Abstract
Metastatic spread to the central nervous system (CNS) is a common and devastating manifestation of major cancer types. Its incidence is associated with poor prognosis manifested by neurological deterioration leading to diminished quality of life and an extremely short median survival. CNS metastasis is becoming an increasingly important clinical problem. This is especially the case for certain types of cancers for which effective treatments of visceral disease are available. As a result of the present limitations in treating CNS metastases, this manifestation of tumor progression remains an unmet clinical need. Despite its significance, our general understanding of the mechanisms that regulate the brain-metastatic phenotype is currently meager. Both the analysis of mechanistic aspects of brain metastasis and the development of effective treatments necessitate the use of appropriate in vivo models that recapitulate the interaction of the tumor cells with the microenvironment of the brain. Here we review the available preclinical models of CNS metastasis and their use as tools to advance knowledge of the biology of the disease (with the aim of identifying relevant molecular determinants, prognostic biomarkers, and therapeutic targets) as well as examining effective approaches for treatment.
Keywords: Preclinical metastasis models, CNS, Experimental metastasis, Spontaneous metastasis, Therapy
1. Introduction
Metastatic dissemination to the central nervous system (CNS) is a common and devastating manifestation of major cancer types (i.e. lung, breast, melanoma and renal cancer). The incidence of CNS metastases can be as high as 80%, as is the case of malignant melanoma [1–3] and is invariably associated with dismal prognosis manifested by progressive neurological deterioration and a short median survival, often measured in weeks [4]. Historically, metastasis to non-CNS organs has been the primary source of morbidity and mortality [1]. However, as our ability to detect and treat systemic visceral metastatic disease improves, treatment of CNS metastases is becoming an increasingly important issue [5–7]. Indeed this is already relevant for certain malignancies where therapies that are effective in controlling, for a time, systemic metastatic disease are currently available [8] but which eventually result in relapses in the CNS. These relapses are likely, at least in part, due to the impermeability of the blood–brain barrier (BBB) to most presently available therapeutic agents. In this respect, the CNS has been noted as a sanctuary site for the development of metastatic disease [9]. Thus, the successes obtained with a number of new drugs and therapies, such as trastuzumab for the treatment of erbB-2/Her2 positive breast cancer, that are able to control metastatic disease in sites such as the liver and lungs, allow patients to survive longer, such that they have a greater chance of eventually presenting with CNS metastases [10]. Indeed, the importance of CNS as sanctuary site is underlined by the fact that even when patients achieve long-term remission, half will experience CNS metastases as the only site of relapse [11]. For such patients, the prospects for long-term survival may be defined by the extent to which brain metastases can be controlled [12,13].
Despite the increasing clinical relevance of CNS metastases, therapeutic agents aimed at treating this manifestation of disease are limited. While techniques such as stereostatic radiosurgery or neurosurgery offer treatment options, their use is limited to instances where only one or a few small CNS metastases (<3 cm diameter) are present [2]. If these treatment options fail or are not applicable (e.g. extensive intracranial metastatic disease), chemotherapy is then utilized as the next line of therapy [14]. However, as noted above, the systemic use of chemotherapeutic approaches to treat cerebral metastases is severely limited by the blood–brain barrier (BBB). This is additionally compounded by the current poor understanding of the cellular, molecular and physiologic processes that mediate the brain-metastatic phenotype. The present inability to treat CNS metastases clearly increases the need for developing and using preclinical models of CNS metastases to study the biology and treatment of such lesions [15].
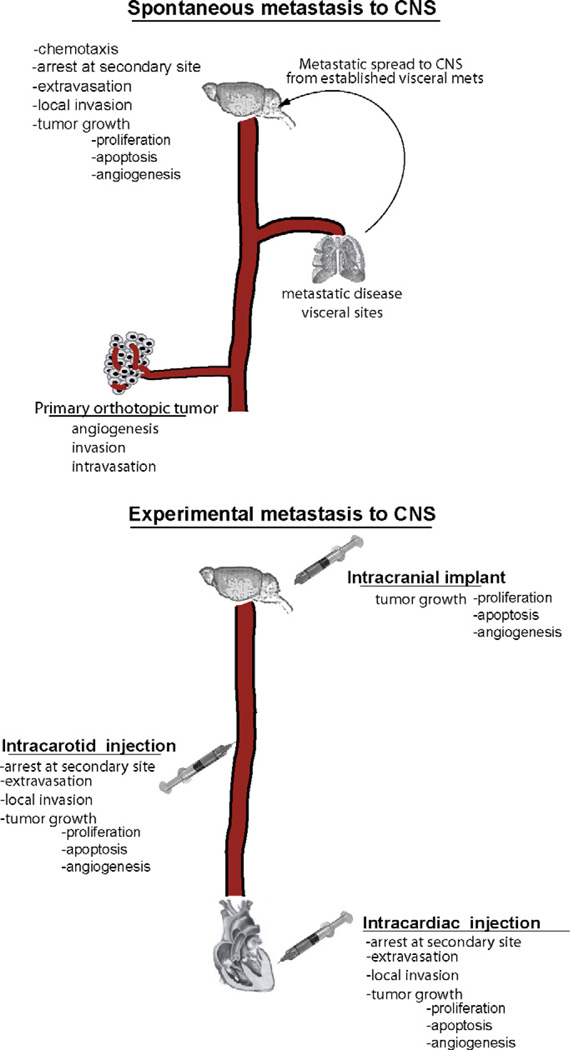
The in vivo metastasis models used to study brain metastatic disease fall into two categories: experimental and spontaneous (Fig. 1). The application of each model depends largely on the question that is being investigated. This question in turn dictates the manner in which the tumor cells are inoculated so as to recreate the desired features of the specific process to be examined [10]. Here we discuss the strengths and limitations of these preclinical models and their potential role in advancing our understanding of the disease and the development of more effective treatments.
Fig. 1.
Comparison of spontaneous and experimental models of CNS metastasis and the steps relevant to the formation of cerebral metastases in each model.
2. Experimental metastasis models of CNS metastasis
Examination of the CNS metastatic process has primarily been achieved through the use of the so-called experimental or ‘artificial’ models of metastasis. These models represent a variation of the traditional metastasis assays in which tumor cells are implanted by means of intravenous injection to target the lung; in this case, cells are delivered to the CNS by means of intracardiac (IC) or intracarotid artery (ICA) injections. The cells are delivered in this manner in an attempt to bypass the pulmonary vascular bed, which is the first capillary bed encountered when cells are delivered intravenously through tail-vein injections. While advantageous in a number of respects (e.g. controlled number of cells delivered that result in the formation of colonies of relatively uniform size and generally short time course for the development of CNS metastases [16]), these models introduce several significant complications (Table 1). For instance, while IC injection allows circumvention of the lungs, it allows dissemination of tumor cells to other visceral sites by means of the arterial circulation. Thus, if cells are competent for growth in other tissues such as the liver or bone marrow, this delivery method may lead to non-CNS metastases which might then become the primary site of concern [10]. More precise targeting of the brain can be achieved by the technically challenging method of ICA injection [17] or through direct intracranial implantation of tumor cells. While introducing a higher level of complexity requiring specialized equipment (stereotactic apparatus), intracranial implantation represents the more direct approach to target the brain parenchyma but which unfortunately bypasses all earlier stages of the metastatic cascade and can thus allow growth of tumor cells within the brain parenchyma which otherwise might not be able to colonize this site [10,17] (Table 2).
