Abstract
There is a significant risk of complication following endovascular abdominal repair (EVAR), including endoleak, graft translocation, thrombosis, and infection. Surveillance imaging is important for detecting EVAR complication. Surveillance modalities include conventional X-ray, computed tomography, magnetic resonance imaging, ultrasound, and conventional angiography, with inherent advantages and drawbacks to each modality. The authors present common complications following EVAR, and recent advances in the key modalities for surveillance.
Keywords: abdominal aortic aneurysm, endovascular aneurysm repair, surveillance imaging, endoleak, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe the role of surveillance imaging, including the advantages and drawbacks of different forms of imaging, following endovascular repair of AAA.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Abdominal aortic aneurysm (AAA) is a major source of morbidity and mortality in the United States, affecting 2 to 4% of individuals at 60 years of age and doubling in prevalence every subsequent decade of life.1 Every year, 4,500 deaths result from untreated AAA, and an additional 1,400 deaths result from treatment complications following AAA repair.2 Endovascular aneurysm repair (EVAR) emerged over 20 years ago as a minimally invasive alternative to open surgical aneurysm graft repair,3 and has been shown to reduce 30-day mortality and lifelong morbidity versus open repair.4 5 Since 2005, EVAR is the dominant treatment choice for the repair of AAA.6 Current manufacturers are now in their fifth and sixth generation of EVAR graft types, allowing for significant advances in the technology as usage continues to expand.7
Trending the rise in utilization of EVAR, there has been an expansion for imaging follow-up in this patient population. Imaging surveillance of EVAR is important due to an overall complication rate of up to 30%8 and an annual rate of aneurysm rupture of 1% after EVAR.9 Complications of EVAR that are detectable by imaging include endoleak the primary contributor to aneurysm enlargement and eventual rupture, as well as graft infection, migration, kinking, and thrombosis. Imaging has been instrumental in detecting and treating these complications. Moreover, recent advances in imaging technology have allowed for improved sensitivity in surveillance while simultaneously reducing patient exposure.
Endoleak
Endoleak deserves particular attention given that it is a unique complication of covered stent grafts in the aorta. Endoleak was a term first proposed by White et al in 1996 following the recent introduction of the EVAR technique in the surgical literature, used at that time to describe incomplete exclusion of the aneurysm sac by the graft.10 Since that time, the classification system for endoleak has been modified to account for the source of blood flow into the space between the aortic lumen and the graft11 (Table 1, Fig. 1).
Table 1. Endoleak categorical classification.
| Type of endoleak | Location of leak |
|---|---|
| Type 1 | Attachment site |
| A | Proximal |
| B | Distal |
| C | Iliac occluder |
| Type 2 | Collateral vessel |
| A | Single vessel |
| B | Multiple vessels |
| Type 3 | Graft failure |
| A | Midgraft puncture |
| B | Junctional |
| C | Other (e.g., suture hole) |
| Type 4 | Porosity of graft wall |
| Type 5 | Endotension |
Source: Reprinted with permission from Shah and Stavropoulos.41
Fig. 1.
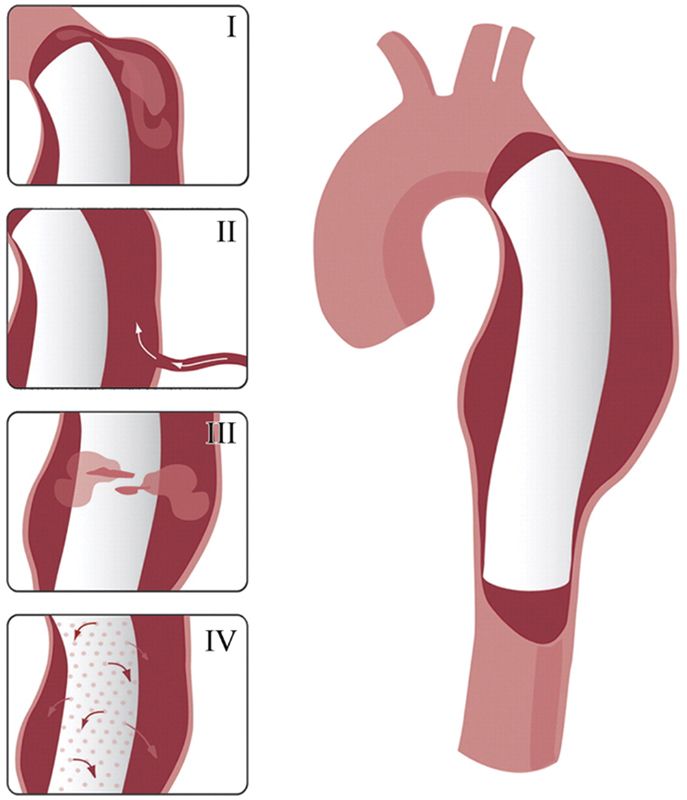
Drawings illustrating the various types of endoleak: type I, attachment site; type II, collateral vessel; type III, graft failure, type IV, graft porosity. (Reprinted with permission from Agarwal P et al. Multidetector CT of thoracic aortic aneurysms. Radiographics 2009; 29:537–552).
