Abstract
Background
At least 14% of Parkinson disease (PD) patients develop impulse control disorders (ICDs). The pathophysiology behind these behaviors and the impact of deep brain stimulation in a real-life setting remains unclear.
Objectives
We prospectively examined the impact of bilateral subthalamic nucleus deep brain stimulation (STN-DBS) on ICDs in PD patients, as well as the relationship between impaired sensorimotor gaiting and impulsivity.
Methods
Patients undergoing bilateral STN-DBS were assessed for ICDs preoperatively and 1-year postoperatively using a validated questionnaire (QUIP-RS). A subset of patients completed the Balloon Analog Risk Task (BART) and auditory pre-pulse inhibition (PPI) testing.
Results
Analysis revealed 12 patients had an improvement in score assessing ICDs (“good responders” – GR; p = 0.006) while 4 had a worse or stable score (“poor responders” – PR; p > 0.05). GR further exemplified a significant decrease in hypersexual behavior (p = 0.005) and binge eating (p = 0.01). Impaired PPI responses also significantly correlated with impulsivity in BART (r = −0.72, p = 0.044).
Discussion
Following bilateral STN-DBS 75% of our cohort had a reduction in ICDs, thus suggesting deep brain stimulation effectively manages ICDs in PD. The role of impaired PPI in predisposition to ICDs in PD warrants further investigation.
Keywords: Deep brain stimulation, subthalamic nucleus, Parkinson’s disease, impulsivity, prepulse inhibition
INTRODUCTION
Impulse control disorders (ICDs) such as pathological gambling, hypersexuality, compulsive shopping, binge eating, punding and dopamine dysregulation syndrome (DDS) are recognized as “behavioral addictions” that develop in at least 14% of PD patients, which can lead to devastating social, familial and financial consequences.[-1–3] Unfortunately, the current treatment options for ICDs are restricted to decreases in dopamine agonists (DA)[4–5] or the addition of psychoactive medications,[2] both of which have disadvantages. Due to such a paucity of treatments, alternative therapies for ICDs such as subthalamic nucleus deep brain stimulation (STN-DBS), have significant implication in PD.[6]
Why ICDs develop in PD patients remains ill-defined. There is some evidence that sensorimotor gating, as measured by pre-pulse inhibition (PPI), may be irregular in patients with PD, schizophrenia, and OCD.[7–10] PPI is known to be disrupted in disorders with basal ganglia dysfunction due to lack of midbrain dopamine.[9,10] A common measure of sensorimotor gating is a startle response.[9] PPI can be induced when a weak sensory event preceding a strong startle-inducing stimulus reduces the magnitude of the startle response.[13–15] Abnormalities signify the inability to properly attend to or ignore environmental stimuli. Iimpaired PPI may lead to ICDs in certain PD patients.
There is mounting evidence that STN-DBS may also reduce ICD behavior in PD patients. Specifically, in a study by Lhommeé et al. (2012), STN-DBS ameliorated DDS in 4 of 4 patients, behavioral addictions in 17/17 patients and compulsive dopaminergic medication use in 9/9 patients.[3] However, in Lhommeé’s study DA reduction was more dramatic than typically occurs in practice.. This study aims to shed light on the role of DBS in ICDs, as well as to examine the role of PPI in impulsivity secondary to PD.
METHODS
Participants
Consecutive patients undergoing bilateral STN-DBS for treatment of PD were offered participation. Those who qualified for surgical treatment completed the Unified Parkinson’s Disease Rating Scale (UPDRS) and neuropsychological testing as part of routine preoperative workup. Patients who did not improve more than 30% on CAPSIT ON/OFF medication testing were not considered acceptable candidates for surgery, as well as those who demonstrated dementia (DRS score ≤ 130) or significant cognitive impairment at baseline testing. Subjects who could not complete testing due to language barriers and/or dementia were excluded from participation in the study. Institutional IRB approval for the study was obtained.
QUIP
After giving informed consent, participants completed an impulsivity questionnaire – Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s disease Rating Scale (QUIP-RS), which was given within one month preoperatively and 1 year postoperatively to track changes in behavior. The QUIP-RS was specifically developed to analyze impulsive behaviors in the Parkinson’s patient population – including both the screening and follow-up of impulsivity in PD patients.[16] The questionnaire was administered in the clinic with a research associate available for assistance (e.g. help filling out questionnaires if writing was difficult due to tremors).