Table 1.
Advantages and disadvantages associated with the preclinical models of brain-metastatic disease.
| Experimental | Spontaneous | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Table 2.
Genes relevant to brain metastasis identified through the use of experimental models of brain.
| Molecule | Model | Observation | Ref |
|---|---|---|---|
| Melanotransferrin | SK-Mel 28 melanoma | Blocking melanotransferrin with mAb L235 reduces development of brain metastases | [30] |
| TCF4 | H2030 and PC9 lung adenocarcinoma | Downregulation resulted in decreased brain metastatic activity | [31] |
| Cox2, HBEGF, ST6GALNAC5 | MDA-MB231 breast cancer | Increased incidence | [25] |
| Stat3 | A375 melanoma | Increased incidence; upregulation of MMP2, VEGF and FGF2 | [32] |
| HER2 | MDA-MB231 breast cancer | Increase size of brain metastases | [33] |
| P75 | 70W melanoma | Upregulated in brain metastatic variant | [34] |
| TIMP-1 | FSL/10 fibrosarcoma | Overexpression decreased brain metastases | [35] |
| MMP2 | ENU1564 adenocarcinoma | MMP inhibition decreased brain metastases | [36] |
| BCL-XL | MDA-MB435 melanoma | Increased incidence of brain metastases | [37] |
| Plasmin | B16F10 melanoma | Increased invasion | [38] |
| Alpha 3beta1 | EBC-1 brain NSCLC | Decreased brain metastases if blocked | [39] |
| Molecules involved in energy pathways | BCM2 breast cancer | Enhanced mitochondrial respiratory chain pathways; reduced susceptibility to oxidative damage | [40] |
| Tgf-beta2 | B16 melanoma | Increased incidence of metastatic lesions in brain parenchyma | [41] |
| MMP2 | MDA-MB-231 | Increased incidence to multiple organs including brain | [42] |
| VEGF | MDA-231 Br | Treatment with VEGF RTKI decreases tumor burden in brain | [43] |
Vascular endothelial growth factor (VEGF), matrix metalloproteinase 2 (MMP2), tissue inhibitor of metalloproteinase 1 (TIMP1), cyclooxygenase 2 (COX2), heparin-binding EGF-like growth factor (HBEGF), transcription factor 4 (TCF4), fibroblast growth factor 2 (FGF2), transforming growth factor beta 2 (TGF-beta2).
Despite the obvious limitations that are inherent to these models, they have become useful tools to examine the biology of the disease and the activity of drugs/biological agents to treat brain metastases. The use of the experimental metastasis model has allowed the generation of brain metastases from human and murine cell lines from various cancer types (e.g. breast cancer (MDA-MB-361), melanoma (70W and A375) and others [18–21]). A number of variants with enhanced or preferential brain metastatic potential (variants of B16F10 mouse melanoma, human melanoma K-1735 as well as MDA-MB-231 human breast cancer cell lines) have been generated and are now used in experimental metastasis studies [10,22]. These models have been useful in improving our understanding of the mechanisms, processes and molecules that may be involved in the cascade leading to CNS metastases, at least with respect to post-intravasation events. In fact, most of the insights about potentially relevant molecular determinant of the brain-metastatic phenotype have been gained through the use of experimental metastasis models as well as through the development of tumor cell lines with enhanced brain metastatic potential (Table 1). These studies have implicated molecules that regulate a variety of molecular processes (e.g. invasion, angiogenesis, adhesion, proliferation). However, no specific pathway/mechanism has been identified as commonly relevant to the brain metastatic phenotype among the diverse cancer types examined (or even within cell lines of the same cancer type). Given the fundamental differences in the nature of the malignancies such as melanoma or breast cancer (e.g. site of primary tumor origin, and characteristic tumorigenic alterations) and the heterogeneity inherent to human tumors, this is not entirely surprising. Thus the involvement of a variety of mechanisms may be seen as an indicator of the complexity of this process. Despite this, relevant molecules have been identified through this type of analysis and which appear to be prevalently altered and significant to brain metastases in specific cancer types (e.g. STAT3 (in melanoma) and HER2 or HBEGF (in breast cancer)). Their influential role in such cases implicates these molecules as targets for therapy, and indeed their usefulness in this respect has been assessed preclinically (and in the case of HER2 also in ongoing clinical trials) [23–25].
As noted above, an important consideration when using brain metastatic cell lines generated by experimental metastasis models is that the manner in which they were generated does not reflect clinical aspects of the kinetics of tumor cells dissemination (i.e. single bolous dose of tumor cells versus the lower and continual release of tumor cells into circulation that takes place in the clinical scenario). In addition, and perhaps more importantly, these models fail to account for the early events in the metastatic cascade and which are clearly involved in clinical disease (e.g. primary tumor growth, angiogenesis, apoptosis, local invasion, intravasation and chemotaxis) [10,26]. As such, cell lines derived through repeated cycles of in vivo selection in the CNS following intracardiac/intravascular inoculation might be less likely to reflect all of the mechanisms and the genetic/epigenetic alterations that take place in clinical disease and which are essential for successful CNS metastasis. This is underscored by the fact that, in some instances, cell lines that are able to form brain metastases in experimental models are unable to spread spontaneously to CNS or do so with limited ability [17,27].
Another important mechanistic consideration that is relevant to the use of these models is whether colonization of CNS is achieved by cells released from the primary tumor, or whether CNS metastases are the product of established visceral metastases in organs such as lungs or lymph nodes in what has been referred previously as “metastasis of metastases” [28]. If this latter step plays a significant role in the metastatic cascade, then it is likely that the successful spread to CNS could be influenced by pathways that are relevant to visceral disease and which will not be addressed when experimental metastasis assays are used or when brain-homing cells are developed through such models. Thus, whether the ability to colonize this site is mediated by brain specific mechanisms or by broad alterations that facilitate growth in multiple organs, including the CNS, constitutes an important question regarding the nature of the brain-metastatic phenotype.
It could be argued that a better understanding of alterations that result in the metastatic phenotype can be gained through the use of models that recapitulate all the steps involved in metastatic cascade (i.e. dissemination from established orthotopic primary tumors that results in the successful formation of a high rate of relevant metastatic lesions [29]). In this respect, spontaneous models of CNS metastasis could be seen as amore clinically relevant choice.