White et al, in their seminal description of endoleak, described what has come to be recognized as a type 1 endoleak. Type I endoleak occurs due to an incomplete attachment site of the proximal or distal graft to the aortic lumen, further classified as type 1a (proximal) or type 1b (distal) at the point of attachment.11 Type 1 endoleak has a prevalence of up to 10% after EVAR, and elevates the risk for aortic rupture.12 Predisposing risk factors for type 1 endoleak include tortuous geometry or heavy atherosclerotic burden at attachment sites,13 14 and advanced EVAR extension techniques using chimney or snorkel grafts that preclude adequate apposition of the graft to the vessel lumen.15 Recent increases in the spatial and temporal resolution of computed tomography (CT) angiography have shown that attachment site suture and metal-ring breaks in the EVAR graft material are responsible for a significant number of delayed type 1 endoleak.16
Type 2 endoleak is the most common type in the treatment of AAA, occurring in 10 to 25% of EVAR.17 Type 2 endoleak results from collateral arterial circulation into the excluded aneurysm sac, most commonly due to the inferior mesenteric artery (IMA) or lumbar arteries in the infrarenal aorta. Further subdivision of type 2 endoleak depends on whether there is one (type 2a) or multiple (type 2b) collateral arteries supplying the sac. Unlike type 1 endoleak, there remains controversy in the degree of contribution of type 2 endoleak to aneurysm enlargement, due to the frequent spontaneous thrombosis and bidirectional flow in and out of the aneurysm sac by the collateral vessels.15 17 However, delayed type 2 endoleak more than 1 year after EVAR is more strongly associated with sac enlargement.18 Risk factors for type 2 endoleak include a larger IMA ostium and a greater number of lumbar arteries present on pre-EVAR imaging, as well as lack of preoperative embolization of the IMA and/or internal iliac arteries in uni-iliac and bi-iliac stent grafts.18 19
Type 3 endoleak is characterized by EVAR graft mechanical failure, in the form of either graft rupture (type 3a) or leakage between contiguous graft components (type 3b). Prevalence of type 3 endoleak is 4% after graft creation and requires urgent endovascular repair given the one-way mechanism of blood flow into the sac.15 Type 4 endoleak is due to porous flow directly through the fabric of the EVAR graft. Almost never seen in newer generation stent grafts, type 4 endoleak is present immediately after graft placement due to the failure of the fabric in earlier versions of EVAR, and is typically of no clinical consequence.17 Type 5 endoleak, referred to as endotension, is a diagnosis of exclusion: one of the primary utilities of imaging surveillance is to exclude type 1–4 endoleak from the differential of endotension. Endotension is the increase in size of the aneurysm sac without detectable endoleak, and as of yet has an indeterminate etiology. Competing theories include ultrafiltration of plasma through the graft, pressure transmittance across the graft into the sac, and insufficiently detected type 1–4 endoleak.20 21
Graft Translocation
EVAR graft migration is one of the most common complications of AAA treatment, occurring in up to 3% of patients.22 Graft migration greater than 10 mm is considered significant.13 Migration may result in attachment site (type 1) endoleak (Fig. 1a), as well as possible branch vessel occlusion, most commonly the renal arteries and internal iliac arteries. Once the graft migrates significantly, there is increased risk for intragraft thrombosis. Predisposing factors for graft migration include infrarenal graft placement, oversizing or undersizing of graft by greater than 30%, and tortuous neck geometry.22 23
EVAR graft kinking has a similar incidence as migration, and frequently may even be due to migration itself if the graft translocates inferiorly with distal attachment sites still intact (Fig. 2b).24 Kinking is also directly associated with limb thrombosis, present in 1 to 5% of EVAR, and is related to nonlaminar flow within the kinked graft (Fig. 2c).25 Kinking may be preempted by avoiding intraprocedural oversizing of the graft to prevent redundancy of the graft fabric and by a linear deployment without torquing within the vessel lumen.24 26
Fig. 2.
