Abstract
Objective
We compared the effectiveness of different highly active antiretroviral therapy (HAART) regimens considering, separately, history of injection drug use (IDU) (yes/no).
Design, methods
A total of 1163 HIV-infected patients initiated HAART between 1 January 2000 and 28 February 2009 in British Columbia, Canada, and were followed until 28 February 2010. HAART effectiveness was measured by the ability to achieve viral suppression below 50 copies/ml at 6 months. We compared HAART regimens containing efavirenz and boosted atazanavir. We developed logistic regression models using different techniques to control for potential confounders.
Results
Among the 1163 patients, 796 (68%) achieved viral suppression at 6 months (32% reporting a history of IDU). Different confounding models yielded very similar odds ratios for achieving viral suppression. Boosted atazanavir-based HAART demonstrated to be the most effective regimen, showing a surprisingly higher benefit for patients with a history of IDU (odds ratios from different models ranged from 1.74–1.95 to 1.45–1.51).
Conclusions
The literature has conflicting results regarding the effectiveness of HAART to treat HIV infection among those with a history of IDU. We have shown that most patients, with and without a history of IDU, were able to achieve viral suppression at 6 months. Boosted atazanavir-based HAART was the most resilient regimen and it was more effective than efavirenz-based HAART among IDUs. Given the limited inclusion of IDU in clinical trials of HAART’s efficacy, a randomized clinical trial comparing different first-line HAART regimens among IDU is warranted based on these results.
Keywords: antiretroviral therapy, boosted atazanavir, boosted protease inhibitor, efavirenz, effectiveness, injection drug use, NNRTI, population-based cohort, viral load, virologic response
The primary goal of highly active antiretroviral therapy (HAART) is to achieve full and long-term suppression of HIV-1 RNA plasma viral load (hereafter viral load) at all stages of HIV disease, even among those infected with multiple drug-resistant HIV [1,2]. As a result, HAART can predictably suppress viral replication, which in turn allows immune reconstitution to take place, and it prevents the emergence of resistance and AIDS-related morbidity and mortality [1-4].
The most commonly recommended first-line HAART regimen typically includes two nucleoside reverse transcriptase inhibitors (NRTIs), or a NRTI and a nucleotide reverse transcriptase inhibitor (NtRTI) and either a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a ritonavir-boosted protease inhibitor (boosted protease inhibitor) [1]. This approach is expected to lead to a full treatment response, namely sustained undetectable viral load and consequent CD4 cell count recovery leading to a remission of the disease, in the vast majority of patients [2]. However, incomplete response to therapy can occur, and the reasons for treatment failure are varied, with incomplete adherence being often a key determinant [2]. Consequently, healthcare providers often take into consideration a patient’s potential for non-adherence such as housing instability, consumption of opioids and alcohol, or the presence of comorbidities when selecting a HAART regimen in this setting [5-9]. Thus, a history of injection drug use (IDU) is a confounder by indication in nonrandomized studies assessing HAART’s effectiveness [9-11].
The literature to date has conflicting results regarding the effectiveness of HAART among IDUs [12-15]. To address this controversy, in this study, we compared the effectiveness of different HAART regimens considering, separately, individuals with and without a history of IDU. HAART effectiveness was measured by the likelihood of achieving viral suppression at 6 months. We hypothesized that there is no difference in clinical response among those with and without a history of IDU.
Methods
Study population
Study data from eligible participants were extracted from the British Columbia Centre for Excellence in HIV/AIDS Drug Treatment Program (DTP)’s monitoring and evaluation system to form the HAART Observational Medical Evaluation and Research (HOMER) cohort. HOMER is a population-based registry of all HIV-positive individuals first prescribed HAART in British Columbia [16]. Treatment-naïve individuals were enrolled between 1 January 2000 and 28 February 2009 and followed until the last available viral load before 28 February 2010. In addition, these individuals were required to have at least 6 months of follow-up time, to be 19 years or older, and to have started HAART consisting of two NRTIs or a NRTI and a NtRTI, and either a NNRTI: efavirenz (EFV), or a boosted protease inhibitor: atazanavir-boosted with a ritonavir dose of 400 mg/day or less (ATA/r).
For all patients in this study, viral loads were determined using the Roche Amplicor Monitor assay (Roche Diagnostics, Laval, Quebec, Canada) using the ultra-sensitive adaptation until 31 January 2008. On 1 February 2008, a new COBAS Ampliprep Taqman HIV-1 assay (Roche Diagnostics Systems, Inc., Laval, Quebec, Canada) replaced Roche’s Amplicor assay. Because of the change in viral load assays, the lower and upper limits of quantification of these assays changed from (50; 100 000) to (40; 1 000 000) copies/ml, and therefore, viral load measurements were re-coded to range from 50 to 100 000 copies/ml to minimize the measurement bias that would have been introduced if the data were left unchanged. Plasma samples were stored chronologically as they were drawn and securely stored (frozen at −20°C) for future use. CD4 cell counts were measured by flow cytometry, followed by fluorescent monoclonal antibody analysis (Beckman Coulter, Inc., Mississauga, Ontario, Canada).