3. Preclinical models of spontaneous CNS metastasis
Spontaneous models of metastatic disease recapitulate the natural multi-step process of cell dissemination from a primary orthotopic tumor leading to the formation of distant metastases in relevant sites that reflect the clinical presentation of the disease [10]. While there are a growing number of such orthotopic spontaneous models of metastasis for various cancer types [44], there has been little success in generating orthotopic models of spontaneous CNS metastasis that would foster studies of the alterations necessary for the brain-metastatic phenotype, especially the early steps of metastasis. Spontaneous metastatic spread to the CNS from a primary tumor has been reported previously [45–47] but few if any of these metastases were harvested and then evaluated to determine if this phenotype was maintained when the cells were transplanted into new recipients. The unfortunate absence of models of spontaneous CNS metastasis has hampered efforts to gain a clearer view of the process/molecules that regulate the progression from malignant to brain-metastatic disease, and in turn the development of effective or improved therapeutic approaches for treatment of CNS metastases [8,28]. Thus far, very few reports have outlined the development of cell lines capable of spontaneously metastasizing to CNS from a primary tumor in a heritable manner [48]. One of the few that exist makes use of a variant of the murine B16 melanoma cell line (named G3.5), which was generated through successive rounds of selection of brain metastases following intravenous inoculation. This model only succeeded in generating leptomeningeal metastases and thus does not recapitulate the clinical disease which presents largely with metastases in the brain parenchyma [49]. Lower incidence of spontaneous brain metastases (15%) has been noted in another syngenic model using the murine mammary cell line 4T1 [50]. Using as similar in vivo selection protocol, Bos et al. [25], reported the generation of a human breast cancer cell line (CN34-BrM2) that is capable of metastasizing spontaneously from a primary mammary tumor in about 42% of mice. Other recent efforts report the generation of models of spontaneous CNS metastasis by human melanoma, named 131/4-5B1 and 131/4-5B2 [51] and human breast cancer cell lines derived from the MD-MB-231 and named 164/8-1B (Man and Kerbel, unpublished results). The procedure used to isolate these melanoma brain-metastatic cell lines replicates the selection mechanisms that often lead to brain metastases in cancer patients (i.e. control of systemic metastases allows sufficient time for occult single cell/micrometastases to develop into relevant macrometastases), and as such, these cell lines are more likely to recapitulate the alterations that mediate this phenotype in clinical disease (Fig. 2). This model of spontaneous CNS melanoma metastases has been thus far used to identify genes that may contribute an important role in the metastatic spread to this site. These results emphasize alterations in expression of a number of molecules that mediate proliferation, locomotion, and neural development. Among these, endothelin receptor B (EDNRB), which is commonly expressed in melanoma, is highlighted as a molecule that can induce higher incidence of CNS metastases in this cancer type (Cruz-Munoz and Kerbel, unpublished results). Similar models of spontaneous CNS metastases are needed for other cancer types for which this is a common manifestation, such as lung cancer. In addition, where availability permits, a direct comparison of brain-metastatic cell lines derived through experimental implantation (IC) or spontaneous metastasis may be warranted to examine whether common mechanisms exist between these two modalities (e.g. compare experimental (MDA-231Br) versus spontaneous metastatic (164/8-1B) cell lines, both of which are derived from the MDA-MB-231 parental line).
Fig. 2.
Schematic representation of protocol used for isolation of highly metastatic variant 113/6-4L and the effect of metronomic CTX/Vbl chemotherapy in the model of advanced metastatic disease. (A) Parental unselected human WM239A melanoma cells were implanted subdermally and the primary tumors that developed were resected when they reached a size of approximately 400 mm3. Four to six months following resection, lungs were excised from mice and adapted for cell culture leading to the subsequent derivation of the 113/6-4L cell line. Implantation of 113/6-4L cells resulted in high metastatic load in lungs in 6 weeks post-primary tumor resection. (B) In the 113/6-4L spontaneous metastatic model, the combination of CTX and Vbl resulted in significant control ov visceral metastatic disease and increased survival from 99.5 to 180 days post-primary tumor resection. (C) Twenty percent of mice that survived long-term cyclophosphamide and vinblastine therapy in the 113/6-4L model of advanced metastatic disease showed the presence of brain metastases. From these metastases, 131/4-5B1 and 131/4-5B2 cell lines were then isolated. Adapted from Cruz-Munoz et al. [51].
While highly relevant in terms of mechanistic studies, the use of spontaneous models of CNS metastasis also introduces a number of complications when used to examine the efficacy of novel therapeutic agents (Table 1). For instance, in the case of the melanoma model noted above [51], the incidence of brain metastases may only become evident after a long time following tumor cell inoculation and may ultimately present as parenchymal metastases of various sizes and locations. Additionally, because the incidence of CNS metastases is about 50% [25,51], the use of such models for therapeutic studies may require the use of larger numbers of mice than would be required with experimental models in which the incidence of disease is close to 100%.
4. Preclinical models of brain metastatic disease as tools to examine novel therapeutic approaches
4.1. Special considerations regarding models
4.1.1. Mechanisms
Preclinical experimental and spontaneous models of brain metastasis play an important role in enhancing our understanding of this devastating manifestation of malignant progression [10]. Careful design of any study aimed at elucidating the mechanisms that regulate this phenotype or assessing the efficacy of new treatments must involve serious consideration of the advantages and disadvantages inherent to each model (Table 1). Given the very limited and incomplete understanding of the processes and molecules that regulate the brain-metastatic phenotype, the rationale for testing of new drugs against CNS metastases has not always been dictated by rational targeting of relevant molecules/pathways. In order to develop more effective treatments against CNS metastases, identification of molecular targets that are relevant to the brain-metastatic phenotype would clearly be helpful. It is likely that these targets may not be completely brain-specific and that an overlap might exist between molecules that influence visceral and cerebral metastatic disease ([25,32], Cruz-Munoz and Kerbel, unpublished results). Nevertheless, potential therapeutic targets identified using such models require validation of its relevance in clinical disease so as to ensure that these alterations are not specific to the brain-metastatic cell lines. In some instances, molecules that are potentially relevant to brain-metastatic disease are identified through the use of tissue microarray or immunohistochemical analysis of clinical samples (for example [52–54]). Such analysis has implicated underexpression of tumor suppressor nm23 [55] and upregulation of a receptor for hyaluranic and osteopontin-CD44 [56] as influential alterations to brain-metastatic disease. These molecules represent viable leads that can be further examined using preclinical models to ascertain whether these play a significant role in the brain-metastatic phenotype [33] or whether such alterations are coincidental changes. For the purposes of using preclinical models to examine the therapeutic efficacy of inhibitors against particular targets, it is essential to fully characterize the preclinical model itself (i.e. cell line) in order to ensure that these targets are present [57].
