Abstract
Cutaneous leishmaniasis is a disease characterized by ulcerating skin lesions, the resolution of which requires an effective, but regulated, immune response that limits parasite growth without causing permanent tissue damage. While mechanisms that control the parasites have been well studied, the factors regulating immunopathologic responses are less well understood. IL-22, a member of the IL-10 family of cytokines, can contribute to wound healing, but in other instances promotes pathology. Here we investigated the role of IL-22 during leishmania infection, and found that IL-22 limits leishmania-induced pathology when a certain threshold of damage is induced by a high dose of parasites. Il22 -/- mice developed more severe disease than wild-type mice, with significantly more pathology at the site of infection, and in some cases permanent loss of tissue. The increased inflammation was not due to an increased parasite burden, but rather was associated with the loss of a wound healing phenotype in keratinocytes. Taken together, these studies demonstrate that during cutaneous leishmaniasis, IL-22 can play a previously unappreciated role in controlling leishmania-induced immunopathology.
Introduction
Cutaneous leishmaniasis is a major neglected tropical disease affecting about 12 million people globally [1]. The spectrum of clinical manifestations in cutaneous leishmaniasis ranges from self-limiting nodules to non-healing ulcers with a highly inflammatory immune response, and the disease is caused by several different species of leishmania that reside within phagocytic cells. Control of the parasites requires IFN-γ produced by CD4+ Th1 cells [2]. However in spite of a Th1 response, some patients exhibit severe non-healing lesions [3,4]. Thus, in addition to controlling the parasites, regulating the inflammatory response is essential for disease control. TNF-α [5,6], IL-1β [7,8] and IL-17 [9,10] have all been implicated in promoting pathology in leishmaniasis, and damage caused by cytolytic CD8 T cells can also contribute to these immunopathologic responses[11–14]. IL-10 can regulate some of these immunopathologic responses [10,15]. Since drug treatment is often ineffective [5], and no human vaccine exists for the disease, a better understanding of the factors that mediate lesion resolution is essential to help develop new immunotherapies for the disease.
Recently, members of the IL-10 subfamily have been identified as key players in the wound healing process [16–18]. IL-22 is a prominent member of this family, and can instruct non-immune cells, such as epithelial cells and fibroblasts, to proliferate, migrate, and mend the extracellular matrix after injury [19,20]. These functions are important in maintaining surface barrier integrity and protection against subsequent infections. Additionally, IL-22 has been shown to induce the production of antimicrobial peptides from epithelial cells in order to maintain a balanced commensal population and prevent dysbiosis [21–23]. However, while IL-22 is important for tissue protection and contributes to wound healing in the skin, gut, and lungs [20,24,25], it can also be pathogenic in other inflammatory conditions, such as psoriasis [26]. These pathologic responses are mediated by some of the same functions of IL-22 that are protective, including uncontrolled proliferation and the production of inflammatory molecules [26–29]. Why IL-22 is protective in some situations and pathologic in others is unclear, but may depend on the amount of IL-22 produced, as well as the presence of other inflammatory cytokines such as IL-17 [29,30].
Like in some patients, the lesions of C57BL/6 mice normally heal after L. major infection. In order to determine if IL-22 contributes to resolution of a leishmanial infection, we infected Il22 -/- mice with Leishmania major and L. braziliensis and monitored the course of infection. We found that Il22 -/- mice exhibited increased tissue pathology compared with infections in wild-type mice. The absence of IL-22 did not influence the parasite burden, but rather led to higher levels of keratin 6a and keratin 16, both of which have been implicated in inhibiting the wound healing capabilities of keratinocytes [31,32]. We discovered that a role for IL-22 was only evident with high doses of parasites, suggesting that a threshold of inflammation might have to be reached before IL-22 contributed to tissue protection. Taken together, our results demonstrate a previously unknown role for IL-22 in limiting pathology during leishmania infection.
Materials and Methods
Ethics statement
This study was conducted according to the principles specified in the Declaration of Helsinki and under local ethical guidelines (Ethical Committee of the Maternidade Climerio de Oliveira, Salvador, Bahia, Brazil; and the University of Pennsylvania Institutional Review Board). This study was approved by the Ethical Committee of the Federal University of Bahia (Salvador, Bahia, Brazil) (010/10) and the University of Pennsylvania IRB (Philadelphia, PA) (813390). All patients provided written informed consent for the collection of samples and subsequent analysis. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee, University of Pennsylvania Animal Welfare Assurance Number A3079-01.
Mice
Female C57BL/6 mice 6–8 weeks old were purchased from the National Cancer Institute (Frederick, MD). B6.IL22 (Il22 -/-) were donated by Pfizer (Cambridge, MA). All mice were maintained in specific pathogen-free facilities at the University of Pennsylvania. Prior to infection, mice were anesthetized using a ketamine and xylazine mixture and monitored until mice were fully awake. At the end of the experiments, mice were humanely euthanized using carbon dioxide inhalation. All procedures were performed in accordance with the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
Parasite and infection
L. major (WHO /MHOM/IL/80/Friedlin wild-type L. major) and L. braziliensis (MHOM/BR/01/BA788) [33] promastigotes were grown to the stationary phase in Schneider’s Drosophila medium (GIBCO BRL, Grand Island, NY, USA) supplemented with 20% heat-inactivated fetal bovine serum (FBS, Invitrogen USA), 2 mM l-glutamine, 100 U of penicillin and 100 μg of streptomycin per mL. Infective-stage promastigotes (metacyclics) were isolated from 4–5 day old (L. major) and 7 day old (L. braziliensis) stationary culture by density gradient separation by Ficoll (Sigma) [34]. Mice were inoculated intradermally in the ear with 10 uL of PBS containing 2 x 106 L. major metacyclic promastigotes. In some experiments mice were infected with a low does of parasite (2 x 103) or a super-high dose of parasites (2 x 107). Lesion development was measured weekly by ear thickness with a digital caliper (Fisher Scientific). Mice were also assessed for pathology, using the following score system: no lesion (0), swelling/redness (1), deformation of the ear pinna (2), ulceration (3), partial tissue loss (4), and total tissue loss (5). Parasite burden in lesion tissues was assessed using a limiting dilution assay as previously described [35]. Freeze-thawed antigen (FTAg) was obtained from stationary-phase promastigotes of L. major. Soluble leishmanial antigen (SLA) was prepared from L. braziliensis parasites are previously described[36].
Patients and recall assays
All cutaneous leishmaniasis patients were seen at the health post in Corte de Pedra, Bahia, Brazil, which is a well-known area of L. braziliensis transmission. The criteria for diagnosis were a clinical picture characteristic of cutaneous leishmaniasis in conjunction with parasite isolation or a positive delayed-type hypersensitivity response to Leishmania antigen, plus histological features of cutaneous leishmaniasis. In all cases, the immunological analysis was performed before therapy. For cell culture and IL-22 measurement, peripheral blood mononuclear cells (PBMCs) were obtained from heparinized venous blood layered over a Ficoll-Hypaque gradient (GE Healthcare), then washed and resuspended in RPMI1640 medium with 10% heat inactivated human AB serum (Sigma) at a concentration of 3 x 106 cells/mL. These cells were added to 24-well plates and were kept unstimulated or were stimulated with soluble leishmania antigen (5 ug/mL) for 96 h at 37C in 5% CO2. The supernatants were collected and stored frozen until analyzed for cytokines. IL-22 was measured by enzyme-linked immunosorbent assay (Pfizer).