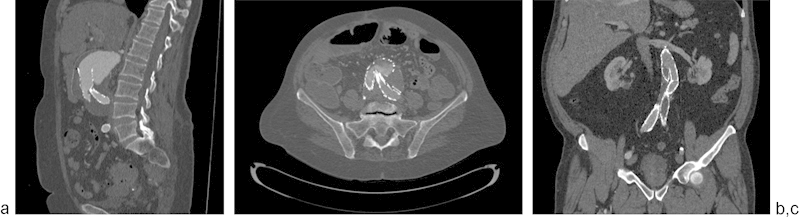
(a) Graft migration with type 1 endoleak (arrow), (b) graft kinking with turbulent flow in lumen, (c) graft kinking with thrombosis of left iliac limb (arrow).
Graft Infection
Infection is a relatively uncommon complication of EVAR, affecting less than 1% of grafts,27 but carrying a disproportionately larger mortality rate of 6 to 11%.28 29 Infection presents with systemic symptoms such as fever, malaise, and leukocytosis, as well as directly related symptoms such as back pain and an increasingly pulsatile aneurysm. Several underlying factors are known to contribute to graft infection, most notably procedure-related contamination of the prosthesis,27 29 as well as secondary contamination due to local infection of subjacent viscera or bacteremia.30 Air within the aneurysm sac and/or periaortic inflammatory stranding are indicators of EVAR infection on CT imaging (Fig. 3a). Aortoenteric fistula is a rare but significant complication of EVAR leading to infection and a direct connection from the aorta into bowel. Contribution by a previously infected or inflammatory aneurysm is the most common reason for developing fistulization into the bowel or genitourinary system31 (Fig. 3b).
Fig. 3.
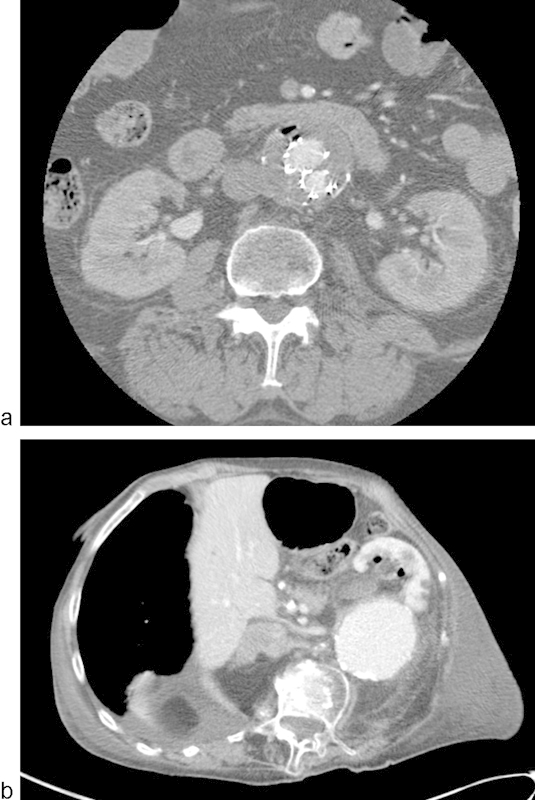
(a) Graft infection with air in the sac (arrow); (b) graft infection with aorto-ureteric fistula and pyelonephritis.
