Abstract
Childhood obesity is associated with biologic and behavioral characteristics that may impact bone mineral density (BMD) and structure. The objective was to determine the association between obesity and bone outcomes, independent of sexual and skeletal maturity, muscle area and strength, physical activity, calcium intake, bio-markers of inflammation, and vitamin D status. Tibia and radius peripheral quantitative CT scans were obtained in 91 obese (BMI > 97th percentile) and 51 non-obese adolescents (BMI > 5th and <85th percentiles). Results were converted to sex- and race-specific Z-scores relative to age. Cortical structure, muscle area and muscle strength (by dynamometry) Z-scores were further adjusted for bone length. Obese participants had greater height Z-scores (p < 0.001), and advanced skeletal maturity (p < 0.0001), compared with non-obese participants. Tibia cortical section modulus and calf muscle area Z-scores were greater in obese participants (1.07 and 1.63, respectively, both p < 0.0001). Tibia and radius trabecular and cortical volumetric BMD did not differ significantly between groups. Calf muscle area and strength Z-scores, advanced skeletal maturity, and physical activity (by accelerometry) were positively associated with tibia cortical section modulus Z-scores (all p < 0.01). Adjustment for muscle area Z-score attenuated differences in tibia section modulus Z-scores between obese and non-obese participants from 1.07 to 0.28. After multivariate adjustment for greater calf muscle area and strength Z-scores, advanced maturity, and less moderate to vigorous physical activity, tibia section modulus Z-scores were 0.32 (95% CI −0.18, 0.43, p = 0.06) greater in obese, vs. non-obese participants. Radius cortical section modulus Z-scores were 0.45 greater (p = 0.08) in obese vs. non-obese participants; this difference was attenuated to 0.14 with adjustment for advanced maturity. These findings suggest that greater tibia cortical section modulus in obese adolescents is attributable to advanced skeletal maturation and greater muscle area and strength, while less moderate to vigorous physical activities offset the positive effects of these covariates. The impact of obesity on cortical structure was greater at weight bearing sites.
Keywords: Adolescence, Bone mineral density, Obesity, Quantitative computed tomography, Muscle strength
Introduction
Adolescence is a critical period for bone accrual, and peak bone mass has long-term implications for fracture risk [1,2]. Recent data show that 17% of U.S. children and adolescents are obese [3]. However, the impact of excess adiposity on acquisition of trabecular and cortical volumetric bone mineral density (vBMD) and cortical structure during growth and development has not been well established. Prior studies in adolescents produced conflicting results with some reporting a positive association between adiposity and bone outcomes, [4–10] while others reported an absent or negative association [11–18]. More recent studies suggest that the association with bone outcomes may vary according to fat distribution [19–21]. These discrepancies may be related to differences in skeletal sites, as well as varying approaches to adjust for known differences in lean body mass, maturation and stature between obese and non-obese adolescents.
Initial studies of bone outcomes in children and adolescents with obesity were limited by the use of dual-energy x-ray absorptiometry (DXA) methods that failed to distinguish between trabecular and cortical density and structure, were confounded by differences in bone size, and were subject to errors introduced by variability in overlying fat mass [22]. In contrast, quantitative computerized tomography (QCT) provides three dimensional measures of trabecular and cortical vBMD and cortical dimensions that are highly correlated with bone strength [23] and are less subject to errors introduced by overlaying fat mass [22].
Prior QCT studies examined the associations of body composition with trabecular and cortical vBMD and cortical dimensions in cohorts of children and adolescents with a low prevalence of obesity [14,15, 17–21,24]. In those that provided sufficiently detailed information on body mass index (BMI, kg/m2) distributions, the proportions with a BMI greater than the 95th percentile for age ranged from 8 to 15% [15, 17,20,21]. In the remainder, the BMI data suggested that even a lower proportion were obese. Furthermore, three of these studies were limited to mature adolescents, ages 17 to 20 years, [14,19,24] while another cohort was limited to pre- and peripubertal females, 8 to 13 years of age [17,21]. Last, these studies did not include comprehensive assessment of lifestyle factors (such as calcium intake or objective measures of physical activity), or biological factors (such as advanced skeletal maturity, greater muscle mass and strength, or inflammation) that may differ between obese and non-obese children and impact bone health.
A more recent peripheral QCT (pQCT) study examined 51 obese adolescent males and matched controls [10]. The study demonstrated that obesity was associated with advanced bone maturation, greater muscle mass and strength, and greater trabecular vBMD and cortical dimensions in the tibia and radius. After adjusting for bone age, the cortical bone and muscle differences in the tibia remained significant while the differences in the radius did not. The authors did not report comparisons of bone outcomes in the obese and non-obese participants adjusted for the differences in muscle mass or strength; therefore, it is not known if the differences in bone outcomes were explained by differences in biomechanical loading. During growth, cortical bone modeling optimizes bone strength; therefore, greater cortical dimensions in obese adolescents may have life-long implications for fracture risk [25,26].
The aims of this study were to compare measures of trabecular and cortical vBMD and cortical section modulus in the radius and tibia in obese adolescents and non-obese controls, and to identify variables that may explain the association between obesity and bone outcomes, such as sexual and skeletal maturity, muscle area and strength, physical activity, calcium intake, biomarkers of inflammation, and vitamin D status.
Materials and methods
Design and participants
This cross-sectional study was performed at the initiation of a weight loss randomized clinical trial (NCT00609713) and included non-obese controls who participated at baseline only. The protocol was approved by the Institutional Review Board of the Children’s Hospital of Philadelphia and informed consent was obtained from all participants. Obese and non-obese subjects were recruited at The Children’s Hospital of Philadelphia and across the greater Philadelphia area using flyers, newspaper and radio advertisements, and referral from local clinicians.
Inclusion criteria for obese participants were age > 10 years and <15 years in order to capture the period of peak bone mineral accretion velocity, [27] and a BMI above the 97th percentile for sex and age [28]. Participants were excluded for a BMI Z-score greater than +3.00 SD to avoid severe co-morbidities or for a body weight greater than 136 kg (the limit of the DXA table). Participants with syndromic or secondary obesity were excluded. The inclusion criteria for the non-obese controls were age > 10 and <18 years and a BMI > 5th and <85th percentiles. The controls were recruited across a broader age range in order to achieve sufficient overlap in height and bone age between obese and non-obese controls, given the anticipated advanced maturity of the obese participants.
Exclusion criteria for both groups included reported developmental delay requiring special education, depression, psychosis, eating disorders, orthopedic problems interfering with moderate to vigorous physical activity, diabetes, polycystic ovary syndrome, cumulative lifetime systemic corticosteroid use exceeding three months, use of anticonvulsivants, weight loss medications (including diet supplements), and any other medications or chronic conditions that could interfere with the intervention or bone health. Other exclusion criteria were weight loss of at least 5% over the preceding six months, participation in another weight loss program, cigarette smoking, and, for females, sexual activity without contraception.