Preparation of dermal sheets
The dorsal and ventral sides of the mouse ear were split mechanically and placed dermis side down in a 24 wells plate in RPMI 1640 containing 0.25 mg/mL of Liberase TL (Roche, Diagnostics Corp.) and 10 μg/mL DNase I (Sigma-Aldrich). Ears were incubated for 90 min at 37°C in a 24-well plate. Dermal cell suspensions were prepared by dissociation on 70- um cell strainer (Falcon) in PBS containing 0.05% BSA and 20 μM EDTA.
In vitro restimulation and cytokine measurements
For measurements of antigen-specific cytokine production in the mouse, the retroauricular lymph node was removed, mechanically dissociated, and single cell suspensions were prepared. Cells were resuspended in RPMI 1640 supplemented with 10% of FBS, 2 mM l-glutamine, 100 U of penicillin and 100 μg of streptomycin per mL and 0.05 μM of β-mercaptoethanol. 4 x106 cells per mL were plated in 24-well plates. Cells were incubated at 37°C in 5% CO2 with 20 x106 L.major or L. braziliensis FTAg/mL. Supernatants were harvested 72 h after stimulation and assayed using a sandwich enzyme-linked immunosorbent assay (ELISA) for IFN-γ (eBioscience), IL-17 (eBioscience), and IL-22 (Pfizer). Cytokine concentrations were calculated from standard curves with a detection limit of 0.030 ng/mL.
Antibodies and flow cytometry
Single cell suspensions from the ear were obtained as described above. For analysis of surface markers and intracellular cytokines, some cells were incubated for 4 h with 10 μg/mL of brefeldin A, 50 ng/mL of PMA and 500 ng/mL ionomycin (Sigma-Aldrich). Before staining, cells were incubated with an anti-Fcγ III/II receptor and 10% rat-IgG in PBS containing 0.1% BSA. Cells were stained for dead cells (Invitrogen) and surface markers (CD4, CD8β [BioLegend], CD45, Ly6G, CD11b [eBioscience]) followed by fixation with 2% of formaldehyde. The data were collected using LSRII flow cytometer (BD) and analyzed using FlowJo software (Tree Star).
RNA isolation, purification, and quantitative real-time PCR
Total RNA was extracted from ear tissue samples in 700uL of RLT lysis buffer (Qiagen). The sample was homogenized using a tissue homogenizer (FastPrep-24, MP Biomedical), and total RNA was extracted according to the recommendations of the manufacturer and further purified using the RNeasy Mini kit (QIAGEN). RNA was reverse transcribed using high capacity cDNA Reverse Transcription (Applied Biosystems). Real-time RT-PCR was performed on a ViiA 7 Real-Time PCR System (Applied Biosystems). Relative quantities of mRNA for several genes was determined using SYBR Green PCR Master Mix (Applied Biosystems) and by the comparative threshold cycle method, as described by the manufacturer. mRNA levels for each sample were normalized to Ribosomal protein S11 gene (RPS11). Primers were designed using Primer Express software (version 2.0; Applied Biosystems); Rps11, forward, 5’-CGTGACGAAGATGAAGATGC-3’ and reverse, 5’-GCACATTGAATCGCACAGTC-3’; Krt5, forward, 5'-TTTGCCTCCTTCATCGACA-3' and reverse, 5'-CGGATCCAGGTTCTGCTTTA-3'; Krt14, forward, 5'-ATCGAGGACCTGAAGAGCAA-3' and reverse, 5'-TCGATCTGCAGGAGGACATT-3'; Krt6a, forward, 5'-GAGGAGAGGGAGCAGATCAA-3' and reverse, 5'-CACTTGGTGTCCAGGACCTT-3'; Krt16, forward, 5'-TTGAGGACCTGAAGAGCAAGA-3' and reverse, 5'-CCTGGCATTGTCAATCTGC-3'; Il22, 5'-ATGAGTTTTTCCCTTATGGGGAC-3' and reverse, 5'-GCTGGAAGTTGGACACCTCAA-3'; Il22bp, forward, 5'-TCAGCAGCAAAGACAGAAGAAAC-3' and reverse, 5'-GTGTCTCCAGCCCAACTCTCA-3'; Ifng, forward, 5'-GACTGTGATTGCGGGGTTGT-3' and reverse, 5'-GGCCCGGAGTGTAGACATCT-3'; Il4, forward, 5'-ATGGAGCTGCAGAGACTCTT-3' and reverse, 5'-AAAGCATGGTGGCTCAGTAC-3'; Il17, forward, 5'-CATGAGTCCAGGGAGAGCTT-3' and reverse, 5'-GCTGAGCTTTGAGGGATGAT-3'; Il12p40, forward, 5'-TTGAAAGGCTGGGTATCGGT-3' and reverse, 5'-GAATTTCTGTGTGGCACTGG-3', Tnfa, forward, 5'-TCACTGGAGCCTCGAATGTC-3' and reverse, 5'-GTGAGGAAGGCTGTGCATTG-3'; Il6, forward, 5’-ACAGAAGGAGTGGCTAAGGA-3’ and reverse, 5’-CACCATGGAGCAGCTCAG- 3’; Il10, forward, 5'-TGTCCAGCTGGTCCTTTGTT-3’ and reverse, 5'-ACTGCACCCACTTCCCAGT-3'; Tgfb, forward, 5’-CGCTGCTACTGCAAGTCAGA-3’ and reverse, 5’-GGTAGCGATCGAGTGTCCA-3’; Il27p28, forward, 5'-GATTGCCAGGAGTGAACCTG-3' and reverse, 5'-CGAGGAAGCAGAGTCTCTCAG-3'; Il1a, forward, 5’-TTGGTTAAATGACCTGCAACA-3’ and reverse, 5’-GAGCGCTCACGAACAGTTG-3’; Il1b, forward, 5’-TTGACGGACCCCAAAAGAT-3’ and reverse, 5’- GATGTGCTGCTGCGAGATT-3’.
Microbiome collection, sequencing, and analysis
Two independent experiments were performed using littermates as controls, with n = 9–10 mice per cohort for a total of 9 Il22 -/- mice, 3 Il22 +/- mice, and 7 Il22 +/+ mice. Microbiota was collected from the ear of the mouse using a swab (Catch-all Sample Collection Swab, Epicentre) moistened in Yeast Cell Lysis Buffer (from MasterPure Yeast DNA Purification Kit; Epicentre). DNA was isolated from swab specimens and amplification of the 16S-V4 region was performed as previously described [37]. Sequencing of 16S rRNA amplicons was performed at the Penn Next Generation Sequencing Core using the Illumina MiSeq platform with 150 bp paired-end ‘V2’ chemistry.
