Highlights
-
•
First in vitro culture system for Anaplasma centrale.
-
•
A. centrale infected and grew in two out of 32 tick cell lines tested.
-
•
Potential for safer and more ethical bovine anaplasmosis vaccine.
Keywords: Anaplasmosis, Anaplasma centrale, Vaccine, Tick cell line, In vitro culture
Abstract
Anaplasma centrale has been used in cattle as a live blood vaccine against the more pathogenic Anaplasma marginale for over 100 years. While A. marginale can be propagated in vitro in tick cell lines, facilitating studies on antigen production, immunisation and vector-pathogen interaction, to date there has been no in vitro culture system for A. centrale. In the present study, 25 cell lines derived from 13 ixodid tick species were inoculated with the Israeli vaccine strain of A. centrale and monitored for at least 12 weeks by microscopic examination of Giemsa-stained cytocentrifuge smears. Infection of 19 tick cell lines was subsequently attempted by transfer of cell-free supernate from vaccine-inoculated tick cells. In two separate experiments, rickettsial inclusions were detected in cultures of the Rhipicephalus appendiculatus cell line RAE25 28–32 days following inoculation with the vaccine. Presence of A. centrale in the RAE25 cells was confirmed by PCR assays targeting the 16S rRNA, groEL and msp4 genes; sequenced PCR products were 100% identical to published sequences of the respective genes in the Israeli vaccine strain of A. centrale. A. centrale was taken through three subcultures in RAE25 cells over a 30 week period. In a single experiment, the Dermacentor variabilis cell line DVE1 was also detectably infected with A. centrale 11 weeks after inoculation with the vaccine. Availability of an in vitro culture system for A. centrale in tick cells opens up the possibility of generating a safer and more ethical vaccine for bovine anaplasmosis.
1. Introduction
Anaplasma centrale, first isolated from a heifer in South Africa in 1909 (Theiler, 1911; cited by Herndon et al., 2013), has been used as a live blood vaccine to protect against bovine anaplasmosis caused by Anaplasma marginale for over 100 years. Currently the A. centrale vaccine is used to protect cattle in several African, South American and Middle Eastern countries including Israel. Production of the vaccine involves infecting splenectomised cattle with A. centrale stabilate and harvesting large volumes of blood from them when the rickettsaemia reaches a suitable level (OIE, 2014). Live blood vaccines have a number of disadvantages including risk of co-transmission of other ruminant pathogens, risk of haemolytic disease in calves born to vaccinated dams and requirement for a stringent cold chain. While an in vitro culture system for A. marginale in cell lines derived from the tick Ixodes scapularis has been available for nearly two decades (Munderloh et al., 1996), and has resulted in exponential progress in knowledge and understanding of this pathogen, to date it has not been possible to propagate A. centrale in vitro. Ability to cultivate A. centrale in vitro would open up the possibility of producing vaccine antigen without the need to splenectomise, infect and exsanguinate cattle.
The present study was carried out with the aim of establishing in vitro culture of the Israeli vaccine strain of A. centrale in one or more tick cell lines, taking advantage of the availability in the Tick Cell Biobank (http://www.pirbright.ac.uk/research/Tickcell/Default.aspx) of multiple cell lines derived from five ixodid tick genera.
2. Materials and methods
2.1. Tick cell lines
A panel of 32 tick cell lines derived from 14 ixodid tick species (Table 1) were tested for ability to support infection and replication of A. centrale. The cell lines were grown at either 28 °C or 32 °C in sealed flat-sided culture tubes (Nunc) containing 2.2 ml of complete culture medium (L-15, H-Lac, L-15B, L-15B300, L-15/MEM, L-15/H-Lac, L-15/L-15B or L-15/H-lac/L-15B as described previously (Munderloh and Kurtti, 1989; Munderloh et al., 1999; Bell-Sakyi, 2004). Prior to infection with A. centrale the supernatent medium was removed from each tube, the cell monolayer was washed once with 1 ml of L-15B medium supplemented with 10% FCS, 10% TPB, 0.1% bovine lipoprotein (MP Biomedicals), 2 mM L-glutamine, 15 mM HEPES and 0.1% NaHCO3 (ACGM) to remove traces of antibiotics and 2 ml of ACGM was added to the tube. For cultures receiving blood vaccine, ACGM was further supplemented with 5 μg/ml Amphotericin B (ACGMA).
Table 1.
Tick cell lines tested for ability to support growth of Anaplasma centrale. The original references for the tick cell lines are cited by Alberdi et al. (2012) except where indicated. A. centrale was inoculated (X) as either diluted vaccine or as clarified supernate from already-infected tick cells.
