnea [16]. A PLMS index ≥ 5/h of sleep was considered elevated [20]. An arousal was an abrupt shift in electroencephalographic frequency ≥ 3 s separated by ≥ 10 s of stable sleep. When associated with a PLMS, the arousal was scored as a PLMS arousal and the number per hour of sleep was the PLM arousal index. All PSGs were interpreted by a physician board certified in sleep medicine (TM).
Activity monitors
Activity monitors (Physical Activity Monitor—Restless Leg, PAM-RL; Philips, Inc., Murryville, PA) were worn concurrently with PSG, fastened around the ankle with a Velcro strap over each TA at bedtime and removed in the morning. A marker button on each unit was pressed to mark the scoring period when getting into bed at night and out of bed in the morning (time in bed), the manufacturer-recommended scoring period. The PAM-RL is an activity monitor designed for the measurement of limb movements (rather than sleep). It samples movement at 40 times per second with a tri-axial accelerometer and can record data for up to 5 consecutive days. An up/down sensor in the monitor allows for discrimination between activity in the standing (up) and lying (down) positions, improving specificity for scoring PLMS. An automated scoring algorithm (PAM-RL Software Version 7.6.2) identifies PLMS-like movements based on diagnostic criteria [16]. Only default settings on the scoring program were used in this study.
Activity monitoring was continued at home for three consecutive days by 16 children. Telephone calls were placed to families each evening to review instructions including a reminder to put on the equipment at bedtime, to place the activity monitors on the correct leg in the correct position, and to push the marker button and complete a sleep diary at bedtime and wake time.
Questionnaire and clinical measures
A demographic and medical history questionnaire included questions on medications, pain, SCD subtype and presence of conditions confounding or comorbid with RLS or PLMS. A daily sleep diary was completed concurrently with PLMS recording, in which was recorded bedtime, time out of bed, pain and pain medication use. Four parent-report questions were used to screen for RLS in children [14]. These included “uncomfortable feeling in the legs or urge to move the legs,” “uncomfortable feelings in the legs that start or get worse when sitting or lying down,” “uncomfortable feelings in the legs that get better or go away if the legs are moved,” and “uncomfortable feelings in the legs that get worse in the evening or at night.” Children with 3–4 of these symptoms were classified as probable RLS, 1–2 symptoms as possible RLS and no symptom as no RLS [21]. Serum total iron, ferritin and high-sensitivity C-reactive protein (CRP) were drawn before (~5 PM) and after PSG (~7:30 AM).
This study was approved by the Institutional Review Board of The Children’s Hospital of Philadelphia and the University of Pennsylvania. Informed consent was obtained from the parent/legal guardian after a full explanation of procedures, and children aged 7 and older signed their assent. Subjects were financially compensated for their participation.
Statistical Analysis
To test the strength of agreement of PLMS measured by activity monitor and PSG, Lin Concordance Correlation Coefficients (ρc) were calculated [22]. The ρc contains a measure of precision and accuracy. A ρc < 0.90 indicates poor strength of agreement between methods, 0.90–0.95 moderate agreement and 0.95–0.99 substantial agreement [23]. The Bland Altman method was employed to calculate the mean bias (mean difference) and 95% limits of agreement between methods [24]. A receiver operator characteristic (ROC) curve tested the sensitivity and specificity of ankle activity monitoring to correctly classify elevated PLMS at two cut-points: ≥ 5/h and ≥ 10/h. Bias analyses (sensitivity, specificity, accuracy, positive predictive value and negative predictive value) were used to compare three PSG and activity monitor PLMS thresholds (≥ 5/h, ≥ 10/h and ≥ 15/h). These diagnostic thresholds were further tested with Matthew’s Correlation Coefficient (MCC) and kappa coefficient. The MCC is a test of correlation between observed and predicted binary classifications that takes into account true and false positives and negatives. MCC values range from +1 (best possible prediction) to −1 (worst possible prediction) [25]. The kappa coefficient is a conservative measure of agreement that takes into account agreement occurring by chance. A kappa value of 0.21–0.40 indicates fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.81–1.00 near-perfect agreement [26].
Paired t-test or Mann-Whitney tested group differences and Wilcoxon Signed Rank test tested within-subject differences over time and between methods. Spearman correlation (r) and Spearman partial correlation (pr) tested associations. A Bland Altman coefficient of repeatability (CR), the ability of two methods to measure a phenomenon equivalently across time, was calculated over 2 nights of concurrent PSG and ankle activity monitoring. A smaller CR indicates better repeatability and less error variance between methods [27]. Analyses were performed with the Statistical Package for the Social Sciences, version 17.0 for Windows (SPSS, Inc., Chicago, IL), except the pr (SAS Statistical Software, version 9.2, SAS Institute, Inc., Cary, NC) and PLMS periodicity analyses (Excel 2007, Microsoft Corp., Redmond, WA, U.S.A.). Alpha was set at p = 0.05 and was corrected for multiple comparisons (0.05/17 IMI, p = 0.003; 0.05/8 hours of sleep, p = 0.006; 0.05/10 PLMS duration intervals, p = 0.005).
RESULTS
A description of the sample is shown in Table 1. Medications included those for management of SCD (penicillin, hydroxyurea, folic acid), asthma (albuterol, fluticasone), allergies (cetirizine, loratadine) and pain (ibuprofen, acetaminophen and opioids, most commonly codeine). Opioids, which can suppress PLMS and RLS symptoms [28] were taken by 15 (75%) children at least occasionally, although 9 of these children took them less than once per month. No children were on medications likely to induce PLMS such as selective serotonin reuptake inhibitors or tricyclic antidepressants [29].
