Abstract
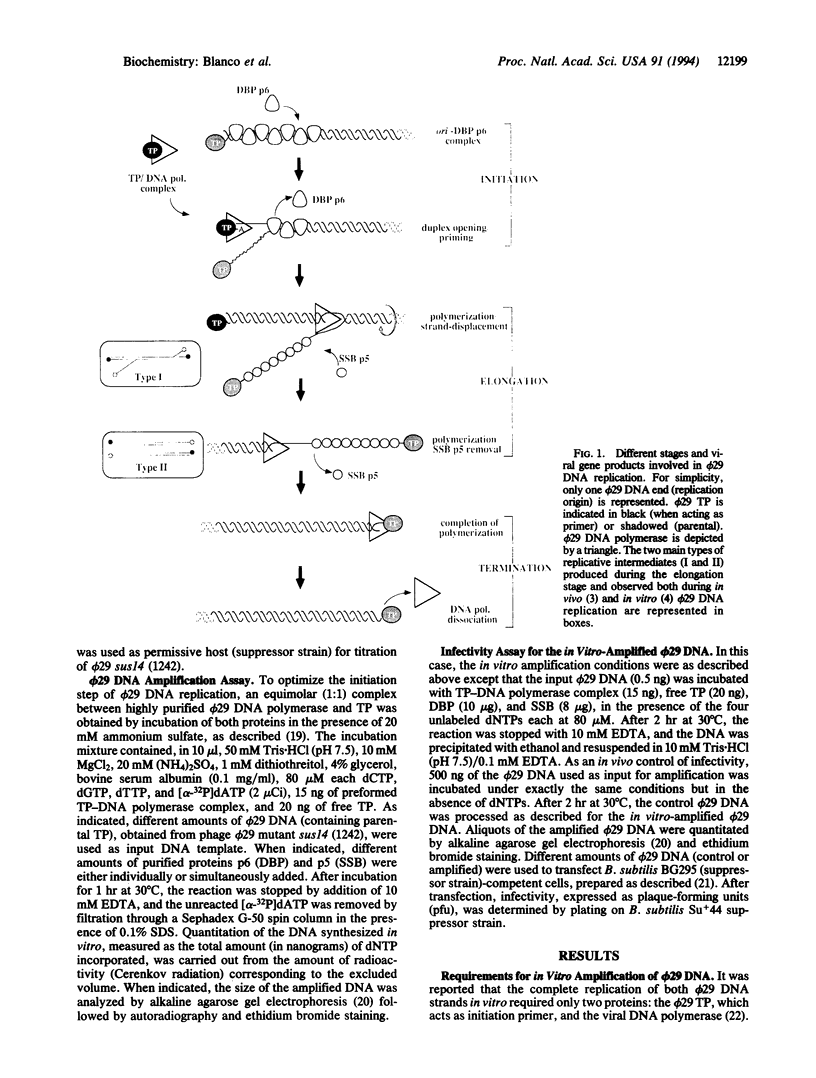
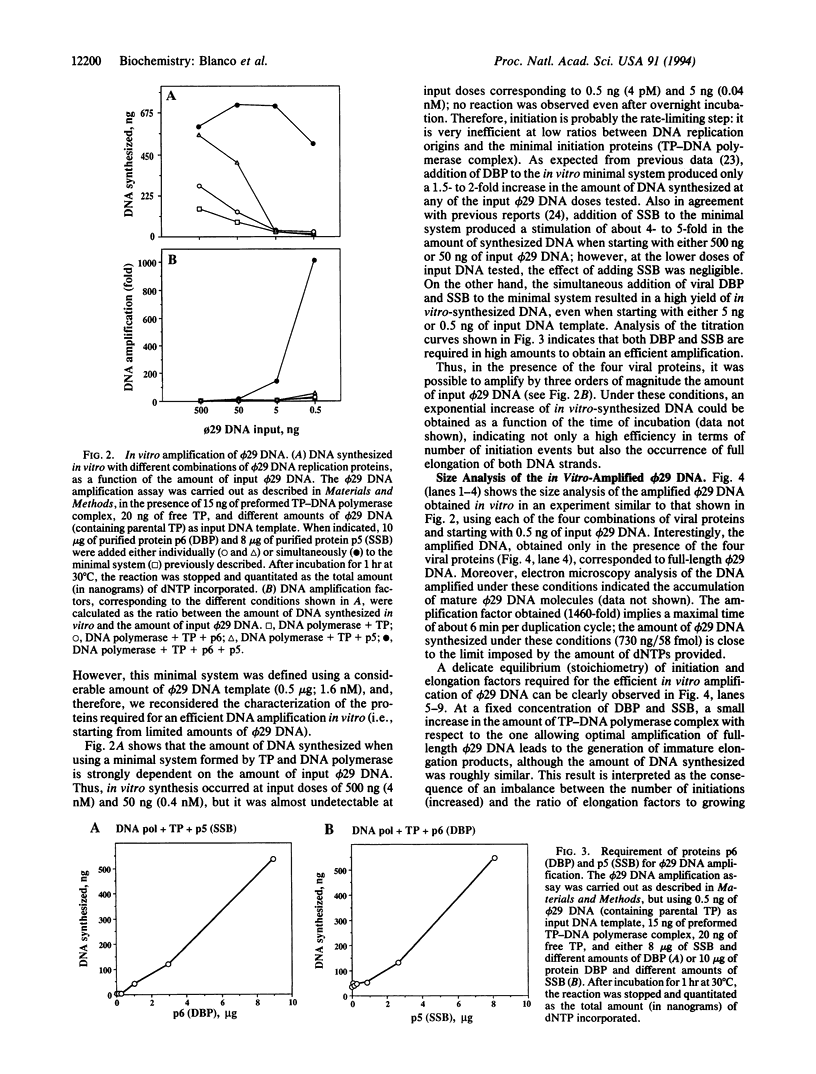
By using appropriate amounts of four bacteriophage phi 29 DNA replication proteins--terminal protein, DNA polymerase, protein p6 (double-stranded DNA-binding protein), and protein p5 (single-stranded DNA-binding protein)--it has been possible to amplify limited amounts of the 19,285-bp-long phi 29 DNA molecule by three orders of magnitude after 1 hr of incubation at 30 degrees C. Moreover, the quality of the amplified material was demonstrated by transfection experiments, in which infectivity of the synthetic (amplified) phi 29 DNA, measured as the ability to produce phage particles, was identical to that of the natural phi 29 DNA obtained from virions. The results presented in this paper establish some of the requisites for the development of isothermal DNA amplification strategies based on the bacteriophage phi 29 DNA replication machinery that are suitable for the amplification of very large (> 70 kb) segments of DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes W. M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Bernad A., Esteban J. A., Salas M. DNA-independent deoxynucleotidylation of the phi 29 terminal protein by the phi 29 DNA polymerase. J Biol Chem. 1992 Jan 15;267(2):1225–1230. [PubMed] [Google Scholar]
- Blanco L., Bernad A., Lázaro J. M., Martín G., Garmendia C., Salas M. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J Biol Chem. 1989 May 25;264(15):8935–8940. [PubMed] [Google Scholar]
- Blanco L., Bernad A., Salas M. Transition from initiation to elongation in protein-primed phi 29 DNA replication: salt-dependent stimulation by the viral protein p6. J Virol. 1988 Nov;62(11):4167–4172. doi: 10.1128/jvi.62.11.4167-4172.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization and purification of a phage phi 29-encoded DNA polymerase required for the initiation of replication. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5325–5329. doi: 10.1073/pnas.81.17.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Characterization of a 3'----5' exonuclease activity in the phage phi 29-encoded DNA polymerase. Nucleic Acids Res. 1985 Feb 25;13(4):1239–1249. doi: 10.1093/nar/13.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco L., Salas M. Replication of phage phi 29 DNA with purified terminal protein and DNA polymerase: synthesis of full-length phi 29 DNA. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6404–6408. doi: 10.1073/pnas.82.19.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrascosa J. L., Camacho A., Moreno F., Jiménez F., Mellado R. P., Viñuela E., Salas M. Bacillus subtilis phage phi29. Characterization of gene products and functions. Eur J Biochem. 1976 Jul 1;66(2):229–241. doi: 10.1111/j.1432-1033.1976.tb10512.x. [DOI] [PubMed] [Google Scholar]