BART and PPI
A subset of patients consented to additional testing consisting of the Balloon Analogue Risk Task (BART) and auditory prepulse inhibition testing (PPI). Both were performed preoperatively ON medications. The BART is a computer game validated to test impulsivity.[17,18] The goal of the participant is to collect as much virtual money as possible by pumping up 30 balloons at a rate of 5 cents per pump. The more pumps, the more potential money they will earn, however, if the balloon pops, they will lose the money. At any point, they may choose to stop pumping, press “collect $$$” and that money will be safe while they complete the rest of the 30 balloons. The balloon randomly pops between 1 and 128 pumps. The two measurements included for analysis were the average adjusted pump count and the total number of explosions.
In PPI, to elicit the startle response, subjects were exposed to a strong startling tone (115dB) in the presence and absence of a pre-startle “warning tone” (74dB). The orbicularis oculi contractile response was concurrently recorded via EMG (Nicolet Viking Quest EMG Machine), and the response data was analyzed for maximal response per auditory stimuli. EMG activity (Filter 80–2,500 Hz) was continuously recorded from the right orbicularis oculi and digitized (sampling frequency 1,000 Hz). The EMG continues recording filter for PPI were: interview scope sweep 100 ms/D, sensitivity 0.2 mV/D, main scope sweep 10 ms/D, input sensitivity 30 uv/D. PPI was assessed by measuring the reduction of the startle response. Patients with severe head tremors that interfered with EMG recordings were excluded, as well as those with hearing impairments. Auditory PPI results were correlated with the BART impulsivity results using Pearson’s correlation analysis.
RESULTS
Demographics
The mean age of our subjects at the time of surgery was 57.63 years (range = 34 – 73) with an average disease duration of 12.19 years prior to surgical intervention (range = 6 – 20). There were 10 male participants and 6 female participants. Table 1 outlines demographics as well as the lead locations and programmed stimulation settings of patient devices.
Table 1.
Patient demographics.
| Patient | Gender | Age of Onset |
Age at surgery |
Duration of PD at surgery |
Lead Location Relative to MC (X= lateral, Y= A–P, Z= vertical) Units = millimeters |
Contacts | Programming Settings Frequency; Pulse Width; Voltage |
LED (mg) Pre-op Post-op |
DA (mg) Pre-op Post-op |
UPDRS- III Pre-op Post-op |
