Abstract
Background
Supplementary motor area (SMA) syndrome occurs after surgery involving the SMA and is characterized by contralateral hemiparesis with or without speech impairment (dependent on involvement of the dominant SMA), which is transient and characteristically resolves over the course of weeks to months.
Objective
Recurrent SMA syndrome after repeat craniotomy has not been previously described. In this manuscript, we describe the presentation and clinical course of patients who developed recurrent SMA syndrome after redo resection of tumors involving the SMA.
Methods
We performed a retrospective review of 15 patients who underwent repeated resection of low grade glioma from the superior and middle frontal gyrus (SFG, MFG). Of these patients we identified six cases of recurrent SMA syndrome.
Results
Six patient had a documented SMA syndrome occurring after initial and subsequent resection of tumor in proximity to the SMA. Intraoperative localization of eloquent motor and language cortex was achieved in each patient using a combination of somatosensory evoked potentials (SSEPs) and electrocortical stimulation mapping. Location of tumor and extent of resection was examined with magnetic resonance (MR) imaging.
Conclusion
This series demonstrates that recurrent SMA syndrome occurs in patients undergoing repeat resection of tumors involving the SMA. The presence of recurrent SMA syndrome provides support for reorganization of SMA function to adjacent ipsilateral cortex after resection. Patients with recurrent neoplasms of the SMA should be counseled on the possibility of recurrent SMA syndrome.
Keywords: Motor Cortex, Supplementary Motor Area, Supplementary Motor Area Syndrome, Glioma, Brain Tumor
Introduction
Lesions involving the supplementary motor area and Brodmann area 6 (SMA; BA6) commonly result in a well described clinical presentation known as the SMA syndrome1–7. As initially reported in 1977 by Laplane et al., SMA syndrome is characterized by immediate and transient postoperative contralateral motor deficit with possible speech impairment after surgery involving the dominant SMA/BA63. The SMA syndrome is reported most commonly following tumor resection2, 4 or resection for epilepsy3, but reports of similar motor neglect are also associated with certain vascular lesions such as anterior cerebral artery infarcts of the medial frontal lobes8, 9. The impairments seen with SMA syndrome are almost universally transient and typically resolve over the course of days to weeks2, 10. Though classically described as akinetic mutism2, motor impairments can range from mild hypokinesia to severe hemiparesis; the severity of the syndrome does not seem to correlate with duration of recovery10.
Despite numerous clinical reports in the literature2, 4, 6, the pathophysiology of SMA syndrome is poorly understood and the mechanism underlying the syndrome’s characteristic reversibility is unclear. Several pathophysiologic mechanisms have been proposed, including cerebral edema, regional ischemia, retraction injury, and most commonly, removal and subsequent reorganization of the SMA function itself6, 10. Preoperative localization of the SMA by functional magnetic resonance imaging (fMRI) demonstrates that resection of the functionally active SMA is highly associated with development of the SMA syndrome6. Reorganization of the SMA has been postulated as a potential mechanism of neurologic recovery in the SMA syndrome6, but no correlative reports based on functional imaging or electrophysiological assessment exists. While multiple examples of repeated resection of the SMA are found in the literature1, 4, 11, 12, development of recurrent SMA syndrome, as well as its subsequent recovery, has not been described. This report presents the clinical characteristics of a series of patients with gliomas involving the SMA who demonstrated recurrent SMA syndrome following repeat resection.
Clinical Materials and Methods
Patient Selection
We retrospectively reviewed all patients undergoing repeat craniotomy for tumor resection with glial neoplasms involving SMA cortex. In this retrospective review, there were 15 patients with redo craniotomy for gliomas involving the SFG. We defined SMA syndrome as a new postoperative hemiparesis or severe motor speech hesitancy that completely or nearly-completely resolved within six months. Patients were excluded if an alternate cause for their neurologic deficit was apparent (seizure, infarct, damage to primary motor or language cortex).
Imaging Assessment
Magnetic resonance imaging to include T1, T2, FLAIR and DWI sequences with and without contrast were obtained pre- and postoperatively and reviewed by a board-certified neuroradiologist. Primary motor cortex was identified on MR imaging using the method described by Berger et al.13 and each tumor was localized to the medial superior frontal gyrus (SFG) anterior to primary motor cortex.