Table 1.
Description of the Sample
| Variable | N (%) |
|---|---|
| Sickle cell disease genotype | |
| SS | 15 (75) |
| SC | 4 (20) |
| S-Beta Thalassemia | 1 (5) |
| Sex, male | 10 (50) |
| Obstructive sleep apnea | 4 (25) |
| RLS classification* | |
| No | 10 (50) |
| Possible | 0 |
| Probable | 8 (40) |
| Transfusion history | |
| Occasional | 12 (60) |
| Multiple | 4 (20) |
| Mean ± SD (range) | |
| Age, years | 10.2 ± 4.4 (4.3 – 17.9) |
| BMI-z score | −0.1 ± 1.0 (−1.7 – 2.2) |
| Pain episodes (n, past 12 mo) | 23.6 ± 35.9 (0 – 144) |
| Mean sleep SpO2, % | 96.4 ± 2.5 (89.1 – 99.0) |
| Nadir sleep SpO2, % | 89.8 ± 4.9 (78.0 ± 98.0) |
| % total sleep time with SpO2 < 90% | 4.9 ± 20.6 (0 – 92.2) |
| PLMS index total, N/h | 8.8 ± 13.2 (0 – 53.4) |
Responses missing for 2 subjects; BMI, body mass index (kg/m2); RLS, restless legs syndrome.
Agreement Between Measures
Lin concordance correlations were examined between the PLMS index measured by PSG and activity monitor on the right and left ankle, the dominant and nondominant ankle, the ankle recording the highest PLMS, and the average of both ankles. The strongest correlation was with PLMS recorded on the dominant ankle (ρc = 0.571), so subsequent analyses used PLMS recorded on the dominant ankle. Bland Altman analyses indicated that the activity monitor overestimated PLMS compared to PSG, mean bias 8.1 ± 10.7, 95% limits of agreement −12.9, 29.1 (Figure 1). The activity monitor both overestimated and underestimated PLMS, with a difference between the two methods ranging from −8.6 to 42.0 PLMS/h. Sleep efficiency (r = −0.711, p = 0.001) and minutes of wake after sleep onset (r = 0.696, p = 0.001) were significantly associated with the difference in estimation of PLMS between methods.
Figure 1.
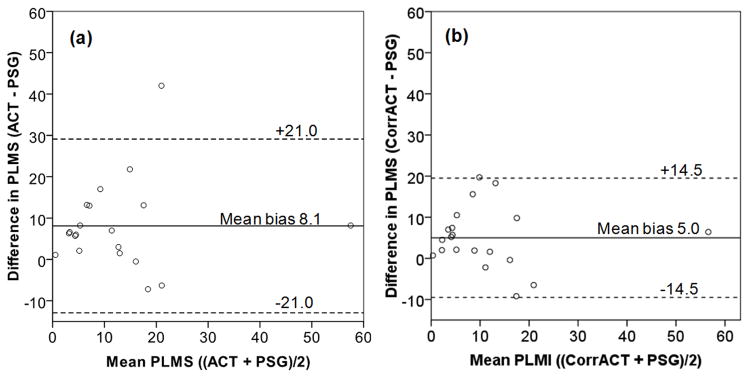
Bland Altman plot of agreement in the measurement of periodic limb movements in sleep (PLMS) between polysomnography (PSG) and activity monitor (ACT). Solid horizontal line indicates the amount ACT overestimated PSG (mean bias); dashed lines represent the 95% limits of agreement between methods. (a) Wide 95% limits of agreement (−12.9, 29.1) of ACT with PSG suggest that unadjusted ACT estimation of PLMS is too variable to be useful as a method of measurement. (b) Correcting ACT for sleep period time, compared to time in bed, decreased the mean bias and 95% limits of agreement (−9.5, 19.5).
PLMS measured by activity monitor were then rescored to better reflect their occurrence during sleep. Only the period from sleep onset to morning waking (sleep period time) identified by electroencephalographic tracing on PSG was scored (corrected activity monitor PLMS index), to test whether this would improve agreement between methods. Correlation between PSG and corrected activity monitor PLMS indices, ρc = 0.766, was an improvement over the uncorrected comparison. Mean bias decreased to 5.0 ± 7.4, 95% limits of agreement −9.5, 19.5. At a diagnostic threshold for elevated PLMS by PSG of 5/h, specificity of an activity monitor threshold of PLMS 5/h was poor, identifying all but one child as having elevated PLMS. The ROC area under the curve (AUC = 0.802, p = 0.025) identified 13.7 PLMS/h as the best activity monitor cut-point to classify PLMS ≥ 5/h, and 8.9 as the best cut-point for the corrected activity monitor PLMS index. Cut-points at 10 PLMS/h were 13.7 (AUC = 0.791, p = 0.036) and 9.9 (AUC = 0.835, p = 0.016) for the activity monitor and corrected activity monitor PLMS indexes, respectively. Bias, MCC and kappa analysis by three PSG and activity monitor PLMS thresholds are compared in Table 2. The MCC was strongest for the correlation of PSG and corrected activity monitor PLMS index cut-points ≥ 10 PLMS/h, with substantial agreement by kappa analysis. Considered together with the Bland Altman analyses, these results suggested that the combination of two modifications, use of a cut-point of PLMS ≥ 10/h for both methods and correction of PLMS activity monitor scoring for sleep period time, provided the best overall identification of elevated PLMS.
Table 2.