Viral load suppression was defined as two consecutive viral load measurements below 50 copies/ml (coded as yes vs. no). Patients were then classified as being suppressed at 6 months if the date of viral load suppression was 6 months or less and not suppressed, otherwise. It is important to mention that we gave a 3-month grace period to obtain the viral load measurements to make sure that patients who have started HAART with very high viral loads are not penalized, and to accommodate delays in measuring their viral load for various reasons.
Epidemiological analysis
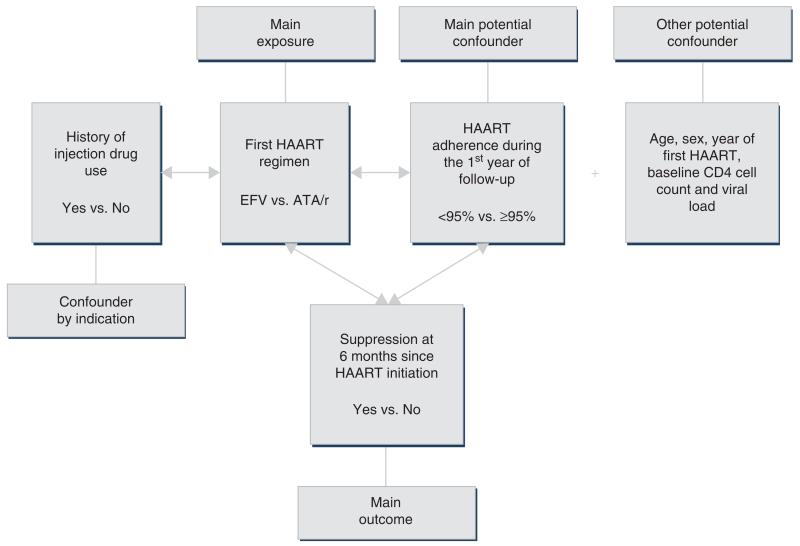
The theoretical model being considered is described in Fig. 1. In this model, having a history of IDU was considered a confounder by indication, and therefore, we controlled for this by initially stratifying the data by history of IDU.
Fig. 1. Theoretical epidemiological model being tested.
ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; EFV, efavirenz.
First HAART regimen containing EFVor ATA/r was the main exposure in this analysis. Acknowledging that treatment changes over time, we decided to use an ‘intention-to-treat’ approach due to the complexity in the data and in the interpretation of results. Adherence measured during the first year was the primary confounder. The choice for this adherence, instead of only measuring it during the first 6 months, relied on the fact that HAART regimens in our program are given to patients at 1, 2 or 3-month intervals depending on how well they are coping with their treatment. Therefore, to avoid patients being penalized for their prescription refill scheduling, we decided, a priori, to incorporate adherence measured over a full year into our analyses. The rate of adherence, or refill compliance [2,3], was defined as the number of days of antiretroviral drugs dispensed divided by the number of days of follow-up (expressed as a percentage). This approach has been found to be independently associated with short and long-term outcomes [2,15,17]. The other potential confounders measured at baseline included age, sex, CD4 cell count, viral load (log10 transformed), and year of first HAART (2000–2004, 2005–2006, and 2007–2009).
Statistical analyses
In bivariable analyses, categorical variables were compared using the Fisher’s exact test and continuous variables were compared using the Wilcoxon rank-sum test. In multivariable analysis, we have built logistic regression models using different methodologies to control for confounding [18-27]. Among these methodologies, we included a confounder selection technique published by our group based on the work by Maldonado and Greenland [20,21], and methods based on propensity scores [22-25] and on the inverse-probability-of-treatment-weighted (IPTW) estimator [26,27]. For means of comparison, we have also included the results for the univariable and multivariable logistic regression models without any control for confounding. Please refer to the appendix for a more detailed description of each of these methods (http://links.lww.com/QAD/A221). Note that for these analyses all reported P values were two-sided, significance level α was set at 0.05, and they were performed using SAS software (version 9.2; SAS Institute Inc., Cary, North Carolina, USA).
Results
Description of study population
A total of 1163 patients started HAART between 1 January 2000 and 28 February 2009 and were followed until the date of the last viral load before 28 February 2010. Table 1a shows the baseline characteristics stratified by history of IDU, and in this retrospective cohort, those with a history of IDU (N = 433; 37%) were more likely to be female (60%; P < 0.0001), to be less than 95% adherent during the first year on HAART (56%; P < 0.0001), to have started HAART between 2000 and 2004 (46%; P < 0.0001), and to have a lower baseline CD4 cell count (170 vs. 200 cells/μl; 25th–75th percentile: 110–230 vs. 120–280 cells/μl; P < 0.0001).