GR v. PR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | 59 | 13 | L: 11.75 left, 2.5 posterior, 4.71 inferior R: 11.68 right, 2.10 posterior, 4.07 inferior |
L: C+, 2- Monopolar R: C+, 1- Monopolar |
L: 140; 60; 3.5 R: 140; 60; 1.7 |
2175 1900 |
3 0 |
82 22 |
GR |
| 2 | M | 43 | 59 | 16 | L: 11.75 left, 2.17 posterior, 4.39 inferior R: 9.18 right, 3.26 posterior, 6.34 inferior |
L: STN1: 2-, 0-, 1+ STN2: C+, 0- Interleaved R: C+, 2- Monopolar |
L: STN1: 125; 90; 4.5 STN2: 125; 90; 3.5 R: 140; 60; 4.0 |
1842.5 1030 |
9 4 |
41 9 |
GR |
| 3 | M | 50 | 60 | 10 | L: 11.49 left, 1.11 posterior, 7.29 inferior R: 10.95 right, 1.70 posterior, 5.91 inferior |
L: C+, 2- Monopolar R: C+, 1- Monopolar |
L: 140; 60; 3.2 R: 130; 60; 2.3 |
1100 525 |
0 0 |
31 13 |
GR |
| 4 | F | 34 | 54 | 20 | L: 10.96 left, 4.26 posterior, 4.39 inferior R: 11.43 right, 0.82 posterior, 3.90 inferior |
L: C+, 1-, 2- Double monopolar R: C+, 2- |
L: 140; 60; 3.3 R: 140; 60; 3.1 |
2230 540 |
24 6 |
44 10 |
PR |
| 5 | M | 43 | 58 | 15 | L: 11.68 left, 3.92 posterior, 7.86 inferior R: 9.73 right, 3.29 posterior, 5.14 inferior |
L: STN1: C+, 3-, 2- STN2: C+, 1- Interleaved R: C+, 1- Monopolar |
L: STN1: 125; 90;4.0 STN2: 125; 60; 3.6 R: 140; 60; 3.1 |
940.5 570 |
0 0 |
34 5 |
GR |
| 6 | M | 40 | 54 | 14 | L: 12.35 left, 4.04 posterior, 6.56 inferior R: 9.41 right, 4.35 posterior, 6.84 inferior |
L: C+, 3- Monopolar R: C+, 3- Monopolar |
L: 170; 90; 3.2 R: 150; 80; 2.8 |
1800 1400 |
1.5 0 |
30 - |
GR |
| 7 | M | 54 | 63 | 9 | L: 12.17 left, 2.22 posterior, 5.15 inferior R: 9.95 right, 3.76 posterior, 4.19 inferior |
L: C+, 1- Monopolar R: C+, 0-, 1- Double monopolar |
L: 140; 60; 3.5 R: 140; 60; 3.8 |
637.5 600 |
2 0 |
50 30 |
GR |
| 8 | F | 55 | 69 | 14 | L: 11.52 left, 2.60 posterior, 3.91 inferior R: 10.13 right, 2.26 posterior, 2.55 inferior |
L: 1-, 2+ 1 bipolar R: 1-, 2+ 1 bipolar |
L: 120; 100; 5.3 R: 100; 100; 5.5 |
857.5 440 |
0.375 2 |
43 27 |
GR |
| 9 | M | 40 | 48 | 8 | L: 11.14 left, 2.27 posterior, 7.59 inferior R: 9.14 right, 4.19 posterior, 7.04 inferior |
L: C+, 3-, 2- Double monopolar R: 1+, 3-, 2+ Bipolar |
L: 140; 60; 3.9 R: 140; 60; 3.7 |
1430 1000 |
0 0 |
32 23 |
PR |
| 10 | F | 45 | 57 | 12 | L: 12.57 left, 0.67 posterior, 4.59 inferior R: 10.01 right, 2.22 posterior, 7.11 inferior |
L: STN1: C+, 2- STN2: C+, 3- Interleaved R: 3-, 2+ Bipolar |
L: ST1: 125; 60; 3.8 STN2: 125; 60; 2.0 R: 130; 90; 5.1 |
550 250 |
0 0 |
38 16 |
GR |
| 11 | F | 40 | 50 | 10 | L: 11.13 left, 2.09 posterior, 6.16 inferior R: 11.61 right, 2.67 posterior, 3.85 inferior |