| Tick species | Cell line | Culture medium/incubation temperature | Inoculum |
|
|---|---|---|---|---|
| From vaccine | From tick cells | |||
| Amblomma americanum | AAE2 | L-15B300/32 °C | X | |
| AAE12 | L-15B300/32 °C | X | ||
| Amblyomma variegatum | AVL/CTVM13 | L-15/L-15B/32 °C | X | |
| AVL/CTVM17 | L-15/H-Lac/L-15B/32 °C | X | ||
| Dermacentor albipictus | DALBE3 | L-15B300/32 °C | X | |
| Dermacentor andersoni | DAE15 | L-15B300/32 °C | X | X |
| DAE100T | L-15B300/32 °C | X | X | |
| Dermacentor nitens | ANE58 | L-15B300/32 °C | X | |
| Dermacentor variabilis | DVE1 | L-15B300/32 °C | X | X |
| Hyalomma anatolicum | HAE/CTVM8 | L-15/H-Lac/32 °C | X | |
| HAE/CTVM9 | L-15/MEM/32 °C | X | ||
| Ixodes ricinus | IRE/CTVM19 | L-15/28 °C | X | |
| Ixodes scapularis | IDE2 | L-15B300/32 °C | X | X |
| IDE8 | L-15B/32 °C | X | X | |
| ISE6 | L-15B300/32 °C | X | X | |
| ISE18 | L-15B300/32 °C | X | ||
| Rhipicephalus appendiculatus | RAE/CTVM1 | L-15/28 °C | X | X |
| RAN/CTVM3 | H-Lac/28 °C | X | ||
| RAE25a | L-15B/32 °C | X | X | |
| RA243 | L-15/32 °C | X | X | |
| Rhipicephalus evertsi | REE/CTVM29 | L-15/28 °C | X | |
| REE/CTVM31 | L-15/MEM/28 °C | X | ||
| REN/CTVM32b | L-15/H-Lac/28 °C | X | ||
| Rhipicephalus sanguineus | RSE8 | L-15/L-15B/32 °C | X | |
| RML-RSEc | L-15/MEM/28 °C | X | ||
| Rhipicephalus (Boophilus) decoloratus | BDE/CTVM16 | L-15/28 °C | X | X |
| Rhipicephalus (Boophilus) microplus | BME/CTVM2 | L-15/28 °C | X | X |
| BME/CTVM5 | L-15/MEM/28 °C | X | ||
| BME/CTVM6 | L-15/28 °C | X | ||
| BME/CTVM23 | L-15/32 °C | X | X | |
| BME/CTVM30 | L-15/MEM/28 °C | X | ||
| BmVIII-SCC | L-15/MEM/32 °C | X | ||
Bell-Sakyi (unpublished); derived from developing adult R. evertsi ticks kindly supplied in 2010 by Dr. Ard Nijhof, then of Utrecht Centre for Tick-borne Diseases, Utrecht University, The Netherlands.
Previously deposited in the Tick Cell Biobank as D. variabilis embryo-derived cell line RML-15 (Yunker et al., 1981). However sequencing of a fragment of the 16S rRNA gene (Black and Piesman, 1994) revealed that the cell line was actually derived from R. sanguineus (data not shown). Three embryo-derived R. sanguineus cell lines were established in the same laboratory as RML-15: RML-21, 22 and 23 (Yunker et al., 1984, 1987). As it is now impossible to determine which of the three cell lines was used in the present study, it is here designated RML-RSE.
2.2. Inoculation of tick cell lines with Anaplasma centrale-infected bovine erythrocytes
The Israeli A. centrale blood vaccine comprising bovine erythrocytes with A. centrale rickettsaemia of 20%, cryopreserved with 5% DMSO as 1.8 ml aliquots containing 1 × 108 infected erythrocytes, was prepared at the Kimron Veterinary Institute and stored in the vapor phase of a liquid nitrogen refrigerator prior to and following transfer on dry ice to the Pirbright Institute. For inoculation onto tick cell lines, a vial of vaccine was thawed rapidly by immersion in a 37 °C water bath and the contents were immediately diluted in 9 ml of ACGMA at room temperature. Aliquots of 0.6–0.7 ml were immediately added to tubes of tick cells in ACGMA, the contents of each tube was mixed by gentle rocking 2–3 times, and the cultures were incubated at 28 °C or 32 °C.
2.3. Maintenance and light microscopical analysis of tick cell lines inoculated with A. centrale
The medium of inoculated cultures was changed after 48 h by removal and replacement of 1.0–1.5 ml ACGMA. Thereafter, medium was changed weekly and, after the second week, ACGMA was replaced with ACGM. Cultures were examined weekly for at least 12 weeks by inverted microscope prior to the medium change for evidence of microbial contamination, general cell health and signs of Anaplasma infection. Giemsa-stained cytocentrifuge smears were prepared at 2–3 week intervals from approx. 50 μl of resuspended cells and examined at 500× and 1000× (oil immersion) for presence of A. centrale bacteria. Photomicrographs were taken using a CCD digital camera attached to a Zeiss Axioskop microscope and Zeiss Axiovision software.
2.4. Subculture of A. centrale within and between tick cell lines
Subcultures were carried out onto a fresh cell culture of the same tick cell line by transfer of 0.3–0.5 ml of supernatent medium without centrifugation. For subculture into different tick cell lines, supernatent medium from tick cell cultures previously inoculated with material containing A. centrale was clarified by centrifugation at 1500 × g for 5 min to remove intact cells, and 0.3–0.5 ml of clarified supernate was added to fresh cultures of the recipient cell line. All subcultures were incubated at 32 °C regardless of the normal incubation temperature of the recipient cell line.
3. Molecular analysis
DNA was extracted from residual A. centrale blood vaccine and from tick cell cultures using a DNeasy blood and tissue kit (Qiagen) following the manufacturer’s instructions for Gram-negative bacteria. DNA extracts were used as templates for PCRs targeting fragments of the 16S rRNA and groEL (HSP60) genes for Ehrlichia and Anaplasma detection (Schouls et al., 1999; Lew et al., 2003). Furthermore, a multiplex PCR assay for A. centrale and A. marginale msp4 gene fragments (Shkap et al., 2008) was also performed. Lastly, a PCR specific for amplification of the A. marginale msp1-α gene (Shkap et al., 2002) was carried out. A negative control containing water instead of template DNA was included in all PCRs. PCR primer pairs, sizes of the amplicons (bp) and annealing temperatures used in the assays are shown in Table 2. All the PCRs were performed as described by the respective authors.
Table 2.