Measurements
Tibia and radius bone outcomes were measured by pQCT using a Stratec XCT-2000 device (Orthometrix, Inc., White Plains, NY) with a 12-detector unit, 0.4 mm voxel size, 2.3 mm slice thickness, 25 mm/s scan sped, and software version 5.5. Tibia and radius length were measured with a sliding caliper (Rosscraft, Surrey, BC, Canada). A scout view was obtained to guide placement of the reference line at the medial proximal border of the distal growth plate in participants with open growth plates and at the medial proximal border of the endplate in participants with fused growth plates. The reference line was placed at the growth plate, as opposed to the endplate, to minimize heterogeneity introduced by variability in epiphysis and physis dimensions. Trabecular vBMD was assessed at the 3% site in the midregion of the metaphysis. Cortical vBMD (mg/mm3), section modulus (mm3), and periosteal and endosteal circumference (mm) were assessed in the diaphysis at 38% in the tibia and 30% in the radius. Section modulus provides a composite measure of the effects of cortical periosteal and endosteal dimensions on bone strength. Previous studies show pQCT measures of cortical section modulus (R2 = 0.78, p < 0.0001) [23] and trabecular vBMD (R2 = 0.56, p < 0.05) [29] were correlated with bone failure moment in mechanical testing. Calf muscle and subcutaneous fat cross-sectional area (mm2) were obtained at the 66% site, at the approximate site of maximum calf circumference. A recent publication in athletes reported that the difference in cortical structure between the dominant and non-dominant leg was evident at the 38% site but not the 66% site [30]. Quality assessment was monitored using daily scans of the manufacturer’s phantom. All scans were reviewed by a single investigator (BSZ) for technical quality. The coefficient of variation (CV) ranged from 0.5 to 1.6% for tibia pQCT outcomes in children and adolescents in our laboratory.
Weight (0.1 kg) was measured with the subjects wearing scrubs or light clothing on a digital electronic scale (Seca, Munich, Germany), and stature (0.1 cm) on a stadiometer (Holtain, Crymych, UK). All measurements were performed in triplicate and the mean was used in the analyses. BMI was converted into a sex-specific Z-score relative to age using the U.S. reference population recommended by the Centers for Diseases Control and Prevention [28]. Pubertal status was determined using the validated self-assessment questionnaire developed by Morris and Udry [31]. The self-assessment questionnaire was completed by the subject with assistance from the parent if needed. Self-assessment of breast Tanner stage may be unreliable in obese girls; therefore we relied on pubic hair assessment in both sexes for consistency. Bone age was determined by comparing a left hand wrist radiograph to the Greulich and Pyle standard. Maturity was calculated as bone age minus chronological age (years); a positive value represented advanced maturity.
Calf muscle strength was measured using Biodex Multi-Joint System 3 Pro (Biodex Medical Systems, Inc. Shirley, NY) after a five-minute warm-up period on a treadmill. Peak isometric torque (ft–lbs) was measured for ankle dorsiflexion at 20°, with a CV of 4.3%, as previously described [32]. The tibialis anterior attaches directly to the tibia (the bone of interest in this study) and causes dorsiflexion of the ankle. We recently demonstrated that muscle torque measured in dorsiflexion was independently and significantly associated with tibia cortical section modulus, but measures obtained in plantarflexion were not [33]. Maximal handgrip strength (kg) was measured with a handgrip dynamometer (Takei, Tokyo, Japan). The subject stood upright with the shoulder adducted holding the dynamometer, not touching the trunk. The handle was adjusted to the participant’s hand size. Three maximal effort trials lasting 4 to 5 s interspersed with 60-second rests were performed and the highest value retained for analysis.
Physical activity was measured using an ActiGraph GT1M accelerometer (ActiGraph, LLC, Fort Walton Beach, FL) worn on the right side attached to a waist belt. Subjects were instructed to wear the device during waking hours for seven days (including two weekend days), and to record each time the device was removed and replaced. The results were considered usable if participants provided at least 8 h of accelerometer data on at least six days, including one weekend day. The average number of activity counts per minute as well as the percentage of time wearing the accelerometer spent in sedentary, light, moderate, vigorous and moderate to vigorous physical activity per period wearing the device was determined using validated activity thresholds [34].
Dietary and supplement calcium intake was assessed by a validated limited food-frequency questionnaire [35]. A non-fasting blood sample was collected to measure serum high sensitivity CRP (hsCRP, mg/L) and 25-hydroxy-vitamin D [25(OH)D; ng/mL] concentrations. hsCRP was measured using Human C-Reactive Protein/CRP Quantikine ELISA kit (R&D Systems, Minneapolis, MN) with a coefficient of variation (CV) of 8%. 25(OH)D was measured by a chemiluminescence immuno-assay using the DiaSorin LIAISON assay (Heartland Assays, Ames, IA) with a CV of 5%.
Statistical analyses
The age related patterns of changes in bone density and structure show distinct sex and ancestry group differences [36]. Therefore, all pQCT outcomes and muscle strength were converted to sex and ancestry group (African American or non-African American) -specific Z-scores relative to age using the LMS method (LMS Chartmaker version 2.3) [37] and our reference data in more than 650 healthy children and adolescents [36]. The LMS method accounts for the non-linearity, heteroscedasticity, and skew of bone and body composition data with age. Reference participants were not excluded for overweight or obesity. The proportion of reference participants 10 to 18 years of age that were overweight or obese (BMI > 85th percentile) was 30% overall, and was 20% and 38% in non-Hispanic White and African American participants, respectively. In comparison, the prevalence of overweight/ obesity in US non-Hispanic white and African American adolescents in 2011–2012 was 31% and 40%, respectively [38]. There was no evidence of an obese-by-ancestry group interaction in the multivariate models described below.
Tibia cortical dimensions, calf muscle and fat cross-sectional areas, and muscle strength in dorsiflexion were significantly correlated with tibia length and radius cortical dimensions and handgrip strength were significantly correlated with radius length. Therefore, these outcomes were first converted into Z-scores for age, then further adjusted for tibia/radius length for age Z-score, similar to the method proposed by Zemel et al. [39]. This adjustment method accounts for the fact that, relative to peers of the same age, sex and ancestry group, individuals with greater bone length have a greater section modulus, muscle and fat area, and muscle strength. Cortical and trabecular vBMD were converted to ancestry group- and sex-specific Z-scores for age. Trabecular and cortical vBMD were not associated with bone length; therefore, further adjustments for bone length were not indicated.
