Abstract
A hallmark of the antigen-specific B and T lymphocytes of the adaptive immune system is their capacity to “remember” pathogens long after they are first encountered, a property that forms the basis for effective vaccine development. However, studies in mice have provided strong evidence that some naive T cells can develop characteristics of memory T cells in the absence of foreign antigen encounters. Such innate memory T cells may develop in response to lymphopenia or the presence of high levels of the cytokine IL-4, and have also been identified in unmanipulated animals, a phenomenal referred to as “virtual memory.” While the presence of innate memory T cells in mice is now widely accepted, their presence in humans has not yet been fully validated. In this issue of the European Journal of Immunology, Jacomet et al. [Eur. J. Immunol. 2015. 45:XXX-XXX] provide the best evidence to date for innate memory T cells in humans. These findings may contribute significantly to our understanding of human immunity to microbial pathogens and tumors.
Keywords: CD8+ T cells, human, memory T cells, immunological memory, innate-like T cells
Immunologists have traditionally divided the immune system into innate and adaptive branches. Innate immune cells express conserved receptors that bind molecular patterns contained within a variety of microorganisms, whereas adaptive immune cells express diverse antigen receptors that bind with pathogen-specific antigens. A critical component of the adaptive immune system is its capacity to remember prior encounters with the same antigen, a property referred to as immunological memory, which forms the basis for the efficacy of vaccines. However, some cells don’t fit neatly within the typical innate or adaptive immune cell designations. For example, natural killer (NK) cells, which are considered part of the innate immune system, can develop long-lived and highly specific memory to hapten-based contact antigens and diverse viral antigens (reviewed in [1]). Conversely, several subsets of innate-like B and T lymphocytes have been identified that express antigen-specific receptors, yet exhibit many characteristics of innate immune cells such as a rapid response to antigenic stimulation and the absence of a recall response to cognate antigen (reviewed in [2]). Natural killer T (NKT) cells, a subset of T cells that recognize glycolipid antigens bound with the MHC class I-related protein CD1d (reviewed in [3]), belong to this family of innate B and T lymphocytes, which has also been referred to as “inbetweeners” (reviewed in [4]). Yet another variation on this theme is that some naïve antigen-specific T cells, especially CD8+ T cells, can acquire the characteristics and functions of memory T cells in the absence of previous encounters with a foreign antigen (for excellent reviews on this topic, see [5, 6]). Whereas such “innate memory” T cells can be induced in response to alterations in the environment, they have also been identified in mice under steady-state conditions, in which case they have been dubbed “virtual memory” T cells [6–8]. Although the concept of innate memory T cells is now well established in mice, whether an equivalent T-cell population exists in humans remains to be fully validated. The paper by Jacomet et al. [9] in this issue of the European Journal of Immunology provides the best evidence yet for the existence of innate memory T cells, including virtual memory T cells, in humans.
Two general pathways for the generation of innate memory T cells in the absence of a foreign antigen have been identified in mice. Adoptive transfer studies of naïve donor T cells into lymphopenic recipient animals demonstrated that the transferred cells not only proliferated in the absence of antigen stimulation but also adopted surface markers such as CD45RO, CD44, and CD122 (IL-2Rβ), and functions such as rapid IFN-γ secretion, characteristic of memory T cells [6, 10, 11]. While some of these T cells recognized commensal microorganisms and therefore are not innate memory T cells but “conventional” or “true” memory T cells (Figure 1), others acquired this phenotype even when transferred into germ-free lymphopenic animals [12]. These memory-phenotype CD8+ T cells rapidly produced IFN-γ and became cytotoxic in response to TCR stimulation or innate cytokine (IL-12 plus IL-18) activation [12]. Lymphopenia-induced innate memory T-cell generation was shown to be dependent on TCR signals resulting from binding with low affinity self-peptide/MHC ligands, which may be qualitatively different from the high-affinity TCR signals induced by foreign antigens [13]. This process also required factors such as IL-7, which is thought to be a main driver of lymphopenia-induced T-cell proliferation [14], and IL-15, which is thought to play a critical role in the maintenance of the memory phenotype in CD8+ T cells [15] (Figure 1). IL-7 receptor (IL-7R) signaling promotes induction of the T-box transcription factor 21 (T-bet) in these cells, which induces CD122 (a component of the IL-15R) and thus sensitizes the cells to the effects of IL-15 to induce the T-box transcription factor eomesodermin (Eomes) [16]. This results in a transcription factor expression pattern very similar to that of conventional memory T cells. Lymphopenia may be induced in certain infections or treatments that impair the immune system, but it is less clear how such a situation would arise under normal physiological conditions. It has been suggested, however, that the neonatal immune system in mice is characterized by partial lymphopenia [17]. A significant population of memory phenotype T cells, which may represent virtual memory T cells, has been observed in neonatal animals [7].