Table 1. Patient characteristics stratified by (a) history of injection drug use and (b) first HAART regimen.
| (a) History of injection drug use Factors |
History of injection drug use |
||
|---|---|---|---|
| No (N = 730) | Yes (N = 433) | P value | |
| Sex | |||
| Male | 650 (67%) | 315 (33%) | <0.0001 |
| Female | 80 (40%) | 118 (60%) | |
| HAART regimen | |||
| EFV-based | 391 (67%) | 223 (36%) | 0.5045 |
| ATA/r-based | 339 (62%) | 210 (38%) | |
| First year adherence to HAART | |||
| >95% | 604 (69%) | 274 (31%) | <0.0001 |
| <95% | 126 (44%) | 159 (56%) | |
| First year of HAART | |||
| 2000–2004 | 109 (54%) | 93 (46%) | <0.0001 |
| 2005–2006 | 220 (59%) | 151 (41%) | |
| 2007–2009 | 401 (68%) | 189 (32%) | |
| Age (years) | 43 (37–51) | 42 (36–49) | 0.0646 |
| Baseline CD4 cell count (cells/μl) | 200 (120–280) | 170 (110–230) | <0.0001 |
| Baseline viral load (log10 copies/ml) | 4.9 (4.5–5.0) | 4.9 (4.5–5.0) | 0.9446 |
| (b) First HAART regimen Factors |
No history of injection drug use | History of injection drug use | |||
|---|---|---|---|---|---|
| HAART regimen |
|||||
| ATA/r-based (N = 391) | EFV-based (N = 339) | ATA/r-based (N = 223) | EFV-based (N = 210) | P value | |
| Sex | |||||
| Male | 345 (36%) | 305 (32%) | 176 (18%) | 139 (14%) | <0.0001 |
| Female | 46 (23%) | 34 (17%) | 47 (24%) | 71 (36%) | |
| First year adherence to HAART | |||||
| >95% | 322 (37%) | 282 (32%) | 152 (17%) | 122 (14%) | <0.0001 |
| <95% | 69 (24%) | 57 (20%) | 71 (25%) | 88 (31%) | |
| First year of HAART | |||||
| 2000–2004 | 46 (23%) | 63 (31%) | 34 (17%) | 59 (29%) | <0.0001 |
| 2005–2006 | 160 (43%) | 60 (16%) | 90 (24%) | 61 (16%) | |
| 2007–2009 | 185 (31%) | 216 (37%) | 99 (17%) | 90 (15%) | |
| Age (years) | 43 (36–51) | 43 (36–51) | 42 (36–49) | 43 (37–49) | 0.2890 |
| Baseline CD4 cell count (cells/μl) | 190 (90–260) | 230 (150–290) | 170 (110–230) | 170 (110–230) | <0.0001 |
| Baseline viral load (log10 copies/ml) | 5.0 (4.6–5.0) | 4.9 (4.4–5.0) | 5.0 (4.5–5.0) | 4.9 (4.4–5.0) | 0.0121 |
ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; EFV, efavirenz.
At the time of first HAART, 549 (47%) patients were prescribed an EFV-based HAART and 614 (53%) an ATA/r-based HAART (Table 1b). Those on an EFV-based HAARTwere more likely to have started HAART between 2000 and 2004 (60%; P < 0.0001), to have a higher baseline CD4 cell count if these patients had no history of IDU (230 vs. 190 cells/μl; 25th–75th percentile: 150–290 vs. 90–260 cells/μl; history of IDU – same values for both regimen groups: 170; 25th–75th percentile: 110–230 cells/μl; P < 0.0001), and a lower baseline viral load (no history of IDU: 4.9 vs. 5.0 log10 copies/ml; 25th–75th percentile: 4.4–5.0 vs. 4.6–5.0 log10 copies/ml; history of IDU: 4.9 vs. 5.0 log10 copies/ml; 25th–75th percentile: 4.4–5.0 vs. 4.5–5.0 log10 copies/ml; P = 0.0121).
Results from models without confounding adjustment
In total, 796 (68%) patients achieved viral suppression at 6 months, with 542 (68%) reporting no history of IDU and 254 (32%) reporting having such a history. Once the data were stratified by history of IDU, the univariable (crude model) and the multivariable models estimated an odds ratio (OR) of experiencing viral suppression at 6 months for individuals firstly given an ATA/r-based HAART vs. an EFV-based HAART, respectively, of 1.66 [95% confidence interval (CI) 1.18–2.33] and 1.51 (95% CI 1.04–2.18) for those without a history of IDU, and 1.51 (95% CI 1.03–2.23) and 1.95 (95% CI 1.25–3.04) for those with a history of IDU (Table 2).
Table 2. Comparison of the estimates for the effect of first HAART regimen and history of injection drug use on the probability of achieving viral suppression at 6 months according to different methods to control for confounding.
| Odds ratio (95% confidence interval) |
||
|---|---|---|
| No history of injection drug use | History of injection drug use | |
| Crude model | ||
| First HAART regimen (ATA/r vs. EFV) | 1.66 (1.18–2.33) | 1.51 (1.03–2.23) |
| Different modeling techniques | ||
| First HAART regimen (ATA/r vs. EFV) | ||
| Multivariable model | 1.51 (1.04–2.18) | 1.95 (1.25–3.04) |
| Confounder model | 1.51 (1.05–2.17) | 1.95 (1.27–2.98) |
| Regression adjusted using propensity score | 1.45 (1.01 –2.07) | 1.76 (1.17–2.63) |
| Regression adjusted using quintiles of the propensity score | 1.43 (1.00–2.04) | 1.77 (1.18–2.66) |
| Regression adjusted using IPTW | 1.46 (1.15–1.86) | 1.74 (1.32–2.28) |
ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; EFV, efavirenz; IPTW, inverse probability of treatment weighted.