Comparisons between obese and non-obese subject characteristics were performed using chi-square and t-tests, or non-parametric tests if continuous variables were not normally distributed. The impact of variables that potentially explained the association between obesity and bone outcomes was evaluated as follows. First, unadjusted comparisons of the primary bone variable Z-scores (trabecular vBMD, cortical vBMD and cortical section modulus) were completed between obese and non-obese subjects. Second, each variable that could potentially contribute to differences in bone outcomes between groups was compared between obese and non-obese subjects (Table 1). Third, each variable that could potentially contribute to differences between groups was tested for its association with the bone Z-scores in univariate analyses in obese and non-obese participants combined (Table 2). Tested covariates included calf muscle area Z-score, puberty status (Tanner 1 or 2 vs. 3 to 5), advanced maturity (bone age-chronological age, yr), muscle strength Z-scores, moderate to vigorous physical activity (% of time accelerometer worn), total physical activity (per 100 count/min), calcium intake (per 100 mg/day), plasma hsCRP (per mg/L), and 25(OH) vitamin D (per 10 ng/mL). Fourth, the impact of adjustment for each covariate on group differences between obese and non-obese subjects was examined in separate models (Table 3): if the regression coefficient for the obese vs. non-obese variable changed by more than 10% after addition to the model of one of these variables, this variable was considered to be a clinically significant factor that contributes to the difference between obese and non-obese subjects [40]. Finally, all variables that were identified as clinically significant were introduced in a single model with the corresponding bone outcome to test if they fully explained the difference between obese and non-obese subjects (Table 4). Secondary analyses examined associations with periosteal and endosteal circumferences in order to determine if group differences in section modulus were due to differences in the periosteal and/or end-osteal components of cortical structure. Multiplicative interaction terms were used to identify potential interactions of obesity with sex or race. There were no significant interactions.
Table 1.
Characteristics of obese and non-obese participants.
| Obese (N = 91) | Non-obese (N = 51) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, year | 12.2 (1.2) | 14.5 (2.0) | <0.0001 |
| Sex, n (%) female | 59 (65%) | 32 (63%) | 0.8 |
| Race, n (%) African American | 56 (62%) | 31 (61%) | 0.9 |
| Anthropometry and maturation | |||
| Weight, kg | 85.7 (1.8) | 53.2 (1.5) | <0.0001 |
| Weight Z-score | 2.64 (0.43) | 0.17 (0.69) | <0.0001 |
| Height, cm | 158.5 (8.2) | 163.4 (11.3) | <0.01 |
| Height Z-score | 1.00 (0.92) | 0.40 (0.96) | <0.001 |
| BMI, kg/m2 | 33.9 (4.9) | 19.7 (2.0) | <0.0001 |
| BMI Z-score | 2.39 (0.22) | −0.01 (0.61) | <0.0001 |
| Puberty, n Tanner 1 or 2 (%) | 26 (30.2) | 8 (15.7) | 0.06 |
| Bone age, year | 13.7 (1.5) | 14.7 (2.4) | <0.01 |
| Advanced skeletal maturity, year | 1.5 (1.0) | 0.2 (1.1) | <0.0001 |
| Muscle strength and physical activity | |||
| Ankle muscle strength, ft–lbs | 22.6 (6.4) | 21.2 (7.7) | 0.10 |
| Ankle muscle strength Z-score | 0.24 (0.09) | −0.51 (0.13) | <0.0001 |
| Handgrip, kg | 22.4 (5.3) | 25.1 (8.0) | 0.02 |
| Handgrip Z-score | −0.1 (1.10) | −0.28 (1.16) | 0.19 |
| Moderate to vigorous physical activity, % | 0.64 (0.28, 1.27) | 1.19 (0.69, 1.97) | <0.01 |
| Total physical activity, counts/min | 254 (185,363) | 239 (182, 316) | 0.5 |
| pQCT lower leg muscle and fat area | |||
| Calf muscle area, mm2 | 6608 (1034) | 5797 (1180) | 0.0001 |
| Calf muscle area Z-score | 1.19 (0.98) | −0.44 (0.88) | <0.0001 |
| Calf subcutaneous fat area, mm2 | 5117 (1198) | 1931 (806) | <0.0001 |
| Calf subcutaneous fat area Z-score | 2.06 (0.61) | −0.36 (0.81) | <0.0001 |
| Dietary intake | |||
| Calcium intake, mg/day | 1204 (708) | 1311 (728) | 0.5 |
| Laboratory parameters | |||
| hsCRP, mg/L | 2.05 (0.96, 4.01) | 0.19 (0.11, 0.49) | <0.0001 |
| 25(OH) vitamin D, ng/mL | 20.1 (9.9) | 23.1 (10.3) | 0.08 |
| Tibia QCT bone outcomes | |||
| Cortical section modulus, mm3 | 1802 (342) | 1592 (486) | <0.01 |
| Cortical section modulus Z-score | 0.76 (0.75) | −0.31 (0.78) | <0.0001 |
| Cortical periosteal circumference, mm | 71.4 (4.7) | 68.1 (6.9) | 0.003 |
| Cortical periosteal circumference Z-score | 0.78 (0.86) | −0.30 (0.80) | <0.0001 |
| Cortical endosteal circumference, mm | 37.3 (5.7) | 35.7 (5.7) | 0.10 |
| Cortical endosteal circumference Z-score | 0.11 (1.19) | −0.24 (0.97) | 0.07 |
| Trabecular volumetric BMD, mg/cm3 | 265 (33) | 263 (34) | 0.8 |
| Trabecular volumetric BMD Z-score | 0.51 (1.10) | 0.17 (1.08) | 0.08 |
| Cortical volumetric BMD, mg/cm3 | 1106 (41) | 1132 (55) | <0.001 |
| Cortical volumetric BMD Z-score | −0.04 (1.00) | −0.04 (1.12) | 0.7 |
| Radius QCT bone outcomes | |||
| Cortical section modulus, mm3 | 215 (128) | 234 (176) | 0.5 |
| Cortical section modulus Z-score | 0.43 (1.36) | −0.02 (1.59) | 0.08 |
| Cortical periosteal circumference, mm | 34.0 (3.3) | 34.2 (4.6) | 0.8 |
| Cortical periosteal circumference Z-score | 0.40 (1.15) | −0.11 (1.29) | 0.02 |
| Cortical endosteal circumference, mm | 16.4 (3.8) | 15.3 (3.9) | 0.12 |
| Cortical endosteal circumference Z-score | 0.12 (1.25) | −0.35 (1.19) | 0.13 |
| Trabecular volumetric BMD, mg/cm3 | 251 (39) | 245 (39) | 0.4 |
| Trabecular volumetric BMD Z-score | 0.29 (1.19) | 0.10 (1.21) | 0.4 |
| Cortical volumetric BMD, mg/cm3 | 1126 (33) | 1154 (41) | <0.001 |
| Cortical volumetric BMD Z-score | 0.02 (1.07) | 0.16 (1.03) | 0.5 |
Data are presented as n (%), mean (SD), or median (interquartile range).