Surveillance Imaging Modalities
Radiology
Conventional X-ray radiography was the first functional imaging modality,32 and is still a useful conjunctive modality in the surveillance of EVAR. Unlike the slice-by-slice presentation of cross-sectional modalities such as CT and magnetic resonance imaging (MRI), X-ray instead provides an overview of the graft as a whole. X-ray displays EVAR position relative to easily identifiable bony landmarks, and reveals graft wire frame integrity.33 34 With proper patient positioning, estimation of endograft position and potential fracture/kinking is reliable to a high degree of accuracy, at a fraction of the cost of cross-sectional modalities such as CT and MRI.35 Conventional radiography has a significantly lower radiation exposure than CT and does not suffer from the metallic coil–related artifacts that are present in ultrasound and MRI.36 However, radiography remains only a complementary modality to cross-sectional imaging because it cannot measure the size of the aneurysm sac surrounding the graft, as well as being unable to detect soft tissue complications such as endoleak and infection.
Computed Tomography
CT angiography (CTA) is the mainstay for surveillance imaging in the post-EVAR patient population. The authors recommend post-EVAR CTA imaging at intervals of 30 days, 6 months, and 1 year postprocedure. If no complications are detected in the first year, then CT imaging is advised annually for the lifetime of the patient. CTA in the authors' protocol is standardized to begin with a noncontrast unenhanced phase, followed by thin-section arterial contrast phase images, and terminates with delayed-phase images. Unenhanced phase images are helpful to delineate high density in the aneurysm sac, such as calcification within mural thrombus, prior coil material, or surgical clips, from a true endoleak seen on later phase images (Fig. 4a, b). Measurements of the aorta, including maximum sac diameter, are completed using 3D multiplanar reformat software to allow measurement perpendicular to the centerline of the aortic lumen for preciseness and interobserver reproducibility.
Fig. 4.
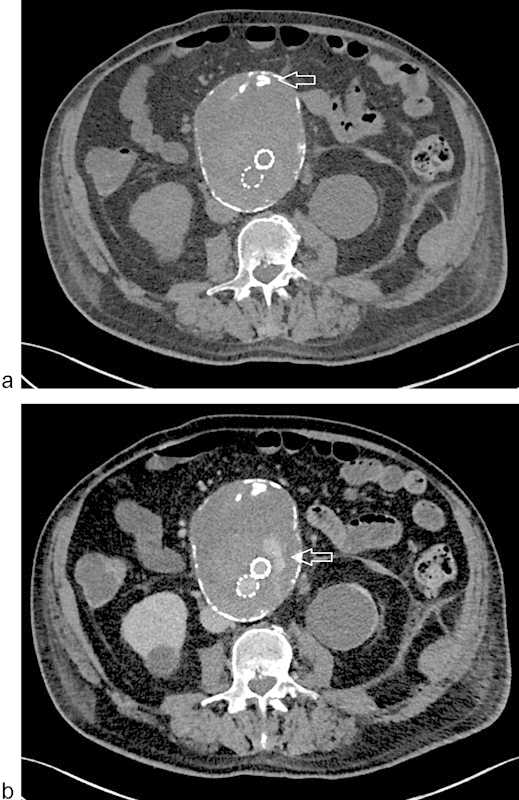
CTA of EVAR. (a) Misleading high attenuation due to neointimal calcium seen on unenhanced imaging (arrow); (b) true endoleak revealed on delayed imaging (arrow).