Understanding the propensity of receiving ATA/r or EFV by history of injection drug use
Table 3a shows the breakdown of patient’s characteristics by viral load suppression at 6 months and history of IDU. Men, those who started HAART on an EFV-based HAART, with higher baseline CD4 cell count, lower baseline viral load and who were at least 95% adherent within the first year were more likely to achieve suppression. Note that for patients without a history of IDU, starting HAART in 2007–2009 was associated with higher chances of achieving viral suppression at 6 months. The period of higher chance of achieving viral suppression at 6 months for those with a history of IDU was 2000–2004. Table 3b shows which of these factors were most influential in achieving viral suppression at 6 months. We fitted different univariable logistic regression and obtained the area under the receiver operating curve for each factor. We observed that the most influential factors were baseline viral load and CD4 cell count and first year adherence, for those with and without a history of IDU. The remaining factors had very similar influence on the main outcome.
Table 3. Patient characteristics (a) and their influence (b) in explaining the achievement of suppression at 6 months (through separate univariable logistic regressions).
| (a) | |||||
|---|---|---|---|---|---|
| No history of injection drug use |
History of injection drug use |
||||
| Achieved suppression at 6 months since HAART initiation | P value | ||||
|
|
|||||
| Factors | No (N = 188) | Yes (N = 542) | No (N = 179) | Yes (N = 254) | |
| Sex | |||||
| Male | 163 (17%) | 487 (51%) | 129 (13%) | 186 (19%) | <0.0001 |
| Female | 25 (13%) | 55 (28%) | 50 (25%) | 68 (34%) | |
| HAART regimen | |||||
| EFV-based | 70 (13%) | 269 (49%) | 76 (14%) | 134 (24%) | 0.0035 |
| ATA/r-based | 118 (19%) | 273 (44%) | 103 (17%) | 120 (20%) | |
| First year adherence to HAART | |||||
| >95% | 134 (15%) | 470 (54%) | 77 (9%) | 197 (22%) | <0.0001 |
| <95% | 54 (19%) | 72 (25%) | 102 (36%) | 57 (20%) | |
| First year of HAART | |||||
| 2000–2004 | 26 (13%) | 83 (41%) | 39 (19%) | 54 (27%) | 0.0022 |
| 2005–2006 | 58 (16%) | 162 (44%) | 65 (17%) | 86 (23%) | |
| 2007–2009 | 104 (18%) | 297 (50%) | 75 (13%) | 114 (19%) | |
| Age (years) | 44 (38–52) | 43 (36–51) | 42 (35–48) | 43 (37–49) | 0.0805 |
| Baseline CD4 cell count (cells/μl) | 180 (80–260) | 215 (130–280) | 150 (90–220) | 185 (120–240) | <0.0001 |
| Baseline viral load (log10 copies/ml) | 5.0 (4.9–5.0) | 4.9 (4.4–5.0) | 5.0 (4.7–5.0) | 4.9 (4.3–5.0) | <0.0001 |
| (b) | |||
|---|---|---|---|
| Influence of each Factor on the achievement of suppression at 6 months since HAART initiation – AUC (rank) |
|||
| Factors | Overall | No history of IDU | History of IDU |
| History of injection drug use | 0.584 (4) | NA | NA |
| Sex | 0.525 (6) | 0.516 (6) | 0.506 (7) |
| HAART regimen | 0.554 (5) | 0.562 (4) | 0.551 (4) |
| First year adherence to HAART | 0.632 (1) | 0.577 (3) | 0.673 (1) |
| First year of HAART | 0.516 (7) | 0.509 (7) | 0.517 (6) |
| Age (years) | 0.504 (8) | 0.534 (5) | 0.537 (5) |
| Baseline CD4 cell count (cells/μl) | 0.592 (3) | 0.585 (2) | 0.583 (3) |
| Baseline viral load (log10 copies/ml) | 0.627 (2) | 0.662 (1) | 0.592 (2) |
ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; AUC, area under the receiver operating curve; EFV, efavirenz.