Pre-processing and community characterization of 16S rRNA sequence data
Sequence pre-processing followed methods previously described [37], but modified by subsampling at 11,000 sequences per sample. QIIME 1.6.0[38] was used for initial stages of sequence analysis. Sequences were clustered into OTUs (operational taxonomic units, a proxy for ‘species’) using UCLUST [39] at 97% sequence similarity. Bacterial diversity was calculated using the following alpha diversity indices: 1) Shannon diversity index; 2) Faith’s phylogenetic distance (PD); and 3) Chao I species estimation; and 4) number of observed OTUs. Relative abundance of bacteria was calculated based on taxonomic classification of sequences using the RDP classifier [40] at a confidence threshold of 0.8. Microbiome data was analyzed with the R statistical software environment (www.r-project.org). Statistical significance was determined using two-sample Wilcoxon tests and corrected for multiple comparisons by FDR where appropriate.
Statistical analysis
Results represent means ± SEM. Data were analyzed using Prism 5.0 (GraphPad Software, San Diego, CA). Statistical significance was determined by one-way ANOVA when comparing more than two groups and by an unpaired two-tailed Student’s t test to compare means of lesion sizes, parasite burdens, and cytokine production from different groups of mice. Statistically significant differences were defined as * when p values <0.05.
Results
Leishmania infections induce the production of IL-22
Since IL-22 can have tissue protective effects, we investigated whether IL-22 might help control pathology during infection with leishmania. We first asked whether infection with leishmania parasites led to an increase in IL-22 production. C57BL/6 (wild-type) mice were infected with L. major and were euthanized at 3 days, 2 weeks or 5 weeks after infection. Cells from the draining lymph nodes were stimulated with leishmanial antigen and cytokine levels were assessed. As expected during infection with L. major, IFN-γ and IL-17 were produced in an antigen dependent manner (Fig 1A and 1B). As early 3 days after infection there was an antigen specific production of IL-22, which was maintained at 2 and 5 weeks post-infection (Fig 1C). Because we know CD4+ T cells can be a major source of IL-22 [27,41], we wanted to determine if CD4+ cells contributed to the antigen-specific production of IL-22 during L. major infection. Thus, C57BL/6 mice were infected with L. major and depleted of CD4+ cells in vivo using a neutralizing antibody 2 days prior to sacrificing the mice. Cells were harvested from the draining lymph nodes at 3 days post-infection and cultured with media alone or with L. major antigen for 72 hours. Antigen stimulated cells from anti-CD4 treated mice produced significantly less IL-22 than untreated mice (Fig 1D), demonstrating that the production of IL-22 is dependent on the presence CD4+ T cells. We also observed the production of IL-22 from cells of mice infected with another species of the parasite, L. braziliensis (data not shown). To determine if patients infected with L. braziliensis parasites also produced IL-22, peripheral blood mononuclear cells (PBMCs) from leishmaniasis patients were isolated and cultured with leishmanial antigen. Similar to cells from mice, PBMCs from infected patients, but not healthy subjects, produced IL-22 in response to stimulation with leishmanial antigen (Fig 1E), suggesting that IL-22 may be important in human patients as well as in experimental murine infections.
Fig 1. IL-22 is induced during leishmania infections.
C57BL/6 mice were intradermally infected with 2 x 106 L. major promastigotes metacyclics in the ear. Cells from the draining lymph nodes of infected mice were isolated and cultured for 72 hours with media or leishmania antigen. Supernatants were collected and (A) IFN-γ (B) IL-17, and (C) IL-22 release was measured by ELISA. (D) C57BL/6 mice were intradermally infected with 2 x 106 L. major promastigotes metacyclics in the ear and two days later treated with anti-CD4. Mice were euthanized on day 3 and cells from the draining lymph nodes were isolated and cultured for 72 hours with media or leishmania antigen to analyze IL-22 production by ELISA. (E) PBMCs from healthy subjects and L. braziliensis infected patients were cultured for 72 hours with media or L. braziliensis antigen. Supernatants were collected and analyzed for IL-22 release by ELISA. Data are representative of at least 3 independent experiments, with 3–5 mice per group. Error bars indicate mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
IL-22 limits pathology during leishmania infection independent of parasite control
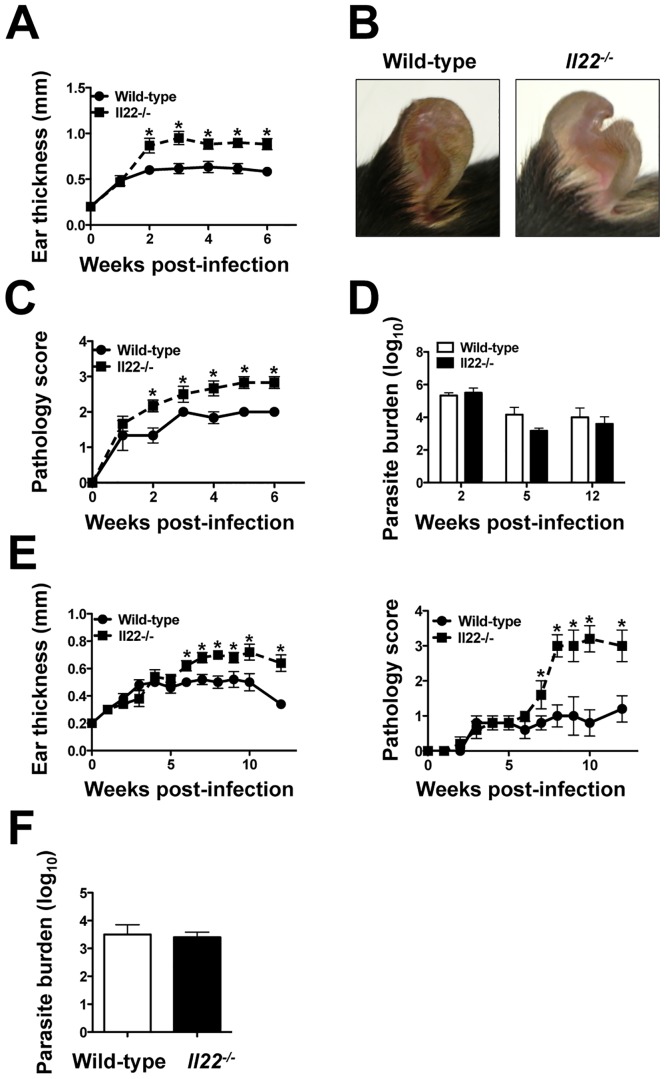
To determine if IL-22 plays a protective role during the course of infection with leishmania, C57BL/6 and Il22 -/- mice were infected with L. major and the disease monitored. Il22 -/- mice exhibited larger lesions compared with wild-type mice (Fig 2A). We noticed that in addition to greater swelling, the ears of Il22 -/- mice often exhibited more severe pathology than wild-type mice, and in some cases led to tissue loss at the site of infection (Fig 2B). To quantify these changes, we employed a scoring system that better captures the pathology associated with leishmania infection. As seen in Fig 2C, Il22 -/- mice exhibited greater pathology than wild-type mice infected with L. major. To determine if the increased pathology observed following L. major infections was due to higher parasite levels in Il22 -/- mice, we assessed the parasite burden in wild-type and Il22 -/- mice at 2, 5 and 12 weeks of infection, and found no significant differences (Fig 2D).
Fig 2. IL-22 limits pathology during leishmania infection.
