Abstract
Type 2 endoleak (T2EL) is the most common complication following endovascular aneurysm repair (EVAR) for abdominal aortic aneurysms. The management of T2ELs is controversial due to the relatively low incidence of negative outcomes when secondary intervention is avoided. Some studies challenge this practice as demonstrated by adverse events following conservative treatment of T2ELs. Evidence has shown that the preoperative computed tomographic angiogram can predict the development of T2EL based on a patient's arterial anatomy, specifically vessels associated with increased rates of post-EVAR endoleak development. Preoperative embolization of those aortic branch vessels associated with T2ELs has shown decreased rates of postoperative complications and may result in a decreased need for surveillance and reintervention.
Keywords: abdominal aortic aneurysm, endovascular aneurysm repair, type 2 endoleak, inferior mesenteric artery embolization, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the predictive factors for the development of type 2 endoleak following EVAR, and role of preoperative inferior mesenteric artery embolization.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Ruptured abdominal aortic aneurysms (AAAs) result in approximately 15,000 deaths annually.1 Patients with AAAs measuring 5.5 cm or larger are recommended to undergo aneurysm repair to reduce the high risk of mortality from rupture. Endovascular aneurysm repair (EVAR) is now a widely accepted treatment for AAA.2 EVAR has been associated with a significant improvement in 30-day postoperative mortality compared with the original repair technique performed via an open transabdominal or retroperitoneal approach.3 4 5 The postoperative course following EVAR is associated with increased complication rates compared with the open approach. Following EVAR, endoleaks complicate 10 to 50% of cases that result in an increased need of repeat intervention.6 Due to high complication rates, lifelong radiographic surveillance is currently recommended following EVAR. Identification of an endoleak and/or increased aneurysm growth may prompt repeat intervention to prevent aneurysm rupture.7 Preoperative computed tomographic (CT) angiogram evaluation can identify anatomical risk factors predisposing to complications, including patent aortic branch vessels associated with type 2 endoleaks (T2ELs) formation.8 Preoperative embolization of these vessels has shown decrease rates of T2EL and aneurysm sac growth.
Endoleak Following Endovascular Aneurysm Repair
Endoleaks are defined as the persistence or reconstitution of perigraft blood flow within the aortic aneurysm sac. Morbidity and mortality rates vary widely based on the mechanism of endoleak. Timely detection and accurate subclassification are important to determine management strategies and prognosis.
The main indication for repeat intervention following EVAR is identification of aneurysm sac enlargement, as this finding is most associated with the risk of sac rupture. The presence of an endoleak has been shown to be the primary predictive factor in aneurysm sac enlargement.6 Four subtypes (1–4) of endoleaks have been described.9 The subtypes can be categorized into high-pressure or low-pressure endoleak. A type 1 endoleak results from a failure to seal at the proximal or distal graft limb attachment site. This subtype occurs in up to 10% of all endoleaks. T2ELs form from retrograde filling of the aneurysm sac through patent aortic branches within the extent of the graft. Type 3 endoleaks develop from leakage through a junctional site of modular endograft components or directly from a disruption of the endograft fabric itself. Type 4 endoleaks, also referred to as endotension, occur in the setting of aneurysm sac enlargement without angiographic demonstration of arterial blood extravasation. Type 4 endoleaks are rarely encountered with modern endografts. High-pressure (type 1 and 3) endoleaks expose the aneurysm sac to systolic arterial pressure, and by consensus intervention is required due to the high risk of aneurysm sac rupture. Type 2 and type 4 endoleaks expose the aneurysm sac to relatively low diastolic arterial pressure associated with less risk of aneurysm rupture. Identification of a low-pressure endoleak may or may not prompt repeat intervention.
Current postoperative management involves close aneurysm surveillance in response to the high incidence of complications following EVAR. The medical community lacks a universally accepted consensus for long-term endoleak surveillance (i.e., intervals of imaging and patient selection) and appropriate use of repeat intervention that satisfies concerns for patient safety and resource utilization.