Given the high spatial resolution of CTA compared with ultrasound and magnetic resonance angiography (MRA), CT is a superior modality for characterizing mechanical graft complications such as kinking, fracture, or migration. Soft tissue complications of infection such as perigraft fluid, inflammation, and abscess formation are also more readily interpreted on CT.27 28 37
The primary indicator of underlying clinically relevant endoleak on CT is enlargement in the aneurysm sac diameter. Indeed, unenhanced phase CT alone is a viable measure of sac size, and certain authors advocate the use of unenhanced CT as the unequivocal initial screening modality, with further arterial and delayed phase imaging completed only if an increase in sac diameter is noted real-time.38 Once sac enlargement is detected, the arterial phase CTA is highly sensitive for detecting endoleak as a potential cause, with a sensitivity exceeding 92% compared with 63% seen with digital subtraction angiography.39 Endoleak appears as contrast opacification within the excluded aneurysm sac. The magnitude of endoleak attenuation on CTA is clinically important in delineating the type of endoleak: type I and III endoleaks are nearly always visualized as high attenuation noted in adjacent arterial structures on arterial phase images, while type II endoleak will appear faint early and eventually highly attenuate on delayed images.13 Delayed phase imaging may reveal additional endoleak not visualized on arterial phase imaging, such as so-called low-flow endoleak that do not briskly enhanced on earlier timed images.40
While CT has established itself as the gold standard of surveillance imaging due to its high image quality, high sensitivity for detecting complications, widespread accessibility, and relatively low cost, significant concerns exist related to ionizing radiation exposure and intravascular contrast loading.41 42 Radiation reduction measures can be taken by reducing the number of phases; authors have proposed unenhanced-only protocols to detect changes in sac size40 and delayed phase–only protocols to detect an endoleak without an arterial phase image.43 In current-generation CT scanners, the enhanced arterial and delayed phases may be completed with lower tube voltage down to 80 kVp based on the preimaging topogram of patient body habitus, versus a standard high-dose setting of 120 kVp.44 Surveillance imaging interval may also safely be lengthened to every 2 to 3 years if the aneurysm sac has not grown and is less than 4 cm in size.45 Newer CT software allows for iterative reconstruction, a dose-saving reconstruction algorithm over the classical filtered back-projection technique. Moreover, the model-based iterative reconstruction (MBIR) has over a 73% dose reduction over even standard low-dose adaptive statistical iterative reconstruction (ASIR) while maintaining diagnostic accuracy of CTA.46 Lastly, the emerging technology of dual energy CT (DECT) imaging provides virtual unenhanced images by subtracting two separate simultaneously acquired energy beams, resulting in further dose saving and improving upon beam-hardening artifacts seen at lower beam energies47 (Fig. 5a,b).
Fig. 5.
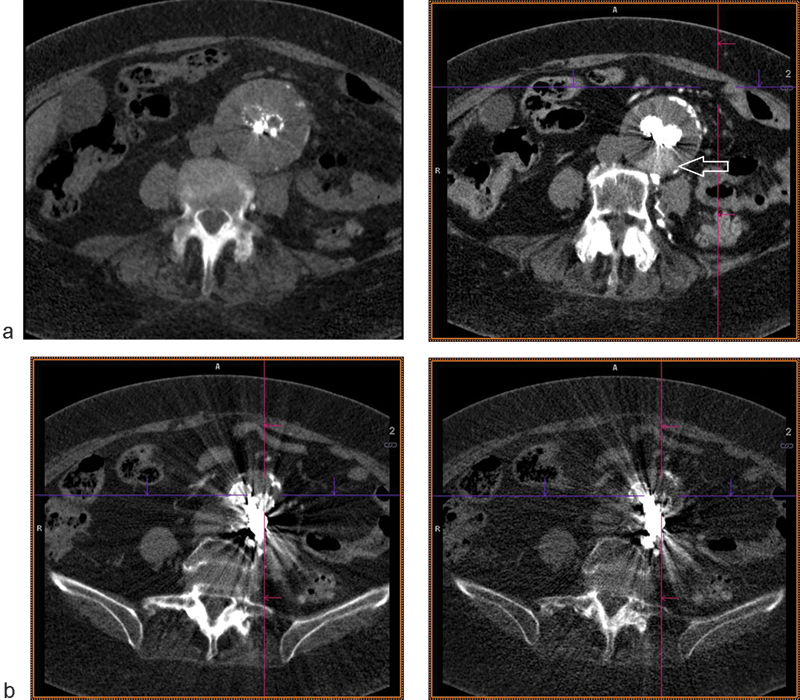
DE-CTA of EVAR. (a) Dose saving from virtual unenhanced reconstruction shown on left; posterior endoleak shown on delayed image on right (arrow). (b) Reduction in coil photon attenuation with monoenergetic beam reconstruction at low (left) and high (right) keV settings.