L: C+, 1- Monopolar R: C+, 2- Monopolar |
L: 140; 60; 2.0 R: 140; 60; 1.7 |
4500 1800 |
0 0 |
55 8 |
GR |
| 12 | F | 48 | 59 | 11 | L: 14.29 left, 2.26 anterior, 3.34 inferior R: 10.02 right, 0.98 anterior, 2.90 inferior |
L: C+, 0- Monopolar R: C+, 1- Monopolar |
L: 140; 60; 1.6 R: 140; 60; 3.3 |
1950 1800 |
0 0 |
43 6 |
GR |
| 13 | M | 60 | 73 | 13 | L: 11.48 left, 1.10 posterior, 6.74 inferior R: 10.01 right, 1.82 posterior, 3.45 inferior |
L: C+, 0- Monopolar R: C+, 0- Monopolar |
L: 140; 60; 3.7 R: 140; 60; 2.1 |
1048 475 |
1.5 2.5 |
26 29 |
PR |
| 14 | M | 28 | 34 | 6 | L: 10.97 left, 4.24 posterior, 5.56 inferior R: 8.61 right, 3.66 posterior, 5.83 inferior |
L: STN1: C+, 0- STN2: 1-, 2+ Interleaved R: C+, 9- Monopolar |
L: STN1:125; 60; 2.8 STN2:125; 60; 2.5 R:125; 90; 3.6 |
1240 760 |
12 8 |
31 7 |
GR |
| 15 | F | 50 | 66 | 16 | L: 10.80 left, 2.89 posterior, 6.53 inferior R: 10.56 right, 2.26 posterior, 5.59 inferior |
L: STN1: 2+, 1- STN2: C+, 3- Interleaved R: STN1: C+, 1- STN2: C+, 3- Interleaved |
L: STN1: 125; 60; 2.2 STN2: 125; 60; 1.3 R: STN1: 125; 60; 2.2 STN2: 125; 60; 1.5 |
1496.5 1180 |
0 6 |
16 14 |
PR |
| 16 | M | 51 | 49 | 8 | L: 12.86 left, 0.47 posterior, 6.59 inferior R: 9.48 right, 0.46 posterior, 4.20 inferior |
L: STN1: C+, 2- STN2: C+, 3- Interleaved R: STN1: C+, 2- STN2: C+, 3- Interleaved |
L: STN1: 125; 60; 4.3 STN2: 125; 90; 2.8 R: STN1: 125; 60; 4.5 STN2: 125; 60; 2.5 |
1200 550 |
1 2 |
35 7 |
GR |
Note – STN 1, STN 2 denote interleaved stimulation. See discussion for explanation.
Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale (QUIP-RS)
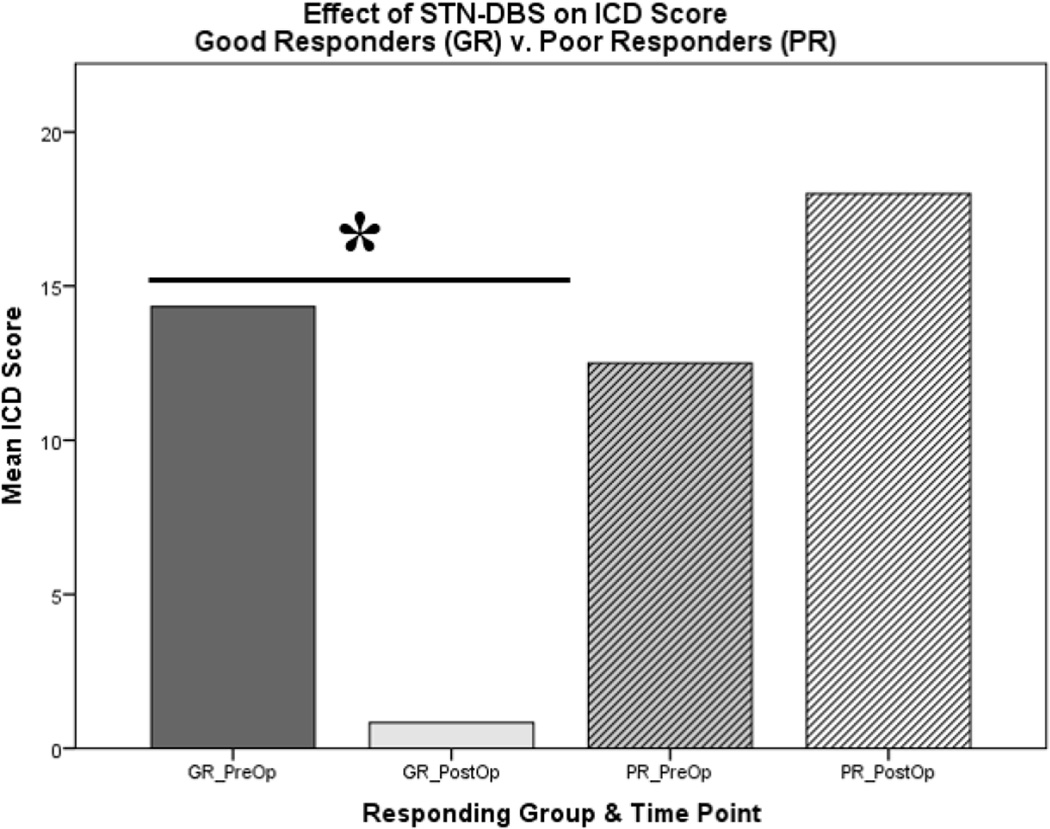
Analysis of the QUIP-RS results showed that 12 patients had an improvement in ICD score (“good responders (GR)”) while 4 had a worse or stable score (“poor responders (PR)”) (Figure 1) with no obvious demographic differences (See supplementary material- Table 2). The 12 GRs had a significant decrease in ICD score (pre-operative 11.75 ± 2.62 to postoperative M = 3.42 ± 1.00); (t(11) = 3.36, p = 0.006), while the 4 PRs had insignificant increases in scores (preoperative 12.50 ± 5.55 to postoperative M = 18.00 ± 1.78); (t(3) = −1.29, p = 0.288). This can be visualized in figure 1. When examining specific impulsive behaviors in GR patients, analyses revealed a significant decrease in hypersexual behavior (t(11) = 3.55, p = 0.005) and binge eating (t(11) = 3.08, p = 0.01) following STN DBS (Supplementary-Figure 2).