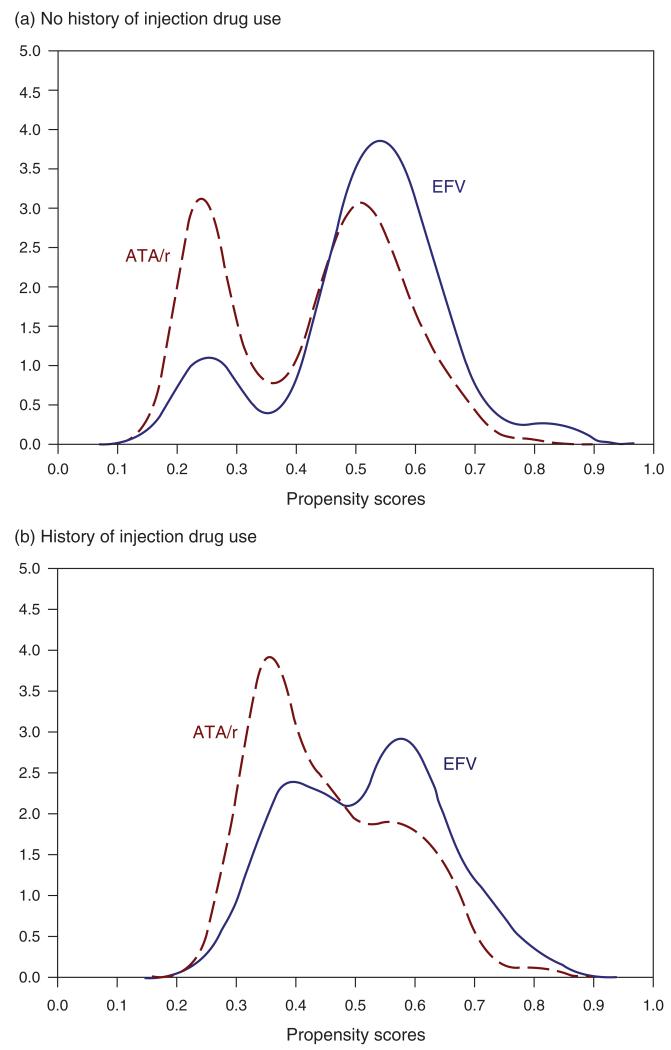
The logistic regression model used to obtain the propensity scores estimated that, for those without a history of IDU, the probability that patients would have received ATA/r-based HAART based on their observed baseline variables was 0.4224 (i.e. overall mean propensity score) and 0.5128 for those who received EFV-based HAART. For those with a history of IDU, the estimated overall mean propensity scores for receiving ATA/r and EFV were, respectively, 0.4530 and 0.5189.
Figure 2 shows the probability density functions of the distribution of propensity scores for those with and without a history of IDU. The overlap in both curves is quite significant, and both distributions are multimodal. The distribution of propensity scores for patients without a history of IDU, based on their baseline age, sex, CD4 cell count, viral load, adherence, and year of first HAART, showed that these patients had high probabilities of being assigned either EFV or ATA/r in their first HAART, and those with a history of IDU, based on the same set of baseline variables, were more likely to have been given a EFV-based HAART as their first HAART regimen.
Fig. 2. Estimated probability density function of the propensity scores for EFV-based HAART vs. ATA/r-based HAART, stratified by history of injection drug use.
(a) No history of injection drug use; (b) history of injection drug use. ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; EFV, efavirenz; X-axis, propensity scores; Y-axis, density distribution.
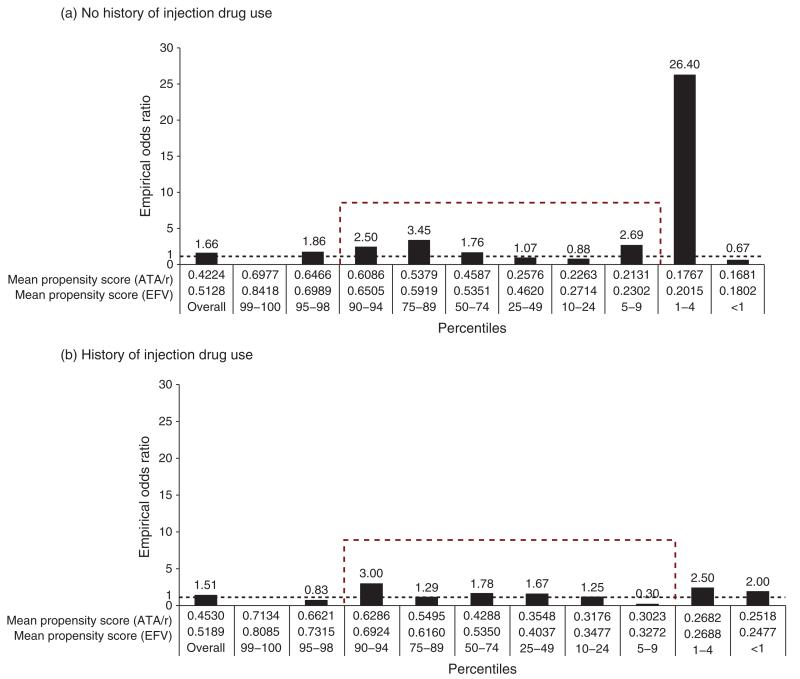
Figure 3 presents the distribution of the empirical OR of achieving suppression at 6 months according to the percentiles of the propensity score by history of IDU and third drug in the first HAART regimen (ATA/r or EFV). By removing the propensity scores in the tail of the distribution (<5% and ≥95% percentiles), there was no clear trend on the distribution of the empirical ORs for those without a history of IDU across the percentiles of the propensity scores. However, for those with a history of IDU, the probability of achieving suppression at 6 months increased as the propensity score comparing those first prescribed ATA/r vs. EFV (reference category) increased. In this case, the empirical OR increased from 0.30 (5th–9th percentile) to 3.00 (90th–94th percentile).