Type 2 Endoleak
T2EL is the most frequent complication encountered following EVAR, estimated to occur in 8 to 45% of cases. Diagnosis is typically made on completion angiogram or initial follow-up imaging. Several studies have demonstrated a high rate of spontaneous resolution of T2EL, ranging from 40 to 67% of patients managed conservatively.10 11 12 13 14 15 16 17 Other retrospective study outcomes challenge the practice of conservative management with findings of increased morbidity, including aneurysm rupture, when intervention is not performed. In a review of AAA ruptures following EVAR, 160 of the 235 patients with ruptured aneurysms were attributed to endoleak; of these cases, 14% were attributed to a T2EL alone.18
A large systematic review19 demonstrated that aneurysm rupture following an isolated T2EL was noted in less than 1% of all T2EL. Aneurysm sac expansion greater than 5 mm, a common threshold for initiating intervention, was absent in greater than one-third of these ruptures. This review also noted that T2ELs that fail to resolve spontaneously after 6 months are associated with increased long-term complications including sac enlargement, repeat intervention with a high rate of conversion to open surgical approach, and rupture. Another large study demonstrated no correlation of T2EL with an increased risk of aneurysm rupture.13
The opposing findings of many well-designed studies have created controversy regarding the management of T2ELs. The majority of studies have found that conservative management of T2EL is likely a safe approach due to low rates of major adverse events. Other studies have reported that T2ELs pose a significant risk for aneurysm rupture, and therefore promote closer surveillance and a lower threshold for initiating aggressive interventional management.
Several recent studies have added helpful information to guide postoperative management decisions. A retrospective review of 103 patients without endoleak on an immediate postoperative CT angiogram did not develop significant complications or require reintervention in the first 3 years. These finding suggest that those patients with normal early postoperative angiography may benefit from a less strict surveillance schedule that may lead to decreased medical costs, radiation, intravenous contrast exposure, and patient anxiety.20 Early postoperative CT angiography demonstrating sac enlargement has also shown value for predicting the future need for repeat intervention. Additionally, detailed aneurysm sac features, including endoleak volume, nidus maximal diameter, and quantity of patent aortic side branches, help to identify patients who will benefit from early T2EL intervention.21 Another patient series reported that patients with delayed endoleaks noted after 1 year following EVAR represented the majority of all endoleaks. Of these patients with delayed endoleak development, there was a significant association with increased aneurysm sac diameter. These finding suggest that a relaxed long-term screening strategy may overlook delayed endoleaks, which will delay detection of significant complication rates.22 Larger studies are needed to identify specific patient factors predisposing to early versus late T2EL, and those who benefit from early aggressive management.
Predictive Factors of Type 2 Endoleaks
A review of preoperative CT angiography has defined anatomical risk factors associated with the development of postoperative T2EL. Risk of T2EL was found to significantly increase based on the number of patent aortic side branch vessels found within the proposed AAA graft site. The presence of two patent L4 lumbar arteries, two patent L3 lumbar arteries, and a patent inferior mesenteric artery (IMA) was associated with a significantly increased incidence of post-EVAR T2EL (Fig. 1); a patent IMA was associated with the highest risk of a T2EL.23
Fig. 1.
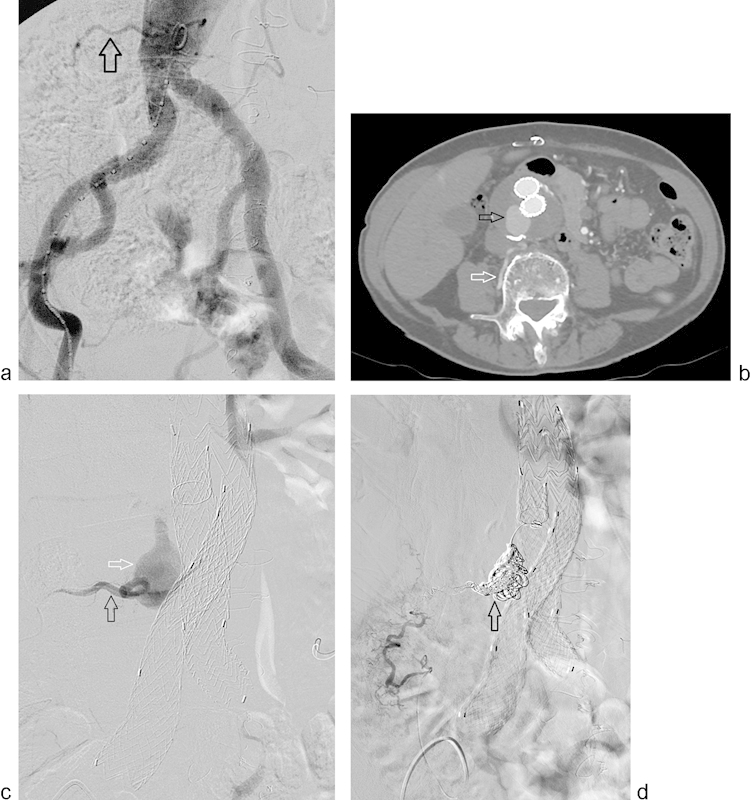
Pre-EVAR planning angiogram (a) demonstrates a patent right lumbar artery (arrow). Postoperative year-4 surveillance CT angiogram (b) shows a patent right lumbar artery (white arrow) and type 2 endoleak (black arrow) with significant growth of the aneurysm sac. Selective right lumbar angiogram (black arrow) (c) shows filling of the aneurysm sac (white arrow). Completion angiogram (d) following successful coil embolization (arrow) of the aneurysm sac and right lumbar artery.