4.1.2. Therapy
Development of effective therapeutic treatments against CNS metastases presents a challenging situation as it requires not only that the therapeutic agent be active against the tumor cell population but also posses the ability to cross the BBB [4,10,13]. The natural function of the BBB is to protect the brain from exposure to toxins [58]. This function, however, also prevents most chemotherapeutic agents currently available from crossing into the circulation of the brain parenchyma. The number of systemic chemotherapeutic agents with significant penetration of the BBB is currently very limited [59,60], and even when such agents are used (such as temozolomide for the treatment of metastatic melanoma in the brain), they offer only very limited improvement in overall survival [61]. Since the BBB appears to be compromised in larger intracerebral tumors, the lack of permeability is likely to be a more relevant concern for intracranial micrometastases in which the BBB is more intact and impermeable [62]. In this respect, studies have noted higher drug levels in intracranial tumors, especially necrotic lesions, than the neighboring normal brain parenchyma; however, these levels are still lower than those achieved in extracranial tumors [58]. Clearly, there is a need for the development of new agents with enhanced ability to penetrate into the brain parenchyma. Since, in vitro models do not replicate the properties of the intact BBB, there is an increasing need to utilize in vivo models of metastatic brain disease for the development of such agents [10] and to test strategies to enhance the transport of therapeutic agents across the BBB in a manner that can achieve a significant effect on brain metastases. For instance, improved delivery of therapeutics into the brain has been achieved by coating drugs with nanoparticles. Such efforts have made use of liposomes (composed of one or more phospolipid bilayers) or micellar nanoparticles (amphiphilic polymers with a hydrophobic inner core) to facilitate increased permeability of agents such as doxorubicin, and this has resulted in improved survival of mice bearing intrancranial tumors [63–65]. Additional approaches have been developed to overcome the BBB by temporarily disrupting its integrity. In this respect, MRI-guided ultrasound disruption of the BBB has been shown to induce a significant increase in the delivery of antibodies, such as trastuzumab, which due to their large molecular size are impeded from entering the brain when administered by systemic infusion [66]. Given the efficacy of trastuzumab against HER-2 positive breast cancer, such an approach could provide an effective means to treat CNS metastases in HER-2 positive breast cancer [67,68].
Another methodology aimed at increasing permeability of chemotherapeutic agents across the CNS, and which has shown promise in the preclinical setting, is inhibition of BBB efflux transporters such as P-glycoprotein (P-gp) [69,70]. Unfortunately, P-gp inhibitors also target the drug metabolizing cytochrome P4503A (CYP3A) leading to alterations in clearance of chemotherapeutic drugs and increased toxicity [69,71]; a limitation that will have to be addressed before continuing with clinical assessment of this approach, if it is used at all. Similar preclinical successes have been observed by inducing disruption of BBB by means of hypotonic solutions such as mannitol [72]. This technique will be examined in a clinical trial utilizing mannitol plus methotrexate and carboplatin with/without trastuzumab in the treatment of breast cancer CNS metastases (Clinicaltrials.gov; NCT00397501).
Unfortunately, in most pre-clinical studies, even when anti-metastatic efficacy is examined, treatment is initiated very soon after implantation of the tumor cell lines. In effect, this represents a model of adjuvant therapy against minimal (microscopic) metastatic disease and thus does not reflect the much more challenging situation typically seen patients enrolled in clinical trials (i.e. those with advanced metastatic disease with cerebral metastases large enough to be detected by imaging, typically of 5–10 mm in diameter) [21,33,73]. Certainly control of early disease is of great importance since this is likely to translate into improved quality of life and prolonged survival of patients with cerebral metastases [33]. Nevertheless, careful preclinical validation of anti-tumor efficacy for any given agent should require testing against established macroscopic metastases [57,73]. Such studies will likely prove a significant challenge and potentially lead to a high rate of failures. However, these preclinical setbacks are preferable to introducing untested therapies on patients in clinical trials, for which proof of activity against established cerebral metastases has not been shown; this is particularly relevant given the large number of clinical trials presently ongoing testing therapies for treatment of CNS metastatic disease (clinicaltrials.gov). In addition, preclinical examination of drug efficacy in both adjuvant and advanced metastatic disease settings will lead to more informed decisions in subsequent clinical trials; allowing the use of the drug in the setting in which it is more likely to lead to an improved outcome.
4.2. Chemotherapeutic approaches evaluated in preclinical CNS metastasis models
Given the aforementioned issues associated with poor drug penetration into the brain parenchyma, it was noted early on that cerebral metastases represent a unique scenario and that as such preclinical models of CNS metastatic disease represent an indispensable tool for the initial screening of potentially effective chemotherapeutic agents [85]. In the absence of these models, the choice of agents aimed at treating CNS metastases may not be determined in the most rational or optimal manner (based on efficacy against viable and relevant targets in brain metastases of specific cancer types) [10].
Experimental models of CNS metastasis involving direct inoculation of tumor cells have been the default methodology used to examine novel therapeutic approaches. A large number of such studies have made use of murine and human melanoma cell lines and their use as tools to examine novel therapeutic approaches has been reviewed previously [10]. In these cases, as well as studies focusing on other cancer types, the predominant modality involves intracranial implantation of tumor cells. Typically, in these experiments treatment is initiated shortly after implantation (typically 3 days). As mentioned previously, initiating treatment at such early time points may in fact target mostly or entirely micrometastatic disease and not the more clinically relevant advanced disease.