Table 2.
Univariate associations of (A) tibia and (B) radius bone outcomes with obesity and with each variable chosen a priori for potential contribution to the differences between obese and non-obese participants.
| Section modulus Z-score
|
Trabecular vBMD Z-score
|
Cortical vBMD Z-score
|
||||
|---|---|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value | |
| A. Tibia | ||||||
| Obese vs. non-obese | 1.07 (0.81 to 1.33) | <0.0001 | 0.34 (−0.04, 0.72) | 0.08 | 0.00 (−0.36, 0.36) | 0.9 |
| Calf muscle area Z-score | 0.55 (0.46 to 0.64) | <0.0001 | 0.16 (0.01, 0.31) | 0.03 | 0.03 (−0.12, 0.18) | 0.07 |
| Tanner 3–5 vs. 1–2 | 0.23 (−0.13 to 0.47) | 0.2 | 0.44 (0.01, 0.86) | 0.045 | 0.51 (0.11, 0.91) | 0.01 |
| Advanced skeletal maturity, per year | 0.37 (0.25 to 0.48) | <0.0001 | 0.23 (0.08, 0.38) | 0.002 | 0.26 (0.12, 0.40) | <0.0001 |
| Ankle strength Z-score | 0.51 (0.37 to 0.65) | <0.0001 | 0.09 (−0.10, 0.28) | 0.4 | −0.05 (−0.23, 0.13) | 0.6 |
| Moderate to vigorous Physical Activity, per % | 0.00 (−0.16 to 0.15) | 0.95 | −0.09 (−0.28, 0.10) | 0.3 | −0.03 (−0.22, 0.15) | 0.7 |
| Total physical activity, per 100 counts per min | 0.23 (0.08 to 0.38) | <0.01 | −0.09 (−0.28, 0.10) | 0.3 | −0.08 (−0.27, 0.10) | 0.4 |
| Calcium intake, per 100 mg/day | 0.017 (−0.005 to 0.039) | 0.12 | 0.02 (0.00, 0.05) | 0.1 | 0.00 (−0.02, 0.03) | 0.9 |
| hsCRP, per mg/L | 0.01 (−0.03 to 0.05) | 0.7 | 0.04 (0.00, 0.08) | 0.07 | 0.04 (0.00, 0.08) | 0.07 |
| 25(OH) vitamin D, per 10 ng/mL | 0.05 (−0.11 to 0.20) | 0.6 | 0.08 (−0.10, 0.27) | 0.4 | −0.04 (−0.21, 0.14) | 0.7 |
| B. Radius | ||||||
| Obese vs. non-obese | 0.45 (−0.05, 0.96) | 0.08 | 0.19 (−0.23, 0.62) | 0.4 | −0.13 (−0.50, 0.23) | 0.5 |
| Tanner 3–5 vs. 1–2 | 0.07 (−0.52, 0.66) | 0.8 | 0.10 (−0.39, 0.59) | 0.7 | 0.11 (−0.32, 0.53) | 0.6 |
| Advanced skeletal maturity, per year | 0.30 (0.09, 0.50) | <0.01 | 0.08 (−0.10, 0.25) | 0.4 | 0.12 (−0.03, 0.27) | 0.12 |
| Handgrip strength Z-score | 0.44 (0.23, 0.65) | <0.001 | 0.21 (0.03, 0.39) | 0.02 | −0.10 (−0.26, 0.06) | 0.2 |
| Moderate to vigorous physical activity, per % | 0.08 (−0.15, 0.31) | 0.5 | −0.21 (−0.43, 0.00) | 0.05 | 0.00 (−0.18, 0.19) | 0.9 |
| Total physical activity, per 100 counts per min | 0.15 (−0.08, 0.37) | 0.2 | −0.14 (−0.35, 0.08) | 0.2 | −0.11 (−0.30, 0.08) | 0.2 |
| Calcium intake, per 100 mg/day | 0.04 (0.00, 0.07) | 0.03 | 0.02 (−0.01, 0.05) | 0.3 | 0.00 (−0.03, 0.03) | 0.9 |
| hsCRP, per mg/L | 0.00 (−0.06, 0.07) | 0.9 | 0.04 (−0.01, 0.09) | 0.09 | 0.03 (−0.01, 0.07) | 0.2 |
| 25(OH) vitamin D, per 10 ng/mL | 0.21 (−0.04, 0.47) | 0.10 | −0.03 (−0.25, 0.19) | 0.8 | 0.04 (−0.14, 0.22) | 0.6 |
Table 3.
Difference in cortical section modulus Z-score between obese and non-obese participants unadjusted, and adjusted separately for each variable potentially contributing to differences between groups.
| Adjustment variable | Difference (95% CI) in section modulus Z-score between obese and non-obese participants | p-value for obese vs. non-obese difference | Percent change in obesity point estimate with adjustment |
|---|---|---|---|
| None | 1.07 (0.81 to 1.33) | <0.0001 | Not applicable |
| Calf muscle area Z-score | 0.28 (−0.01 to 0.57) | 0.06 | −74% |
| Puberty | 1.05 (0.78 to 1.33) | <0.0001 | −1.8% |
| Advanced skeletal maturity | 0.83 (0.53 to 1.13) | <0.0001 | −22.6% |
| Muscle strength Z-score | 0.81 (0.55 to 1.07) | <0.0001 | −24.7% |
| Moderate to vigorous physical activity | 1.12 (0.82 to 1.42) | <0.0001 | +16.7%a |
| Total physical activity | 0.93 (0.66 to 1.21) | <0.0001 | −3.1%a |
| Calcium intake | 1.13 (0.86 to 1.39) | <0.0001 | +4.9% |
| hsCRP concentration | 1.15 (0.85 to 1.45) | <0.0001 | +7.1% |
| 25(OH)vitamin D concentration | 1.03 (0.76 to 1.31) | <0.0001 | −3.6% |
Comparison with unadjusted obesity point estimate of 0.96 among 125 participants with accelerometer data
Table 4.