Figure 1.
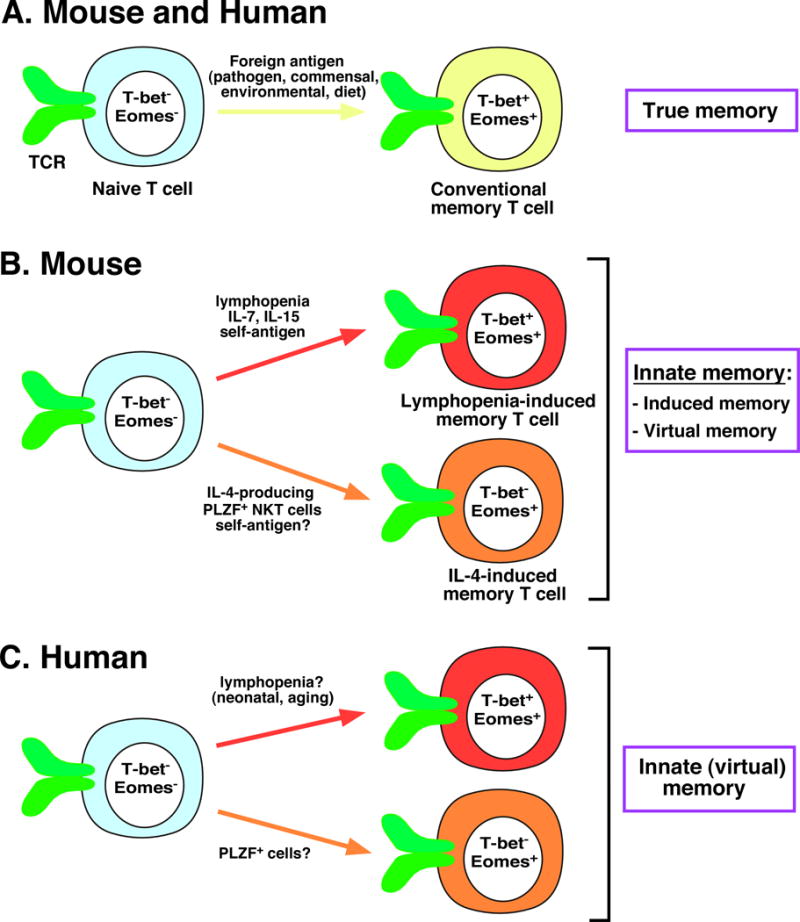
Pathways for the generation of distinct memory T-cell populations in mice and humans. (A) Naïve T lymphocytes in both mice and humans can differentiate into conventional, or “true” memory T cells in response to foreign antigens. (B) Alternatively, naïve T cells in mice can adopt a memory phenotype independently of foreign antigens to become innate memory T cells. Such cells may be induced following lymphopenia with the assistance of self-antigens and cytokines such as IL-7 and IL-15 to become lymphopenia-induced memory T cells (top), or in response to the cytokine IL-4 produced by PLZF+ NKT cells to become IL-4-induced memory T cells (bottom). Both of these alternative pathways of memory cell generation may occur in response to alterations in the environment, called induced innate memory, or under steady-state conditions, called virtual memory. (C) The new study from Jacomet et al. [9] validates the presence of innate (predominantly virtual) memory T cells in humans, which may be generated in response to either lymphopenia (top) or IL-4 (bottom). Precise mechanisms for the generation of these cells in humans remain to be determined. Differences in the expression of critical transcription factors between naïve T cells and distinct subsets of memory T cells are depicted.