Preclinical efficacy has been noted in experimental CNS metastasis models with therapies such as those using the dual EGFR and HER2 targeting inhibitor (lapatinib) [23], an histone deacytelase HDAC inhibitor (vorinostat) [9,75], a VEGFR-2 TKI (AZD2171) [79], and sagolipone (Zk-EPO, a low-molecular-weight epothilone) [74] (Table 3). The use of lapatinib at dosages of 30 or 100 mg per kg of body weight was associated with a significant decrease in the emergence of macro-metastases generated by the HER2 overexpressing cell line (231-BR-HER2); however, the incidence of micro-metastases remained unaffected. These results suggest that such an agent could be used in the adjuvant setting to prevent the outgrowth of large cerebral metastases, a role that seems to be implicated by clinical evidence [86]. Indeed, the clinical efficacy of lapatinib in the adjuvant and neo-adjuvant setting is currently being investigated (adjuvant lapatinib and/or trastuzumab treatment optimisation-ALTTO trial) [87]. Similarly, the use of vorinostat to treat triple-negative breast cancer pre-clinically (MDA-MB-231) was also shown to induce a significant reduction in the number of large metastases, but only when treatment was initiated shortly after tumor cell implantation (by intracardiac injection of cells), suggesting efficacy of this agent in the adjuvant setting but, again, little if any effect on established cerebral metastases [9]. On the other hand, using the highly brain- and bone-metastatic DU145/RasB1 prostate adenocarcinoma cell line, AZD2171 was capable of mediating a significant decrease in vascular volume in established large cerebral metastases which was also accompanied by anti-tumor activity when treating small tumors [79]. Unfortunately, in these studies, the overall effect of the various treatments on survival was not examined. Despite this limitation, these preclinical results support the rationale of ongoing clinical trials using such agents against CNS metastases. Certainly with respect to lapatinib, previous clinical evidence seems to support the results obtained in the aforementioned preclinical studies and show that this drug (when used in conjunction with capecitabine) can reduce the incidence and delay the onset of CNS disease [88–90]. Accordingly, many phase I and II trials are underway to examine the efficacy of lapatinib, alone or in combination with other agents for the treatment of breast cancer cerebral metastases, e.g. lapatinib plus capecitabine or lapatinib plus temozolomide (clinicaltrials.gov identifiers: NCT00967031 and NCT00614978, respectively). Additional ongoing clinical trials involve the testing of vorinostat plus radiation therapy (NCT00838929) to treat breast cancer CNS metastases or vorinostat plus stereotactic radiosurgery for the treatment of brain metastases in non-small cell lung cancer (NCT00946673). Similarly, other studies are ongoing to examine the efficacy of AZD2171 in conjunction with whole brain radiation therapy (NCT00937482) for treatment of CNS metastases associated with various tumor types (breast, colorectal, kidney, lung cancers and melanoma) or the efficacy of sagolipone (ZK-EPO) for the treatment of breast cancer brain metastases (NCT00496379).
Table 3.
Examples of preclinical models of CNS metastasis used to examine the efficacy of chemotherapeutic approaches. ACNU, (1-4-amino-2-methyl-5-pyrimidinyl)-methyl-3-(2-chloroethyl)-3-nitrosourea hydrochloride; CCNU, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea; CTX, cyclophosphamide; MTX, methotrexate.
| Model | Agent | Effect | Ref. |
|---|---|---|---|
| MDA-MB-435 melanoma and patient-derived Lu7187 and Lu7466 NSCLC intracranial implant | Sagopilone (ZK-EPO) | Significant inhibition of intracranial tumors | [74] |
| MDA-MB-231 breast cancer intracranial implant | Vorinostat + irradiation | Combination with radiation resulted in increased median survival | [75] |
| MDA-MB231 (231-Br) breast cancer intracardiac injection | Angiopep-2 paclitaxel conjugate (ANG1005) | Improved delivery into intracranial tumors | [76] |
| EMT6 breast cancer intracranial implant | BCNU in combination with biodegradable polymers | Significant prolongation of survival | [77] |
| B16 melanoma intracranial implant | Poly(ADP-ribose)glycohydrolase inhibitor GPI16552 + temozolomide | Reduced intracaranial tumor growth and prolonged survival | [78] |
| DU145/RasB1 prostate adenocarcinoma intracardiac injection | VEGFR2 RTKI (AZD2171) | Decreased brain metastases vascular bed | [79] |
| E0771breast cancer intracranial implant | Interleukin-2 secreting fibroblasts | Significant prolongation of survival | [80] |
| MDA-MB231 (231-Br) breast cancer intracardiac injection | vorinostat | Prevents formation of brain metastases | [9] |
| Various tumor types intracranial implant | Campothecin analogues | Inhibit growth of melanoma and NSCLC | [81] |
| MCF-7/HER2-18 breast cancer intracranial implant | Herceptin | Intracerebral microinfusion of Herceptin increases median survival | [82] |
| Walker 256 carcinosarcoma intracardiac injection and intracranial implant | ACNU, CCNU, CTX, MTX | ACNU, CCNU and CTX increases median survival in intracranial implant, all increase median survival in intracardiac model | [83] |
| B16 melanoma intracranial implant | IFN-alpha immunotherapy + STAT-3 inhibitor (WP1193) | Significant increase in median survival by combination | [24] |
| MDA-MB-231-BR (231-BR-HER2) intracardiac | lapatinib | Decrease in number of large cerebral metastases | [23] |
| MDA-MB-468 and Nt2.5luc breast cancer intracranial | Clostridium perfringens enterotoxin | Increased median survival | [84] |
Overall, these agents do not target molecules known to be exclusive to the brain-metastatic phenotype. The identification of molecules that appear to play a more defined or dominant role with respect to brain-metastatic disease now presents the possibility of more viable and specific therapeutic targets. For instance, STAT3 activity has been noted to be higher in melanoma brain metastatic cells [32]. Accordingly, its inhibition by means of STAT3 inhibitors WP1066 or WP1193 (in combination with IFN-alpha immunotherapy) was found to induce a significant increase in median survival of mice with intracranially implanted B16 melanoma cells [24,91]. It should be pointed out, however, that the effectiveness of these agents was not attributed to STAT3 inhibition alone but also to immune-modulatory effects associated with blockade of this pathway. The identification of the role of STAT3 and its subsequent use as a therapeutic target represents a paradigm for making similar efforts with respect to targeting other molecules implicated in preclinical models as important contributors to CNS metastasis.
5. Future prospects
Treatment of brain metastases is an unmet and increasingly significant therapeutic challenge. Preclinical models of brain metastases are potentially valuable tools to examine the biology of CNS metastatic disease and to examine the efficacy of new approaches for treatment. A large number of clinical trials are presently underway to examine the efficacy of several new therapeutic strategies to treat brain metastases. However, it would be helpful to first conduct proof of activity studies in relevant preclinical models to examine the ability of any given drug to cross the BBB and induce a beneficial effect for the treatment of established CNS metastases before undertaking such trials. The use of preclinical models may also help to define the effect of any given drug in various clinical settings (e.g. adjuvant versus advanced metastatic disease), thus hopefully revealing the most efficacious manner in which to use these agents [13].
The continuing use of these models to examine the underlying molecular mechanisms that regulate the brain-metastatic phenotype may in turn also provide potential biomarkers which could help to identify patients who are at increased risk of developing CNS metastasis and for which earlier and more aggressive therapies might be useful. Such markers may also represent novel targets for therapeutic approaches. Given both the delicate nature and the importance of the organ in question, identification of target molecules that are differentially expressed between cerebral metastases and adjacent normal brain tissue would improve our ability to treat metastatic disease while sparing the CNS [84]. In addition, the use of these preclinical models may facilitate studies of strategies to develop techniques aimed at increasing drug permeability across the BBB.
Acknowledgments
W. Cruz-Munoz is supported by a fellowship from The Canadian Cancer Society Research Institute of Canada/Terry Fox Foundation. R.S. Kerbel’s research is supported by grants from the National Institute of Health-USA (CA-41233), the Canadian Institutes for Health Research (CIHR), The Canadian Cancer Society Research Institute, The Ontario Institute for Cancer Research, and a Tier I Canada Research Chair in Tumor Biology, Angiogenesis and Antiangiogenic Therapy.