Electrophysiologic Assessment
All patients underwent electrophysiological monitoring to confirm the location of primary motor cortex at the time of surgery. Median nerve somatosensory evoked potentials (SSEPs) were used to localize the central sulcus and in turn somatosensory cortex and motor cortex. Primary motor cortex localization was confirmed using bipolar stimulation to elicit motor responses. During resection, bipolar stimulation was performed intermittently to identify crucial descending motor fibers. Cortical stimulation mapping was likewise performed to identify eloquent language cortex (i.e. Broca’s area and Wernicke’s area) in those patients where resection would put postoperative language function at risk.
Results
In our series, six patients developed SMA syndrome after both initial and repeated tumor resection. No patients who had SMA syndrome after initial resection did not develop recurrent SMA syndrome upon redo craniotomy. Initial clinical presentation consisted of seizure in all patients. Initial pathology from first resection was oligodendroglioma in 2 patients, mixed oligoastrocytoma in 2 patients, astrocytoma in one patient, and glioblastoma in one patient. (Table 1) A gross total resection was obtained in four (67%) patients; involvement of eloquent cortex limited resection in the other two. Postoperative transient neurologic deficit consistent with SMA syndrome was demonstrated in all patients when the resection boundaries encompassed the tumor involving the SFG (Table 1). Symptoms generally demonstrated improvement by post-operative day 4 with a median duration of 2.5 weeks.
Table 1.
Patient characteristics
| Initial Surgery | Repeat Surgery | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Age/Sex | Handedness | Chief Complaint |
Location | Neuromonitoring | Pathology | Adjuvant Treatment |
Age | Chief Complaint |
Pathology | Adjuvant Treatment |
| 1 | 32 / F | R | Seizure | L SFG | SSEP, DCS/motor mapping |
Oligodendroglioma (II) |
IMRT | 37 | Headache | Treated glioma with malignant transformation |
TMZ, BCNU, carboplatin, bevicizumab |
| 2 | 48 / M | R | Seizure | L SFG | SSEP, DCS/motor mapping |
Oligodendroglioma (II) |
IMRT | 55 | MRI recurrence |
Treated glioma with malignant transformation |
IMRT, TMZ bevicizumab irinotecan, tamoxifen |
| 3 | 32 / F | R | Seizure | L SFG | SSEP, DCS/motor mapping |
Astrocytoma (II) | IMRT, TMZ |
34 | MRI recurrence |
Anaplastic oligodendroglioma (III) vs glioblastoma (IV) |
Carboplatin, bevicizumab |
| 4 | 50 / F | R | Seizure | R SFG, PCG |
Unknown (OSH) | Mixed oligoastrocytoma (II) |
None | 56 | Increased seizure frequency |
Oligoastrocytoma (II) |
Proton beam IMRT |
| 5 | 70 / M | R | Seizure | R SFG, MFG, PCG |
SSEP, DCS/motor mapping |
Glioblastoma (IV) | IMRT, TMZ |
70 | MRI recurrence |
Treated glioblastoma |
Carboplatin |
| 6 | 30 / M | R | Seizure | R SFG | SSEP, DCS/motor mapping |
Mixed oligoastrocytoma (II) |
None | 39 | MRI recurrence |
Anaplastic oligodendroglioma (III) |
IMRT, TMZ |
F: female; M: male; R: right; L: left; SFG: superior frontal gyrus; MFG: middle frontal gyrus; SSEP: somatosensory evoked potentials; DCS: direct cortical stimulation mapping; Oligo: oligodendroglioma; GA: gemistocytic astrocytoma; IMRT: intensity modulated radiation therapy; TMZ: temozolamide; BCNU: carmustine.
Tumor recurrence was identified via surveillance MRI in four (67%) patients, increased seizure frequency in one patient and increased headaches in one patient. Two patients had persistent neurologic deficit after initial craniotomy. During repeat craniotomy, subtotal resection was performed in four (67%) patients given tumor involvement in eloquent cortex. Again, all patients demonstrated post-operative neurologic deficit consistent with SMA syndrome with a median duration of 2 weeks (Table 2). One patient who had developed only a motor SMA syndrome after her first resection experienced both motor and speech involvement after her repeat craniotomy. The two patients who had previously developed SMA syndrome with motor and speech symptoms again experienced both after redo resection. Further details of three representative cases are presented below.
Table 2.