(A) C57BL/6 (wild-type) and Il22 -/- mice were intradermally infected with 2 x 106 L. major promastigote metacyclics and euthanized at various time-points after infection. The lesions were assessed by measuring ear thickness for 6 weeks. (B) Pictures were taken at 5 weeks post-infection. (C) Lesion pathology was determined based on a pathology score. (D) Number of parasites in the lesions was quantified using a limiting assay at 2, 5, and 12 weeks post-infection. (E) Wild-type and Il22 -/- mice were intradermally infected with 2 x 106 L. braziliensis promastigote metacyclics and lesions were assessed by measuring ear thickness and given a pathology score for 12 weeks and (F) parasite numbers were quantified using a limiting dilution assay in the lesions at 12 weeks post-infection. Data are representative of at least 2 independent experiments, with 3–5 mice per group. Error bars indicate mean ± SEM, *p < 0.05.
L. braziliensis parasites are known to induce a particularly strong inflammatory response in patients, and also cause mucosal leishmaniasis, the most severe form of the disease [42]. Therefore, we asked if IL-22 regulated the lesion resolution in this infection as well. We infected wild-type and Il22 -/- mice with L. braziliensis and followed the course of infection. As with L. major, L. braziliensis infected Il22 -/- mice had significantly larger lesions than wild-type mice with more pathology, but no differences in the number of parasites within the lesions (Fig 2E and 2F).
IL-22 maintains wound-healing capabilities in the skin during L. major infection
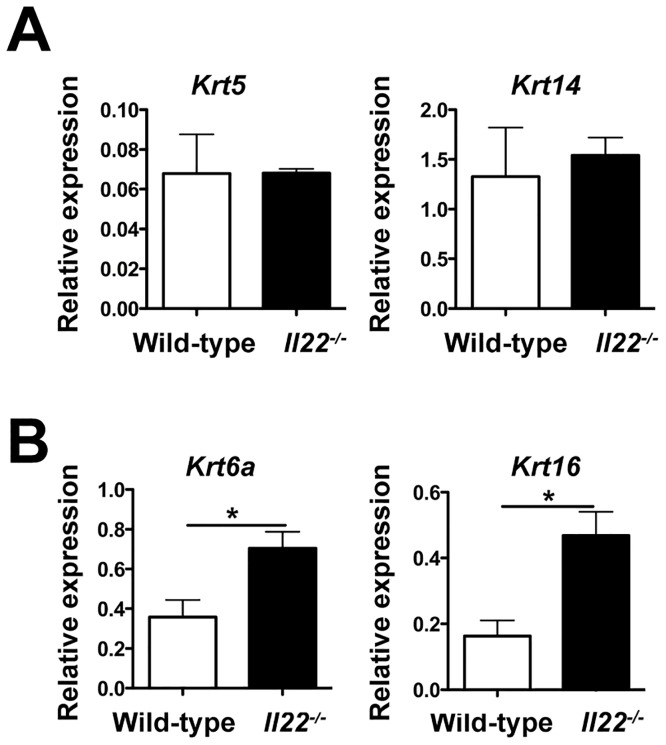
The resolution of a leishmanial lesion is analogous to wound healing, which requires keratinocyte proliferation and differentiation [43]. Therefore, we analyzed the expression of several genes at the peak of infection to assess keratinocyte functions in the lesions of wild-type and Il22 -/- mice. We observed no difference in the expression of keratin 5 and keratin 14, both of which are expressed in proliferating keratinocytes, between wild-type and Il22 -/- mice (Fig 3A). We then decided to look at other keratins which are upregulated in chronic wounds and can inhibit the ability of keratinocytes to efficiently heal wounds and damage [31,32]. We observed that Il22 -/- mice had higher expression of keratin 6a and keratin 16 (Fig 3B), both of which are known to inhibit keratinocytes migration. Thus, one role of IL-22 during cutaneous leishmaniasis may be to promote wound healing capabilities of keratinocytes by regulating the expression of keratins involved in migration and differentiation.
Fig 3. IL-22 regulates the expression of skin repair genes during L. major infection.
(A-B) Wild-type and Il22 -/- mice were intradermally infected with 2 x106 L. major promastigote metacyclics and RNA was isolated from the lesions at 5 weeks post-infection to assess gene expression. Data are represented as relative expression to housekeeping gene rps11 and are representative of at least 2 independent experiments, with 3–5 mice per group. Error bars indicate mean ± SEM, *p < 0.05.
The requirement for IL-22 depends on parasite burden and inflammation
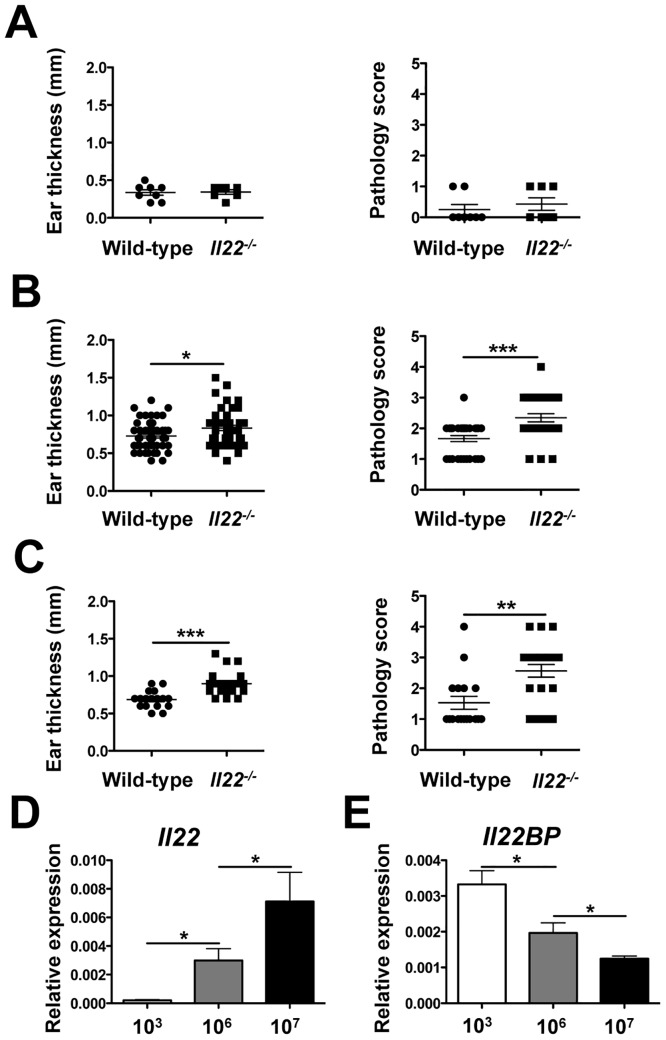
Recently, it was reported that IL-22 does not play a role during a low dose of infection with L. major [44]. Our results, taken together with other findings prompted us to consider the possibility that IL-22 might only be required when a threshold of inflammation and tissue damage was present. To test this hypothesis, we infected mice with a super high dose of parasites (2 x 107), an intermediate dose (2 x 106), and with a low dose of parasites (2 x 103), and followed the course of infection. Because we noticed some variability between experiments, we decided to pool data from multiple experiments and compare pathology at the peak of infection. Similar to recent findings in which mice were infected with a low dose of parasites [44], we observed no difference in the lesion size or pathology between wild-type and Il22 -/- mice when infected with 2 x 103 parasites (Fig 4A). On the other hand, Il22 -/- mice infected with 2 x 106 and 2 x 107 parasites had more pathology than their wild-type counterparts (Fig 4B and 4C). We euthanized these animals at 5 weeks post-infection and assessed their parasite burdens. As expected from the results described above, no differences were observed in the parasite burden between wild-type and Il22 -/- mice (data not shown). We then measured levels of IL-22 expression in the lesions, and found significantly higher expression of Il22 mRNA when mice were infected with more parasites (Fig 4D). The IL-22 binding protein (IL-22BP) is a soluble receptor that inhibits IL-22 signaling through its receptors [45]. Thus, we examined the expression of Il22BP in wild-type mice infected with L. major infection. Unlike IL-22, IL-22BP was expressed at significantly lower levels when mice were infected with more parasites (Fig 4E). These results suggest that following infection with high numbers of parasites IL-22 is induced to a greater extent and less inhibited by IL-22BP, and that IL-22 helps regulate the pathology associated with a higher parasite burden.