Comparison of bias, correlation and agreement on classification of elevated periodic limb movements in sleep (PLMS) at different activity monitor and polysomnography thresholds
| Sensitivity | Specificity | PPV | NPV | Accuracy | MCC | Kappa | |
|---|---|---|---|---|---|---|---|
| PLMS ≥ 5/h by Polysomnography | |||||||
| ACT PLMS ≥ 5/h | 1.00 | 0.08 | 0.42 | 1.00 | 0.45 | 0.187 | 0.068 |
| CorrACT PLMS ≥ 5/h | 1.00 | 0.25 | 0.47 | 1.00 | 0.55 | 0.343 | 0.211 |
| ACT PLMS ≥ 10/h | 1.00 | 0.58 | 0.62 | 1.00 | 0.75 | 0.599† | 0.528† |
| CorrACT PLMS ≥ 10/h | 0.88 | 0.67 | 0.64 | 0.89 | 0.75 | 0.533* | 0.510* |
| ACT PLMS ≥ 15/h | 0.50 | 0.75 | 0.57 | 0.69 | 0.65 | 0.257 | 0.255 |
| CorrACT PLMS ≥ 15/h | 0.50 | 0.75 | 0.57 | 0.69 | 0.65 | 0.257 | 0.255 |
|
| |||||||
| PLMS ≥ 10/h by Polysomnography | |||||||
| ACT PLMS ≥ 5/h | 1.00 | 0.08 | 0.37 | 1.00 | 0.40 | 0.168 | 0.055 |
| CorrACT PLMS ≥ 5/h | 1.00 | 0.23 | 0.41 | 1.00 | 0.50 | 0.308 | 0.174 |
| ACT PLMS ≥ 10/h | 1.00 | 0.54 | 0.54 | 1.00 | 0.70 | 0.538* | 0.450* |
| CorrACT PLMS ≥ 10/h | 1.00 | 0.69 | 0.64 | 1.00 | 0.80 | 0.664‡ | 0.612‡ |
| ACT PLMS ≥ 15/h | 0.57 | 0.77 | 0.57 | 0.77 | 0.70 | 0.341 | 0.341 |
| CorrACT PLMS ≥ 15/h | 0.57 | 0.77 | 0.57 | 0.77 | 0.70 | 0.341 | 0.341 |
|
| |||||||
| PLMS ≥ 15/h by Polysomnography | |||||||
| ACT PLMS ≥ 5/h | 1.00 | 0.06 | 0.21 | 1.00 | 0.25 | 0.115 | 0.026 |
| CorrACT PLMS ≥ 5/h | 1.00 | 0.19 | 0.24 | 1.00 | 0.35 | 0.210 | 0.085 |
| ACT PLMS ≥ 10/h | 1.00 | 0.44 | 0.31 | 0.44 | 0.55 | 0.367 | 0.237 |
| CorrACT PLMS ≥ 10/h | 1.00 | 0.56 | 0.36 | 0.56 | 0.65 | 0.452* | 0.340* |
| ACT PLMS ≥ 15/h | 0.75 | 0.75 | 0.43 | 0.75 | 0.75 | 0.419 | 0.390 |
| CorrACT PLMS ≥ 15/h | 0.75 | 0.75 | 0.43 | 0.75 | 0.75 | 0.419 | 0.390 |
p < 0.05
p < 0.01
p<0.005.
ACT, PLMS measured by activity monitor; CorrACT, PLMS measured by ACT corrected to include sleep period time only; MCC, Matthew’s Correlation Coefficient; NPV, negative predictive value; PPV, positive predictive value.
Feasibility of Home Measurement of PLMS
There were no complaints of discomfort with the equipment. One child wore the activity monitors on only 3 of the 4 study nights. The missing night was due to forgetting to put the activity monitors on at bedtime. The remainder wore them on all 4 nights for the duration of time in bed. Batteries were inadvertently removed from one set of activity monitors by study staff, ending the study after the first night. Afterwards, battery compartments were securely taped with no further incidents.
PLMS Variability, Repeatability and Time Structure
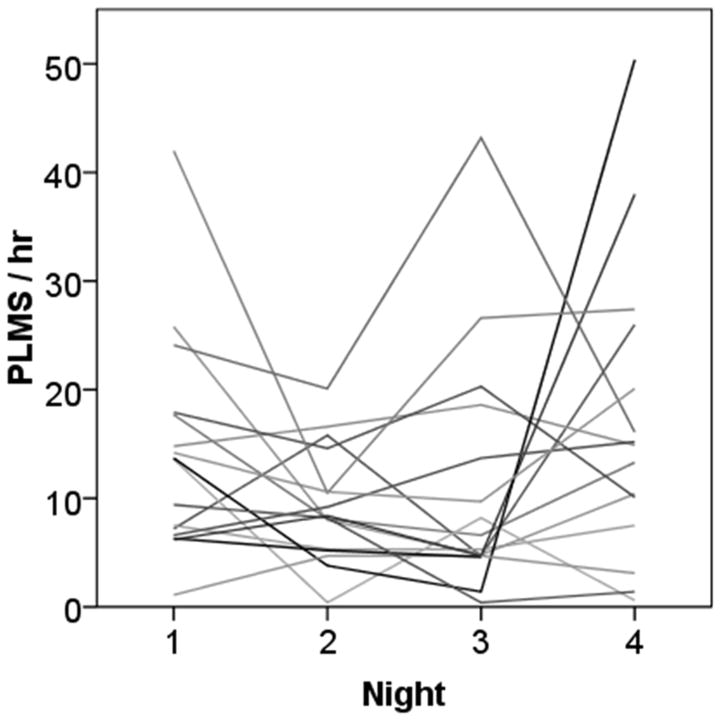
Individual subject PLMS indexes measured by activity monitor over 4 nights are shown in Figure 2. The change in PLMS across nights ranged from 1.7 to 49.0 PLMS/h, with a mean change in the PLMS index among individual subjects of 16.1 ± 13.4 PLMS/h. Variance in PLMS indices across nights increased as the mean PLMS index increased (Figure 3). Mean bias in PLMS measured over 2 nights of activity monitoring and PSG was 5.4 (CR 16.9), confidence interval −11.5, 22.3, which improved to a mean bias 3.5 (CR 12.5) with correction for sleep period time.