Clinical characteristics of initial and recurrent SMA syndrome
| Initial Presentation | Repeat Presentation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Pre- operative deficit |
Extent of Resection |
Clinical Manifestation |
Duration | Residual deficit | Duration between surgery |
Extent of Resection |
Clinical Manifestation |
Duration | Residual deficit |
| 1 | None | GTR | R hemiparesis | 2 weeks (partial); 6 weeks (total) |
None | 4 yr | STR | R hemiparesis with mild dysarthria |
2 weeks (both) | Mild RLE hemiparesis |
| 2 | None | GTR | R hemiparesis, mutism |
2 weeks (motor); 6 weeks (speech) |
None | 7 yr | STR | R hemiparesis and speech delay |
2 weeks (both) | None |
| 3 | None | GTR | R hemiparesis, mutism |
3 weeks | Mild speech hesitancy |
1.25 yr | GTR | R hemiparesis and speech delay |
5 weeks (motor); 8 weeks (speech) |
Mild word finding difficulties |
| 4 | None | STR | L hemiparesis | < 1 month (OSH) |
Minimal L hemiparesis |
6 yr | STR | L hemiparesis | 2 weeks | Mild L hemiparesis |
| 5 | None | STR | L hemiparesis | 2 weeks | None | 0.5 yr | STR | L hemiparesis | 3 weeks | None |
| 6 | None | GTR | L hemiparesis | 1 week | None | 9 yr | GTR | L hemiparesis | 2 weeks | None |
R: Right; L: Left, GTR: Gross Total Resection; STR: Subtotal Resection; yrs: years; mo: months; wks: weeks; LE: lower extremity; DF: dorsiflexion; PF: plantarflexion
Case Illustrations
Case 1
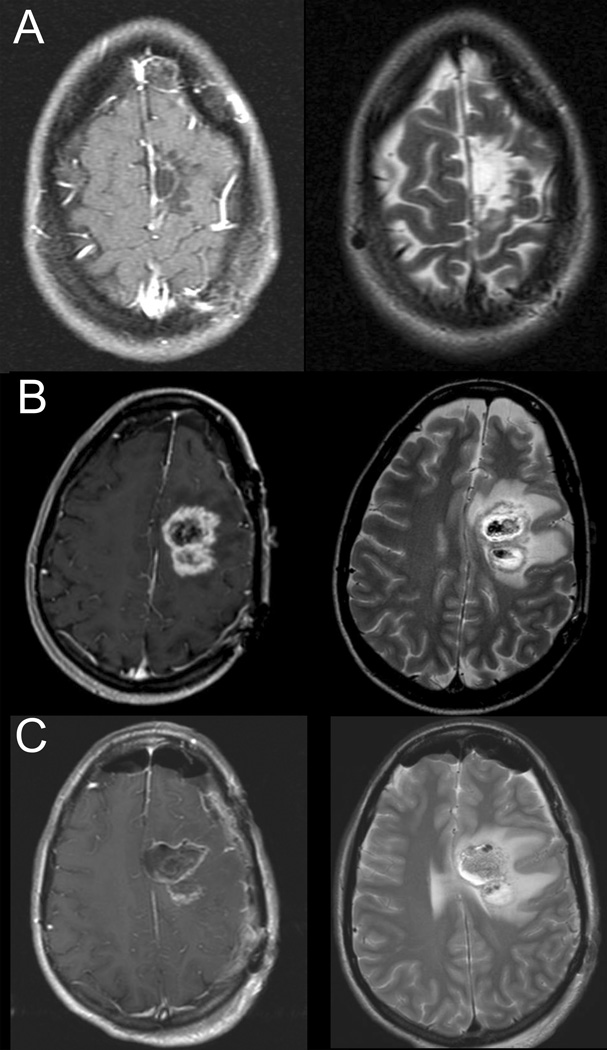
A 32 year-old right-handed woman presented with her first seizure and was found to have an abnormality in the medial left SFG on MR imaging. The abnormality was hyperintense on T2 and FLAIR and was non-enhancing. It measured 2.7 × 2.0 × 2.6 cm. She underwent craniotomy with motor mapping for a gross total resection (Figure 1A). Postoperatively, the patient had a dense right hemiparesis with partial improvement after 2 weeks and complete resolution 6 weeks after surgery. Pathology was consistent oligodendroglioma (II) and the patient underwent subsequent fractionated radiotherapy for a total of 6120 cGy.
Figure 1.
Post-contrast T1-weighted (left panel) and T2-weighted (right panel) MR images for Case 1: A) MR imaging following first operation, B) MR imaging prior to second operation demonstrating tumor recurrence, C) Postoperative MR imaging showing subtotal resection.