Figure 1.
Significant decrease of ICD score in good responders (GR) to STN-DBS, in comparison to poorly responding (PR) patients. (N = 16)
Treatment Differences
In an effort to determine differences between the GR and PR subsets, we examined total daily levodopa equivalent dose (LED) and dopamine agonist dose (DA). There was a significant decrease in LED over time (t(15) = 4.62, p = 0.0003), but no difference between groups. Similarly there was no difference in total DA dose between groups (p = 0.257), lead location and/or stimulation parameters. Two of 4 of the PRs had increases in DA versus 2 of 12 GRs. Of the two PRs that did not have DA increases, one patient had a pre-existing abuse history of cocaine, amphetamines, marijuana and hallucinogens, suggesting addictive predispositions preceding PD. The other patient developed a unique sensory wearing off phenomenon characterized by upper abdominal pain, which was only responsive to levodopa after surgery (despite continued programming and other medications). QUIP showed de novo appearance gambling, punding and hobbyism, and compulsive use of PD medication. In real life, he did not gamble or exhibit hobbyism.
Of note, 3 of 4 non-responders had double monopolar settings, in comparison to 5 of 12 GRs. Only one PR had interleaved stimulation programming in contrast to 5 of 12 GRs. Monopolar stimulation was more commonly used in GR as compared to PR (82.4% v. 17.6% respectively).
Pre-pulse inhibition and impulsivity
Analysis of the PPI and BART data from the separate subset of patients (N = 8) revealed a significant correlation between both overall PPI responses and total number of balloon explosions in the BART (r = −0.72, p = 0.044), as well as responses to non-startling auditory stimuli and total number of balloon explosions in the BART (r = −0.77, p = 0.026) (supplementary -Figure 3). There were also trends between responses to non-startling stimuli and total number of balloon pumps in the BART (r = −0.67, p = 0.069).
DISCUSSION
Reduction in ICDs
We examined how STN-DBS affects impulsivity in PDas well as the possible influence of abnormal sensorimotor gating. We demonstrate that 75% of patients (12/16) have improvement in ICDs following surgery. This prospective study is the first to examine the effect of STN- DBS on ICDs prospectively in routine clinical settings.
Our findings are in agreement with the finding that STN DBS coupled with aggressive DA reduction results in ICD reduction.[3] Other previous reports regarding the impact of STN-DBS on impulsivity in PD have been inconsistent.[19–21] One retrospective study, showed a decrease in pathological gambling following bilateral STN-DBS, and while a general trend supporting this finding was found in our study the change did not reach statistical significance.[22] Conversely, several case reports have documented the development of ICDs de novo following surgery.[23–25]
In our study, there is a variable response observed to STN-DBS. In two PRs, DA dose increased postoperatively.. Why the two GRs with DA increase did not see the same rise in ICDs suggests that it is not DAs alone that results in postoperative ICDs. When examining stimulation parameters in GR v. PR, there are many variables that may account for the differences in response. First, inadvertent stimulation of limbic and associated territories may account for increases in impulsivity. In contrast to delineation of striatal territories, the borderlines between the functionally segregated territories in the STN overlap.26,27. With this model, one can argue overflow of stimulation into unintended areas of the STN causing unwanted side-effects.