- Chai S., Szepan U., Lüder G., Trautner T. A., Alonso J. C. Sequence analysis of the left end of the Bacillus subtilis bacteriophage SPP1 genome. Gene. 1993 Jul 15;129(1):41–49. doi: 10.1016/0378-1119(93)90694-x. [DOI] [PubMed] [Google Scholar]
- Cheng S., Fockler C., Barnes W. M., Higuchi R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5695–5699. doi: 10.1073/pnas.91.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J. A., Salas M., Blanco L. Fidelity of phi 29 DNA polymerase. Comparison between protein-primed initiation and DNA polymerization. J Biol Chem. 1993 Feb 5;268(4):2719–2726. [PubMed] [Google Scholar]
- Garmendia C., Bernad A., Esteban J. A., Blanco L., Salas M. The bacteriophage phi 29 DNA polymerase, a proofreading enzyme. J Biol Chem. 1992 Feb 5;267(4):2594–2599. [PubMed] [Google Scholar]
- Gutiérrez C., Sogo J. M., Salas M. Analysis of replicative intermediates produced during bacteriophage phi 29 DNA replication in vitro. J Mol Biol. 1991 Dec 20;222(4):983–994. doi: 10.1016/0022-2836(91)90589-x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J., García J. A., Blanco L., Salas M. Cloning and template activity of the origins of replication of phage phi 29 DNA. Gene. 1986;43(1-2):1–11. doi: 10.1016/0378-1119(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Hermoso J. M., Méndez E., Soriano F., Salas M. Location of the serine residue involved in the linkage between the terminal protein and the DNA of phage phi 29. Nucleic Acids Res. 1985 Nov 11;13(21):7715–7728. doi: 10.1093/nar/13.21.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez F., Camacho A., De La Torre J., Viñuela E., Salas M. Assembly of Bacillus subtilis phage phe29. 2. Mutants in the cistrons coding for the non-structural proteins. Eur J Biochem. 1977 Feb 15;73(1):57–72. doi: 10.1111/j.1432-1033.1977.tb11291.x. [DOI] [PubMed] [Google Scholar]
- Landegren U. Molecular mechanics of nucleic acid sequence amplification. Trends Genet. 1993 Jun;9(6):199–204. doi: 10.1016/0168-9525(93)90119-3. [DOI] [PubMed] [Google Scholar]
- Martín G., Lázaro J. M., Méndez E., Salas M. Characterization of the phage phi 29 protein p5 as a single-stranded DNA binding protein. Function in phi 29 DNA-protein p3 replication. Nucleic Acids Res. 1989 May 25;17(10):3663–3672. doi: 10.1093/nar/17.10.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín G., Salas M. Characterization and cloning of gene 5 of Bacillus subtilis phage phi 29. Gene. 1988 Jul 30;67(2):193–201. doi: 10.1016/0378-1119(88)90396-4. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Mellado R. P., Vinuela E., Salas M. Isolation of a strong suppressor of nonsense mutations in Bacillus subtilis. Eur J Biochem. 1976 May 17;65(1):213–223. doi: 10.1111/j.1432-1033.1976.tb10408.x. [DOI] [PubMed] [Google Scholar]