Footnotes
Conflict of interest
None.
References
- 1.Lin NU, Winer EP. Brain metastases: the HER2 paradigm. Clin Cancer Res. 2007;13(6):1648–1655. doi: 10.1158/1078-0432.CCR-06-2478. [DOI] [PubMed] [Google Scholar]
- 2.Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30(6):515–520. doi: 10.1016/j.ctrv.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Gore E, Choy H. Non-small cell lung cancer and central nervous system metastases: should we be using prophylactic cranial irradiation? Semin Radiat Oncol. 2004;14(4):292–297. doi: 10.1016/j.semradonc.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Wong ET, Berkenblit A. The role of topotecan in the treatment of brain metastases. Oncologist. 2004;9(1):68–79. doi: 10.1634/theoncologist.9-1-68. [DOI] [PubMed] [Google Scholar]
- 5.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 6.Soffietti R, Ruda R, Trevisan E. Brain metastases: current management and new developments. Curr Opin Oncol. 2008;20(6):676–684. doi: 10.1097/CCO.0b013e32831186fe. [DOI] [PubMed] [Google Scholar]
- 7.Aragon-Ching JB, Zujewski JA. CNS metastasis: an old problem in a new guise. Clin Cancer Res. 2007;13(6):1644–1647. doi: 10.1158/1078-0432.CCR-07-0096. [DOI] [PubMed] [Google Scholar]
- 8.Schackert G, Price JE, Zhang RD, Bucana CD, Itoh K, Fidler IJ. Regional growth of different human melanomas as metastases in the brain of nude mice. Am J Pathol. 1990;136(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- 9.Palmieri D, Lockman PR, Thomas FC, Hua E, Herring J, Hargrave E, et al. Vorinostat inhibits brain metastatic colonization in a model of triple-negative breast cancer and induces DNA double-strand breaks. Clin Cancer Res. 2009;15(19):6148–6157. doi: 10.1158/1078-0432.CCR-09-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cranmer LD, Trevor KT, Bandlamuri S, Hersh EM. Rodent models of brain metastasis in melanoma. Melanoma Res. 2005;15(5):325–356. doi: 10.1097/00008390-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Tarhini AA, Agarwala SS. Management of brain metastases in patients with melanoma. Curr Opin Oncol. 2004;16(2):161–166. doi: 10.1097/00001622-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Ewend MG, Williams JA, Tabassi K, Tyler BM, Babel KM, Anderson RC, et al. Local delivery of chemotherapy and concurrent external beam radiotherapy prolongs survival in metastatic brain tumor models. Cancer Res. 1996;56(22):5217–5223. [PubMed] [Google Scholar]
- 13.Maher EA, Mietz J, Arteaga CL, DePinho RA, Mohla S. Brain metastasis: opportunities in basic and translational research. Cancer Res. 2009;69(15):6015–6020. doi: 10.1158/0008-5472.CAN-08-4347. [DOI] [PubMed] [Google Scholar]
- 14.Arslan C, Dizdar O, Altundag K. Systemic treatment in breast-cancer patients with brain metastasis. Expert Opin Pharmacother. 2009;11(7):1089–1100. doi: 10.1517/14656561003702412. [DOI] [PubMed] [Google Scholar]
- 15.Mayer M. A patient perspective on brain metastases in breast cancer. Clin Cancer Res. 2007;13(6):1623–1624. doi: 10.1158/1078-0432.CCR-06-2842. [DOI] [PubMed] [Google Scholar]
- 16.Khanna C, Hunter K. Modeling metastasis in vivo. Carcinogenesis. 2005;26(3):513–523. doi: 10.1093/carcin/bgh261. [DOI] [PubMed] [Google Scholar]
- 17.Schackert G, Fidler IJ. Development of in vivo models for studies of brain metastasis. Int J Cancer. 1988;41(4):589–594. doi: 10.1002/ijc.2910410419. [DOI] [PubMed] [Google Scholar]
- 18.Zhang RD, Fidler IJ, Price JE. Relative malignant potential of human breast carcinoma cell lines established from pleural effusions and a brain metastasis. Invasion Metastasis. 1991;11(4):204–215. [PubMed] [Google Scholar]
- 19.Ishikawa M, Fernandez B, Kerbel RS. Highly pigmented human melanoma variant which metastasizes widely in nude mice: including to skin and brain. Cancer Res. 1988;48(17):4897–4903. [PubMed] [Google Scholar]
- 20.Engebraaten O, Fodstad O. Site-specific experimental metastasis patterns of two human breast cancer cell lines in nude rats. Int J Cancer. 1999;82(2):219–225. doi: 10.1002/(sici)1097-0215(19990719)82:2<219::aid-ijc12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Palmieri D, Chambers AF, Felding-Habermann B, Huang S, Steeg PS. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13(6):1656–1662. doi: 10.1158/1078-0432.CCR-06-2659. [DOI] [PubMed] [Google Scholar]
- 22.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167(4):913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gril B, Palmieri D, Bronder JL, Herring JM, Vega-Valle E, Feigenbaum L, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100(15):1092–1103. doi: 10.1093/jnci/djn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong LY, Gelbard A, Wei J, Reina-Ortiz C, Wang Y, Yang EC, et al. Inhibition of p-STAT3 enhances IFN-alpha efficacy against metastatic melanoma in a murine model. Clin Cancer Res. 2010;16(9):2550–2561. doi: 10.1158/1078-0432.CCR-10-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66(9):4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 27.Hiroishi S, Morimoto J, Taniguchi Y, Mori H. Multiple metastases of mammary carcinoma cell lines isolated from feral mouse. Cancer Lett. 1995;92(1):83–86. doi: 10.1016/0304-3835(95)03757-n. [DOI] [PubMed] [Google Scholar]
- 28.Fidler IJ, Schackert G, Zhang RD, Radinsky R, Fujimaki T. The biology of melanoma brain metastasis. Cancer Metastasis Rev. 1999;18(3):387–400. doi: 10.1023/a:1006329410433. [DOI] [PubMed] [Google Scholar]
- 29.Talmadge JE, Singh RK, Fidler IJ, Raz A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol. 2007;170(3):793–804. doi: 10.2353/ajpath.2007.060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rolland Y, Demeule M, Fenart L, Béliveau R. Inhibition of melanoma brain metastasis by targeting melanotransferrin at the cell surface. Pigment Cell Melanoma Res. 2009;22(1):86–98. doi: 10.1111/j.1755-148X.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen DX, Chiang AC, Zhang XH, Kim JY, Kris MG, Ladanyi M, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138(1):51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, et al. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66(6):3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 33.Palmieri D, Bronder JL, Herring JM, Yoneda T, Weil RJ, Stark AM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. doi: 10.1158/0008-5472.CAN-06-3316. [DOI] [PubMed] [Google Scholar]