Four years after her initial surgery she began to complain of increasing headaches. MRI imaging revealed two areas of focal enhancement concerning for tumor recurrence and malignant transformation (Figure 1B). Repeat craniotomy with motor mapping was performed with a subtotal resection secondary to tumor involvement in primary motor cortex (Figure 1C). Final pathology was treated glioma with multiple mitoses and increased anaplasia consistent with malignant transformation. Postoperatively, the patient again experienced dense right hemiparesis as well as mild dysarthria. On postoperative day 14 the patient’s symptoms had resolved to mild weakness of dorsiflexion and plantarflexion on the right side. She underwent treatment with temozolamide post-operatively but had continued progression that was resistant to BCNU, carboplatin and bevicizumab and she succumbed to her disease 6 years after her original diagnosis and 12 months after her repeat surgery.
Case 2
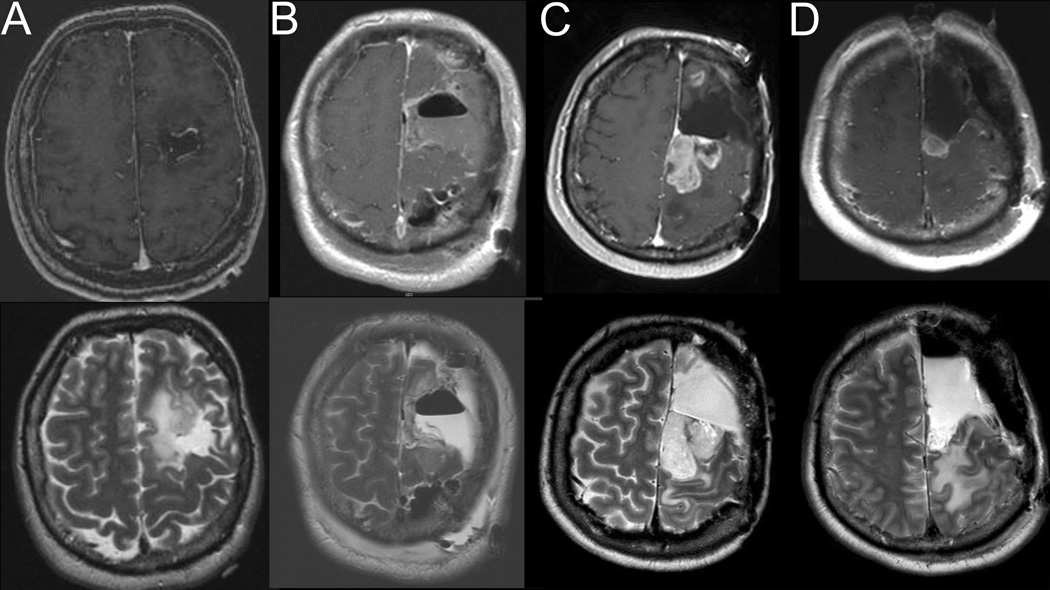
A 48-year-old right-handed man presented after surveillance scanning demonstrated a recurrence of a previously resected left frontal gemistocytic astrocytoma (II) at an outside institution. Notably his initial symptom before his first resection was seizure and he had not experienced a SMA syndrome after this initial resection as the tumor was chiefly in the middle frontal gyrus. He did not receive any post-operative adjunct therapy. His recurrence, however, clearly extended into the SFG (Figure 2A). Thus, at our institution he underwent craniotomy with cortical and subcortical motor mapping resulting in gross total resection (Figure 2B). There was residual supplementary motor cortex (SMC) tissue present after the initial resection (Figure 2B). The patient developed post-operative SMA syndrome consisting of right hemiparesis and mutism. On post-operative day 14 his right hemiparesis had resolved completely and he had only mild speech hesitancy. The patient’s symptoms resolved completely on 6 week post-operative follow-up. Interestingly despite the initial pathology revealing a gemistocytic astrocytoma, the final pathology after this resection was an oligodendroglioma (II). He was treated with adjunctive external beam radiotherapy at an outside institution.
Figure 2.
Post-contrast T1-weighted (top panel) and T2-weighted (bottom panel) MR images for Case 2: A) MR imaging prior to second operation (first operation at our institution) showing tumor recurrence, B) Postoperative MR imaging showing gross total resection after second operation, C) MR images demonstrating tumor recurrence in the left superior and middle frontal gyri, D) Postoperative MR imaging demonstrating subtotal tumor resection.