Figure 2.
Night-to-night variability in activity monitor measurement of periodic limb movements in sleep (PLMS) across 4 nights. Each line on the plot represents one case.
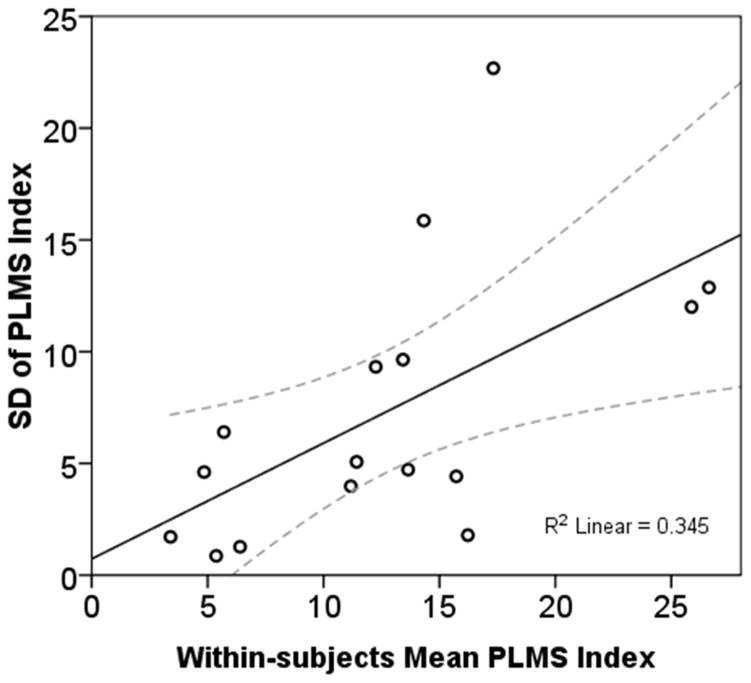
Figure 3.
Individual subjects’ mean periodic limb movements in sleep (PLMS) index measured by ankle activity monitors over 4 nights plotted against the standard deviation (SD). Solid line indicates the regression line of best fit; dotted lines the 95% confidence interval. The plot suggests that as mean PLMS across 4 nights increased, their night-to-night variability increased.
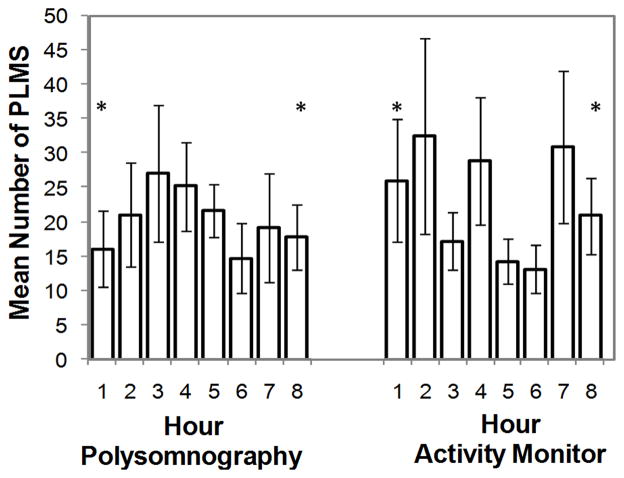
Fifteen PSGs demonstrated PLMS and were included in IMI, hour of sleep and PLMS duration comparisons between methods, for a total of 1302 individual PLMS by PSG and 1881 by corrected activity monitor. Individual-subject IMI were binned by 5 second intervals (5–<10 s, 10–<15 s, …, 80–<85 s, 85–90 s), PLMS by hour of sleep (1–8) and PLMS duration by second (0.5–1.4 s, 1.5–2.4 s, …, 8.5–9.4 s, 9.5–10 s). Methods for binning have been described elsewhere [8]. Mean IMI by PSG (38.8 s ± 25.8 s) peaked at 20–35 s, similar to previous findings [8]. In contrast, mean IMI measured by activity monitor (32.7 s ± 20.6 s) peaked at 10–15 s and differed significantly between methods at IMI 5–10 s (p = 0.003) and IMI 10–15 s (p =0.001) (Figure 4). Polysomnographic PLMS binned by hour of sleep peaked at hour 3 then decreased. In contrast, activity monitor PLMS showed no pattern across hour of sleep (Figure 5), and were significantly more frequent than by PSG at hours 1 (p = 0.002) and 8 (p = 0.005). Duration of PLMS peaked at 2 s for both methods. Mean PLMS duration was shorter by PSG than by activity monitor (mean 2.6 s ± 1.5 s and 3.3 s ± 2.1 s, respectively), with PLMS of 4 s through 9 s occurring significantly more frequently by activity monitor than PSG (each p < 0.005) (Figure 6).
Figure 4.