Full model adjusted for all variables considered to contribute significantly to the group difference for the difference in tibia cortical section modulus Z-score (SD) between obese and non-obese subjects.
| Variable | β (95% CI) | p-value |
|---|---|---|
| Obese vs. non-obese | 0.32 (−0.01 to 0.64) | 0.06 |
| Calf muscle area Z-score | 0.35 (0.21 to 0.49) | <0.0001 |
| Advanced skeletal maturity, per year | 0.06 (−0.05 to 0.17) | 0.3 |
| Muscle strength Z-score | 0.21 (0.05 to 0.36) | <0.01 |
| Moderate to vigorous physical activity, per percent | 0.21 (0.10 to 0.32) | <0.0001 |
All analyses were performed using Stata/SE 12.0 (StataCorp LP, College Station, TX) and a two-sided p-value of 0.05 was considered statistically significant. The assumptions of the fitted regression models were assessed graphically and with statistical tests.
Results
Anthropometry, maturation and body composition
Characteristics of the obese and non-obese participants are summarized in Table 1. As expected, obese participants had significantly greater height Z-scores and more advanced skeletal maturity, compared with non-obese participants. The obese and non-obese participants had sufficiently overlapping ranges of height (1.41 to 1.81 and 1.39 to 1.89 m, respectively) and bone age (10–18 and 9–19 years, respectively), facilitating adjustment for these two important covariates. Calf muscle area Z-scores were markedly greater in obese compared with non-obese participants with mean values of 1.19 and −0.44, respectively.
The mean BMI Z-score in the non-obese controls was −0.01 while the mean BMI Z-score in the reference population that informed the generation of pQCT Z-scores was +0.43. The fact that the non-obese controls were limited to those with a BMI < 85th percentile accounts for the fact that the Z-scores for calf muscle and fat area, and muscle strength by dynamometry and handgrip were less than zero in these non-obese controls, compared with reference participants.
Lifestyle variables and laboratory results
Accelerometer data were available in 125 participants. Seventeen participants were given accelerometers but were not included in the physical activity analyses because they did not have enough data (n = 13), lost or failed to return the accelerometer (n = 2) or the device malfunctioned (n = 2). Participants who did vs. did not contribute accelerometry data did not differ in age, sex, race, BMI Z-score, or tibia or radius section modulus, cortical vBMD, or trabecular vBMD Z-scores. Accelerometer-based measures of total physical activity did not differ between groups; however, the obese subjects engaged in significantly less moderate to vigorous physical activity. Both groups engaged in very limited moderate to vigorous physical activity, on average less than 2% of the time wearing the accelerometer. Calcium intake did not differ between groups. Serum hsCRP levels were significantly greater in obese participants. Serum 25(OH)D levels were higher in the non-obese participants; however, this differences was not statistically significant.
Bone density and structure
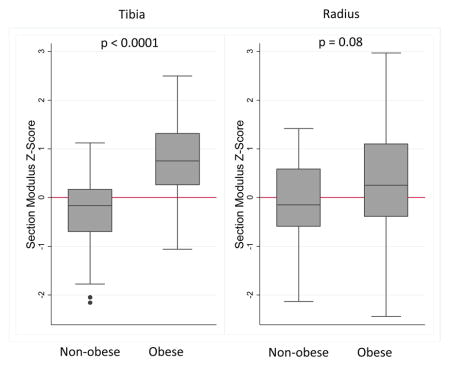
The average section modulus Z-score of the obese subjects was a full SD higher than the non-obese subjects in the tibia (Table 1). This difference was due to greater periosteal circumference Z-scores in the obese participants. In contrast, the cortical section modulus Z-score was only 0.45 SD greater in the radius in obese compared with non-obese subjects. While the difference in radius section modulus Z-score was not significant (p = 0.08), the periosteal circumference Z-score was significantly greater in the obese subjects. Cortical vBMD Z-scores did not differ between obese and non-obese subjects in the tibia or radius. Trabecular vBMD Z-scores were greater in obese compared with non-obese participants in the tibia and radius; however, the differences were not significant.
The first row of Table 2 summarizes the unadjusted differences in tibia cortical section modulus, trabecular vBMD, and cortical vBMD Z-scores between the obese and non-obese participants, consistent with the results shown in Table 1. The subsequent rows show the associations of each possible contributing variable with each of the three tibia bone outcomes in the obese and non-obese participants combined. Greater calf muscle area Z-score, more advanced skeletal maturity, greater muscle strength Z-score, and greater total physical activity were associated with greater tibia cortical section modulus Z-scores. The second portion of the table shows the same analyses in the radius. More advanced skeletal maturity and greater strength Z-score were also associated with greater cortical section modulus Z-scores in the radius. Higher Tanner stage (3–5 vs. 1–2) and more advanced skeletal maturity were associated with greater trabecular and cortical vBMD for age Z-scores in the tibia only. Calcium intake was associated with trabecular vBMD Z-score in the radius only. hsCRP and vitamin D levels were not associated with any of the bone Z-scores in the tibia or radius in univariate analyses.
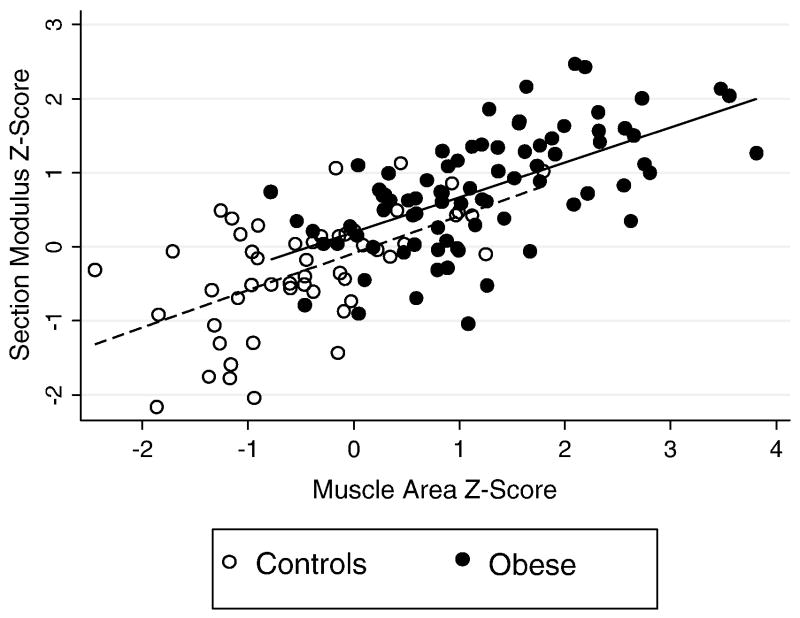
Table 3 summarizes the differences in tibia section modulus between the obese and non-obese groups, separately adjusted for each possible contributing variable. Adjustment for calf muscle area Z-score had the greatest impact, reducing the group difference in section modulus Z-score between obese and non-obese participants from 1.07 to 0.28, representing a 74% reduction in the point estimate of the obesity effect. This difference in section modulus Z-score between obese and non-obese participants was only marginally significant (p = 0.06) after adjustment for muscle area Z-score. Fig. 1 illustrates the greater section modulus and calf muscle area Z-scores in obese vs. non-obese participants, the positive associations between these measures, and the marginally greater section modulus relative to muscle area Z-score in the obese participants. Adjustment for the advanced maturity or greater muscle strength in the obese participants in separate regression models also attenuated the difference between obese and non-obese subjects by more than 10%. Adjustment for the significantly lower moderate to vigorous physical activity in the obese participants (a potential risk factor for smaller cortical section modulus), increased the group difference by 16.7% and moderate to vigorous physical activity was positively and significantly (p = 0.007) associated with cortical section modulus Z-score in the model adjusted for the obese vs. non-obese covariate. The other variables tested did not change the coefficient of the difference between groups by more than 10% and therefore were not considered clinically significant intermediates in the association between obesity and section modulus. Similar changes in coefficients were observed in models using tibia periosteal circumference Z-scores instead of section modulus Z-scores (data not shown).