The preoperative finding of occluded L3 or L4 lumbar arteries was associated with a decreased incidence of T2EL, suggesting a protective effect. Mural thrombosis greater than 50% of the diameter of the sac was not associated with an increased or decreased risk. Prior studies have reported a protective effect of an aortic thrombus–covered perimeter of 67% or greater. Identification of aortic side branch vessel patency, regardless of size, was satisfactory to determine T2EL risk in one study.24 In a separate study of 120 patients who underwent EVAR, orifice size of the IMA was measured retrospectively from preoperative CT angiography. Fifty percent of the patients with transient T2EL and 100% of patients with persistent endoleak had an IMA orifice greater than 2.5 mm. In patients without endoleak, only 24% were found to have an IMA orifice of greater than 2.5 mm.25 These findings suggest that a size greater than 2.5 mm or vessel patency, regardless of orifice size, may predict preoperative risk for a T2EL. Anatomical predictors allow a clinician to identify patients who may benefit from more frequent postoperative surveillance or preoperative intervention to decrease the risk of endoleak.
Preoperative Embolization
Currently, there is no consensus for ideal surveillance duration or timing of intervention, as there is uncertain clinical significance of T2EL. A valid solution to this dilemma is to decrease the likelihood of T2EL development prior to EVAR. Preoperative EVAR aortic branch vessel embolization has shown to be a reliable, feasible, and safe modality for decreasing the incidence of T2EL. Preoperative embolization of a patent IMA has been shown to significantly decrease this incidence by 15%, and to decrease aneurysm sac volume by 21%.26 Additionally, the technical success rate of IMA embolization approaches 100%.26 27 28 Lumbar artery embolization has significantly lower reported success rates due to increased procedural time and technical challenges. Studies suggest that the IMA is an ideal pre-EVAR embolization target due to the higher risk of T2EL associated with a patent IMA compared with a patent lumbar artery. Prospective randomized studies comparing preoperative EVAR IMA and lumbar embolization have not yet been performed.
Pre-EVAR embolization performed to prevent development of a T2EL may be preferable to managing a postoperative endoleak with transarterial feeding vessel embolization techniques, which have shown unsatisfactory success rates approaching only 60%.29 30 Although preoperative embolization exposes the patient to additional operative risks, the benefit of reduced postoperative complications may outweigh any downside. Decreasing the incidence of significant outcomes, including T2EL formation and aneurysm size increase, may lessen the need to perform invasive and technically challenging repeat interventions later in a patient's course. Larger long-term studies are needed to investigate whether preoperative embolization of patent perigraft vessels predisposing to T2EL safely allows less frequent imaging and decrease risk of repeat intervention, aneurysm rupture, and mortality.
Inferior Mesenteric Artery Embolization Operative Technique
Candidates for IMA embolization prior to EVAR are identified by a patent IMA on routine preoperative CT angiography. Per the authors' institutional standard preoperative EVAR workup, patients undergo conventional angiography of the infrarenal aorta to establish longitudinal measurements and again demonstrate a patent IMA during calibrated flush aortography. A 5F calibrated pigtail catheter with 1-cm radiopaque markers is used for flush aortography via a transfemoral approach. Aortography protocol includes anteroposterior and lateral projections at the level of the renal arteries, as well as right and left anterior oblique projections of the pelvis. The IMA is then selectively catheterized using a 5F reverse-curve selective catheter, followed by a selective angiogram to confirm patency and identify the origin of the left colic artery. Using a coaxial technique, a 3F microcatheter is advanced into the IMA proximal to the origin of the left colic artery. Interlocking 0.018-inch platinum microcoils are sequentially deployed in the proximal IMA, proximal to the origin of the left colic artery. Interlocking coils are preferred over pushable coils in this procedure because precise IMA delivery is necessary to avoid bowel ischemia. Intermittent angiography is performed until complete vascular stasis is achieved (Fig. 2). IMA embolization is routinely performed on an ambulatory basis 1 day prior to EVAR.31
Fig. 2.