Also 3 PR patients were stimulated with 1 or 2 medially located active contacts. Increased charge needed to control motor symptoms may have elicited impulsivity based on spreading of current to the medial and anterior limbic areas.
Further, there were more double monopolar settings in PR as opposed to more interleaving in GRs. Interleaved stimulation allows for two programs to be used in an alternating fashion on the same lead and has been shown to reduce side effects.28 Theoretically, interleaving shapes the current to reduce spread, whereas double monopolar stimulation spreads the current. This finding suggests that interleaved stimulation might reduce ICDs in PD.
Mechanisms of Impulsivity
We also showed a significant negative correlation between impaired PPI and impulsivity on BART, suggesting an important disruption in sensorimotor information gating. Disorders with impaired PPI including PD all generate abnormalities in cortico-striato-pallido-pontine and cortico-striato-pallido-thalamic circuitry, which notably modulate PPI.[8–10] Therefore, neurodegeneration associated with PD is likely to affect both information gating and reward processing. PPI is known to be improved by DAs.[9] Furthermore, lower PPI is associated with increased distractibility,[9] and impairments have been linked to increased impulsivity and risk-taking behaviors.[28] Our study adds to the literature by showing that reduced PPI correlates with impulsivity in PD patients. However, despite the novelty of these present findings we acknowledge small sample size and possible epiphenomenon. Future studies of PPI and impulsivity in PD, and more so, how STN-DBS can impacts PPI are needed.
In conclusion, this study provides prospective data indicating STN-DBS improves ICDs in the majority of PD patients. Due to of the lack of treatment options for ICDs in PD patients, these results have encouraging clinical implications.
Supplementary Material
Acknowledgments
Conflict of interest: Ms. Lucy Gee received funding from NIH grant 5 T35 HL 071483. Dr. Julie Pilitsis is a consultant for Medtronic, St. Jude and Boston Scientific and receives grant support from Medtronic, Boston Scientific, St. Jude and NIH 1R01CA166379. Dr. Adolfo Ramirez-Zamora is a consultant for TEVA neuroscience and received clinical trial support from Boston Scientific. Dr. Eric Molho is a consultant for Lundbeck, US World Meds and Merz and has received speaking honoraria from US World Meds. He receives grant support from the Cure Huntington Disease Initiative, Kyowa, Teva, US World Meds, Auspex, Acadia, Merz, and Boston Scientific.
REFERENCES
- 1.Leeman R, Potenza M. Impulse control disorders in Parkinson’s disease: Clinical characteristics and implications. Neuropsychiatry. 2011;2:133–137. doi: 10.2217/npy.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weintraub D, Nirenberg MJ. Impulse control and related disorders in Parkinson’s disease. Neurodegenerative Diseases. 2013;11:63–71. doi: 10.1159/000341996. [DOI] [PubMed] [Google Scholar]
- 3.Lhommée E, Klinger H, Thobois S, Schmitt E, Ardouin C, Bichon A, Krack P. Subthalamic stimulation in Parkinson’s disease: Restoring balance of motivated behaviours. Brain. 2012;135:1463–1477. doi: 10.1093/brain/aws078. [DOI] [PubMed] [Google Scholar]
- 4.Emad E. Mechanisms of deep brain stimulation for obsessive compulsive disorder: Effects upon cells and circuits. Frontiers in Integrative Neuroscience. 2012;6:1–14. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frank M, Samanta J, Moustafa A, Sherman S. Hold your horses: Impulsivity, deep brain stimulation, and medication in Parkinson’s disease. Journal of Neurology Neurosurgery & Psychiatry. 2007;78:517–519. [Google Scholar]
- 6.Rodriguez-Oroz M, López-Azcárate J, Garcia-Garcia D. Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain. 2010 doi: 10.1093/brain/awq301. awq301. [DOI] [PubMed] [Google Scholar]