He subsequently developed enhancing recurrence and was given temozolamide by his neuro-oncologist. He follow up scans after chemotherapy continued to demonstrate growth in the enhancing portion of the lesions, with the largest being a 2.3 × 1.6 × 2.3 cm enhancing mass in the left superior and middle frontal gyri extending back into motor cortex (Figure 2C). Thus 7 years later he again underwent a second repeat craniotomy with cortical and subcortical motor mapping for near total resection (Figure 2D) and again had an SMA syndrome classified by right hemiparesis and significant speech delay. These deficits resolved after 2 weeks. Pathology after this resection was treated glioma with high mitotic activity, endothelial proliferation and multifocal necrosis consistent with malignant transformation. He underwent 5400 cGy intensity-modulated radiotherapy and 12 cycles of temozolamide. His tumor progressed and he was started on bevicizumab, irinotecan, and later carboplatin. This was unsuccessful as was a rechallenge with bevicizumab with the addition of tamoxifen. He was transitioned to hospice 18 years after initial diagnosis and nearly 22 months after his last surgery.
Case 3
A 32-year-old right-handed woman initially presented with seizure and was found on MRI to have a left frontal lesion. She underwent stereotactic biopsy with pathology demonstrating fibrillary astrocytoma (II) for which she underwent radiotherapy at an outside institution.
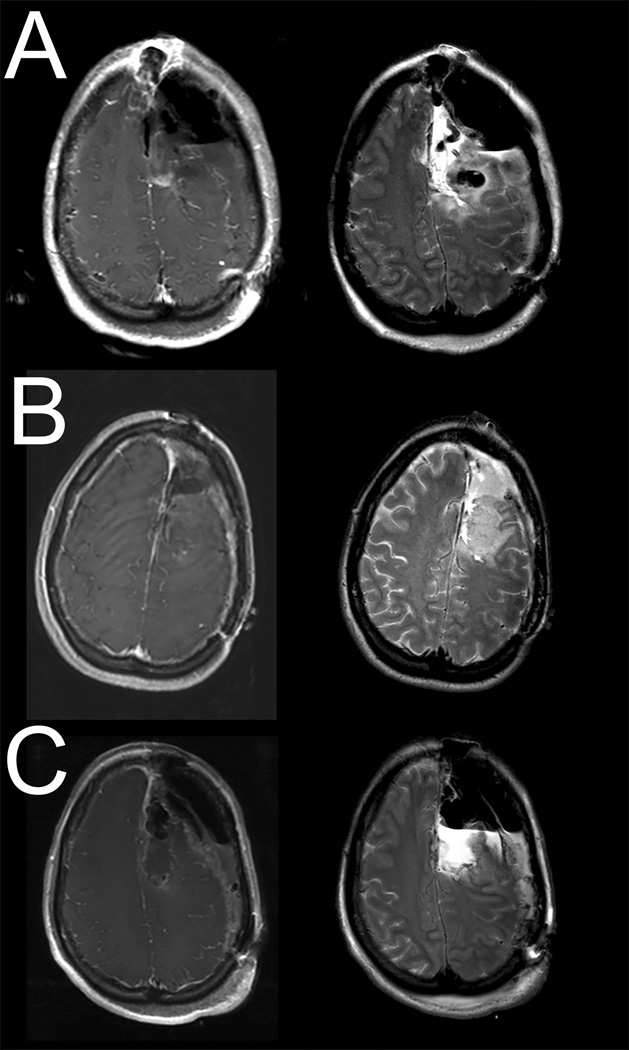
Eight years later, she presented to our institution with radiographic tumor progression. She underwent awake craniotomy with motor and language mapping with gross total resection of the enhancing component of the tumor (Figure 3A). There was residual tissue noted in the SMC postoperatively (Figure 3B). Postoperatively, the patient had right hemiparesis and mutism. Her hemiparesis had improved significantly by two weeks and had resolved completely three weeks following surgery. Her mutism had improved to mild speech hesitancy after 3 weeks and nearly resolved completely after 8 weeks. Pathology was anaplastic oligodendroglioma (III) vs secondary glioblastoma (IV) and received adjunct temozolamide.
Figure 3.
Post-contrast T1-weighted (left panel) and T2-weighted (right panel) MR images for Case 3: A) Postoperative MR imaging from initial craniotomy for resection, B) Surveillance MR imaging showing tumor recurrence, C) Postoperative MR images from second resection demonstrating gross total resection.