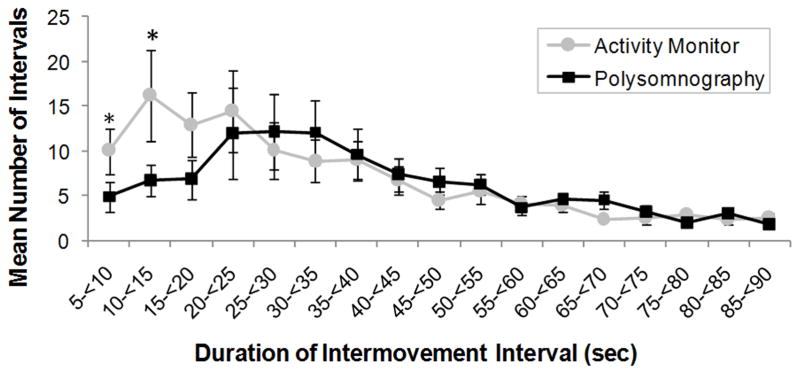
Distribution of the mean number of periodic limb movements in sleep (PLMS) inter-movement intervals (IMI) measured by polysomnography (black squares) and activity monitor corrected for sleep period time (gray circles). Error bars represent standard error of the mean. Asterisks represent significant within-subjects differences between methods (p ≤ 0.003).
Figure 5.
Distribution of periodic limb movements in sleep (PLMS) by hour of sleep measured by polysomnography and activity monitor corrected for sleep period time. Values are shown as mean (columns) and standard error (error bars). Asterisks represent significant within-subjects differences between methods (p < 0.006).
Figure 6.
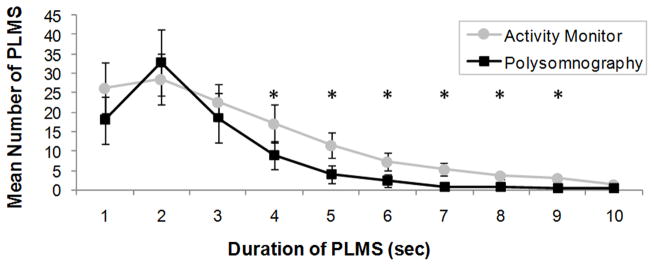
Distribution of the mean periodic limb movements in sleep (PLMS) duration measured by polysomnography (black squares) and activity monitor corrected for sleep period time (gray circles). Error bars represent standard error of the mean. Asterisks represent significant within-subjects differences between methods (p < 0.005). PLMS by Polysomnography versus Activity Monitor in Children
Correlates of PLMS
Results of serum iron, ferritin and CRP between PLMS groups (PLMS < 5/h vs. ≥ 5/h) are shown in Table 3. One child, transfused the morning prior to study participation, was excluded from analysis. Neither pre- nor post-PSG iron was associated with the PLMS index, nor was the circadian change in iron. Controlling for individual subject inflammation with the respective (pre- or post-PSG) CRP, only the post-PSG ferritin was associated, positively, with the PLMS index (pr = 0.515, p = 0.034). Pre- and post-PSG CRP were independently associated with the PLMS index (r = 0.699, p = 0.001 and r = 0.626, p = 0.005, respectively). The PLMS index was not associated with the number of RLS symptoms or RLS classification.
Table 3.
Comparison of laboratory values between children with sickle cell disease having normal and elevated periodic limb movements in sleep
| PLMS < 5/h | PLMS ≥ 5/h | |||
|---|---|---|---|---|
|
| ||||
| Laboratory values | Mean ± SD | Range | Mean ± SD | Range |
| Iron (Pre-PSG), mcg/dL | 92.3 ± 39.6 | 42 – 193 | 67.8 ± 12.0* | 53 – 90 |
| Iron (Post-PSG), mcg/dL | 123.3 ± 31.7 | 61 – 162 | 120.6 ± 38.2 | 72 – 173 |
| Delta-iron, mcg/dL | 29.2 ± 34.0 | −31 – 82 | 52.9 ± 33.9 | 15 – 98 |
| Ferritin (Pre-PSG), ng/dL | 71.7 ± 39.4 | 31.4 – 129.0 | 164.2 ± 87.7† | 31.9 – 301.0 |
| Ferritin (Post-PSG), ng/dL | 89.2 ± 47.8 | 35.4 – 163.0 | 216.4 ± 86.3† | 34.1 – 291.0 |
| Delta-Ferritin, ng/dL | 17.5 ± 16.1 | 0.8 – 49.3 | 52.2 ± 45.5 | −10.0 – 110.0 |
| CRP (Pre-PSG), mcg/mL | 1.72 ± 1.65 | 0.37 – 5.84 | 5.24 ± 2.81† | 2.15 – 10.30 |
| CRP (Post-PSG), mcg/mL | 2.39 ± 3.71 | 0.35 – 12.50 | 4.56 ± 2.43* | 2.01 – 8.99 |
| Delta- CRP, mcg/mL | 0.67 ± 2.15 | −0.40 – 6.66 | −0.68 ± 0.86 | −2.07 – 0.49 |
p < 0.05
p < 0.01.
Tests of group differences by t-test or Mann Whitney test. Delta indicates the change in value from pre- to post-polysomnography (PSG); CRP, high sensitivity C-reactive protein; PLMS, periodic limb movements in sleep.
DISCUSSION
Measurement of PLMS over several consecutive nights and serial measurement in high-risk individuals are needed to better quantify the risk factors for PLMS and their long-term consequences. We found poor agreement with PSG when PLMS measured by activity monitor were analyzed according to the manufacturer recommendation of scoring time in bed, and improved agreement when scored during sleep period time. Use of a modified threshold of ≥ 10/h for both methods improved identification of elevated PLMS by activity monitor. Activity monitors were well tolerated and used reliably, even by the youngest children. Finally, there were some significant differences in periodicity of PLMS between methods.