Fig. 3. Distribution of the probability of achieving viral suppression at 6 months for those patients who started on an EFV-based HAART or on an ATA/r-based HAART according to the percentiles of the propensity score for the entire study population stratified by history of injection drug use.
ATA/r, atazanavir boosted with 400 mg/day or less of ritonavir; EFV, efavirenz.
Results from different confounder models
We compared the ORs according to the different methods of controlling for confounding by history of IDU (Table 2). The four methods resulted in very similar ORs for achieving viral suppression, with those initiating on an ATA/r-based HAART having higher odds than those first given an EFV-based HAART, with a higher benefit for those with a history of IDU. For patients without a history of IDU, the ORs ranged from 1.43 (method: regression adjusted using quintiles of the propensity scores; 95% CI 1.00–2.04) to 1.51 (method: confounder model; 95% CI 1.05–2.17), and for those with a history of IDU it ranged from 1.74 (method: regression adjusted using IPTW; 95% CI 1.32–2.28) to 1.95 (method: confounder model; 95% CI 1.27–2.98). Note that the confounder model and the multivariable model yield almost identical ORs, probably due to the significant overlap in the density distributions. By removing the propensity scores in the tail of the distribution (<5% and ≥95% percentiles) for both group of patients, the estimated ORs by the different methods (univariable and multivariable models) became more similar and those starting on ATA/r-based HAART still had higher OR of being suppressed at 6 months than those initially given an EFV-based HAART, regardless of history of IDU.
Discussion
Our results show a higher effectiveness of ATA/r over EFV-based HAART in achieving short-term viral suppression, among those with and without a history of IDU. EFV-based HAART has been for sometime the simplest of the HAART regimens available, as it requires fewer pills and it can be taken once a day without dietary restrictions. Some argue that these regimens are better suited to individuals who may have substantial adherence challenges (i.e. homelessness, addiction, mental illness, etc.). However, our results suggest that this notion may need to be re-evaluated in a clinical trial among IDUs, as we found that EFV-based HAART was associated with inferior short-term virologic outcomes, in part due to inferior adherence by these patients, regardless of history of IDU. It should also be mentioned that patients starting on ATA/r-based HAARTwere in general sicker, but had similar demographics as the ones starting on an EFV-based HAART.
Note that the comparison of the efficacy of boosted protease inhibitors and NNRTIs has been done in previous randomized clinical trials. There were several trials comparing lopinavir/r vs. EFV, very few comparing ATA/r and EFV, and some recent ones comparing newer boosted protease inhibitors and EFV [28-33]. The results are quite puzzling since several of these trials were inconclusive (i.e. regimens were equivalent, noninferior, or nonsuperior). In other trials, which showed EFV to be superior to boosted protease inhibitors, they used as boosted protease inhibitor lopinavir/r instead of ATA/r. Thus, we believe that although our results were based on observational data and caution in the interpretation of results is warranted, these results should not be dismissed and yet further investigated as a valuable first-line option for populations with potential adherence issues.
Several factors might explain the results in this study, such as drug tolerability, potency, side-effects and choice of backbone (given the large number of drug combinations), which were not controlled for in these analyses, and therefore could have potentially introduced residual bias in our results. A further limitation is that, in this study, we used history of IDU instead of current use, and as such, we were not able to assess the impact of ongoing drug use on viral suppression. Note that there were other confounders not available to us that should have been controlled for, giving room to the possibility of residual confounding, which is a reality in any observational study and is an important limitation when medications are being compared. Finally, several adherence studies have shown that women tend to be more adherent than men in taking HAART. However, in British Columbia, the HIV epidemic among women has been concentrated among IDUs and the epidemic among men has been concentrated among men who have sex with men. Thus in our study, as expected, women were more likely to have a history of IDU, and those with such a history were more likely to be less than 95% adherent.
We, however, believe that this study also has important advantages. Clinically, patients were closely followed by physicians and their medications and laboratory monitoring were provided free of charge, thus enabling us to eliminate some bias if patients were required to pay for their treatment. We were able to control for adherence, and patients did not exhibit drug resistance at baseline to either regimen, thus also eliminating these factors as possible explanation for our results. Also, our sample size was large and given that we have based our analysis on data from a registry of patients, our results reflect and represent British Columbia’s patient population demographics, and they can be generalized to other settings. Statistically, it was interesting to observe the consistency in the results produced by different confounder-adjustment techniques. This study demonstrated that regardless of how noble a particular methodology is, if the theoretical framework was not properly thought of or incorrectly modeled, we would most likely draw incorrect conclusions in our study. Once we identified the different sources of confounding, this study has shown that all individuals had a higher likelihood of achieving viral suppression at 6 months if initially given ATA/r-based HAART. Note that the confounder model selection previously published by our group seems to overestimate the OR and produce results very similar to the multivariable model, whereas the remaining models based on propensity scores or IPTW produced very similar results.