Surveillance MRI 15 months post-op showed tumor recurrence in the previous resection cavity (Figure 3B) and she underwent craniotomy with motor mapping for gross total resection of the enhancing portion of the tumor (Figure 3C). She had severe right hemiparesis more pronounced in the upper extremity as well as mutism immediately after surgery. Her hemiparesis had improved significantly by 2 weeks post-op and resolved by 5 weeks. She additionally had severe speech difficulty post-op with near-total improvement 8 weeks after surgery with some mild residual word-finding difficulty Pathology was anaplastic oligodendroglioma (III) versus secondary glioblastoma (IV). She had tumor recurrence 10 months after surgery and was started on carboplatin and bevacizumab; this was unfortunately complicated by delayed breakdown of her craniotomy incision. She succumbed to her disease 12 months after her last surgery and 11 years after initial diagnosis.
Discussion
Supplementary motor area (SMA) is an anatomically well-defined area of the dorsomedial frontal lobe (Brodmann’s area 6c) that plays an important role in generating complex voluntary motor and speech behaviors14, 15. The SMA itself comprises approximately 10% of all corticospinal neurons16 and has afferent input from neurons in the globus pallidus internus (GPi) via the thalamus, cerebellum and primary motor cortex and efferent output to the striatum17 and primary motor cortex. This neuronal feedback loop modulates motor activity via the basal ganglia14.
Evidence from electrophysiological and functional neuroimaging studies supports the SMA playing a critical and complex role in initiating and modulating voluntary movements14. Direct electrical stimulation of the SMA has been performed in both non-human primates18, 19 and humans20–22; stimulation in non-human primates results in movements of the head, trunk, hindlimb, forelimb, and orofacial musculatures with greater complexity and involvement of more muscle groups when compared to those seen after stimulation of primary motor cortex18, 19. Similarly, stimulation in humans results in generation of complex motor behavior, such as changes in posture or vocalization 20–22. Stimulation and lesioning studies support somatotopic organization of the SMA in humans with the posterior SMA correlating to the lower extremities and the anterior SMA correlating to the face22. Additionally, electroencephalography targeting the SMA demonstrates an increasing negative potential just prior to movement onset23; similar changes in neuronal population electrical activity were seen on ECoG recording of mesial SFG in human subjects24. These electrophysiologic studies are correlated with recent evidence demonstrating fMRI signal in SMA immediately prior to activation of primary motor cortex25.
Damage to the SMA results in a characteristic syndrome of transient neurologic deficit primarily manifested as contralateral hemiparesis or hemiplegia with or without a speech deficit (dependent on the involvement of the dominant SMA) 2, 5. Our series adds further support for this phenomenon and demonstrates the possibility of recurrent SMA syndrome after repeated tumor resection. The neurologic deficits associated with SMA syndrome in our patients were always transient and typically showed total or near-total improvement within weeks, corresponding the timing of recovery in the literature4. In our patients, the time course of recovery did not seem to be significantly different between initial and recurrent SMA syndrome.
Despite the stereotyped clinical presentation, the underlying pathophysiology of the SMA syndrome and the mechanism of its characteristic reversal remains unclear. One proposed mechanism is that surgical trauma, namely retraction, resection, or post-operative cerebral edema, results in transient dysfunction of primary motor cortex, supplementary motor cortex, and in some cases eloquent language cortex. However, this explanation seems unable to adequately explain the SMA syndrome. If these symptoms are solely due to post-operative irritation and edema than they would be expected to resolve faster, as has been described for improvement of motor deficits secondary to edema involving primary motor cortex after surgery 2. Notably, recovery of the motor and speech deficits seen in SMA syndrome follow a similar time course, despite the location of motor and speech function being remote from both each other and the site of cortical resection as identified during intra-operative electrophysiologic monitoring4. This suggests that SMA syndrome results from direct resection of the functional cortex in the SMA itself, as supported by anatomical and functional MRI correlating resection of medial SFG and development of the SMA syndrome10.