PCR primer pairs and conditions used in this study. W = T or A; Y = T or G; R = G or A; M = A or C; N = G or A or T or C.
| Target organism | Target gene | Primer sequence 5′ → 3′ | Amplicon size (bp) | Annealing temp. (°C) | Reference |
|---|---|---|---|---|---|
| Ehrlichia/Anaplasma | 16S rRNA | 16S8FE: GGAATTCAGAGTTGGATCMTGGYTCAG B-GA1B: CGGGATCCCGAGTTTGCCGGGACTTCTTCT |
468 | 67–57 (touchdown) | Schouls et al., 1999 |
| groEL | HSPC: AAATGGCGAATGTTGTWGTYAC HSPB: TTARAARCCRCCCATRCCRCCCATGCC |
1650 | 60 | Lew et al., 2003 | |
| A. centrale | msp4* | F: CATGGGGCATGAATCTGTG R: AATTGGTTGCAGTGAGCGC |
395 | 53 | Shkap et al., 2008 |
| A. marginale | msp4* | F: CATCTCCCATGAGTCACGAAGTGGC R: GCTGAACAGGAATCTTGCTCC |
761 | 53 | Shkap et al., 2008 |
| msp1α | F: GCATTACAACGCAACGCTT R: ACCTTGGAGCGCATCTCTT |
515-687 | 56 | Shkap et al., 2002 | |
*Both genes are targeted in the same multiplex PCR.
Positive PCR products were purified using a High Pure PCR Product Purification kit (Roche Life Science) following the manufacturer’s instructions. Purified amplification products were sequenced in the forward and reverse directions, and homology searches were performed in the NCBI database using the BLAST search programme (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Sequences were aligned using the European Bioinformatics Institute multisequence software ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2) for multiple sequence alignment.
4. Results
4.1. Isolation of centrale from blood vaccine inoculum
Twenty-five tick cell lines (Table 1) were inoculated with thawed, diluted A. centrale blood vaccine derived from one of two different cryovials in separate experiments. Cytocentrifuge smears prepared from undiluted inoculum showed numerous intrerythrocytic rickettsial bodies (Fig. 1A). Of the tick cell lines, all survived the inoculation except BME/CTVM6 in which all cells died within 24 h. In all cases, the inoculum formed a loose plasma clot incorporating some of the tick cells from the monolayer; this clot did not appear to have any deleterious effect on the cultures, and was gradually broken up during resuspension for preparation of cytocentrifuge smears.
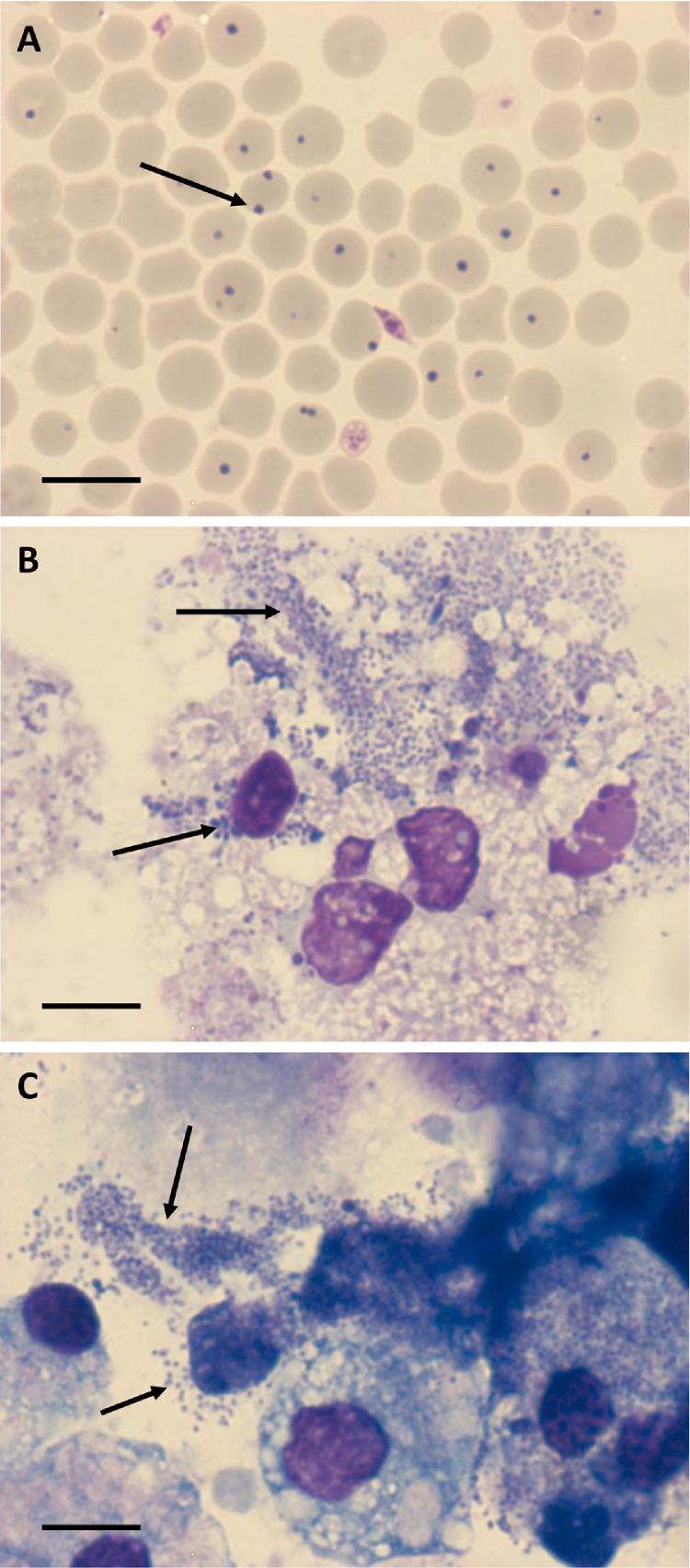
Fig. 1.