Failure to achieve short-term suppression of viral load has negative patient and public health implications. From a patient standpoint, failing at 6 months on an EFV-based regimen maybe associated with a lower likelihood of suppression at a later disease stage, given the increased risk of emergence of resistance [1]. The higher barrier to resistance posed by the boosted protease inhibitor regimens, such as ATA/r, offers an advantage in a setting in which less than perfect adherence may be more frequent. Given the increased recognition of the added secondary preventive value of HAART in decreasing the rate of HIV transmission, these findings are also of potential relevance to the roll out of ‘Treatment as Prevention’ strategies, particularly in settings in which viral monitoring is not readily available and continued drug supply may be unreliable [34].
In summary, this observational study has shown that individuals, regardless of having or not a history of IDU, had better virologic outcomes if initiated on an ATA/r-based HAART than those who initiated on EFV. Given that suppression at 6 months is a crucial outcome for individuals on antiretroviral therapy, and it has been shown to have important consequences to the availability of future treatment options, closer monitoring of patients is vital to maximize short-term viral suppression, especially in settings in which most individuals are on EFV-based HAART.
Acknowledgements
We thank the staff from the British Columbia Centre for Excellence in HIV/AIDS, especially Benita Yip, for their assistance and commitment to maintain a state of the art database and, our patients for participating in our study.
Funding: This work was supported by the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research through Fellowship Awards to Dr Lima.
Role of the sponsors: The funding sources had no role in the choice of methods, the contents or form of this work, or the decision to submit the results for publication.
Footnotes
Conflicts of interest
J.S.G.M. has received funding from Merck, Gilead, and ViiV Healthcare to support research into Treatment as Prevention.
Author contributions: Study concept and design: V.D.L.; acquisition of data: V.D.L.; analysis and interpretation of data: V.D.L.; drafting of the manuscript: V.D.L.; critical revision of the manuscript for important intellectual content: V.D.L., B.N., E.W., T.K., W.Z., K.C., J.S.G.M.; statistical analysis: V.D.L.; obtained funding: V.D.L.; administrative, technical, or material support: J.S.G.M.; study supervision: J.S.G.M.
Ethical approval: The Centre’s HIV/AIDS Drug Treatment program has received ethical approval from the University of British Columbia Ethics Review Committee at its St. Paul’s Hospital site. The program also conforms with the province’s Freedom of Information and Protection of Privacy Act.
References
- 1.Hammer SM, Eron JJ, Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. International AIDS Society-USA. Antiretro-viral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 2.Hull MW, Lima VD, Hogg RS, Harrigan PR, Montaner JS. Epidemiology of treatment failure: a focus on recent trends. Curr Opin HIV AIDS. 2009;4:467–473. doi: 10.1097/COH.0b013e328331d353. [DOI] [PubMed] [Google Scholar]
- 3.Lima VD, Hogg RS, Harrigan PR, Moore D, Yip B, Wood E, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 4.Lima VD, Hogg RS, Montaner JS. Expanding HAART treatment to all currently eligible individuals under the 2008 IAS-USA Guidelines in British Columbia, Canada. PLoS One. 2010;5:e10991. doi: 10.1371/journal.pone.0010991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood E, Kerr T, Tyndall MW, Montaner JS. A review of barriers and facilitators of HIV treatment among injection drug users. AIDS. 2008;22:1247–1256. doi: 10.1097/QAD.0b013e3282fbd1ed. [DOI] [PubMed] [Google Scholar]
- 6.Krüsi A, Wood E, Montaner J, Kerr T. Social and structural determinants of HAART access and adherence among injection drug users. Int J Drug Policy. 2010;21:4–9. doi: 10.1016/j.drugpo.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Ding L, Landon BE, Wilson IB, Wong MD, Shapiro MF, Cleary PD. Predictors and consequences of negative physician attitudes toward HIV-infected injection drug users. Arch Intern Med. 2005;165:618–623. doi: 10.1001/archinte.165.6.618. [DOI] [PubMed] [Google Scholar]
- 8.Cescon AM, Cooper C, Chan K, Palmer AK, Klein MB, Machouf N, et al. Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multisite Canadian cohort. HIV Med. 2011;12:352–360. doi: 10.1111/j.1468-1293.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 9.McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, Rebeiro PF, et al. Drug use and receipt of highly active antiretroviral therapy among HIV-infected persons in two U.S. clinic cohorts. PLoS One. 2011;6:e18462. doi: 10.1371/journal.pone.0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walker AM. Confounding by indication. Epidemiology. 1996;7:335–336. [PubMed] [Google Scholar]
- 11.Garcia de la Hera M, Davo MC, Ballester-Añón R, Vioque J. The opinions of injecting drug user (IDUs) HIV patients and health professionals on access to antiretroviral treatment and health services in Valencia, Spain. Eval Health Prof. 2011;34:349–361. doi: 10.1177/0163278711401743. [DOI] [PubMed] [Google Scholar]
- 12.Werb D, Mills EJ, Montaner JS, Wood E. Risk of resistance to highly active antiretroviral therapy among HIV-positive injecting drug users: a meta-analysis. Lancet Infect Dis. 2010;10:464–469. doi: 10.1016/S1473-3099(10)70097-9. [DOI] [PubMed] [Google Scholar]
- 13.Lert F, Kazatchkine MD. Antiretroviral HIV treatment and care for injecting drug users: an evidence-based overview. Int J Drug Policy. 2007;18:255–261. doi: 10.1016/j.drugpo.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Strathdee SA. 12 myths about HIV/AIDS and people who use drugs. Lancet. 2010;376:208–211. doi: 10.1016/S0140-6736(10)61005-7. [DOI] [PubMed] [Google Scholar]
- 15.Malta M, Strathdee SA, Magnanini MM, Bastos FI. Adherence to antiretroviral therapy for human immunodeficiency virus/acquired immune deficiency syndrome among drug users: a systematic review. Addiction. 2008;103:1242–1257. doi: 10.1111/j.1360-0443.2008.02269.x. [DOI] [PubMed] [Google Scholar]
- 16.British Columbia Centre for Excellence in HIV/AIDS [Accessed September 28, 2011];The HAART Observational Medical Evaluation and Research (HOMER) study website. Available at: http://www.cfenet.ubc.ca/our-work/initiatives/homer.