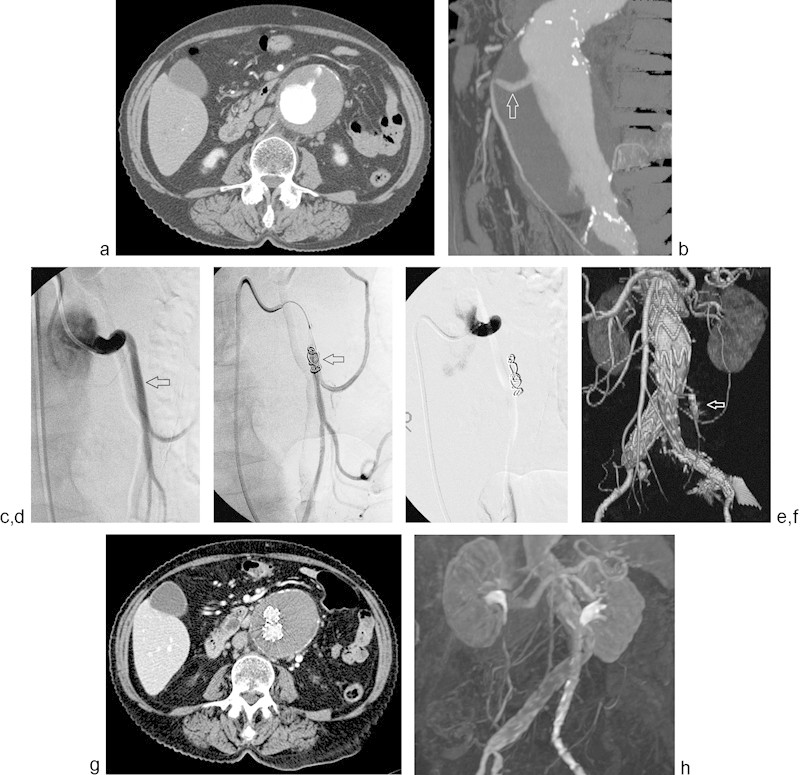
Axial CT angiogram (a) demonstrates a large infrarenal AAA with a widely patent IMA. 3D CT angiogram reconstruction (b) re-demonstrates a patent IMA (arrow). Pre-EVAR angiogram (c) demonstrates a patent IMA (arrow). Platinum coils were deployed with intermittent angiography (arrow) (d) until complete IMA stasis was achieved (e). CT angiogram 3D reconstruction (f) obtained 1 month post-EVAR demonstrates occlusion of the IMA (arrow) and absence of endoleak. Surveillance CT (g) and MR (h) angiogram, obtained at 2 and 3 years postoperatively, respectively, again demonstrate freedom from type 2 endoleak or sac enlargement.
Major complications following IMA embolization are infrequent. Most minor complications are due to nonlocalized abdominal pain that resolves with intravenous hydration and observation. Postembolization abdominal pain is hypothesized to be related to a transient steal syndrome from newly dilated collateral vessels preferentially supplying the descending and sigmoid colon; the risk of mesenteric ischemia is limited by the proximal location of IMA embolization. Proximal embolization allows collateral pathways, including the arc of Riolan and the marginal artery of Drummond, to remain functional. Complications resulting in mesenteric ischemia are associated with high mortality rates; therefore, evaluation for nonstandard anatomy including evidence of prior colon resection or other procedures resulting in disruption of collateral flow must be thoroughly evaluated during initial patient consultation and prior imaging. If the past history is unclear, a superior mesenteric artery angiogram can be performed for evaluation of middle colic artery patency. Disrupted collateral pathways are considered to be a contraindication to IMA embolization.26
Conclusion
The management of T2EL following EVAR continues to present a dilemma due to uncertain significance and appropriate use of interventional techniques. Conservative management of T2EL has been reported by many authors to be a safe and cost-effective approach; however, several studies oppose this finding, including cases of aneurysm rupture occurring without warning. Multiple opposing studies leading to unclear understanding of T2EL significance demonstrate the need for further research. Anatomic factors predisposing to T2EL, including patent IMA and lumbar arteries, have been identified and shown to decrease complication incidence when preoperatively embolized. In the absence of a consensus for postoperative management, a reasonable approach to improving patient's safety and resource utilization may include decreasing known risk factors of postoperative complications. Preoperative IMA embolization has demonstrated feasibility and shown a decreased incidence T2EL formation. Larger long-term studies are necessary to determine if preoperative embolization results in a reduced need for repeat intervention and postoperative morbidity.