Anaplasma centrale in Giemsa-stained cytocentrifuge smears of (A) thawed, undiluted blood vaccine, (B) RAE25 cells 122 days post inoculation and (C) DVE1 cells 88 days post inoculation. Arrows indicate bacteria. Scale bar = 10 μm.
Only two of the 25 tick cell lines showed evidence of A. centrale infection during the subsequent 12-week observation period: Rhipicephalus appendiculatus embryo-derived RAE25 and Dermacentor variabilis embryo-derived DVE1, both incubated at 32 °C. Small numbers of cell-free or cell-associated atypical Anaplasma-like bacteria were seen in Giemsa-stained cytocentrifuge smears of RAE25 cells inoculated with diluted vaccine from both cryovials after 28–32 days incubation. These gradually became more numerous and increasingly typical in appearance over the subsequent weeks (Fig. 1B). By 12 weeks post inoculation, the cultures were heavily infected and dead and dying cells and abundant extracellular debris were visible by inverted microscope examination of cultures, and by 18 weeks many, though not all, of the RAE25 cells were dead. Typical A. centrale bacterial inclusions were first seen in a few DVE1 cells inoculated with diluted vaccine from one of the two cryovials after 11 weeks in culture. Infected cells (Fig. 1C) gradually became more numerous, reaching an infection rate of 5% at 6 months post inoculation.
4.2. Confirmation of A. centrale identity by PCR
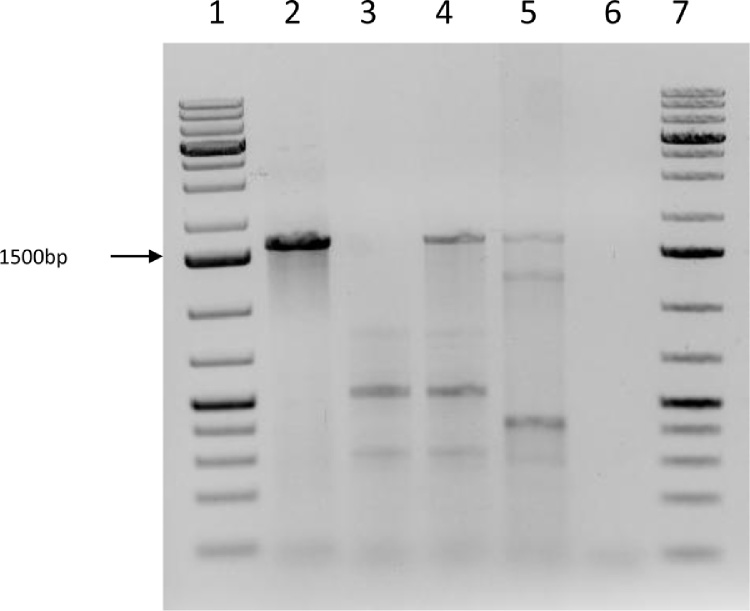
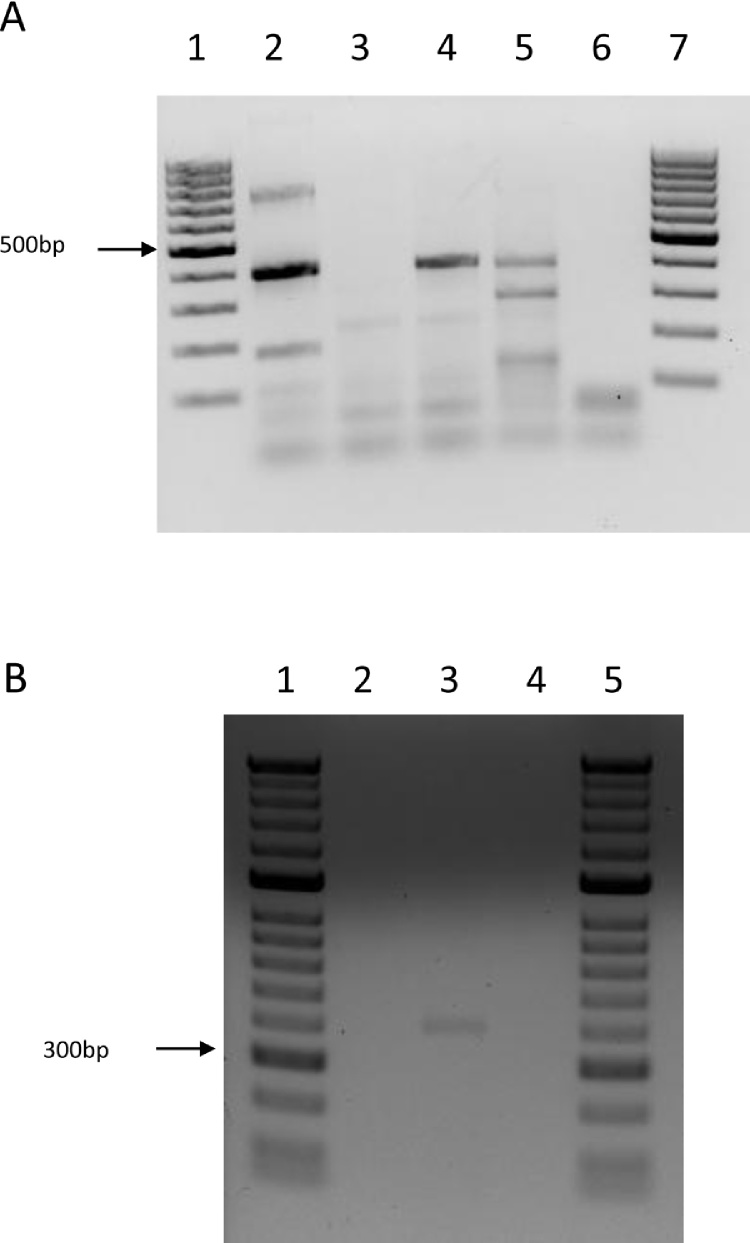
At 6 weeks post inoculation, DNA was extracted from Anaplasma-positive and control uninoculated RAE25 cells, from Anaplasma-inoculated IDE8 cells (an I. scapularis embryo-derived cell line that supports growth of other Anaplasma spp. but in which there was no evidence of replicating bacteria in Giemsa-stained smears) and from residual A. centrale blood vaccine stored at −20 °C in the original cryovials since the day of inoculum preparation. PCR amplification of a fragment of the 16S rRNA gene produced bands of the expected size (468 bp) in the blood vaccine and in the RAE25 and IDE8 cells previously inoculated with diluted A. centrale blood vaccine (Fig. 2). PCR amplification of a fragment of a second Anaplasma gene, groEL, from the same samples produced a very strong band of the expected size (1650 bp) in the vaccine, a fainter band in the A. centrale-inoculated RAE25 cells, and a very faint band in the A. centrale-inoculated IDE8 cells (Fig. 3). A multiplex PCR targeting the msp4 genes of both A. centrale and A. marginale produced bands of the expected size for A. centrale in vaccine and A. centrale-inoculated RAE25 and IDE8 cells in decreasing order of strength, and a very faint band of the expected size for A. marginale in the blood vaccine (Fig. 4A). The PCR targeting the msp1-a gene of A. marginale failed to amplify any PCR product from the vaccine or the A. centrale-inoculated RAE25 and IDE8 cells (data not shown), indicating that the A. centrale blood vaccine was not contaminated with A. marginale. The multiplex PCR was also used at 6 months post inoculation to confirm that the bacteria seen in DVE1 cells inoculated with blood vaccine were A. centrale (Figure 4B). There was no amplification of specific products of the expected size from the uninfected RAE25 or DVE1 cells in any of the PCR assays.