- 34.Menter DG, Herrmann JL, Marchetti D, Nicolson GL. Involvement of neurotrophins and growth factors in brain metastasis formation. Invas Metast. 1994;14(1–6):372–384. [PubMed] [Google Scholar]
- 35.Krüger A, Sanchez-Sweatman OH, Martin DC, Fata JE, Ho AT, Orr FW, et al. Host TIMP-1 overexpression confers resistance to experimental brain metastasis of a fibrosarcoma cell line. Oncogene. 1998;16(18):2419–2423. doi: 10.1038/sj.onc.1201774. [DOI] [PubMed] [Google Scholar]
- 36.Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metast. 2007;24(5):341–351. doi: 10.1007/s10585-007-9071-0. [DOI] [PubMed] [Google Scholar]
- 37.Rubio N, España L, Fernández Y, Blanco J, Sierra A. Metastatic behavior of human breast carcinomas overexpressing the Bcl-x(L) gene: a role in dormancy and organospecificity. Lab Invest. 2001;81(5):725–734. doi: 10.1038/labinvest.3780281. [DOI] [PubMed] [Google Scholar]
- 38.Perides G, Zhuge Y, Lin T, Stins MF, Bronson RT, Wu JK. The fibrinolytic system facilitates tumor cell migration across the blood-brain barrier in experimental melanoma brain metastasis. BMC Cancer. 2006;6:56. doi: 10.1186/1471-2407-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimasu T, Sakurai T, Oura S, Hirai I, Tanino H, Kokawa Y, et al. Increased expression of integrin alpha3beta1 in highly brain metastatic subclone of a human non-small cell lung cancer cell line. Cancer Sci. 2004;95(2):142–148. doi: 10.1111/j.1349-7006.2004.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen EI, Hewel J, Krueger JS, Tiraby C, Weber MR, Kralli A, et al. Adaptation of energy metabolism in breast cancer brain metastases. Cancer Res. 2007;67(4):1472–1486. doi: 10.1158/0008-5472.CAN-06-3137. [DOI] [PubMed] [Google Scholar]
- 41.Zhang C, Zhang F, Tsan R, Fidler IJ. Transforming growth factor-beta2 is a molecular determinant for site-specific melanoma metastasis in the brain. Cancer Res. 2009;69(3):828–835. doi: 10.1158/0008-5472.CAN-08-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tester AM, Waltham M, Oh SJ, Bae SN, Bills MM, Walker EC, et al. Pro-matrix metalloproteinase-2 transfection increases orthotopic primary growth and experimental metastasis of MDA-MB-231 human breast cancer cells in nude mice. Cancer Res. 2004;64(2):652–658. doi: 10.1158/0008-5472.can-0384-2. [DOI] [PubMed] [Google Scholar]
- 43.Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin Exp Metast. 2004;21(2):107–118. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- 44.Cruz-Munoz W, Kerbel RS. Metronomic chemotherapy for treatment of metastatic disease: from preclinical research to clinical trials. In: Lyden D, Welch DR, Psaila B, editors. Cancer metastasis: biologic basis and therapeutics. in press. [Google Scholar]
- 45.Yang M, Jiang P, Sun FX, Hasegawa S, Baranov E, Chishima T, et al. A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res. 1999;59(4):781–786. [PubMed] [Google Scholar]
- 46.Yang M, Jiang P, An Z, Baranov E, Li L, Hasegawa S, et al. Genetically fluorescent melanoma bone and organ metastasis models. Clin Cancer Res. 1999;5(11):3549–3559. [PubMed] [Google Scholar]
- 47.Howard RB, Mullen JB, Pagura ME, Johnston MR. Characterization of a highly metastatic: orthotopic lung cancer model in the nude rat. Clin Exp Metast. 1999;17(2):157–162. doi: 10.1023/a:1006637712294. [DOI] [PubMed] [Google Scholar]
- 48.Alterman AL, Stackpole CW. B16 melanoma spontaneous brain metastasis: occurrence and development within leptomeninges blood vessels. Clin Exp Metastasis. 1989;7(1):15–23. doi: 10.1007/BF02057178. [DOI] [PubMed] [Google Scholar]
- 49.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135(6):807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 50.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68(12):4500–4505. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 52.Hicks DG, Yoder BJ, Short S, Tarr S, Prescott N, Crowe JP, et al. Loss of breast cancer metastasis suppressor 1 protein expression predicts reduced disease-free survival in subsets of breast cancer patients. Clin Cancer Res. 2006;12(22):6702–6708. doi: 10.1158/1078-0432.CCR-06-0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hicks DG, Short SM, Prescott NL, Tarr SM, Coleman KA, Yoder BJ, et al. Breast cancers with brain metastases are more likely to be estrogen receptor negative: express the basal cytokeratin CK5/6, and overexpress HER2 or EGFR. Am J Surg Pathol. 2006;30(9):1097–1104. doi: 10.1097/01.pas.0000213306.05811.b9. [DOI] [PubMed] [Google Scholar]
- 54.Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol. 2005;131(3):191–198. doi: 10.1007/s00432-004-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sarris M, Scolyer RA, Konopka M, Thompson JF, Harper CG, Lee CS. Cytoplasmic expression of nm23 predicts the potential for cerebral metastasis in patients with primary cutaneous melanoma. Melanoma Res. 2004;14(1):23–27. doi: 10.1097/00008390-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Harabin-Słowi0144;ska M, Słowiński J, Konecki J, Mrówka R. Expression of adhesion molecule CD44 in metastatic brain tumors. Folia Neuropathol. 1998;36(3):179–184. [PubMed] [Google Scholar]
- 57.Bibby MC. Orthotopic models of cancer for preclinical drug evaluation: advantages and disadvantages. Eur J Cancer. 2004;40(6):852–857. doi: 10.1016/j.ejca.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Deeken JF, Loscher W. The blood–brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13(6):1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 59.Patel RR, Mehta MP. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res. 2007;13(6):1675–1683. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 60.Motl S, Zhuang Y, Waters CM, Stewart CF. Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet. 2006;45(9):871–903. doi: 10.2165/00003088-200645090-00002. [DOI] [PubMed] [Google Scholar]
- 61.Schadendorf D, Hauschild A, Ugurel S, Thoelke A, Egberts F, Kreissig M, et al. Dose-intensified bi-weekly temozolomide in patients with asymptomatic brain metastases from malignant melanoma: a phase II DeCOG/ADO study. Ann Oncol. 2006;17(10):1592–1597. doi: 10.1093/annonc/mdl148. [DOI] [PubMed] [Google Scholar]
- 62.Stewart DJ. A critique of the role of the blood-brain barrier in the chemotherapy of human brain tumors. J Neurooncol. 1994;20(2):121–139. doi: 10.1007/BF01052723. [DOI] [PubMed] [Google Scholar]