Fig 4. The requirement for IL-22 is parasite dose dependent.
Lesion sizes and pathology scores were compiled from several experiments at 5 weeks post-infection from wild-type and Il22 -/- mice that were intradermally infected with (A) 2 x 103, (B) 2 x 106, or (C) 2 x 107 L. major metacyclics. RNA was isolated from the lesions of wild-type mice infected with L. major to assess (D) Il22 and (E) Il22BP expression. Data are represented as relative expression to housekeeping gene rps11 and are representative of at least 2 independent experiments, with 3–5 mice per group. Error bars indicate mean ± SEM, *p < 0.05,*p < 0.01, ***p < 0.001.
IL-22 does not modulate the skin microbiome at the steady state
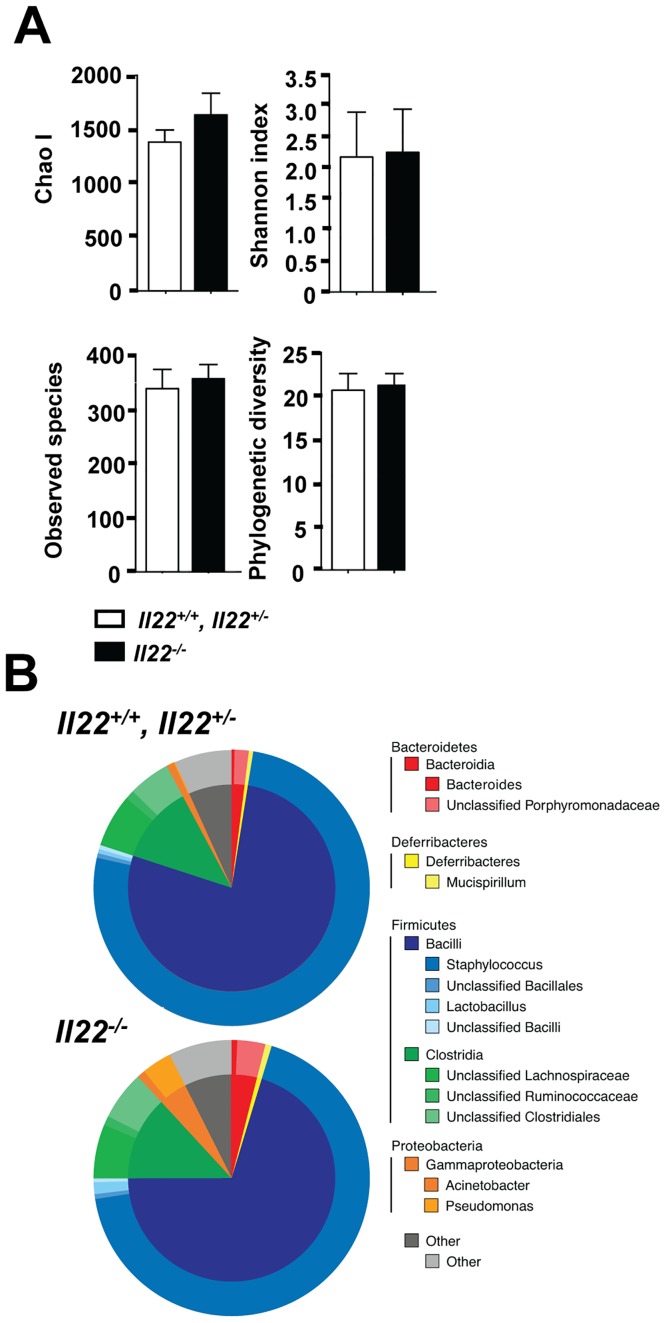
Recent studies indicate that the skin microbiome influences the pathology associated with leishmania infection [46]. Since IL-22 regulates the production of antimicrobial peptides (AMPs) [41,47], we considered the possibility that homeostatic levels of IL-22 might influence AMP levels, resulting in changes in the skin microbiome and consequently disease development. To test this idea, the ears of uninfected Il22 +/+/Il22 +/- and Il22 -/- littermates were swabbed to extract bacterial DNA. 16S ribosomal RNA genomic sequencing was performed and the skin microbiome was analyzed. In two independent experiments (n = 9 Il22 -/- mice and n = 10 control littermate mice) no significant differences in bacterial diversity were observed between littermate controls and Il22 -/- mice (Fig 5A). There were also no differences in the relative abundance of the bacterial communities between controls and Il22 -/- mice (Fig 5B). These findings indicate that Il22 -/- mice do not have a dysbiotic skin microbiome responsible for the increased pathology.
Fig 5. IL-22 does not modulate the skin microbiome at the steady state.
Swabs were collected from Il22 +/+, Il22 +/-, and Il22 -/- cohoused littermates and bacterial DNA was isolated and sequenced. (A) Within sample diversity was calculated using four commonly utilized alpha metrics: Chao I, Shannon Index, Observed Species, and Faith’s Phylogenetic Diversity. (B) The microbiome composition was calculated at multiple phylogenetic levels. The outer ring represents the relative contributions of the 12 most prevalent genera. The inner ring represents the corresponding class for each genus. The remaining genera were compiled into the “Other” category depicted in gray. Data are representative of 2 independent experiments, with 4–5 mice per group.