PLMS Agreement, Repeatability and Variability
The best correlation between PSG and activity monitor was found with the PLMS index measured on the dominant ankle, suggesting that a single unit worn on the dominant ankle would adequately capture PLMS with this device. Activity monitors overestimated PLMS compared to PSG as sleep efficiency decreased and wake after sleep onset increased, suggesting that leg movements during wake were erroneously scored as PLMS. When higher thresholds of ≥ 10 PLMS/h were used and time prior to sleep onset and after morning waking were excluded from analysis, classification of elevated PLMS improved. As the aim of this study was to find a convenient way to study PLMS in the home, we suggest that concurrent wrist actigraphy could be used in the home setting to determine sleep onset and offset. Validation of this method is recommended.
In comparison to our study, Sforza et al. [30] in a study of adults referred to a sleep clinic, found a mean difference between the PAM-RL and PSG of 6.2 ± 17.5, similar to our finding. Investigators also found that the difference between methods was significantly associated with wake time, and that a cut-point of 10 PLMS/h provided a reasonable balance between sensitivity and specificity. Durmer et al. [21] found that the PAM-RL could be used reliably over 5 days in children as young as 2 years of age, similar to our findings. They also found that the devices demonstrated good discriminant validity between groups based on RLS probability, suggesting that the PAM-RL correlated well with PSG.
Our validity estimates for activity monitor versus PSG-measured PLMS are comparable to other validation studies of activity monitors, which have agreement rates between actigraphy and PSG ranging from 72.1% to 96.5%, sensitivity from 86.5% to 98.7% and specificity from 27.7% to 67.1% [31]. Actigraphic estimation of sleep showed the highest stability coefficient over 14 days of measurement [31], a length of time we could not achieve in measuring PLMS due to inherent data storage limits of the PAM-RL. We did, however, find that repeatability across two nights of activity monitoring improved agreement between methods.
In comparing IMI, hour of sleep distribution and PLMS duration between methods, there were notable differences. A periodic peak in IMI at 20–35 s was evident by PSG, while the activity monitor demonstrated a brief peak at the shorter 10–15 s IMI but lacked the periodicity captured by PSG. Ferri and colleagues have reported a bimodal distribution of inter-leg movement intervals, peaking at < 10 s and again at 20–22 s in patients with RLS, where the second peak is suggested to represent the occurrence of true PLMS while the first peak probably represents another type of leg movement [32]. In children with RLS, the periodic peak has been found to be significantly reduced compared to adults, while the earlier peak in IMI has been demonstrated across several disorders [5,33,34].
Our findings confirmed that PLMS in children with SCD demonstrate periodicity, but also suggest that other nonperiodic leg movements, captured by shorter IMI, may at least partially confound identification of true PLMS by activity monitor despite the scoring algorithm. This conclusion is strengthened by several findings. First, increasing the threshold for elevated PLMS to 10/h for both PSG and activity monitor improved the specificity for detecting PLMS, suggesting that scoring rules for PLMS, which include a minimum period length between leg movements of 5 s [16], may be capturing and misclassifying leg movements that are not true PLMS. Second, in the earliest and latest periods of sleep, during which lighter sleep and associated greater movement may be more common, PLMS occurred more frequently by activity monitor than PSG, suggesting that nonperiodic leg movements are being scored as PLMS. Finally, PLMS duration was longer when measured by activity monitor than by PSG, suggesting activity monitors may detect muscle movement in addition to the TA, and incorporate this movement into their scoring of PLMS.
An additional explanation of differences between methods may lie in their method of measurement. Polysomnography measures electrical voltage generated by movement of the TA, while the activity monitor measures any movement of the lateral ankle which may be generated by a single or collective group of muscles that may or may not include the TA. It has been demonstrate in EMG studies of PLMS that only 53% of PLMS are initiated in the TA, and that the TA is involved in only about 75% of PLMS [35]. Therefore, duration of PLMS would be longer when measured from the initiation of any movement, as occurs with the activity monitor, compared to electrical activity initiated in the TA as measured by PSG. Likewise, PLMS might be recorded more frequently by activity monitor, resulting in more frequent PLMS and shorter mean IMI, if periodic movements not involving the TA were additionally accounted for. This effect might be more evident in children than in adults, as their smaller physical size brings the collective lower leg muscles in closer proximity to the activity monitor and might, consequently, enhance detection of muscle movements other than the TA.
Considerable night-to-night variability exists in PLMS [4,36]. In a study of children with attention deficit-hyperactivity disorder who underwent 2–4 nights of PSG, Picchietti et al. found that 60% of children who achieved a cutoff score ≥ 5 PLMS/h on one night had a PLMS index below the cutoff score on the second night [5]. In the present study, no child who underwent consecutive-night PSGs changed classification across 2 nights. However, across 4 nights of activity monitoring, children demonstrated large variability in PLMS, which increased as their mean PLMS index increased across 4 nights. These findings demonstrate the need to measure PLMS over as many nights as possible to capture their variability and better account for the consequences of PLMS when studying health outcomes.