Fig. 2.
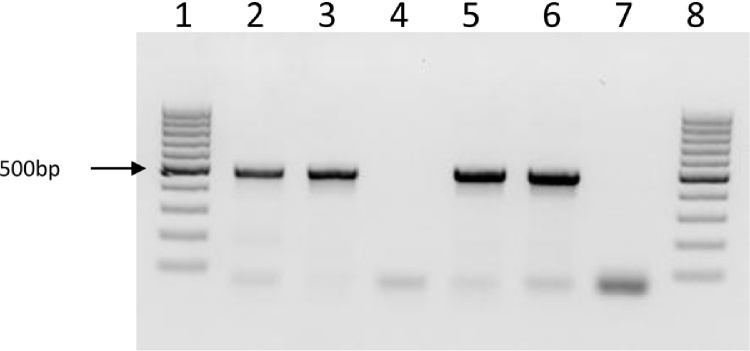
PCR amplification using primers targeting a 468 bp fragment of the 16S rRNA gene with a sequence conserved between Anaplasma and Ehrlichia spp. DNA extracted on day 42 post inoculation. Lanes 1 and 8: molecular weight markers; lane 2: positive control (Ehrlichia ruminantium DNA); lane 3: inoculum (Anaplasma centrale Israeli blood vaccine); lane 4: uninfected RAE25 cells; lane 5: RAE25 cells inoculated with A. centrale; lane 6: IDE8 cells inoculated with A. centrale; lane 7: negative control (no DNA).
Fig. 3.
PCR amplification using primers targeting a 1650 bp fragment of the groEL gene of Anaplasma centrale. DNA extracted on day 42 post inoculation. Lanes 1 and 7: molecular weight markers; lane 2: inoculum (A. centrale Israeli blood vaccine); lane 3: uninfected RAE25 cells; lane 4: RAE25 cells inoculated with A. centrale; lane 5: IDE8 cells inoculated with A. centrale; lane 6: negative control (no DNA).
Fig. 4.
Multiplex PCR amplification using primers targeting fragments of the msp4 genes of Anaplasma marginale (761 bp) and Anaplasma centrale (396 bp). A. DNA extracted on day 42 post inoculation. Lanes 1 and 7: molecular weight markers; lane 2: inoculum (A. centrale Israeli blood vaccine); lane 3: uninfected RAE25 cells; lane 4: RAE25 cells inoculated with A. centrale blood vaccine; lane 5: IDE8 cells inoculated with A. centrale blood vaccine; lane 6: negative control (no DNA). B. DNA extracted 6 months post inoculation. Lanes 1 and 5: molecular weight markers; lane 2: uninfected DVE1 cells; lane 3: DVE1 cells inoculated with A. centrale blood vaccine; lane 4: negative control (no DNA).
All the sequences obtained from the 16S rRNA (426 bp), groEL (1545 bp) and A. centrale msp4 (357 bp) PCR products were identical to each other for each gene, and showed 100% identity with the sequences corresponding to the A. centrale Israeli vaccine strain deposited in GenBank (Table 3). The 16S rRNA sequences were also 100% identical to those of the Australian A. centrale vaccine strain and A. centrale isolated from Rhipicephalus simus ticks in South Africa (Potgieter and Van Rensberg, 1987), while the groEL sequences were 100% similar to the Australian vaccine stain and 99.2% similar to the R. simus-derived strain (Table 3). Unexpectedly, the sequence of the amplicon of the expected size for A. marginale msp4 (703 bp) obtained from the blood vaccine in the multiplex PCR was also homologous to the A. centrale msp4 sequence (Table 3), indicating that the A. centrale vaccine was not contaminated with A. marginale. For all three genes examined, levels of similarity with published sequences of A. marginale strains from Israel, USA and Australia were lower than with the A. centrale strains: 99.1% for 16S rRNA, 97.1–97.4% for groEL and 80.1–83.1% for msp4 (Table 3).