References
- 1.Sakalihasan N, Limet R, Defawe O D. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–1589. doi: 10.1016/S0140-6736(05)66459-8. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch A T, Haskal Z J, Hertzer N R. et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47(6):1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Greenhalgh R M Brown L C Kwong G P Powell J T Thompson S G; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial Lancet 20043649437843–848. [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh R M Brown L C Powell J T Thompson S G Epstein D Sculpher M J; United Kingdom EVAR Trial Investigators. Endovascular versus open repair of abdominal aortic aneurysm N Engl J Med 2010362201863–1871. [DOI] [PubMed] [Google Scholar]
- 5.Lederle F A, Freischlag J A, Kyriakides T C. et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–1542. doi: 10.1001/jama.2009.1426. [DOI] [PubMed] [Google Scholar]
- 6.Schanzer A, Greenberg R K, Hevelone N. et al. Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation. 2011;123(24):2848–2855. doi: 10.1161/CIRCULATIONAHA.110.014902. [DOI] [PubMed] [Google Scholar]
- 7.White G H, Yu W, May J, Chaufour X, Stephen M S. Endoleak as a complication of endoluminal grafting of abdominal aortic aneurysms: classification, incidence, diagnosis, and management. J Endovasc Surg. 1997;4(2):152–168. doi: 10.1177/152660289700400207. [DOI] [PubMed] [Google Scholar]
- 8.Lewin R L Fatterpekar G Doshi A Cohen E Nowakowski F Anatomic risk factors for type 2 endoleak following EVAR: a retrospective review of CT angiography in 184 patients Scientific Abstract presented at: RSNA Annual Meeting; November 27, 2005; Chicago, IL
- 9.Veith F J, Baum R A, Ohki T. et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35(5):1029–1035. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- 10.White G H, Yu W, May J. Endoleak—a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg. 1996;3(1):124–125. doi: 10.1583/1074-6218(1996)003<0124b:>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.White G H, May J, Waugh R C, Chaufour X, Yu W. Type III and type IV endoleak: toward a complete definition of blood flow in the sac after endoluminal AAA repair. J Endovasc Surg. 1998;5(4):305–309. doi: 10.1177/152660289800500403. [DOI] [PubMed] [Google Scholar]
- 12.Baum R A, Stavropoulos S W, Fairman R M, Carpenter J P. Endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2003;14(9 Pt 1):1111–1117. doi: 10.1097/01.rvi.0000085773.71254.86. [DOI] [PubMed] [Google Scholar]
- 13.van Marrewijk C, Buth J, Harris P L, Norgren L, Nevelsteen A, Wyatt M G. Significance of endoleaks after endovascular repair of abdominal aortic aneurysms: The EUROSTAR experience. J Vasc Surg. 2002;35(3):461–473. doi: 10.1067/mva.2002.118823. [DOI] [PubMed] [Google Scholar]
- 14.Baum R A, Carpenter J P, Tuite C M. et al. Diagnosis and treatment of inferior mesenteric arterial endoleaks after endovascular repair of abdominal aortic aneurysms. Radiology. 2000;215(2):409–413. doi: 10.1148/radiology.215.2.r00ma17409. [DOI] [PubMed] [Google Scholar]
- 15.Görich J, Rilinger N, Sokiranski R. et al. Leakages after endovascular repair of aortic aneurysms: classification based on findings at CT, angiography, and radiography. Radiology. 1999;213(3):767–772. doi: 10.1148/radiology.213.3.r99dc04767. [DOI] [PubMed] [Google Scholar]
- 16.Back M R, Bowser A N, Johnson B L, Schmacht D, Zwiebel B, Bandyk D F. Patency of infrarenal aortic side branches determines early aneurysm sac behavior after endovascular repair. Ann Vasc Surg. 2003;17(1):27–34. doi: 10.1007/s10016-001-0327-x. [DOI] [PubMed] [Google Scholar]
- 17.van Marrewijk C J Fransen G Laheij R J Harris P L Buth J; EUROSTAR Collaborators. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up Eur J Vasc Endovasc Surg 2004272128–137. [DOI] [PubMed] [Google Scholar]