The development of contrast nephropathy is a concern in patients with underlying renal insufficiency and those who have had recent intravenous contrast dye load within 24 hours of the CT scan. Similar to measures for radiation reduction, patients at risk for contrast nephropathy may be managed with unenhanced-only scan technique, and if CTA with contrast is then required, the patient may be preloaded with intravenous hydration and n-acetyl cysteine to reduce the risk of developing nephropathy. CT scan technology has advanced to the point where a reduced contrast dose will yield diagnostic image quality; Utsunomyia et al and Schindera et al both showed that a lower dose 80 kVp protocol yield equivalent image quality to higher dose protocols while simultaneously utilizing less contrast.48 49 If the patient truly cannot support a contrast load, then that patient may be managed with a modality such as ultrasound that does not require intravenous contrast.
Magnetic Resonance Imaging
MRA using gadolinium contrast emerged in the late 20th century as an alternative to CTA for post-EVAR imaging,50 and is applicable in the detection of stent graft patency, thrombosis, and aortic rupture. MRA protocol at the authors' institution begins with axial T1-weighted gradient echo images, followed by axial single shot fast spin echo images and pre- and postgadolinium dynamic contrast images. As with CTA imaging, MRA images should be viewed utilizing multiplanar reformat software and maximum intensity projection (MIP) reconstruction. MRA is particularly useful in patients with nitinol stent grafts; stainless steel and nickel alloy grafts cause a large amount of susceptibility artifact that precludes optimal evaluation by magnetic resonance imaging (Fig. 6). The absence of these artifacts is also well studied in patients who have had prior coil embolization with platinum coils.51 52 53
Fig. 6.
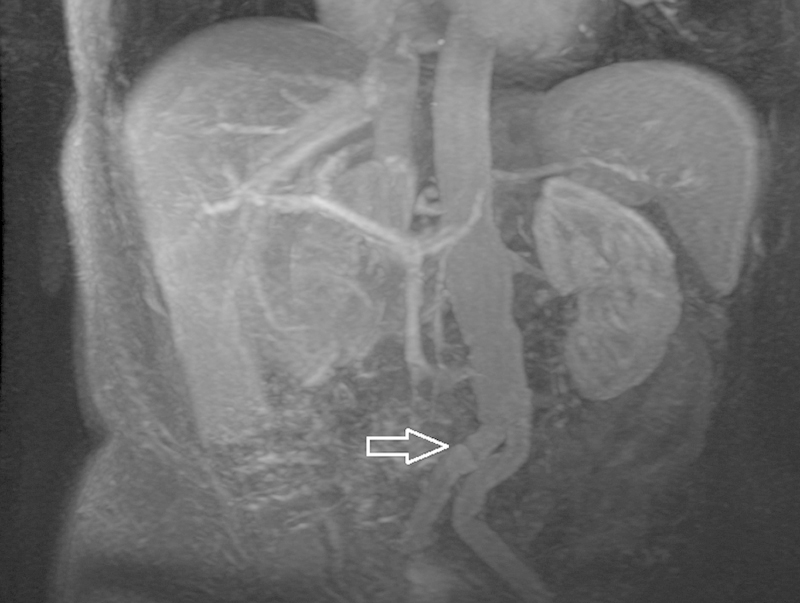
MRA maximum intensity projection of EVAR showing kinking in the iliac limbs (arrow).
In regard to endoleak detection, MRA has been shown to be equivalent to CTA in sensitivity, and in the case of type II endoleak it is superior for patients with newer generation nitinol EVAR grafts.53 In particular, the late gadolinium contrast images are the most sensitive for detecting endoleak,54 akin to the delayed phase images on CTA. Time-resolved MRA (TR-MRA) is a recent advance that allows for increased temporal resolution and improved visualization of contrast dynamics of endoleak accumulation; however, inferior spatial resolution and susceptibility artifacts from the gradient echo sequence cause TR-MRA to function at most as an adjunctive sequence to gadolinium-enhanced MRA for endoleak detection.55 56 Blood pool contrast agents, such as gadofosveset trisodium that binds to albumin, have been shown to supplement the detection for endoleak on MRA, albeit with a time penalty to reach steady-state phase of the blood pool contrast agent in the vasculature.57 58 Emerging technology utilizing four-dimensional phase contrast (PC) MRA can reveal turbulent flow dynamics in the pre- and post-EVAR aorta, a risk factor for graft thrombosis and endoleak.59
MRA affords several advantages over CT-based imaging, including the absence of ionizing radiation and iodinated contrast-based nephropathy. However, in patients with a calculated glomerular filtration rate below 30, a significant risk of nephrogenic systemic fibrosis remains a concern, particularly in dialysis patients.60 In these patients, noncontrast MRA using time-of-flight imaging is appropriate, noting that a specificity of these examinations has been documented as low as 54% for detecting endoleak.61 In addition to the limitation of nonnitinol EVAR graft material to susceptibility artifact, drawbacks to MRA versus CTA are related to longer scan times, greater cost, and lesser accessibility in the community setting.