- 17.Lima VD, Bangsberg DR, Harrigan PR, Deeks SG, Yip B, Hogg RS, et al. Risk of viral failure declines with duration of suppression on highly active antiretroviral therapy irrespective of adherence level. J Acquir Immune Defic Syndr. 2010;55:460–465. doi: 10.1097/QAI.0b013e3181f2ac87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. doi: 10.1093/aje/kwj047. [DOI] [PubMed] [Google Scholar]
- 19.Cook EF, Goldman L. Asymmetric stratification. An outline for an efficient method for controlling confounding in cohort studies. Am J Epidemiol. 1988;127:626–639. doi: 10.1093/oxfordjournals.aje.a114838. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 21.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS. 2007;21:1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 22.Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–145. doi: 10.1146/annurev.publhealth.21.1.121. [DOI] [PubMed] [Google Scholar]
- 23.Mitra N, Heitjan DF. Sensitivity of the hazard ratio to nonignorable treatment assignment in an observational study. Stat Med. 2000;26:1398–1414. doi: 10.1002/sim.2606. [DOI] [PubMed] [Google Scholar]
- 24.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–287. doi: 10.1093/aje/kwg115. [DOI] [PubMed] [Google Scholar]
- 25.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 26.Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 Suppl 2):S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 27.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. AIDS Clinical Trials Group Study A5142 Team Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazzard B, Duvivier C, Zagler C, Castagna A, Hill A, van Delft Y, et al. Phase 2 double-blind, randomized trial of etravirine versus efavirenz in treatment-naive patients: 48-week results. AIDS. 2011;25:2249–2258. doi: 10.1097/QAD.0b013e32834c4c06. [DOI] [PubMed] [Google Scholar]
- 30.Daar ES, Tierney C, Fischl MA, Sax PE, Mollan K, Budhathoki C, et al. AIDS Clinical Trials Group Study A5202 Team Atazanavir plus ritonavir or efavirenz as part of a 3-drug regimen for initial treatment of HIV-1. Ann Intern Med. 2011;154:445–456. doi: 10.1059/0003-4819-154-7-201104050-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda M, Ishisaka M, Ishizuka N, Kimura S, Oka S, Japanese Anti-HIV-1 QD Therapy Study Group Open-label randomized multicenter selection study of once daily antiretroviral treatment regimen comparing ritonavir-boosted atazanavir to efavirenz with fixed-dose abacavir and lamivudine. Intern Med. 2011;50:699–705. doi: 10.2169/internalmedicine.50.4572. [DOI] [PubMed] [Google Scholar]
- 32.Sierra-Madero J, Villasis-Keever A, Méndez P, Mosqueda-Gómez JL, Torres-Escobar I, Gutiérrez-Escolano F, et al. Prospective, randomized, open label trial of Efavirenz vs. Lopinavir/Ritonavir in HIV+ treatment-naive subjects with CD4+<200 cell/mm3 in Mexico. J Acquir Immune Defic Syndr. 2010;53:582–588. doi: 10.1097/QAI.0b013e3181cae4a1. [DOI] [PubMed] [Google Scholar]
- 33.Domingo P, Suárez-Lozano I, Torres F, Teira R, Lopez-Aldeguer J, Vidal F, et al. First-line antiretroviral therapy with efavirenz or lopinavir/ritonavir plus two nucleoside analogues: the SUSKA study, a nonrandomized comparison from the VACH cohort. J Antimicrob Chemother. 2008;61:1348–1358. doi: 10.1093/jac/dkn121. [DOI] [PubMed] [Google Scholar]
- 34.Hirnschall G, Schwartländer B. Treatment 2.0: catalysing the next phase of scale-up. Lancet. 2011;378:209–211. doi: 10.1016/S0140-6736(11)60247-X. [DOI] [PubMed] [Google Scholar]