IL-22 does not regulate inflammatory cell infiltrate, but rather limits tissue damage during L. major infection
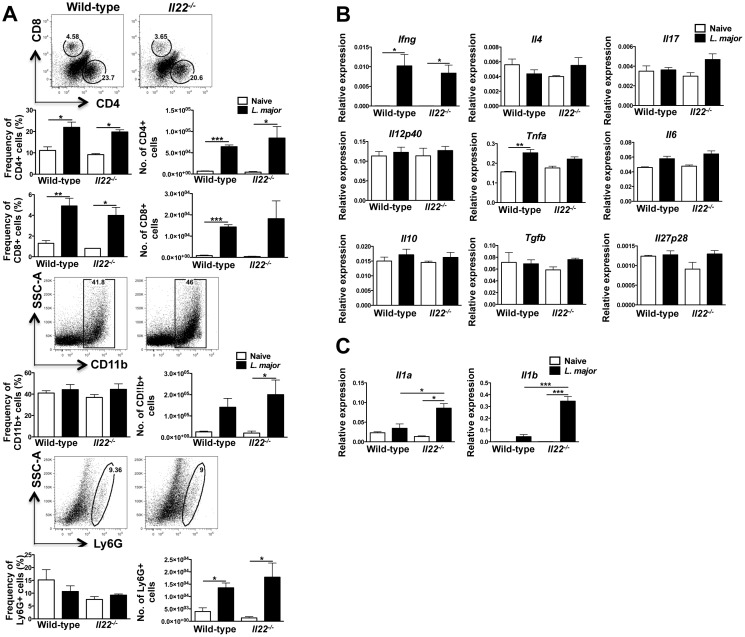
Because we observed more pathology and inflammation in the Il22 -/- mice, we wanted to determine if there was increased inflammatory cell infiltrate in the lesions of these mice. We examined the presence of CD4+ and CD8+ T cells, CD11b+ myeloid cells, and neutrophils in the lesions of L. major infected wild-type and Il22 -/- mice at the peak of infection. While there was an increase over naïve skin in the frequency and numbers of T cells of wild-type and Il22 -/- lesions and in the numbers of myeloid cells, there was no difference in these populations between wild-type and Il22 -/- mice (Fig 6A). We also assessed transcript levels of inflammatory and regulatory cytokines in the lesions of wild-type and Il22 -/- mice. As expected, Ifng levels were increased following infection, and there was a similar increase in wild-type and Il22 -/- mice. There were minimal or no changes in Il4, Il17, Tnfa, Il12a, Il6, Il10, Tgfb and Il27p28 gene expression between naïve skin and leishmanial lesions, and no significant differences between wild-type and Il22 -/- mice (Fig 6B). However, we found that the lesions of Il22 -/- mice had higher expression of Il1a and Il1b compared with wild-type mice (Fig 6C). The expression of these molecules is often observed in inflamed tissue and can be induced and released when cells encounter tissue damage [48]. Although there were no differences in the immune response between wild-type and Il22 -/- mice, increased expression of these damage-associated molecules demonstrates that Il22 -/- mice directly or indirectly regulate their production during infection with L. major.
Fig 6. IL-22 does not alter the immune response during L. major infection.
Wild-type and Il22 -/- mice were intradermally infected with 2 x106 L. major promastigote metacyclics and cells from 5 week old lesions were collected and analyzed by flow cytometry. (A) Representative dot plots and bar graphs depict frequencies and total cell numbers of CD4+, CD8+, CD11b+, and LY6G+ cells. (B-C) RNA was isolated from the lesions of wild-type mice infected with L. major to assess gene expression. Data are represented as relative expression to housekeeping gene rps11 and are representative of at least 3 independent experiments, with 3–5 mice per group. Error bars indicate mean ± SEM, *p < 0.05,*p < 0.01, ***p < 0.001.
Discussion
Our results uncover a previously unknown role for IL-22 during cutaneous leishmaniasis. While a pathologic and inflammatory role for IL-22 has been reported in other cutaneous diseases [26,28,49], we found that IL-22 does not promote increased inflammation during infection with Leishmania spp. Rather, Il22 -/- mice exhibited more tissue damage than wild-type mice when infected with L. major or L. braziliensis, suggesting that IL-22 limits pathology when a threshold of inflammation is reached during leishmaniasis.
Our results demonstrate that the production of IL-22 is dependent on the presence of CD4+ T cells, which have previously been shown to produce IL-22 [27,41]. However, γδ T cells, NK cells, ILCs and neutrophils are other potential sources of IL-22 that might contribute to the IL-22 observed in these lesions [26,50–52]. Interestingly, the production of IL-22 appeared to be dose-dependent, such that mice infected with higher doses of L. major expressed higher levels of IL-22 in the lesions. Inflammation and damage in other models of disease have been shown to induce IL-22 expression [21,27,29,53,54], consistent with our findings that higher doses of L. major elicit more inflammation and higher expression of IL-22. Conversely, we observed a decrease in the expression of the IL-22 antagonist, IL-22BP, in mice with higher doses of the parasite. This inverse relationship of IL-22/IL-22BP regulating tissue damage has also been observed during Hepatitis C and schistosome infections [55]. Thus, we hypothesize that having a high IL-22/IL-22BP ratio is required to limit pathology.
In order to determine whether the immune response was influenced by the absence of IL-22, we assessed cytokine responses within leishmanial lesions of Il22 -/- mice. Changes in the balance of Th1 and Th2 cytokines is often associated with increased susceptibility to L. major, but since there were no differences in the parasite burden it was not surprising that the mRNA levels of Ifng, Tnfa, Il12p40 and Il4 were similar in both wild-type and Il22 -/- mice. Moreover, there were no differences in the cellular infiltrate of T cells and myeloid cells in the lesions of wild-type and Il22 -/- mice. These results prompted us to consider other ways in which IL-22 can provide tissue protection during inflammation.
L. major infection leads to the development of ulcerated lesions that eventually resolve due to tissue remodeling at the infection site [56–58]. IL-22 promotes wound healing by increasing epithelial cell proliferation, decreasing the differentiation of keratinocytes and inducing anti-apoptotic molecules in keratinocytes [19,59–61]. Thus, one way IL-22 may enhance wound healing in leishmaniasis is by regulating L. major induced keratinocyte death. Additionally, IL-22 stimulates fibroblasts to produce extracellular matrix proteins, as well as increases the differentiation of myofibroblasts that help to contract wounds [20], and both of these functions could be critical in the resolution of leishmanial lesions. In this study, we found another mechanism in which IL-22 contributes to wound healing and tissue repair. Keratinocyte proliferation and differentiation are critically regulated processes during wound repair [43]. Upon injury, activated keratinocytes migrate to close the wound, while basal keratinocytes proliferate at the basement membrane [62,63]. In order for a cell to proliferate and repair the basement membrane, differentiation must be halted [62]. IL-22 can induce proliferation, but also down-regulate keratinocyte differentiation and keratin expression [19]. Thus, we decided to examine the expression of various proliferation and differentiation markers. While the proliferation markers, keratin 5 and keratin 14 were unaffected by the absence of IL-22, the lesions of Il22 -/- mice expressed higher levels of keratins 6a and 16. These genes are induced in keratinocytes upon injury and are maintained during reepithiliazation. However, the intensity in expression levels of these keratins is important because their overexpression can lead to defects in keratinocyte migration and wound closure [31]. The higher expression of keratins 6a and 16 observed in chronic wounds is consistent with our data showing that Il22 -/- mice have a defect in wound repair during L. major infection. Interestingly, lower expression of keratin 16 or deletion of keratin 6a can enhance keratinocyte migration [32], which may explain the eventual lesion resolution in wild-type mice with lower expression of these keratins. Keratinocyte differentiation and migration are key to wound healing, and thus our results suggest that IL-22 may be important in regulating these processes through keratins 6a and 16 in order to efficiently resolve leishmanial lesions.