Ultrasound
Ultrasound offers a low-cost, nonradiation-based alternative to cross-sectional imaging modalities in the surveillance of EVAR. Both grayscale and Doppler techniques have been described in the literature, with aortic diameter measurements equivalent, and in one study more precise, than measurements using CTA.62 63 Similar to TR-MRA, color Doppler ultrasound can reveal the directionality of endoleak because of its sensitivity for flowing blood.62 Spectral Doppler allows the analysis of waveforms within regions of endoleak to document arterial inflow. Contrast-enhanced ultrasound (CEUS) using nontargeted microbubbles can offer supplementation to CTA or unenhanced ultrasound in a problem-solving role. Millen et al demonstrated that CEUS used after CTA or unenhanced ultrasound in 539 patients resulted in an additional 10 patients who required secondary intervention for endoleak.63 Ultrasound microbubble contrast is not nephrotoxic, and although at this current time untargeted microbubble contrast agents are approved by the FDA in the United States, targeted applications such as the evaluation of endoleak in the abdomen remain unapproved for clinical use per FDA recommendation.64
Unlike the standardized approach to CTA examination, ultrasound studies are heterogeneous due to a significantly higher degree of interoperator variability in skill and technique, as well as suffering from attenuation artifacts in large body habitus patients.65 Due to these heterogeneities, ultrasound has a high specificity for endoleak in up to 95% but overall sensitivity as low as 70% when using CTA as a gold standard.66 67 Overall views of EVAR graft integrity, and complications such as graft migration and kinking, are also poorly evaluated by ultrasound.68 As such, at the authors' institution, ultrasound remains an adjunctive modality for problem-solving in difficult cases, i.e., for differentiating true low-flow endoleak from endotension, rather than a first-line imaging modality unless there are contraindications to CTA or MRA.
Conventional Angiography
Digital subtraction angiography (DSA) has been an integral component to EVAR dating to the development and placement of the first EVAR grafts by Parodi et al in 1991.3 As early as the late 1990s, however, CTA and MRA have shown increased sensitivity for detecting complications during post-EVAR placement surveillance, including endoleak,39 41 with DSA falling out of favor due to the relatively high radiation, cost, and procedural time. Moreover, DSA is an invasive technique that requires direct arterial puncture and has a low but inherent risk for complications including pseudoaneurysm, arteriovenous fistulae, retroperitoneal hemorrhage, and arterial thrombosis.69
DSA at the current time is appropriate in the treatment management of endoleak found on prior noninvasive imaging, or for preprocedural planning for EVAR revision or extension. Both transarterial- and translumbar-approach endoleak embolization are preceded by intravascular DSA, which can reveal the directionality of the leak and very frequently the culprit inflow vessel that may not be depicted on the noninvasive imaging70 71 (Fig. 7a). Postprocedure DSA allows the physician to bypass immediate follow-up noninvasive imaging (Fig. 7b).
Fig. 7.
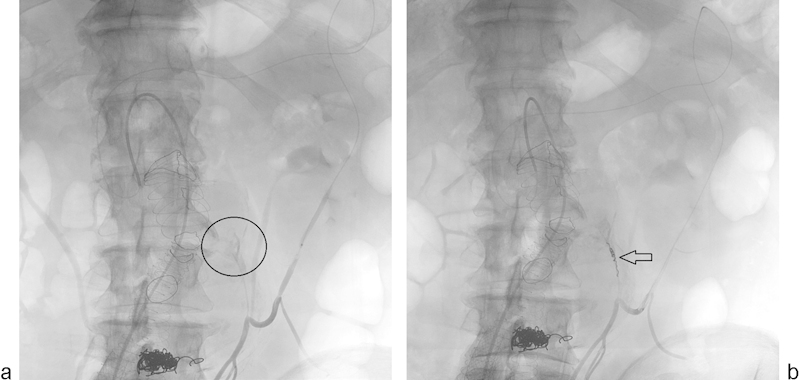
Conventional angiography of EVAR. (a) Previously embolized endoleak via translumbar approach shows residual type 2 endoleak supplied via the IMA (circle), here injected through the marginal artery from an SMA approach; (b) successful coil embolization of the IMA with resolution of endoleak (arrow).