Table 3.
Similarity of the partial genes amplified in the present study with Anaplasma centrale and Anaplasma marginale sequences deposited in GenBank.
| Anaplasma spp. (GenBank accession no.) | 16S rRNA % identity (bp) | groEL % identity (bp) |
msp4 % identity (bp) |
|
|---|---|---|---|---|
| Small amplicon | Large amplicon | |||
| A. centrale str. Israel (CP001759) | 100 (426/426) | 100(1545/1545) | 100(357/357) | 100(703/703) |
| Anaplasma centrale strain vaccine from Australia (AF414868, AF414867) | 100 (426/426) | 100(1545/1545) | – | – |
| A. centrale from South Africa from Rhipicephalus simus (AF414869, AF414866) | 100 (426/426) | 99.2(1532/1545) | – | – |
| A. marginale from Israel non-tailed (AF414875, AF414861, AY786993) | 99.1(422/426) | 97.3(1503/1545) | 82.1(293/357) | 81.8(570/703) |
| A. marginale from Israel tailed (AF414876, AF414862, AY786994) | 99.1(422/426) | 97.3(1503/1545) | 82.1(293/357) | 80.1(568/703) |
| A.marginale strain St. Maries from USA (CP000030) | 99.1(422/426) | 97.1(1500/1545) | 82.1(293/357) | 81.2(571/703) |
| A.marginale strain Florida from USA (CP001079) | 99.1(422/426) | 97.2(1501/1545) | 82.4(294/357) | 81.4(572/703) |
| A. marginale strain Gypsy Plains from Australia (CP006846) | 99.1(422/426) | 97.4(1505/1545) | 82.1(293/357) | 83.1(584/703) |
| A.marginale strain Dawn from Australia (CP006847) | 99.1(422/426) | 97.4(1505/1545) | 82.1(293/357) | 81.5(573/703) |
4.3. A. centrale subculture and transfer between different tick cell lines
A. centrale from RAE25 cells was successfully subcultured into fresh RAE25 cells 64 days after culture initiation and, at the time of writing, has been maintained in RAE25 cells through three passages over a 210 day-period. Cell-free supernate from infected RAE25 cells was inoculated onto 18 heterologous tick cell lines (Table 1) including 10 cell lines that failed to become infected following inoculation with A. centrale blood vaccine. Of these, only DVE1 cells became detectably infected with A. centrale during the 12-week observation period, as determined by microscopic examination.
5. Discussion
For the first time, A. centrale has been successfully propagated in vitro in tick cell lines derived from the ixodid species R. appendiculatus and D. variabilis. Amplification and sequencing of three Anaplasma genes confirmed that the bacteria growing in the tick cell lines was indeed A. centrale and not A. marginale. Interestingly, isolation from intraerythrocytic stages of A. centrale was not achieved in any of the cell lines derived from I. scapularis ticks that have been successfully used to isolate and cultivate a wide range of intracellular arthropod-borne bacteria of the genera Anaplasma, Ehrlichia and Rickettsia (reviewed by Bell-Sakyi et al., 2007), Cardinium (Kurtti et al., 1996) and Neoehrlichia (Munderloh et al., 2007). While vaccine-inoculated IDE8 cells were PCR-positive 6 weeks post inoculation, presumably as a result of residual bacterial DNA or non-viable bacteria as reported for tick cell lines inoculated with mammalian stages of Ehrlichia ruminantium (Bell-Sakyi, 2004), no microscopic evidence of bacterial replication was seen at any time in any of the I. scapularis cell lines inoculated with either A. centrale blood vaccine or with A. centrale transferred from infected RAE25 cells.
The R. appendiculatus cell line RAE25 supports growth of several tick-borne bacteria including E. ruminantium (Bell-Sakyi, 2004), Ehrlichia canis, Ehrlichia mineirensis and Anaplasma phagocytophilum (author’s unpublished results) and a spotted fever group Rickettsia (Munderloh et al., 1998), but has not been reported previously to be a suitable cell line for isolation of Anaplasmataceae bacteria from mammalian cells. The D. variabilis cell line DVE1 supports growth of Rickettsia peacockii (Kurtti et al., 2005) and E. canis (author’s unpublished results); in both cases the bacteria were transferred from already-infected cell lines derived from different tick species, making the present study the first report of successful isolation of an intracellular bacterium directly from mammalian cells into DVE1. In contrast to E. ruminantium which, once established in IDE8 cells was readily transferred into heterologous tick cell lines refractory to infection with mammalian stages of the bacterium (Bell-Sakyi, 2004), in the present study it was only possible to transfer A. centrale infection between tick cell lines that were susceptible to infection with the intraerythrocytic stages.
Natural tick transmission of A. centrale has only been reported once; a previously tick-free splenectomised ox infested with 80 adult R. simus collected in the field in South Africa contracted A. centrale infection (Potgieter and Van Rensberg, 1987). Descendants of the same ticks (120–130 adults infected as nymphs) transstadially transmitted A. centrale between splenectomised cattle (n = 2), although it is unclear whether this was the field isolate of A. centrale or the vaccine strain. A single splenectomised ox inoculated with ground-up unfed adult R. simus, previously fed as nymphs on a calf that had contracted the A. centrale vaccine strain in utero, developed patent A. centrale infection. The authors failed to transmit A. centrale between splenectomised cattle by interrupted feeding of the one-host ticks Rhipicephalus (Boophilus) decoloratus and Rhipicephalus (Boophilus) microplus (Potgieter and Van Rensberg, 1987).