- 63.Yang H. Nanoparticle-mediated brain-specific drug delivery: imaging, and diagnosis. Pharm Res. 2010;27(9):1759–1771. doi: 10.1007/s11095-010-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madhankumar AB, Slagle-Webb B, Wang X, Yang QX, Antonetti DA, Miller PA, et al. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8(3):648–654. doi: 10.1158/1535-7163.MCT-08-0853. [DOI] [PubMed] [Google Scholar]
- 65.Inoue T, Yamashita Y, Nishihara M, Sugiyama S, Sonoda Y, Kumabe T, et al. Therapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor models. Neuro Oncol. 2009;11(2):151–157. doi: 10.1215/15228517-2008-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340(4):1085–1090. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 67.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc Natl Acad Sci USA. 2006;103(31):11719–11723. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hynynen K. Ultrasound for drug and gene delivery to the brain. Adv Drug Deliv Rev. 2008;60(10):1209–1217. doi: 10.1016/j.addr.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joo KM, Park K, Kong DS, Song SY, Kim MH, Lee GS, et al. Oral paclitaxel chemotherapy for brain tumors: ideal combination treatment of paclitaxel and P-glycoprotein inhibitor. Oncol Rep. 2008;19(1):17–23. [PubMed] [Google Scholar]
- 70.Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhänel M, Spruss T, et al. Transport of paclitaxel (Taxol) across the blood–brain barrier in vitro and in vivo. J Clin Invest. 2002;110(9):1309–1318. doi: 10.1172/JCI15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breedveld P, Beijnen JH, Schellens JH. Use of P-glycoprotein and BCRP inhibitors to improve oral bioavailability and CNS penetration of anticancer drugs. Trends Pharmacol Sci. 2006;27(1):17–24. doi: 10.1016/j.tips.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Barth RF, Yang W, Bartus RT, Rotaru JH, Ferketich AK, Moeschberger ML, et al. Neutron capture therapy of intracerebral melanoma: enhanced survival and cure after blood-brain barrier opening to improve delivery of boronophenylalanine. Int J Radiat Oncol Biol Phys. 2002;52(3):858–868. doi: 10.1016/s0360-3016(01)02734-1. [DOI] [PubMed] [Google Scholar]
- 73.Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived-but they can be improved. Cancer Biol Ther. 2003;2(4) Suppl. 1:S134–S139. [PubMed] [Google Scholar]
- 74.Hoffmann J, Fichtner I, Lemm M, Lienau P, Hess-Stumpp H, Rotgeri A, et al. Sagopilone crosses the blood–brain barrier in vivo to inhibit brain tumor growth and metastases. Neuro Oncol. 2009;11(2):158–166. doi: 10.1215/15228517-2008-072). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baschnagel A, Russo A, Burgan WE, Carter D, Beam K, Palmieri D, et al. Vorinostat enhances the radiosensitivity of a breast cancer brain metastatic cell line grown in vitro and as intracranial xenografts. Mol Cancer Ther. 2009;8(6):1589–1595. doi: 10.1158/1535-7163.MCT-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA, et al. Uptake of ANG1005: a novel paclitaxel derivative, through the blood–brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26(11):2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ewend MG, Sampath P, Williams JA, Tyler BM, Brem H. Local delivery of chemotherapy prolongs survival in experimental brain metastases from breast carcinoma. Neurosurgery. 1998;43(5):1185–1193. doi: 10.1097/00006123-199811000-00093. [DOI] [PubMed] [Google Scholar]
- 78.Tentori L, Leonetti C, Scarsella M, Muzi A, Vergati M, Forini O, et al. Poly(ADP-ribose) glycohydrolase inhibitor as chemosensitiser of malignant melanoma for temozolomide. Eur J Cancer. 2005;41(18):2948–2957. doi: 10.1016/j.ejca.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 79.JuanYin J, Tracy K, Zhang L, Munasinghe J, Shapiro E, Koretsky A, et al. Noninvasive imaging of the functional effects of anti-VEGF therapy on tumor cell extravasation and regional blood volume in an experimental brain metastasis model. Clin Exp Metastasis. 2009;26(5):403–414. doi: 10.1007/s10585-009-9238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deshmukh P, Glick RP, Lichtor T, Moser R, Cohen EP. Immunogene therapy with interleukin-2-secreting fibroblasts for intracerebrally metastasizing breast cancer in mice. J Neurosurg. 2001;94(2):287–292. doi: 10.3171/jns.2001.94.2.0287. [DOI] [PubMed] [Google Scholar]
- 81.Potmesil M, Vardeman D, Kozielski AJ, Mendoza J, Stehlin JS, Giovanella BC. Growth inhibition of human cancer metastases by camptothecins in newly developed xenograft models. Cancer Res. 1995;55(23):5637–5641. [PubMed] [Google Scholar]
- 82.Grossi PM, Ochiai H, Archer GE, McLendon RE, Zalutsky MR, Friedman AH, et al. Efficacy of intracerebral microinfusion of trastuzumab in an athymic rat model of intracerebral metastatic breast cancer. Clin Cancer Res. 2003;9(15):5514–5520. [PubMed] [Google Scholar]
- 83.Hasegawa H, Shapiro WR, Posner JB. Chemotherapy of experimental metastatic brain tumors in female Wistar rats. Cancer Res. 1979;39(7 Pt 1):2691–2697. [PubMed] [Google Scholar]
- 84.Kominsky SL, Tyler B, Sosnowski J, Brady K, Doucet M, Nell D. Clostridium perfringens enterotoxin as a novel-targeted therapeutic for brain metastasis. Cancer Res. 2007;67(17):7977–7982. doi: 10.1158/0008-5472.CAN-07-1314. [DOI] [PubMed] [Google Scholar]
- 85.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30(9):2394–2400. [PubMed] [Google Scholar]
- 86.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 87.Tomasello G, de Azambuja E, Dinh P, Snoj N, Piccart-Gebhart M. Jumping higher: is it still possible? The ALTTO trial challenge. Expert Rev Anticancer Ther. 2008;8(12):1883–1890. doi: 10.1586/14737140.8.12.1883. [DOI] [PubMed] [Google Scholar]
- 88.Gluck S, Castrellon A. Lapatinib plus capecitabine resolved human epidermal growth factor receptor 2-positive brain metastases. Am J Ther. 2009;16(6):585–590. doi: 10.1097/MJT.0b013e31818bee2b. [DOI] [PubMed] [Google Scholar]
- 89.Metro G, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2010 doi: 10.1093/annonc/mdq434. (ahead of pub). [DOI] [PubMed] [Google Scholar]
- 90.Sutherland S, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases – the UK experience. Br J Cancer. 2010;102(6):995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kong LY, Abou-Ghazal MK, Wei J, Chakraborty A, Sun W, Qiao W, et al. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin Cancer Res. 2008;14(18):5759–5768. doi: 10.1158/1078-0432.CCR-08-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]