- Moreno F. Suppressor-sensitive mutants and genetic map of Bacillus subtilis bacteriophage phi 29. Virology. 1974 Nov;62(1):1–16. doi: 10.1016/0042-6822(74)90298-0. [DOI] [PubMed] [Google Scholar]
- Méndez J., Blanco L., Esteban J. A., Bernad A., Salas M. Initiation of phi 29 DNA replication occurs at the second 3' nucleotide of the linear template: a sliding-back mechanism for protein-primed DNA replication. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9579–9583. doi: 10.1073/pnas.89.20.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana R., Lázaro J. M., Blanco L., García J. A., Méndez E., Salas M. Overproduction and purification of protein P6 of Bacillus subtilis phage phi 29: role in the initiation of DNA replication. Nucleic Acids Res. 1985 May 10;13(9):3083–3100. doi: 10.1093/nar/13.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñalva M. A., Salas M. Initiation of phage phi 29 DNA replication in vitro: formation of a covalent complex between the terminal protein, p3, and 5'-dAMP. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5522–5526. doi: 10.1073/pnas.79.18.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Rottländer E., Trautner T. A. Genetic and transfection studies with B, subtilis phage SP 50. I. Phage mutants with restricted growth on B. subtilis strain 168. Mol Gen Genet. 1970;108(1):47–60. doi: 10.1007/BF00343184. [DOI] [PubMed] [Google Scholar]
- Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- Serrano M., Gutiérrez J., Prieto I., Hermoso J. M., Salas M. Signals at the bacteriophage phi 29 DNA replication origins required for protein p6 binding and activity. EMBO J. 1989 Jun;8(6):1879–1885. doi: 10.1002/j.1460-2075.1989.tb03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Salas M., Hermoso J. M. A novel nucleoprotein complex at a replication origin. Science. 1990 May 25;248(4958):1012–1016. doi: 10.1126/science.2111580. [DOI] [PubMed] [Google Scholar]
- Talavera A., Salas M., Viñuela E. Temperature-sensitive mutants affected in DNA synthesis in phage phi29 of Bacillus subtilis. Eur J Biochem. 1972 Dec 4;31(2):367–371. doi: 10.1111/j.1432-1033.1972.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Zaballos A., Salas M. Functional domains in the bacteriophage phi 29 terminal protein for interaction with the phi 29 DNA polymerase and with DNA. Nucleic Acids Res. 1989 Dec 25;17(24):10353–10366. doi: 10.1093/nar/17.24.10353. [DOI] [PMC free article] [PubMed] [Google Scholar]