Fig. 1.
Tibia cortical section modulus Z-score relative to muscle area Z-score in obese and non-obese control participants. This figure illustrates the markedly greater muscle area and section modulus Z-scores in the obese participants, and the positive association observed in both groups. Section modulus Z-score relative to muscle area Z-score was marginally greater in obese compared with non-obese participants (p = 0.06).
Although radius cortical section modulus Z-scores were not significantly different in obese and non-obese participants (p = 0.08), secondary analyses were performed to pursue possible explanations for the different associations of obesity with cortical section modulus in the tibia and radius. Adjustment for advanced skeletal maturity attenuated the differences between obese and non-obese participants from 0.45 to 0.14 (95% CI −0.46 to 0.74, p = 0.6) and adjustment for handgrip strength Z-score attenuated the difference from 0.45 to 0.34 (−0.14 to 0.82; p = 0.17). Among the 125 participants with accelerometer data, adjustment for moderate to vigorous physical activity increased the obesity point estimate from 0.52 to 0.69 (0.18 to 1.20; p < 0.01) while adjustment for total physical activity had negligible effect: adjusted estimate 0.50 (0.026 to 0.98; p = 0.04). Neither moderate to vigorous physical activity nor total physical activity was significant in these models adjusted for obesity status.
Table 4 summarizes the fully adjusted model for tibia cortical section modulus Z-score. After adjustment for greater calf muscle area Z-score, advanced maturity, greater muscle strength Z-score, and less moderate to vigorous physical activity in the obese participants, the section modulus Z-score was 0.32 greater in obese, compared with non-obese participants (p = 0.06) (Table 4). Three different components of mechanical loading (muscle area Z-score, muscle strength, and moderate to vigorous physical activity) were significantly and independently associated with section modulus Z-score in this final model. In a univariate model with the obesity variable only in those with accelerometry data, the R2 was 0.27. Adjustment for muscle area Z-score increased the R2 to 0.52 and further adjustment for all the variables shown in Table 4 increased the R2 to 0.62. When moderate to vigorous physical activity was excluded from the model, the obesity coefficient was 0.18 (−0.12, 0.48; p = 0.23).
In a multivariate model for radius cortical section modulus, adjustment for accelerated maturity eliminated the association with obesity and adjustment for MVPA did not uncover a significant association with obesity.
Discussion
The present study demonstrates that tibia cortical section modulus (adjusted for sex, ancestry group, age, and tibia length) was significantly higher in obese compared to non-obese adolescents, and that this difference was explained by advanced skeletal maturation, greater calf muscle area and greater muscle strength in the obese participants. The significantly less moderate to vigorous physical activity in the obese participants partially offset the positive effects of maturation, muscle mass, and muscle strength on tibia cortical structure. In contrast, radius cortical section modulus was only marginally greater in the obese participants and the difference was eliminated with adjustment for advanced maturation. To our knowledge, this is the first study to examine obese adolescents of both sexes, and to include extensive assessment of lifestyle (calcium intake and accelerometer-based measures of physical activity), and biological factors (inflammation and vitamin D status) that may differ between obese and non-obese adolescents and also impact bone health. An additional unique strength is the use of robust reference data to express the group differences as Z-scores, providing greater perspective on the magnitude of group differences.
Prior studies have examined associations between body composition and QCT measures of cortical bone in children, adolescents and young adults; [13,14,16–20,23,24] however, most included few obese participants. For example, Farr et al. showed a positive correlation between total body fat mass and increases in tibia stress strain index (SSI, a composite measure of cortical section modulus and cortical vBMD) and this association was attenuated with adjustment for total body lean mass. However, only 7.7% of the participants were obese. Similarly, Janicka et al. obtained femur QCT scans in 300 adolescents, 13 to 21 years of age; 12% of females and 15% of males were obese [15]. In multiple linear regression, DXA lean mass but not fat mass, was positively associated with femur cross-sectional area in males and females. These studies did not consider the contributions of other lifestyle, maturation or biologic factors.
To our knowledge, the recent study by Vandewalle was the first pQCT study to focus on obese adolescents but was limited to male subjects [10]. Our study included males and females and did not identify any sex differences in the association of obesity with trabecular or cortical bone outcomes. Similar to our results, the Vanderwalle study demonstrated that obesity was associated with advanced bone maturation, greater tibia cortical dimensions, and greater muscle area and strength compared with age and height matched- or bone age-matched controls. Also similar to our results, the magnitude of the differences in cortical area and periosteal circumference in the obese vs. age-matched control adolescents was much greater in the tibia, compared to the radius. And, when the obese participants were compared with bone aged-matched controls in the Vandewalle study, the differences remained highly significant in the tibia, but were no longer significant in the radius. The authors did not report comparisons of bone outcomes between the obese and non-obese participants adjusted for muscle mass or strength; therefore, it is not known if the tibia differences could be attributed to differences in biomechanical loading. The study included measures of sex hormone in these male participants, demonstrating higher circulating free estrogen levels in the obese participants. Free testosterone and IGF-1 levels did not differ.
When various possible contributing variables were examined separately, calf muscle area was the strongest intermediate between obesity and tibia section modulus; adjustment for this variable resulted in a 74% decrease in the difference between the obese and the non-obese group. Greater muscle strength contributed to the group differences in section modulus, independent of muscle area (Table 4). The greater section modulus in the obese participants was driven by a greater periosteal circumference. This is consistent with numerous studies demonstrating periosteal expansion in response to biomechanical loading (e.g. playing vs. nonplaying arm in tennis players) during growth [41,42]. Of note, Ducher et al. reported that height velocity was a significant determinant of exercise-induced benefits in periosteal dimensions (p < 0.01), concluding that Tanner stages 1 through 3 represent the optimal time to enhance bone mass [41]. Therefore, the positive effect of greater lean mass on cortical expansion in association with obesity may be limited to periods of growth. Although the gains may only occur during growth, cortical enlargement through newly added bone on the periosteal surface increases bone strength dramatically as the bending strength of a unit area of bone is proportional to the fourth power of its distance from the long axis of the bone [43]. Therefore, gains in periosteal circumference may have life-long beneficial effects on bone strength. This is consistent with prior studies attributing life-long sex differences in fracture risk to differences in cortical structure that are established during growth and development [25,26].