If the development of SMA syndrome is secondary to functional interruption of supplementary motor cortex during surgery, then recovery is likely primarily driven by reorganization of the SMA. The bilateral organization and representation of the SMA is thought to account for the reversibility of the SMA syndrome by some1, and indeed the majority of literature investigating functional cortical reorganization describes it as occurring in the homologous functional region on the contralateral side26–29. The observation that repeated resection of the SMA results in recurrent SMA syndrome would suggest that the SMA reorganizes ipsilaterally around the resected SMA. Since the patients described in this manuscript had partial SMA resection (as opposed to complete), it is possible that ipsilateral reorganization was facilitated by the remaining SMA cortex. Ipsilateral reorganization of motor cortex and recruitment of surrounding non-lesioned cortex is not an unknown phenomenon, having been reported following focal ischemic injuries with associated functional improvement30, 31 In patients who undergo complete SMA resection (e.g. for epilepsy), it is possible that a different reorganization mechanism is involved, perhaps involving the contralateral SMA.
Our study is limited by size and additional patients would allow us to examine recurrent SMA syndrome in greater detail. Pre- and post-operative functional MRI would provide important information regarding the functional recovery after SMA syndrome and also help elucidate specific areas as centers for reorganization.
Conclusions
This case series demonstrates that SMA syndrome can recur after repeated resection of tumors in the SMA region. In patients with residual SMA cortex after tumor resection, the SMA can reorganize ipsilaterally around the resected SMA as seen in the patients presented in this manuscript. It is uncertain if a similar mechanism is present in patients who undergo complete resection of the SMA. Neurosurgeons should be aware of the risk for recurrent SMA syndrome when counseling patients about surgery.
Acknowledgments
Funding: Dr. Abel is supported by a grant through the National Institutes of Health (NIH F32-NS087664).
Footnotes
Disclosure: The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
References
- 1.Zentner J, Hufnagel A, Pechstein U, Wolf HK, Schramm J. Functional results after resective procedures involving the supplementary motor area. J Neurosurg. 1996;85(4):542–549. doi: 10.3171/jns.1996.85.4.0542. [DOI] [PubMed] [Google Scholar]
- 2.Rostomily RC, Berger MS, Ojemann GA, Lettich E. Postoperative deficits and functional recovery following removal of tumors involving the dominant hemisphere supplementary motor area. J Neurosurg. 1991;75(1):62–68. doi: 10.3171/jns.1991.75.1.0062. [DOI] [PubMed] [Google Scholar]
- 3.Laplane D, Talairach J, Meininger V, Bancaud J, Orgogozo JM. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci. 1977;34(3):301–314. doi: 10.1016/0022-510x(77)90148-4. [DOI] [PubMed] [Google Scholar]
- 4.Russell SM, Kelly PJ. Incidence and clinical evolution of postoperative deficits after volumetric stereotactic resection of glial neoplasms involving the supplementary motor area. Neurosurgery. 2003;52(3):506–516. doi: 10.1227/01.neu.0000047670.56996.53. discussiom 515-506. [DOI] [PubMed] [Google Scholar]
- 5.Ulu MO, Tanriover N, Ozlen F, Sanus GZ, Tanriverdi T, Ozkara C, Uzan M. Surgical treatment of lesions involving the supplementary motor area: clinical results of 12 patients. Turk Neurosurg. 2008;18(3):286–293. [PubMed] [Google Scholar]
- 6.Rosenberg K, Nossek E, Liebling R, Fried I, Shapira-Lichter I, Hendler T, Ram Z. Prediction of neurological deficits and recovery after surgery in the supplementary motor area: a prospective study in 26 patients. J Neurosurg. 2010;113(6):1152–1163. doi: 10.3171/2010.6.JNS1090. [DOI] [PubMed] [Google Scholar]
- 7.Bannur U, Rajshekhar V. Post operative supplementary motor area syndrome: clinical features and outcome. Br J Neurosurg. 2000;14(3):204–210. doi: 10.1080/026886900408379. [DOI] [PubMed] [Google Scholar]
- 8.Della Sala S, Marchetti C, Spinnler H. Right-sided anarchic (alien) hand: a longitudinal study. Neuropsychologia. 1991;29(11):1113–1127. doi: 10.1016/0028-3932(91)90081-i. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg TE, Schindler RJ, Flanagan NG, Haber LD. Two alien hand syndromes. Neurology. 1992;42(1):19–24. doi: 10.1212/wnl.42.1.19. [DOI] [PubMed] [Google Scholar]