- 18.Schlösser F J, Gusberg R J, Dardik A. et al. Aneurysm rupture after EVAR: can the ultimate failure be predicted? Eur J Vasc Endovasc Surg. 2009;37(1):15–22. doi: 10.1016/j.ejvs.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Sidloff D A, Stather P W, Choke E, Bown M J, Sayers R D. Type II endoleak after endovascular aneurysm repair. Br J Surg. 2013;100(10):1262–1270. doi: 10.1002/bjs.9181. [DOI] [PubMed] [Google Scholar]
- 20.Kirkpatrick V E, Wilson S E, Williams R A, Gordon I L. Surveillance computed tomographic arteriogram does not change management before 3 years in patients who have a normal post-EVAR study. Ann Vasc Surg. 2014;28(4):831–836. doi: 10.1016/j.avsg.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 21.Dudeck O, Schnapauff D, Herzog L. et al. Can early computed tomography angiography after endovascular aortic aneurysm repair predict the need for reintervention in patients with type II endoleak? Cardiovasc Intervent Radiol. 2015;38(1):45–52. doi: 10.1007/s00270-014-0901-6. [DOI] [PubMed] [Google Scholar]
- 22.Zhou W, Blay E Jr, Varu V. et al. Outcome and clinical significance of delayed endoleaks after endovascular aneurysm repair. J Vasc Surg. 2014;59(4):915–920. doi: 10.1016/j.jvs.2013.10.093. [DOI] [PubMed] [Google Scholar]
- 23.Ward T J, Cohen S, Patel R S. et al. Anatomic risk factors for type-2 endoleak following EVAR: a retrospective review of preoperative CT angiography in 326 patients. Cardiovasc Intervent Radiol. 2014;37(2):324–328. doi: 10.1007/s00270-013-0646-7. [DOI] [PubMed] [Google Scholar]
- 24.Brountzos E, Karagiannis G, Panagiotou I, Tzavara C, Efstathopoulos E, Kelekis N. Risk factors for the development of persistent type II endoleaks after endovascular repair of infrarenal abdominal aortic aneurysms. Diagn Interv Radiol. 2012;18(3):307–313. doi: 10.4261/1305-3825.DIR.4646-11.1. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda T, Matsuda H, Sanda Y. et al. CT Findings of Risk Factors for Persistent Type II Endoleak from Inferior Mesenteric Artery to Determine Indicators of Preoperative IMA Embolization. Ann Vasc Dis. 2014;7(3):274–279. doi: 10.3400/avd.oa.14-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward T J, Cohen S, Fischman A M. et al. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol. 2013;24(1):49–55. doi: 10.1016/j.jvir.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Bonvini R, Alerci M, Antonucci F. et al. Preoperative embolization of collateral side branches: a valid means to reduce type II endoleaks after endovascular AAA repair. J Endovasc Ther. 2003;10(2):227–232. doi: 10.1177/152660280301000210. [DOI] [PubMed] [Google Scholar]
- 28.Velazquez O C, Baum R A, Carpenter J P. et al. Relationship between preoperative patency of the inferior mesenteric artery and subsequent occurrence of type II endoleak in patients undergoing endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2000;32(4):777–788. doi: 10.1067/mva.2000.108632. [DOI] [PubMed] [Google Scholar]
- 29.Solis M M, Ayerdi J, Babcock G A. et al. Mechanism of failure in the treatment of type II endoleak with percutaneous coil embolization. J Vasc Surg. 2002;36(3):485–491. doi: 10.1067/mva.2002.126542. [DOI] [PubMed] [Google Scholar]
- 30.Ermis C, Krämer S, Tomczak R. et al. Does successful embolization of endoleaks lead to aneurysm sac shrinkage? J Endovasc Ther. 2000;7(6):441–445. doi: 10.1177/152660280000700603. [DOI] [PubMed] [Google Scholar]
- 31.Axelrod D J, Lookstein R A, Guller J. et al. Inferior mesenteric artery embolization before endovascular aneurysm repair: technique and initial results. J Vasc Interv Radiol. 2004;15(11):1263–1267. doi: 10.1097/01.RVI.0000141342.42484.90. [DOI] [PubMed] [Google Scholar]