In addition to lymphopenia-induced innate memory T cells, a population of memory phenotype CD8+ T cells has been identified in mice that appeared to develop in response to the cytokine IL-4 (reviewed in [6]). This type of innate (virtual) memory CD8+ T cell was abundant in certain mouse strains, such as BALB/c during steady-state conditions, and required the presence of IL-4-producing cells expressing promyelocytic leukemia zinc finger (PLZF), a transcription factor that is characteristically expressed by subsets of innate and innate-like lymphocytes (Figure 1) [18]. Subsequent studies identified a critical role of NKT cells for the generation of innate memory CD8+ T cells in BALB/c mice, which was consistent with the abundance of IL-4-producing NKT cells (called NKT2 cells) in these animals, as compared with mouse strains such as C57BL/6 that harbor few NKT2 cells and where IL-4-induced innate memory CD8+ T cells are sparse [19], except when promoted by the co-stimulatory receptor CD28 [20]. Current thinking holds that IL-4-induced memory T cells may be generated both in the thymus and the periphery during steady-state conditions, and that these cells may be induced in the periphery not only by NKT2 cells but also by strong type 2 immune responses to microbial or environmental antigens [21]. Whether the generation of these cells requires TCR-mediated signals remains to be determined. IL-4-induced memory CD8+ T cells express the transcription factor Eomes, but unlike conventional and lymphopenia-induced memory CD8+ T cells, these cells do not express T-bet [8]. Like lymphopenia-induced memory CD8+ T cells, IL-4-induced memory CD8+ T cells have the ability to rapidly produce IFN-γ in response to TCR engagement or inflammatory cytokines [18].
Studying innate memory T cells in mice is challenging because it is hard to exclude the involvement of microbial, environmental or food antigens in their generation. This becomes particularly problematic when looking for innate memory T cells in humans. One cellular source that has provided a partial solution to this problem is tissues obtained from premature births. Earlier studies provide evidence for memory-phenotype T cells in the spleen and cord blood of human fetuses [22], and for memory-like CD8+ T cells expressing NK-cell receptors in full-term cord blood [23]. Additional studies identified a population of human fetal CD8+ thymocytes that expressed Eomes [24], as well as a population of thymic PLZF-expressing cells [25]. Jacomet et al. [9] put several of these pieces of the puzzle together by demonstrating that full-term human cord blood contains a sizeable population of CD8+ T cells that lack expression of CCR7, a chemokine receptor that enables cells to migrate to lymph nodes, but co-express the memory T cell marker CD45RO and the naïve T cell marker CD45RA. This surface marker expression profile is characteristic of memory T cells with a terminal effector phenotype, often referred to as terminally differentiated effector memory T cells (TEMRA, reviewed in [26]). These cells also expressed Eomes and the NK-cell markers KIR (killer cell Ig-like receptor) and NKG2A. The KIR+NKG2A+Eomes+ memory-phenotype CD8+ T cells produced IFN-γ in response to stimulation with inflammatory cytokines (IL-12 plus IL-18) or nonspecific stimuli (PMA, phorbol myristate acetate, plus ionomycin) [9]. The majority (60%) of these cells also expressed T-bet, which is consistent with the possibility that many of them arise in response to partial lymphopenia associated with the fetal or neonatal immune system in humans. Additionally, the presence of these cells correlated well with expression of PLZF among NKT cells in cord blood, which is consistent with a contribution of IL-4-producing, PLZF-expressing cells to their generation. Regardless of their precise origin, however, these findings provide strong evidence for the presence of innate memory T cells, especially virtual memory T cells, in human cord blood.
Are innate memory T cells present in adult humans? Prior studies identified a subset of CD8+ T cells in human peripheral blood that express NK-cell receptors such as KIR [27–29]. Jacomet et al. [9] further demonstrated that more than half of these adult peripheral cells express Eomes and the majority (60%) of these cells also co-express T-bet. Akin to the virtual memory T cells identified in cord blood, these cells exhibit a terminally differentiated effector cell phenotype and respond to TCR or innate cytokine signals by producing IFN-γ [9]. These cells also exert NK cell-like cytolytic activity in an antibody-dependent manner via the human low affinity Fc receptor CD16, and against MHC class I-deficient target cells. These findings are thus consistent with the presence of innate memory CD8+ T cells in adult humans.