- 10.Krainik A, Lehericy S, Duffau H, Vlaicu M, Poupon F, Capelle L, Cornu P, Clemenceau S, Sahel M, Valery CA, Boch AL, Mangin JF, Bihan DL, Marsault C. Role of the supplementary motor area in motor deficit following medial frontal lobe surgery. Neurology. 2001;57(5):871–878. doi: 10.1212/wnl.57.5.871. [DOI] [PubMed] [Google Scholar]
- 11.von Lehe M, Schramm J. Gliomas of the cingulate gyrus: surgical management and functional outcome. Neurosurg Focus. 2009;27(2):E9. doi: 10.3171/2009.6.FOCUS09104. [DOI] [PubMed] [Google Scholar]
- 12.Tate MC, Kim CY, Chang EF, Polley MY, Berger MS. Assessment of morbidity following resection of cingulate gyrus gliomas. Clinical article. J Neurosurg. 2011;114(3):640–647. doi: 10.3171/2010.9.JNS10709. [DOI] [PubMed] [Google Scholar]
- 13.Berger MS, Cohen WA, Ojemann GA. Correlation of motor cortex brain mapping data with magnetic resonance imaging. J Neurosurg. 1990;72(3):383–387. doi: 10.3171/jns.1990.72.3.0383. [DOI] [PubMed] [Google Scholar]
- 14.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci. 2008;9(11):856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 15.Akkal D, Dum RP, Strick PL. Supplementary motor area and presupplementary motor area: targets of basal ganglia and cerebellar output. J Neurosci. 2007;27(40):10659–10673. doi: 10.1523/JNEUROSCI.3134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inase M, Tokuno H, Nambu A, Akazawa T, Takada M. Corticostriatal and corticosubthalamic input zones from the presupplementary motor area in the macaque monkey: comparison with the input zones from the supplementary motor area. Brain Res. 1999;833(2):191–201. doi: 10.1016/s0006-8993(99)01531-0. [DOI] [PubMed] [Google Scholar]
- 18.Mitz AR, Wise SP. The somatotopic organization of the supplementary motor area: intracortical microstimulation mapping. J Neurosci. 1987;7(4):1010–1021. doi: 10.1523/JNEUROSCI.07-04-01010.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macpherson JM, Marangoz C, Miles TS, Wiesendanger M. Microstimulation of the supplementary motor area (SMA) in the awake monkey. Exp Brain Res. 1982;45(3):410–416. doi: 10.1007/BF01208601. [DOI] [PubMed] [Google Scholar]
- 20.Penfield W, Welch K. The supplementary motor area of the cerebral cortex; a clinical and experimental study. AMA Arch Neurol Psychiatry. 1951;66(3):289–317. doi: 10.1001/archneurpsyc.1951.02320090038004. [DOI] [PubMed] [Google Scholar]
- 21.Van Buren JM, Fedio P. Functional representation on the medial aspect of the frontal lobes in man. J Neurosurg. 1976;44(3):275–289. doi: 10.3171/jns.1976.44.3.0275. [DOI] [PubMed] [Google Scholar]
- 22.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11(11):3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deecke L, Kornhuber HH. An electrical sign of participation of the mesial 'supplementary' motor cortex in human voluntary finger movement. Brain Res. 1978;159(2):473–476. doi: 10.1016/0006-8993(78)90561-9. [DOI] [PubMed] [Google Scholar]
- 24.Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007;27(9):2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weilke F, Spiegel S, Boecker H, von Einsiedel HG, Conrad B, Schwaiger M, Erhard P. Time-resolved fMRI of activation patterns in M1 and SMA during complex voluntary movement. J Neurophysiol. 2001;85(5):1858–1863. doi: 10.1152/jn.2001.85.5.1858. [DOI] [PubMed] [Google Scholar]
- 26.Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- 27.Weiller C, Isensee C, Rijntjes M, Huber W, Muller S, Bier D, Dutschka K, Woods RP, Noth J, Diener HC. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann Neurol. 1995;37(6):723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- 28.Rijntjes M, Weiller C. Recovery of motor and language abilities after stroke: the contribution of functional imaging. Prog Neurobiol. 2002;66(2):109–122. doi: 10.1016/s0301-0082(01)00027-2. [DOI] [PubMed] [Google Scholar]
- 29.Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30(4):749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- 30.Nudo RJ. Functional and structural plasticity in motor cortex: implications for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14(1 Suppl):S57–S76. doi: 10.1016/s1047-9651(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 31.Liepert J, Bauder H, Wolfgang HR, Miltner WH, Taub E, Weiller C. Treatment-induced cortical reorganization after stroke in humans. Stroke. 2000;31(6):1210–1216. doi: 10.1161/01.str.31.6.1210. [DOI] [PubMed] [Google Scholar]