While IL-22 protects against certain pathogens, such as Klebsiella pneumonia and Citrobacter rodentium [21,25], in our study we found no evidence that IL-22 contributes to control of L. major or L. braziliensis, as wild-type and Il22 -/- mice contained the same number of parasites in their lesions. These results are similar to those observed with other parasites, such as toxoplasma or schistosomes [64]. However, this is in contrast to visceral leishmaniasis, where the production of IL-22 has been correlated with increased protection [65,66]. How IL-22 promotes resistance in visceral leishmaniasis is unknown, but it is unlikely to be a direct effect on the parasites, since the IL-22R is not expressed on the cells infected with leishmania [67]. Since stromal cells play a role in immunoregulation in visceral leishmaniasis, one possibility is that stimulation of stromal cells by IL-22 might indirectly influence the development of disease [68].
IL-22 helps maintain barrier function in the skin, but when produced at high levels and/or in the context of other proinflammatory cytokines, such as IL-17, IL-22 promotes increased pathology [29]. The factors that determine whether IL-22 will play a protective or pathologic role remain poorly understood, although it has been suggested that the nature of the inflammatory response may be a determining factor [29]. Our results indicate that one factor determining whether IL-22 is important in protection in the skin may be the degree of damage induced. Thus, when Il22 -/- mice were infected with a high dose of parasites, we routinely saw increased pathology in Il22 -/- mice compared with wild-type mice, while we found no differences in the development of lesions in Il22 -/- mice and wild-type mice when the animals were infected with a low dose of parasites. The latter finding would account for the results of a prior study where IL-22 was reported to have no role in L. major infection [44]. These results suggest that the protective role for IL-22 requires a threshold of inflammation that is reached at high parasite doses in this experimental model. This raises the issue of how our murine studies relate to human leishmaniasis. While the initial dose of parasites transmitted by sandflies is much less than the high doses we have studied here, patients also exhibit significantly more pathology than what occurs in low dose infections in mice. Thus, we hypothesize that in more severe forms of cutaneous leishmaniasis, as often seen following L. braziliensis infection, IL-22 might be induced to ensure that even more severe disease does not develop. Consistent with this was our finding that cells from patients made IL-22 in response to stimulation, indicating that there was sufficient damage in the patients to promote IL-22 production.
Taken together, our results in Il22 -/- mice show that IL-22 limits pathology during cutaneous leishmaniasis and suggest that once a certain threshold of damage is reached, IL-22 is expressed at higher levels and limits subsequent damage by maintaining skin barrier integrity and wound healing capacities. In the absence of IL-22, not only do lesions fail to resolve, but higher expression of the inflammatory molecules IL-1α and IL-1β may lead to even greater tissue destruction. Thus, IL-22 plays an important, and previously unappreciated, role in maintaining skin repair properties and limiting inflammation during cutaneous leishmanial infections.
Supporting Information
(PDF)
Acknowledgments
We would like to thank Ba Nguyen and Amanda Tyldsley for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by National Institutes of Health, RO1AI106842 (PS), AR060873 (EAG), Tropical Medicine Research Centers AI30639 (EMC), and T32AI753216 (CG). All funders played a role in the study design, data collection and analysis, decision to publish, and/or preparation of the manuscript.
References
- 1. Kedzierski L. Leishmaniasis Vaccine: Where are We Today? J Glob Infect Dis. 2010;2: 177–185. 10.4103/0974-777X.62881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179: 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70: 6734–6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63: 70–78. [DOI] [PubMed] [Google Scholar]
- 5. Bafica A, Oliveira F, Freitas LA, Nascimento EG, Barral A. American cutaneous leishmaniasis unresponsive to antimonial drugs: successful treatment using combination of N-methilglucamine antimoniate plus pentoxifylline. Int J Dermatol. 2003;42: 203–207. [DOI] [PubMed] [Google Scholar]
- 6. Antonelli LR, Dutra WO, Almeida RP, Bacellar O, Carvalho EM, Gollob KJ. Activated inflammatory T cells correlate with lesion size in human cutaneous leishmaniasis. Immunol Lett. 2005;101: 226–230. [DOI] [PubMed] [Google Scholar]
- 7. Fernandez-Figueroa EA, Rangel-Escareno C, Espinosa-Mateos V, Carrillo-Sanchez K, Salaiza-Suazo N, Carrada-Figueroa G, et al. Disease severity in patients infected with Leishmania mexicana relates to IL-1beta. PLoS Negl Trop Dis. 2012;6: e1533 10.1371/journal.pntd.0001533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voronov E, Dotan S, Gayvoronsky L, White RM, Cohen I, Krelin Y, et al. IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. Int Immunol. 2010;22: 245–257. 10.1093/intimm/dxq006 [DOI] [PubMed] [Google Scholar]
- 9. Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, von Stebut E. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J Immunol. 2009;182: 3039–3046. 10.4049/jimmunol.0713598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez-Lombana C, Gimblet C, Bacellar O, Oliveira WW, Passos S, Carvalho LP, et al. IL-17 mediates immunopathology in the absence of IL-10 following Leishmania major infection. PLoS Pathog. 2013;9: e1003243 10.1371/journal.ppat.1003243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novais FO, Carvalho LP, Graff JW, Beiting DP, Ruthel G, Roos DS, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS Pathog. 2013;9: e1003504 10.1371/journal.ppat.1003504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crosby EJ, Goldschmidt MH, Wherry EJ, Scott P. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS Pathog. 2014;10: e1003970 10.1371/journal.ppat.1003970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. da Silva Santos C, Attarha S, Saini RK, Boaventura V, Costa J, Khouri R, et al. Proteome Profiling of Human Cutaneous Leishmaniasis Lesion. J Invest Dermatol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Novais FO, Carvalho LP, Passos S, Roos DS, Carvalho EM, Scott P, et al. Genomic Profiling of Human Leishmania Braziliensis Lesions Identifies Transcriptional Modules Associated with Cutaneous Immunopathology. J Invest Dermatol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Faria DR, Gollob KJ, Barbosa J Jr, Schriefer A, Machado PR, Lessa H, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73: 7853–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168: 5397–5402. [DOI] [PubMed] [Google Scholar]
- 17. Sa SM, Valdez PA, Wu J, Jung K, Zhong F, Hall L, et al. The effects of IL-20 subfamily cytokines on reconstituted human epidermis suggest potential roles in cutaneous innate defense and pathogenic adaptive immunity in psoriasis. J Immunol. 2007;178: 2229–2240. [DOI] [PubMed] [Google Scholar]
- 18. Sun DP, Yeh CH, So E, Wang LY, Wei TS, Chang MS, et al. Interleukin (IL)-19 promoted skin wound healing by increasing fibroblast keratinocyte growth factor expression. Cytokine. 2013;62: 360–368. 10.1016/j.cyto.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 19. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005;174: 3695–3702. [DOI] [PubMed] [Google Scholar]
- 20. McGee HM, Schmidt BA, Booth CJ, Yancopoulos GD, Valenzuela DM, Murphy AJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. 2013;133: 1321–1329. 10.1038/jid.2012.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14: 282–289. 10.1038/nm1720 [DOI] [PubMed] [Google Scholar]