Whereas the new study [9] provides the best evidence to date for the existence of innate memory T cells in humans, the origin of these cells remains unclear. In humans it not feasible at present to exclude the involvement of foreign antigens in the generation of T cells with a memory phenotype. Another challenge is to determine the antigen-specificity of these cells, a problem that in mice was typically addressed with TCR transgenic animals. In this context, previous studies have found an expanded population of CD16-expressing effector CD8+ T cells with NK cell-like functions in patients chronically infected with hepatitis C virus [30]. Cells with a similar phenotype have been identified in the blood of healthy donors and in patients with hyperlymphocytosis, and in cultures of Epstein-Barr virus-specific cytotoxic T lymphocytes [31]. Whether these cells represent innate memory T cells remains to be determined and will require further investigation of their phenotype, transcription factor expression and origin.
What are the functions of innate memory T cells? Studies with lymphopenia- and IL-4-induced memory CD8+ T cells in mice have shown that these cells responded more strongly than naïve CD8+ T cells with the same specificity, yet these cells were less effective than foreign antigen-induced memory CD8+ T cells with regard to their proliferative and cytolytic capacity [6]. These cells were able to protect animals against infection by the bacterial pathogen Listeria monocytogenes [32] and against tumors [33, 34], suggesting an important contribution to host immunity. Furthermore, because these cells can respond rapidly to innate cytokines such as IL-12 and IL-18, they might contribute to the inflammatory milieu early during an immune response. Situations in humans where such cells may be generated include the transient lymphopenia induced following certain infections, severe autoimmunity, and various treatments such as chemotherapy, radiotherapy, immunosuppressive drugs, and host conditioning regimens for adoptive transfer of tumor-specific T cells. Thus, the memories captured by innate memory T cells may contribute to a variety of immune responses and the success of several treatments. Interestingly, recent studies have revealed that virtual memory CD8+ T cells constitute the majority of memory CD8+ T cells in aged mice [35–37], which is consistent with the notion that aging is associated with partial T cell lymphopenia (reviewed in [38]). It has been demonstrated that as mice age, this virtual memory population becomes enriched in T cells with high TCR avidity and fast rates of homeostatic proliferation [37]. It was further shown that the impaired ability of these cells to proliferate and induce their effector functions as compared with conventional memory T cells contributes to the decline of immune responses with aging [36]. These findings therefore suggest the possibility that accumulation of virtual memory T cells contributes to the decline in immune function with advancing age.
The identification of innate and virtual memory T cells in humans will undoubtedly lead to increased efforts to explore the origins and functions of these fascinating cells, with the ultimate goal of harnessing them for prophylactic and therapeutic purposes.
Acknowledgments
The author’s work is supported by grants from the National Institutes of Health, the Crohn’s and Colitis Foundation of America, and the National Multiple Sclerosis Society.
Abbreviations
- Eomes
eomesodermin
- KIR
killer cell Ig-like receptor
- PLZF
promyelocytic leukemia zinc finger
- T-bet
T-box transcription factor 21
Footnotes
Conflict of interest: The author declares no financial or commercial conflict of interest.
References
- 1.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 2.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1:177–186. doi: 10.1038/35105052. [DOI] [PubMed] [Google Scholar]
- 3.Van Kaer L. NKT cells: T lymphocytes with innate effector functions. Curr Opin Immunol. 2007;19:354–364. doi: 10.1016/j.coi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Lanier LL. Shades of grey–the blurring view of innate and adaptive immunity. Nat Rev Immunol. 2013;13:73–74. doi: 10.1038/nri3389. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends Immunol. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jameson SC, Lee YJ, Hogquist KA. Innate memory T cells. Adv Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci U S A. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacomet F, Cayssials E, Basbous S, Levescot A, Piccirilli N, Desmier D, Robin A, Barra A, Giraud C, Guilhot F, Roy L, Herbelin A, Gombert JM. Evidence for eomesodermin-expressing innate-like CD8+ KIR/NKG2A+ T cells in human adults and cord blood samples. Eur J Immunol. 2015;45:XXX–XXX. doi: 10.1002/eji.201545539. [DOI] [PubMed] [Google Scholar]