Correlates of PLMS
The correlates of PLMS in children with SCD are not well explored. Low to low-normal values of iron and ferritin have been associated with PLMS in children [37,38]. In contrast, in our sample iron levels were unrelated to PLMS, and ferritin was associated such that subjects with elevated PLMS had higher, rather than lower ferritin levels than those with normal PLMS indexes even after controlling for inflammation with the CRP. These findings may reflect SCD-related pathology such as vaso-occlusion-related inflammation or treatments such as frequent transfusions [39,40]. Due to the small sample size, no conclusions can be drawn. Larger sample studies are needed to elucidate these relationships. Unexpectedly, no association was found between the PLMS index and RLS symptoms [8]. It is possible that responses to our RLS questions may have better described unrecognized SCD-related limb pain than RLS.
Limitations
Our findings should be interpreted in light of several limitations. This study was funded as a pilot, thus our sample size was small, and repeat PSGs were performed on only five children. We did not undertake an epoch-by-epoch comparison of PLMS between the activity monitor and PSG as we could not assure continued alignment of epochs between the two measures throughout the recording period. We therefore cannot confirm that PLMS measured by PSG are the same leg movements that were scored by the activity monitor. Epoch-by-epoch analysis would improve our understanding of PLMS measurement by activity monitoring. Finally, the effect of pathology and treatments specific to children with SCD may limit the generalizibility of these findings to other pediatric populations.
Conclusions
The PAM-RL demonstrated adequate validity in screening for elevated PLMS in children with SCD in comparison to PSG-measured PLMS, when scoring modifications were applied to the interpretation. These modifications included adjustment of the scoring period to incorporate only sleep onset to offset and application of a higher cut-point of 10 PLMS/h for identification of elevated PLMS. The devices were easily used at home, and if combined with wrist actigraphy to identify sleep period time could be useful for longitudinal investigation of PLMS in children with SCD.
However, in order for activity monitors to become a more reliable and valid measure of PLMS, technical improvements are required in activity monitoring technology. These changes should including simultaneous bilateral ankle activity monitoring and actigraphic measurement of sleep, with communication between devices so that wake time can be excluded from analysis and both legs can be accounted for simultaneously in scoring PLMS, in line with established PSG scoring criteria [16]. Extending recording capacity beyond the current 5 days would improve stability of PLMS estimation. Finally, development of a method to link PLMS with associated arousals could provide valuable data, given the finding that PLMS-related arousals may be more important clinically than the absolute PLMS index [1]. The list of negative health outcomes associated with PLMS is growing. There is a vital need for technological innovation that improves screening for and long-term follow-up of this phenomenon.
Acknowledgments
This research was funded by Clinical and Translational Science Award Research Center Junior Investigator Pilot Grant #UL1RR024134 and 2010 American Nurses’ Foundation/Sanofi-Pasteur grant, awarded to Valerie E. Rogers. We gratefully acknowledge James R. Rogers, PhD, for his assistance with PLMS time structure and time of night analyses, the sleep laboratory technicians at The Children’s Hospital of Philadelphia for their expert conduct of overnight sleep studies and ability to put our subjects at ease, and the children with SCD and their families who participated in our study with enthusiasm and good humor.
Contributor Information
Valerie E. Rogers, Email: verogers@son.umaryland.edu, University of Maryland, Department of Family and Community Health, 655 West Lombard Street, Room W215, Baltimore, MD 21201, Office: 410-706-8091, Fax: 410-706-0253.
Paul R. Gallagher, Clinical and Translational Research Center1.
Carole L. Marcus, Sleep Center1.
Kwaku Ohene-Frempong, Department of Hematology1.
Joel T. Traylor, Sleep Center1.
Thornton B. A. Mason, Sleep Center1.
References
- 1.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–97. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walters AS, Rye DB. Evidence continues to mount on the relationship of restless legs syndrome/periodic limb movements in sleep to hypertension, cardiovascular disease, and stroke. Sleep. 2010;33:287. doi: 10.1093/sleep/33.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picchietti DL, England SJ, Walters AS, Willis R, Verrico T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J Child Neurol. 1998;13:588–94. doi: 10.1177/088307389801301202. [DOI] [PubMed] [Google Scholar]
- 4.Hornyak M, Kopasz M, Feige B, Riemann D, Voderholzer U. Variability of periodic leg movements in various sleep disorders: implications for clinical and pathophysiologic studies. Sleep. 2005;28:331–5. [PubMed] [Google Scholar]
- 5.Picchietti MA, Picchietti DL, England SJ, Walters AS, Couvadelli BV, Lewin DS, et al. Children show individual night-to-night variability of periodic limb movements in sleep. Sleep. 2009;32:530–5. doi: 10.1093/sleep/32.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trotti LM, Bliwise DL, Greer SA, Sigurdsson AP, Gudmundsdottir GB, Wessel T, et al. Correlates of PLMs variability over multiple nights and impact upon RLS diagnosis. Sleep Med. 2009;10:668–71. doi: 10.1016/j.sleep.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Rogers VE, Lewin DS, Winnie GB, Geiger-Brown J. Polysomnographic characteristics of a referred sample of children with sickle cell disease. J Clin Sleep Med. 2010;6:374–81. [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers VE, Marcus CL, Jawad AF, Smith-Whitley K, Ohene-Frempong K, Bowdre C, et al. Periodic limb movements and disrupted sleep in children with sickle cell disease. Sleep. 2011;34:899–908. doi: 10.5665/SLEEP.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong FD, Thompson RJ, Jr, Wang W, Zimmerman R, Pegelow CH, Miller S, et al. Cognitive functioning and brain magnetic resonance imaging in children with sickle cell disease. Neuropsychology Committee of the Cooperative Study of Sickle Cell Disease Pediatr. 1996;97:864–70. [PubMed] [Google Scholar]