- 22. Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34: 122–134. 10.1016/j.immuni.2010.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zenewicz LA, Yin X, Wang G, Elinav E, Hao L, Zhao L, et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J Immunol. 2013;190: 5306–5312. 10.4049/jimmunol.1300016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206: 1465–1472. 10.1084/jem.20082683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14: 275–281. 10.1038/nm1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Belle AB, de Heusch M, Lemaire MM, Hendrickx E, Warnier G, Dunussi-Joannopoulos K, et al. IL-22 is required for imiquimod-induced psoriasiform skin inflammation in mice. J Immunol. 2012;188: 462–469. 10.4049/jimmunol.1102224 [DOI] [PubMed] [Google Scholar]
- 27. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445: 648–651. [DOI] [PubMed] [Google Scholar]
- 28. Ma HL, Liang S, Napierata L, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. 2008;118: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sonnenberg GF, Nair MG, Kirn TJ, Zaph C, Fouser LA, Artis D. Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J Exp Med. 2010;207: 1293–1305. 10.1084/jem.20092054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guilloteau K, Paris I, Pedretti N, Boniface K, Juchaux F, Huguier V, et al. Skin Inflammation Induced by the Synergistic Action of IL-17A, IL-22, Oncostatin M, IL-1{alpha}, and TNF-{alpha} Recapitulates Some Features of Psoriasis. J Immunol. 2010. [DOI] [PubMed] [Google Scholar]
- 31. Wawersik MJ, Mazzalupo S, Nguyen D, Coulombe PA. Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Mol Biol Cell. 2001;12: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rotty JD, Coulombe PA. A wound-induced keratin inhibits Src activity during keratinocyte migration and tissue repair. J Cell Biol. 2012;197: 381–389. 10.1083/jcb.201107078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Moura TR, Novais FO, Oliveira F, Clarencio J, Noronha A, Barral A, et al. Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis. Infect Immun. 2005;73: 5827–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp Parasitol. 2001;99: 97–103. [DOI] [PubMed] [Google Scholar]
- 35. Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat Med. 2004;10: 1104–1110. [DOI] [PubMed] [Google Scholar]
- 36. Reed SG, Badaro R, Masur H, Carvalho EM, Lorenco R, Lisboa A, et al. Selection of a skin test antigen for American visceral leishmaniasis. Am J Trop Med Hyg. 1986;35: 79–85. [DOI] [PubMed] [Google Scholar]
- 37. Hannigan GD, Hodkinson BP, McGinnis K, Tyldsley AS, Anari JB, Horan AD, et al. Culture-independent pilot study of microbiota colonizing open fractures and association with severity, mechanism, location, and complication from presentation to early outpatient follow-up. J Orthop Res. 2014;32: 597–605. 10.1002/jor.22578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7: 335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26: 2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 40. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73: 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Oliveira CI, Brodskyn CI. The immunobiology of Leishmania braziliensis infection. Front Immunol. 2012;3: 145 10.3389/fimmu.2012.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276: 75–81. [DOI] [PubMed] [Google Scholar]
- 44. Brosch S, Dietze-Schwonberg K, Lopez Kostka S, Lorenz B, Haak S, Becher B, et al. Disease Control in Cutaneous Leishmaniasis Is Independent of IL-22. J Invest Dermatol. 2014. [DOI] [PubMed] [Google Scholar]
- 45. Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A. 2001;98: 9511–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science. 2012;337: 1115–1119. 10.1126/science.1225152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336: 1321–1325. 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Carta S, Lavieri R, Rubartelli A. Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines? Front Immunol. 2013;4: 123 10.3389/fimmu.2013.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorgammat+ innate lymphocytes and gammadelta T cells initiate psoriasiform plaque formation in mice. J Clin Invest. 2012;122: 2252–2256. 10.1172/JCI61862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carlsen ED, Jie Z, Liang Y, Henard CA, Hay C, Sun J, et al. Interactions between Neutrophils and Leishmania braziliensis Amastigotes Facilitate Cell Activation and Parasite Clearance. J Innate Immun. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xu X, Weiss ID, Zhang HH, Singh SP, Wynn TA, Wilson MS, et al. Conventional NK cells can produce IL-22 and promote host defense in Klebsiella pneumoniae pneumonia. J Immunol. 2014;192: 1778–1786. 10.4049/jimmunol.1300039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, et al. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One. 2011;6: e21799 10.1371/journal.pone.0021799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29: 947–957. 10.1016/j.immuni.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med (Berl). 2009;87: 451–454. [DOI] [PubMed] [Google Scholar]
- 55. Sertorio M, Hou X, Carmo RF, Dessein H, Cabantous S, Abdelwahed M, et al. IL-22 and IL-22 binding protein (IL-22BP) regulate fibrosis and cirrhosis in hepatitis C virus and schistosome infections. Hepatology. 2015;61: 1321–1331. 10.1002/hep.27629 [DOI] [PubMed] [Google Scholar]
- 56. Elso C, Kumar B, Smyth G, Foote S, Handman E. Dissociation of disease susceptibility, inflammation and cytokine profile in lmr1/2 congenic mice infected with Leishmania major. Genes Immun. 2004;5: 188–196. [DOI] [PubMed] [Google Scholar]
- 57. Elso CM, Roberts LJ, Smyth GK, Thomson RJ, Baldwin TM, Foote SJ, et al. Leishmaniasis host response loci (lmr1-3) modify disease severity through a Th1/Th2-independent pathway. Genes Immun. 2004;5: 93–100. [DOI] [PubMed] [Google Scholar]
- 58. Baldwin T, Sakthianandeswaren A, Curtis JM, Kumar B, Smyth GK, Foote SJ, et al. Wound healing response is a major contributor to the severity of cutaneous leishmaniasis in the ear model of infection. Parasite Immunol. 2007;29: 501–513. [DOI] [PubMed] [Google Scholar]
- 59. Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39: 1332–1342. [DOI] [PubMed] [Google Scholar]
- 60. Wolk K, Witte E, Wallace E, Docke WD, Kunz S, Asadullah K, et al. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006;36: 1309–1323. [DOI] [PubMed] [Google Scholar]
- 61. Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, et al. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104: 4260–4268. [DOI] [PubMed] [Google Scholar]
- 62. Usui ML, Underwood RA, Mansbridge JN, Muffley LA, Carter WG, Olerud JE. Morphological evidence for the role of suprabasal keratinocytes in wound reepithelialization. Wound Repair Regen. 2005;13: 468–479. [DOI] [PubMed] [Google Scholar]
- 63. Usui ML, Mansbridge JN, Carter WG, Fujita M, Olerud JE. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J Histochem Cytochem. 2008;56: 687–696. 10.1369/jhc.2008.951194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilson MS, Feng CG, Barber DL, Yarovinsky F, Cheever AW, Sher A, et al. Redundant and pathogenic roles for IL-22 in mycobacterial, protozoan, and helminth infections. J Immunol. 2010;184: 4378–4390. 10.4049/jimmunol.0903416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, et al. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest. 2009;119: 2379–2387. 10.1172/JCI38813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ghosh K, Sharma G, Saha A, Kar S, Das PK, Ukil A. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J Infect Dis. 2013;207: 1016–1025. 10.1093/infdis/jis771 [DOI] [PubMed] [Google Scholar]
- 67. Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21: 241–254. [DOI] [PubMed] [Google Scholar]
- 68. Svensson M, Maroof A, Ato M, Kaye PM. Stromal cells direct local differentiation of regulatory dendritic cells. Immunity. 2004;21: 805–816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.