More recently, Ueti et al. (2007) demonstrated that when adult male Dermacentor andersoni ticks were acquisition-fed for 7 days on A. centrale-infected cattle, incubated off-host for a further 7 days and then transmission-fed, 54% of midguts and 71% of salivary glands from 150 ticks were PCR-positive for A. centrale. However, presence of A. centrale bacteria in these organs was not demonstrated microscopically, and 100 of these ticks failed to transmit A. centrale to either of two susceptible calves. In a subsequent study, Herndon et al. (2013) reported transmission of A. centrale between calves by batches of 500 adult male D. andersoni acquisition-fed as above, while batches of 100 ticks failed to transmit the pathogen, indicating that male D. andersoni ticks were not efficient vectors of A. centrale despite apparently supporting bacterial infection of midgut and salivary glands.
Shkap et al. (2009) failed to experimentally transmit the Israeli vaccine strain of A. centrale by interrupted feeding (as above) of any of adult male or female Hyalomma excavatum, Rhipicephalus (Boophilus) annulatus or Rhipicephalus sanguineus ticks, although A. centrale DNA was detected in salivary glands of 1/10 R. sanguineus ticks transmission fed for 6 days.
In the present study, cell lines derived from five Rhipicephalus species (including two R. sanguineus cell lines, RSE8 and RML-RSE) and four Dermacentor species (including two D. andersoni cell lines, DAE15 and DAE100T) were tested for ability to support A. centrale infection and growth. Of these, there was no evidence of infection in the cell lines derived from tick species previously reported to be experimentally infectable with A. centrale – RSE8, RML-RSE, DAE15 and DAE100T – while one out of four R. appendiculatus and one D. variabilis cell lines did become infected. As far as we know, neither R. appendiculatus nor D. variabilis have been tested for ability to transmit A. centrale; the results of the present study suggest that the vector capacity of these two tick species should be investigated. As there is no cell line derived from R. simus, it was not possible to compare the in vitro susceptibility of cells from this tick species with those of R. appendiculatus.
Availability of continuous in vitro culture systems for other Anaplasma species resulted in a rapid expansion of knowledge on many different aspects of, in particular, A. marginale and A. phagocytophilum, reviewed by Blouin et al. (2002), Bell-Sakyi et al. (2007) and more recently by Passos (2012). The ability to grow A. centrale in tick cells will facilitate a similar expansion of knowledge of this enigmatic bacterium and, importantly, opens up the possibility of producing A. centrale vaccine in an in vitro system that will be much safer and more controllable than the current in vivo system (OIE, 2014). Following this vital but preliminary step of establishing the in vitro culture system, further research will be needed to determine the safety, immunogenicity and efficacy of tick cell-derived A. centrale as a putative vaccine antigen.
Acknowledgements
The authors would like to thank Ulrike Munderloh and Timothy Kurtti of University of Minnesota and Patricia Holman of Texas A&M University for permission to use their tick cell lines. All tick cell lines used in this study are maintained in the Tick Cell Biobank at The Pirbright Institute. LBS is supported by the United Kingdom Biotechnology and Biological Sciences Research Council’s National Capability Grant to the Pirbright Institute. AMP is supported by Fundación Rioja Salud (FRS/PIF-01/10). ELB is funded by the United Kingdom Biotechnology and Biological Sciences Research Council EASTBIO Doctoral Training Partnership and an Eastern Associations Regional Studentship (EARS).
Contributor Information
Lesley Bell-Sakyi, Email: lesley.sakyi@pirbright.ac.uk.
Ana M. Palomar, Email: ampalomar@riojasalud.es.
Emma L. Bradford, Email: dr01elb14@abdn.ac.uk.
Varda Shkap, Email: shkapv@int.gov.il.
References
- Alberdi M.P., Dalby M.J., Rodriguez-Andres J., Fazakerley J.K., Kohl A., Bell-Sakyi L. Detection and identification of putative bacterial endosymbionts and endogenous viruses in tick cell lines. Ticks Tick-borne Dis. 2012;3:137–146. doi: 10.1016/j.ttbdis.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L. Ehrlichia ruminantium grows in cell lines from four ixodid tick genera Ehrlichia ruminantium grows in cell lines from four ixodid tick genera. J. Comp. Pathol. 2004;130:285–293. doi: 10.1016/j.jcpa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bell-Sakyi L., Zweygarth E., Blouin E.F., Gould E.A., Jongejan F. Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol. 2007;23:450–457. doi: 10.1016/j.pt.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Black W.C., Piesman J. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl Acad. Sci. U. S. A. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin E.F., de la Fuente J., Garcia-Garcia J., Sauer J.R., Saliki J.T., Kocan K.M. Applications of a cell culture system for studying the interaction of Anaplasma marginale with tick cells. Anim. Health Res. Rev. 2002;3:57–68. [PubMed] [Google Scholar]
- Herndon D.R., Ueti M.W., Reif K.E., Noh S.M., Brayton K.A., Agnes J.T., Palmer G.H. Identification of multilocus genetic heterogeneity in Anaplasma marginale subsp. centrale and its restriction following tick-borne transmission. Infect. Immun. 2013;81:1852–1858. doi: 10.1128/IAI.00199-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtti T.J., Munderloh U.G. Tick cell culture: characteristics, growth requirements and applications to parasitology. In: Maramorosch, Mitsuhashi, editors. Invertebrate Cell Culture Applications. Academic Press; New York: 1982. pp. 195–232. [Google Scholar]