- 10.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat Rev Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 12.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knudson KM, Hamilton SE, Daniels MA, Jameson SC, Teixeiro E. Cutting edge: The signals for the generation of T cell memory are qualitatively different depending on TCR ligand strength. J Immunol. 2013;191:5797–5801. doi: 10.4049/jimmunol.1300905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Sandau MM, Winstead CJ, Jameson SC. IL-15 is required for sustained lymphopenia-driven proliferation and accumulation of CD8 T cells. J Immunol. 2007;179:120–125. doi: 10.4049/jimmunol.179.1.120. [DOI] [PubMed] [Google Scholar]
- 16.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Campion A, Bourgeois C, Lambolez F, Martin B, Leaument S, Dautigny N, Tanchot C, Penit C, Lucas B. Naive T cells proliferate strongly in neonatal mice in response to self-peptide/self-MHC complexes. Proc Natl Acad Sci U S A. 2002;99:4538–4543. doi: 10.1073/pnas.062621699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yousefi M, Duplay P. CD28 controls the development of innate-like CD8+ T cells by promoting the functional maturation of NKT cells. Eur J Immunol. 2013;43:3017–3027. doi: 10.1002/eji.201343627. [DOI] [PubMed] [Google Scholar]
- 21.Kurzweil V, LaRoche A, Oliver PM. Increased peripheral IL-4 leads to an expanded virtual memory CD8+ population. J Immunol. 2014;192:5643–5651. doi: 10.4049/jimmunol.1301755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrne JA, Stankovic AK, Cooper MD. A novel subpopulation of primed T cells in the human fetus. J Immunol. 1994;152:3098–3106. [PubMed] [Google Scholar]
- 23.Warren HS, Rana PM, Rieger DT, Hewitt KA, Dahlstrom JE, Kent AL. CD8 T cells expressing killer Ig-like receptors and NKG2A are present in cord blood and express a more naive phenotype than their counterparts in adult blood. J Leukoc Biol. 2006;79:1252–1259. doi: 10.1189/jlb.0905536. [DOI] [PubMed] [Google Scholar]
- 24.Min HS, Lee YJ, Jeon YK, Kim EJ, Kang BH, Jung KC, Chang CH, Park SH. MHC class II-restricted interaction between thymocytes plays an essential role in the production of innate CD8+ T cells. J Immunol. 2011;186:5749–5757. doi: 10.4049/jimmunol.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee YJ, Jeon YK, Kang BH, Chung DH, Park CG, Shin HY, Jung KC, Park SH. Generation of PLZF+ CD4+ T cells via MHC class II-dependent thymocyte-thymocyte interaction is a physiological process in humans. J Exp Med. 2010;207:237–246. doi: 10.1084/jem.20091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who’s who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 27.Bjorkstrom NK, Beziat V, Cichocki F, Liu LL, Levine J, Larsson S, Koup RA, Anderson SK, Ljunggren HG, Malmberg KJ. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood. 2012;120:3455–3465. doi: 10.1182/blood-2012-03-416867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huard B, Karlsson L. A subpopulation of CD8+ T cells specific for melanocyte differentiation antigens expresses killer inhibitory receptors (KIR) in healthy donors: evidence for a role of KIR in the control of peripheral tolerance. Eur J Immunol. 2000;30:1665–1675. doi: 10.1002/1521-4141(200006)30:6<1665::AID-IMMU1665>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.van der Veken LT, Diez Campelo M, van der Hoorn MA, Hagedoorn RS, van Egmond HM, van Bergen J, Willemze R, Falkenburg JH, Heemskerk MH. Functional analysis of killer Ig-like receptor-expressing cytomegalovirus-specific CD8+ T cells. J Immunol. 2009;182:92–101. doi: 10.4049/jimmunol.182.1.92. [DOI] [PubMed] [Google Scholar]
- 30.Bjorkstrom NK, Gonzalez VD, Malmberg KJ, Falconer K, Alaeus A, Nowak G, Jorns C, Ericzon BG, Weiland O, Sandberg JK, Ljunggren HG. Elevated numbers of Fc gamma RIIIA+ (CD16+) effector CD8 T cells with NK cell-like function in chronic hepatitis C virus infection. J Immunol. 2008;181:4219–4228. doi: 10.4049/jimmunol.181.6.4219. [DOI] [PubMed] [Google Scholar]
- 31.Clemenceau B, Vivien R, Berthome M, Robillard N, Garand R, Gallot G, Vollant S, Vie H. Effector memory alphabeta T lymphocytes can express FcgammaRIIIa and mediate antibody-dependent cellular cytotoxicity. J Immunol. 2008;180:5327–5334. doi: 10.4049/jimmunol.180.8.5327. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 33.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 35.Chiu BC, Martin BE, Stolberg VR, Chensue SW. Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Zugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24:495–499. doi: 10.1016/j.immuni.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