- 10.de Montalembert M, Maunoury C, Acar P, Brousse V, Sidi D, Lenoir G. Myocardial ischaemia in children with sickle cell disease. Arch Dis Child. 2004;89:359–62. doi: 10.1136/adc.2003.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogan AM, Pit-ten Cate IM, Vargha-Khadem F, Prengler M, Kirkham FJ. Physiological correlates of intellectual function in children with sickle cell disease: hypoxaemia, hyperaemia and brain infarction. Dev Sci. 2006;9:379–87. doi: 10.1111/j.1467-7687.2006.00503.x. [DOI] [PubMed] [Google Scholar]
- 12.Hijmans CT, Fijnvandraat K, Grootenhuis MA, van Geloven N, Heijboer H, Peters MJ, et al. Neurocognitive deficits in children with sickle cell disease: a comprehensive profile. Pediatr Blood Cancer. 2011;56:783–8. doi: 10.1002/pbc.22879. [DOI] [PubMed] [Google Scholar]
- 13.Hassell KL. Population estimates of sickle cell disease in the U. S Am J Prev Med. 2010;38:S512–21. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 15.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2:501–10. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 16.Iber C, Ancoli-Israel S, Chesson A, Quan SF American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Event: Rules, Terminology and Technical Specifications. 1. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 17.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatric Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 18.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Resp Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 19.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–8. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 20.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 21.Durmer JS, Bliwise DL, Rye DB. Ambulatory measurement of periodic leg movements in children with and without restless legs symptoms. Sleep. 2004;27:A113. [Google Scholar]
- 22.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68. [PubMed] [Google Scholar]
- 23.Lin Concordance calculator. [Accessed July 11, 2011];National Institute of Water and Atmospheric Research. 2011 at http://www.niwa.co.nz/our-services/online-services/statistical-calculators/lins-concordance.
- 24.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 25.Baldi P, Brunak S, Chauvin Y, Andersen CA, Nielsen H. Assessing the accuracy of prediction algorithms for classification: an overview. Bioinformatics. 2000;16:412–24. doi: 10.1093/bioinformatics/16.5.412. [DOI] [PubMed] [Google Scholar]
- 26.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–3. [PubMed] [Google Scholar]
- 27.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. The Statistician. 1983;32:307–17. [Google Scholar]
- 28.Karatas M. Restless legs syndrome and periodic limb movements during sleep: diagnosis and treatment. Neurolog. 2007;13:294–301. doi: 10.1097/NRL.0b013e3181422589. [DOI] [PubMed] [Google Scholar]
- 29.Hoque R, Chesson AL., Jr Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J Clin Sleep Med. 2010;6:79–83. [PMC free article] [PubMed] [Google Scholar]
- 30.Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study. Sleep Med. 2005;6:407–13. doi: 10.1016/j.sleep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Van de Water AT, Holmes A, Hurley DA. Objective measurements of sleep for non-laboratory settings as alternatives to polysomnography—a systematic review. J Sleep Res. 2011;20:183–200. doi: 10.1111/j.1365-2869.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 32.Ferri R, Zucconi M, Manconi M, Plazzi G, Bruni O, Ferini-Strambi L. New approaches to the study of periodic leg movements during sleep in restless legs syndrome. Sleep. 2006;29:759–69. [PubMed] [Google Scholar]
- 33.Ferri R, Manconi M, Lanuzza B, Cosentino FII, Bruni O, Ferini-Strambi L, et al. Age-related changes in periodic leg movements during sleep in patients with restless legs syndrome. Sleep Med. 2008;9:790–8. doi: 10.1016/j.sleep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Ferri R, Franceschini C, Zucconi M, Drago V, Manconi M, Vandi S, et al. Sleep polygraphic study of children and adolescents with narcolepsy/cataplexy. Dev Neuropsycol. 2009;34:523–38. doi: 10.1080/87565640903133699. [DOI] [PubMed] [Google Scholar]
- 35.Provini F, Vetrugno R, Meletti S, Plazzi G, Solieri L, Lugaresi E, et al. Motor pattern of periodic limb movements during sleep. Neurology. 2001;57:300–4. doi: 10.1212/wnl.57.2.300. [DOI] [PubMed] [Google Scholar]
- 36.Allen RP. Is one night enough for measurement of periodic limb movements in sleep of restless legs patients? Sleep. 2005;28:296–7. [PubMed] [Google Scholar]
- 37.Simakajornboon N, Gozal D, Vlasic V, Mack C, Sharon D, McGinley BM. Periodic limb movements in sleep and iron status in children. Sleep. 2003;26:735–8. doi: 10.1093/sleep/26.6.735. [DOI] [PubMed] [Google Scholar]
- 38.Bokkala S, Napalinga K, Pinninti N, Carvalho KS, Valencia I, Legido A, et al. Correlates of periodic limb movements of sleep in the pediatric population. Pediatr Neurol. 2008;39:33–9. doi: 10.1016/j.pediatrneurol.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 39.Inati A, Musallam KM, Wood JC, Taher AT. Iron overload indices rise linearly with transfusion rate in patients with sickle cell disease. Blood. 2010;115:2980–1. doi: 10.1182/blood-2009-09-243568. [DOI] [PubMed] [Google Scholar]
- 40.Ezeh C, Ugochukwu CC, Weinstein JIO. Hepcidin, haemoglobin and ferritin levels in sickle cell anaemia. Eur J Haematol. 2005;74:86–8. doi: 10.1111/j.1600-0609.2004.00337.x. [DOI] [PubMed] [Google Scholar]