- Kurtti T.J., Munderloh U.G., Andreadis T.G., Magnarelli L.A., Mather T.N. Tick cell culture isolation of an intracellular prokaryote from the tick Ixodes scapularis. J. Invertebr. Pathol. 1996;67:318–321. doi: 10.1006/jipa.1996.0050. [DOI] [PubMed] [Google Scholar]
- Kurtti T.J., Simser J.A., Baldridge G.D., Palmer A.T., Munderloh U.G. Factors influencing in vitro infectivity and growth of Rickettsia peacockii (Rickettsiales: Rickettsiaceae), an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni (Acari, Ixodidae) J. Invertebr. Pathol. 2005;90:177–186. doi: 10.1016/j.jip.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew A.E., Gale K.R., Minchin C.M., Shkap V., de Waal D.T. Phylogenetic analysis of the erythrocytic Anaplasma species based on 16S rDNA and GroEL (HSP60) sequences of A. marginale, A. centrale, and A. ovis and the specific detection of A. centrale vaccine strain. Vet. Microbiol. 2003;92:145–160. doi: 10.1016/s0378-1135(02)00352-8. [DOI] [PubMed] [Google Scholar]
- Munderloh U.G., Kurtti T.J. Formulation of medium for tick cell culture. Exp. Appl. Acarol. 1989;7:219–229. doi: 10.1007/BF01194061. [DOI] [PubMed] [Google Scholar]
- Munderloh U.G., Hayes S.F., Cummings J., Kurtti T.J. Microscopy of spotted fever rickettsia movement through tick cells. Microsc. Microanal. 1998;4:115–121. [Google Scholar]
- Munderloh U.G., Yabsley M.J., Murphy S.M., Luttrell M.P., Howerth E.W. Isolation and establishment of the raccoon Ehrlichia-like agent in tick cell culture. Vector-borne Zoonot. Dis. 2007;7:418–425. doi: 10.1089/vbz.2007.0640. [DOI] [PubMed] [Google Scholar]
- Munderloh U.G., Blouin E.F., Kocan K.M., Ge N.L., Edwards W.L., Kurtti T.J. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J. Med. Entomol. 1996;33:656–664. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- Munderloh U.G., Jauron S.D., Fingerle V., Leitritz L., Hayes S.F., Hautman J.M., Nelson C.M., Huberty B.W., Kurtti T.J., Ahlstrand G.G., Greig B., Mellencamp M.A., Goodman J.L. Invasion and intracellular development of the human granulocytic ehrlichiosis agent in tick cell culture. J. Clin. Microbiol. 1999;37:2518–2524. doi: 10.1128/jcm.37.8.2518-2524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE . Manual of Diagnostic tests and Vaccines for Terrestrial Animals. OIE World Organisation for Animal Health; Paris: 2014. Bovine anaplasmosis. pp. 589–600. [Google Scholar]
- Passos L.M.F. In vitro cultivation of Anaplasma marginale and A. phagocytophilum in tick cell lines: a review. Rev. Bras. Parasitol. Vet. Jaboticabal. 2012;21:81–86. doi: 10.1590/s1984-29612012000200002. [DOI] [PubMed] [Google Scholar]
- Potgieter F.T., Van Rensberg L. Tick transmission of Anaplasma centrale. Onderstepoort J. Vet. Res. 1987;54:5–7. [PubMed] [Google Scholar]
- Schouls L.M., Van de Pol I., Rijpkema S.G.T., Schot C.S. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 1999;37:2215–2222. doi: 10.1128/jcm.37.7.2215-2222.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkap V., Molad T., Fish L., Palmer G.H. Detection of the Anaplasma centrale vaccine strain and specific differentiation from Anaplasma marginale in vaccinated and infected cattle. Parasitol. Res. 2002;88:546–552. doi: 10.1007/s00436-002-0612-9. [DOI] [PubMed] [Google Scholar]
- Shkap V., Leibovitz B., Krigel Y., Molad T., Fish L., Mazuz M., Fleiderovitz L., Savitsky I. Concomitant infection of cattle with the vaccine strain Anaplasma marginale ss centrale and field strains of A. marginale. Vet. Microbiol. 2008;130:277–284. doi: 10.1016/j.vetmic.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Shkap V., Kocan K., Molad T., Mazuz M., Leibovich B., Krigel Y., Michoytchenko A., Blouin E., de la Fuente J., Samish M., Mtshali M., Zweygarth E., Fleiderovich E.L., Fish L. Experimental transmission of field Anaplasma marginale and the A. centrale vaccine strain by Hyalomma excavatum, Rhipicephalus sanguineus and Rhipicephalus (Boophilus) annulatus ticks. Vet. Microbiol. 2009;134:254–260. doi: 10.1016/j.vetmic.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Theiler, A., 1911. Further investigations into anaplasmosis of South African cattle. First report of the Director of Veterinary Research. Union of South Africa, Johannesburg, South Africa. Cited by Herndon et al. 2013.
- Ueti M.W., Reagan J.O., Knowles D.P., Scoles G.A., Shkap V., Palmer G.H. Identification of midgut and salivary glands as specific and distinct barriers to efficient tick-borne transmission of Anaplasma marginale. Infect. Immun. 2007;75:2959–2964. doi: 10.1128/IAI.00284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker C.E., Cory J., Meibos H. Continuous cell lines from embryonic tissues of ticks (Acari: Ixodidae) In Vitro. 1981;17:139–142. doi: 10.1007/BF02618071. [DOI] [PubMed] [Google Scholar]
- Yunker C.E., Cory J., Meibos H. Tick tissue and cell culture: applications to research in medical and veterinary acarology and vector-borne disease. In: Griffiths, Bowman, editors. Acarolog 6, Vol 2. Ellis Horwood; Chichester: 1984. pp. 1082–1088. [Google Scholar]
- Yunker C.E., Tully J.G., Cory J. Arthropod cell lines in the isolation and propagation of tickborne spiroplasmas. Curr. Microbiol. 1987;15:45–50. [Google Scholar]