Advanced skeletal maturity also contributed to the greater section modulus in obese participants. Obese children have advanced biological maturity, and earlier maturating children have greater bone mass and density in young adulthood. Of note, the effect of accelerated maturation may have been related to maturation effects on muscle area and strength as the effect was no longer significant in the fully adjusted model (Table 4).
The obese children in our study had similar total physical activity levels compared with the normal weight controls; however, a significantly smaller percentage of time was spent in moderate to vigorous physical activity. Physical activity in childhood has been associated with increased bone strength in a variety of observational and intervention studies. These data demonstrated that three different components of biomechanical loading – muscle area, muscle strength, and physical activity – were all significantly and independently associated with tibia cortical section modulus (Table 4). Interestingly, physical activity was not associated with cortical section modulus in the radius.
This study did not demonstrate significant differences in cortical vBMD Z-scores between obese and non-obese adolescents. The tibia trabecular vBMD Z-scores were 0.34 greater in obese vs. non-obese participants; however, the difference was not significant. The lack of differences in cortical vBMD is consistent with the study by Vandewalle et al. [10] however, that study reported modestly greater tibia trabecular vBMD in obese vs. age-matched control participants (239 + 28 vs. 225 + 34 mg/cm3; p < 0.05). The lack of Z-scores hampers comparison of the magnitude of group differences with our study.
The present study has important limitations. First, the cross-sectional design precluded assessment of the impact of concurrent growth and maturation on differences between obese and non-obese participants. In addition, this limitation may account for the lack of associations of bone outcomes with calcium intake, hsCRP levels, or vitamin D levels. Second, it is not possible to demonstrate causality in this observational study that may have been subject to residual confounding. Third, the study did not include measures of growth factors, insulin metabolism, or sex hormones. Fourth, the study did not include measures of muscle area in the forearm. Last, conventional pQCT has insufficient resolution to assess trabecular microarchitecture or cortical porosity. Future studies with high resolution pQCT (HR-PQCT, isotropic voxel size 0.61 to 0.82 μm) are needed to assess these measures of bone quality in obese and non-obese adolescents.
The lack of data on visceral and subcutaneous fat is an additional limitation. Recent studies suggested independent effects of visceral, subcutaneous and intramuscular fat on bone; however, these studies were conducted in cohorts with very few obese participants. Gilsanz et al., obtained QCT measures of femur cortical area and body composition in 100 healthy young women, ages 15 to 25 years, and reported that thigh muscle area and subcutaneous fat were significantly, positively, and independently associated with cortical dimensions, while visceral subcutaneous fat was negatively associated with cortical dimensions [20]. Farr et al. examined 9 to 12 year old females and reported that greater intramuscular fat was associated with lower cortical bone area, independent of subcutaneous fat [44]. In contrast, Deere et al. recently reported that greater intramuscular and subcutaneous fat were both associated with greater periosteal circumference and cortical thickness in a large cohort of 18 year old male and females (median BMI 21.6 kg/m2), with some attenuation after adjustment for muscle area [19]. Therefore, the effects of fat distribution may vary with age and should be explored using a larger sample size across the adolescent age range.
This study had several notable strengths. The inclusion of contemporary non-obese controls with a wider age range than the obese subjects provided obese and non-obese adolescents of overlapping bone ages and heights. This facilitated the assessment of these key determinants of bone health. To our knowledge, this is the first study to include both male and female obese participants and appropriate non-obese controls, and to assess the independent effects of multiple components of biomechanical loading (muscle area, physical activity and muscle strength), accelerated maturation, dietary intake and inflammation at a weight bearing and non-weight bearing site. The study was further strengthened by the use of accelerometry to measure physical activity. Both total and moderate to vigorous physical activities were associated with cortical section modulus in the tibia, but it was the differences in moderate to vigorous physical activity between obese and non-obese participants that contributed to group differences in section modulus.
In conclusion, the present study confirms prior reports that obesity in adolescents is associated with greater muscle mass and strength, and this in turn is associated with greater cortical dimensions and estimates of bone strength. However, this association is much greater in the weight bearing tibia, compared with the radius. This study extends these observations to demonstrate the impact of accelerated maturation, greater muscle strength, and less moderate to vigorous physical activity. These findings suggest that the increased lower extremity fracture risk observed among obese adolescents may be due to abnormal microarchitecture or poor motor proficiency, while the increased upper extremity fracture rates may be due to inadequate compensation for the greater forces with falls [45–47]. Future studies are needed to determine the impact of weight loss interventions on vBMD and cortical structure during growth and development in obese adolescents.
Acknowledgments
The study was supported by the National Institutes of Health grants R01 HD049701, K24 DK076808, and UL1 RR 024134. The authors thank the study subjects, their families, the study staff, and the staff of the Nutrition and Growth Laboratory at the Children Hospital of Philadelphia.
Footnotes
Conflict of interest statement
All authors state that they have no conflicts of interest.
Funding: The study was supported by National Institutes of Health grants R01 HD049701, K24 DK076808, and UL1 RR024134.