- 7.Powell SB, Weber M, Geyer MA. Genetic models of sensorimotor gating: Relevance to neuropsychiatric disorders. Current Topics in Behavioral Neuroscience. 2012;12:251–318. doi: 10.1007/7854_2011_195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoetmulder M, Beirnat HB, Nikolic M, Korbo L, Jennum PJ. Sensorimotor gating deficits in multiple system atrophy: Comparison with Parkinson’s disease and idiopathic REM sleep behavior disorder. Parkinsonism and Related Disorders. 2014;20:297–302. doi: 10.1016/j.parkreldis.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: Normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- 10.Lukhanina E, Berezetskaya N, Karaban I. Paired-pulse inhibition in the auditory cortex in Parkinson’s disease and its dependence on clinical characteristics of patients. Parkinson's Disease. 2011;2011:1–8. doi: 10.4061/2011/342151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumenthal TD, Gescheider GA. Modification of the acoustic startle response by a tactile prepulse: Effects of stimulus onset asynchrony and prepulse intensity. Psychophysiology. 1987;28:296–306. doi: 10.1111/j.1469-8986.1987.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham FK. Control of reflex blink excitability. Neural mechanisms of goal-directed behavior and learning. 1980:511–519. [Google Scholar]
- 15.Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychological review. 1980;87(2):175. [PubMed] [Google Scholar]
- 16.Weintraub M, Stewart B, Shea J. Validation of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s disease (QUIP) Movement Disorders. 2009;24:1461–1467. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalley J, Everitt B, Robbins T. Impulsivity, compulsivity and top down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Rask (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 19.Claassen DO, van den Wildenberg WPM, Ridderinkhof R, Jessup CK, Harrison MB, Wooten K, Wylie SA. The risky business of dopamine agonist in Parkinson’s disease and impulse control disorders. Behavioral Neuroscience. 2011;125(4):492–500. doi: 10.1037/a0023795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, Weintraub D. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Movement Disorders. 2010;25(11):1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu K, Politis M, Piccini P. Parkinson disease and impulse control disorders: a review of clinical features, pathophysiology and management. Postgraduate medical journal. 2009;85(1009):590–596. doi: 10.1136/pgmj.2008.075820. [DOI] [PubMed] [Google Scholar]
- 22.Ardouin C, Voon V, Worbe Y, Abouazar N, Czernecki V, Hosseini H, Krack P. Pathological gambling in Parkinson's disease improves on chronic subthalamic nucleus stimulation. Movement disorders. 2006;21(11):1941–1946. doi: 10.1002/mds.21098. [DOI] [PubMed] [Google Scholar]
- 23.Lu C, Bharmal A, Suchowersky O. Gambling and PD. Archives of Neurology. 2006;63:298. doi: 10.1001/archneur.63.2.298-a. [DOI] [PubMed] [Google Scholar]
- 24.Smeding HMM, Speelman JD, Koning-Haanstra M, Schuurman PR, Nijssen P, Van Laar T, Schmand B. Neuropsychological effects of bilateral STN stimulation in Parkinson disease: A controlled study. Neurology. 2006;66(12):1830–1836. doi: 10.1212/01.wnl.0000234881.77830.66. [DOI] [PubMed] [Google Scholar]
- 25.Smeding HMM, Goudriaan AE, Foncke EMJ, Schuurman PR, Speelman JD, Schmand B. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(5):517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathai A, Smith Y. The Corticostriatal and Corticosubthalamic Pathways: Two Entries, One Target. So What? Frontiers in Systems Neuroscience. 2011;5(64) doi: 10.3389/fnsys.2011.00064. eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes W, Haber S. The Organization of Prefrontal-Subthalamic Inputs in Primates Provides an Anatomical Substrate for Both Functional Specificity and Integration: Implications for Basal Ganglia Models and Deep Brain Stimulation. Journal of Neuroscience. 2013;33(11):4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez-Zamora A, Kahn M, Campbell J, DeLaCruz P, Pilitsis J. Interleaved programming of subthalamic deep brain stimulation to avoid adverse effects and preserve motor benefit in Parkinson’s disease. Journal of Neurology. 2014 doi: 10.1007/s00415-014-7605-3. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 29.Swann AC, Lijffijt M, Lane SD, Steinberg JL, Acas MD, Cox B, Moeller FG. Pre-attentive information processing and impulsivity in bipolar disorder. Journal of Psychiatric Research. 2013;47(12):1917–1924. doi: 10.1016/j.jpsychires.2013.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.