References
- 1.Seeman E. Pathogenesis of bone fragility in women and men. Lancet. 2002;359:1841–50. doi: 10.1016/S0140-6736(02)08706-8. [DOI] [PubMed] [Google Scholar]
- 2.Foley S, Quinn S, Jones G. Tracking of bone mass from childhood to adolescence and factors that predict deviation from tracking. Bone. 2009;44:752–7. doi: 10.1016/j.bone.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States. JAMA pediatr. 2014:1999–2012. doi: 10.1001/jamapediatrics.2014.21. [DOI] [PubMed] [Google Scholar]
- 4.Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am J Clin Nutr. 2004;80:514–23. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- 5.Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36:568–76. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Stettler N, Berkowtiz RI, Cronquist JL, et al. Observational study of bone accretion during successful weight loss in obese adolescents. Obesity. 2008;16:96–101. doi: 10.1038/oby.2007.17. [DOI] [PubMed] [Google Scholar]
- 7.El Hage R, Jacob C, Moussa E, Benhamou CL, Jaffre C. Total body, lumbar spine and hip bone mineral density in overweight adolescent girls: decreased or increased? J Bone Miner Metab. 2009;27:629–33. doi: 10.1007/s00774-009-0074-6. [DOI] [PubMed] [Google Scholar]
- 8.Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J Clin Endocrinol Metab. 2010;95:699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J Clin Endocrinol Metab. 2006;91:2534–41. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandewalle S, Taes Y, Van Helvoirt M, et al. Bone size and bone strength are increased in obese male adolescents. J Clin Endocrinol Metab. 2013;98:3019–28. doi: 10.1210/jc.2012-3914. [DOI] [PubMed] [Google Scholar]
- 11.Weiler HA, Janzen L, Green K, Grabowski J, Seshia MM, Yuen KC. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone. 2000;27:203–7. doi: 10.1016/s8756-3282(00)00314-8. [DOI] [PubMed] [Google Scholar]
- 12.Goulding A, Taylor RW, Jones IE, McAuley KA, Manning PJ, Williams SM. Overweight and obese children have low bone mass and area for their weight. Int J Obes Relat Metab Disord. 2000;24:627–32. doi: 10.1038/sj.ijo.0801207. [DOI] [PubMed] [Google Scholar]
- 13.Nagasaki K, Kikuchi T, Hiura M, Uchiyama M. Obese Japanese children have low bone mineral density after puberty. J Bone Miner Metab. 2004;22:376–81. doi: 10.1007/s00774-004-0498-y. [DOI] [PubMed] [Google Scholar]
- 14.Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr. 2007;86:1530–8. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- 15.Janicka A, Wren TA, Sanchez MM, et al. Fat mass is not beneficial to bone in adolescents and young adults. J Clin Endocrinol Metab. 2007;92:143–7. doi: 10.1210/jc.2006-0794. [DOI] [PubMed] [Google Scholar]
- 16.Rocher E, Chappard C, Jaffre C, Benhamou CL, Courteix D. Bone mineral density in prepubertal obese and control children: relation to body weight, lean mass, and fat mass. J Bone Miner Metab. 2008;26:73–8. doi: 10.1007/s00774-007-0786-4. [DOI] [PubMed] [Google Scholar]
- 17.Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46:977–84. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wey HE, Binkley TL, Beare TM, Wey CL, Specker BL. Cross-sectional versus longitudinal associations of lean and fat mass with pQCT bone outcomes in children. J Clin Endocrinol Metab. 2011;96:106–14. doi: 10.1210/jc.2010-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deere K, Sayers A, Viljakainen HT, et al. Distinct relationships of intramuscular and subcutaneous fat with cortical bone: findings from a cross-sectional study of young adult males and females. J Clin Endocrinol Metab. 2013;98:E1041–9. doi: 10.1210/jc.2013-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94:3387–93. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laddu DR, Farr JN, Laudermilk MJ, et al. Longitudinal relationships between whole body and central adiposity on weight-bearing bone geometry, density, and bone strength: a pQCT study in young girls. Arch osteoporos. 2013;8:156. doi: 10.1007/s11657-013-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–24. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu D, Manske SL, Kontulainen SA, et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18:991–7. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 24.Pollock NK, Laing EM, Hamrick MW, Baile CA, Hall DB, Lewis RD. Bone and fat relationships in postadolescent black females: a pQCT study. Osteoporos Int. 2011;22:655–65. doi: 10.1007/s00198-010-1266-6. [DOI] [PubMed] [Google Scholar]
- 25.Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res Off J Am Soc Bone Miner Res. 2003;18:1766–74. doi: 10.1359/jbmr.2003.18.10.1766. [DOI] [PubMed] [Google Scholar]
- 26.Seeman E. Structural basis of growth-related gain and age-related loss of bone strength. Rheumatology. 2008;47(Suppl 4):iv2–8. doi: 10.1093/rheumatology/ken177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver CM. Adolescence: the period of dramatic bone growth. Endocrine. 2002;17:43–8. doi: 10.1385/ENDO:17:1:43. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Kontulainen SA, Johnston JD, Liu D, Leung C, Oxland TR, McKay HA. Strength indices from pQCT imaging predict up to 85% of variance in bone failure properties at tibial epiphysis and diaphysis. J Musculoskelet Neuronal Interact. 2008;8:401–9. [PubMed] [Google Scholar]
- 30.Anliker E, Sonderegger A, Toigo M. Side-to-side differences in the lower leg muscle-bone unit in male soccer players. Med Sci Sports Exerc. 2013;45:1545–52. doi: 10.1249/MSS.0b013e31828cb712. [DOI] [PubMed] [Google Scholar]
- 31.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 32.Wetzsteon RJ, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103–8. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DY, Wetzsteon RJ, Zemel BS, et al. Muscle torque relative to cross-sectional area and the functional muscle–bone unit in children and adolescents with chronic disease. J Bone Miner Res Off J Am Soc Bone Miner Res. 2014 doi: 10.1002/jbmr.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puyau MR, Adolph AL, Vohra FA, Butte NF. Validation and calibration of physical activity monitors in children. Obes Res. 2002;10:150–7. doi: 10.1038/oby.2002.24. [DOI] [PubMed] [Google Scholar]
- 35.Zemel BS, Carey LB, Paulhamus DR, Stallings VA, Ittenbach RF. Quantifying calcium intake in school age children: development and validation of the Calcium Counts! food frequency questionnaire. Am J Hum Biol. 2010;22:180–6. doi: 10.1002/ajhb.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole TJ. The LMS, method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 38.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 41.Ducher G, Bass SL, Saxon L, Daly RM. Effects of repetitive loading on the growth-induced changes in bone mass and cortical bone geometry: a 12-month study in pre/peri- and postmenarcheal tennis players. J Bone Miner Res. 2011;26:1321–9. doi: 10.1002/jbmr.323. [DOI] [PubMed] [Google Scholar]
- 42.Kontulainen S, Sievanen H, Kannus P, Pasanen M, Vuori I. Effect of long-term impact-loading on mass, size, and estimated strength of humerus and radius of female racquet-sports players: a peripheral quantitative computed tomography study between young and old starters and controls. J Bone Miner Res. 2002;17:2281–9. doi: 10.1359/jbmr.2002.17.12.2281. [DOI] [PubMed] [Google Scholar]
- 43.Seeman E. Periosteal bone formation—a neglected determinant of bone strength. N Engl J Med. 2003;349:320–3. doi: 10.1056/NEJMp038101. [DOI] [PubMed] [Google Scholar]
- 44.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–25. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valerio G, Galle F, Mancusi C, et al. Prevalence of overweight in children with bone fractures: a case control study. BMC Pediatr. 2012;12:166. doi: 10.1186/1471-2431-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res. 2013;471:1199–207. doi: 10.1007/s11999-012-2621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J Bone Miner Res. 2000;15:2011–8. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]