Conclusion
As the next generation of EVAR devices are created, the number of patients amenable to endovascular treatment of AAA will continue to grow. The role of surveillance imaging is an important adjunctive to EVAR, particularly given the breadth of complications. At the current time, at the authors' institution CTA is recommended as the gold standard for EVAR surveillance given its widespread availability and reproducibility. Within the limits of scanner technology, the authors recommend the incorporation of kVp dose reduction, single-phase unenhanced CTA, iterative reconstruction algorithms, and DE-CTA. At institutions where MRA is available, it is a feasible direct alternative in patients with nitinol stents or allergy to iodinated contrast. It is the authors' opinion that ultrasound technology is an adjunctive to other modalities, and at the very least should be incorporated with conventional radiography if used for surveillance. Conventional angiography is limited to therapeutic intervention in patients who have EVAR complications detected on noninvasive imaging surveillance.
References
- 1.Singh K, Bønaa K H, Jacobsen B K, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am J Epidemiol. 2001;154(3):236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 2.McPhee J T, Hill J S, Eslami M H. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001-2004. J Vasc Surg. 2007;45(5):891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Parodi J C, Palmaz J C, Barone H D. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5(6):491–499. doi: 10.1007/BF02015271. [DOI] [PubMed] [Google Scholar]
- 4.Greenhalgh R M Brown L C Kwong G P Powell J T Thompson S G; EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial Lancet 20043649437843–848. [DOI] [PubMed] [Google Scholar]
- 5.Schermerhorn M L, O'Malley A J, Jhaveri A, Cotterill P, Pomposelli F, Landon B E. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358(5):464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 6.Ng T T Mirocha J Magner D Gewertz B L Variations in the utilization of endovascular aneurysm repair reflect population risk factors and disease prevalence J Vasc Surg 2010514801–809., 809.e1 [DOI] [PubMed] [Google Scholar]
- 7.Vandy F, Upchurch G R Jr. Endovascular aneurysm repair: current status. Circ Cardiovasc Interv. 2012;5(6):871–882. doi: 10.1161/CIRCINTERVENTIONS.111.966184. [DOI] [PubMed] [Google Scholar]
- 8.d'Audiffret A, Desgranges P, Kobeiter D H, Becquemin J P. Follow-up evaluation of endoluminally treated abdominal aortic aneurysms with duplex ultrasonography: validation with computed tomography. J Vasc Surg. 2001;33(1):42–50. doi: 10.1067/mva.2001.112215. [DOI] [PubMed] [Google Scholar]
- 9.Vallabhaneni S R, Harris P L. Lessons learnt from the EUROSTAR registry on endovascular repair of abdominal aortic aneurysm repair. Eur J Radiol. 2001;39(1):34–41. doi: 10.1016/s0720-048x(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 10.White G H, Yu W, May J. Endoleak—a proposed new terminology to describe incomplete aneurysm exclusion by an endoluminal graft. J Endovasc Surg. 1996;3(1):124–125. doi: 10.1583/1074-6218(1996)003<0124b:>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Stavropoulos S W, Baum R A. Imaging modalities for the detection and management of endoleaks. Semin Vasc Surg. 2004;17(2):154–160. doi: 10.1053/j.semvascsurg.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Stavropoulos S W, Clark T W, Carpenter J P. et al. Use of CT angiography to classify endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Interv Radiol. 2005;16(5):663–667. doi: 10.1097/01.RVI.0000152386.97448.F1. [DOI] [PubMed] [Google Scholar]
- 13.Stavropoulos S W, Charagundla S R. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007;243(3):641–655. doi: 10.1148/radiol.2433051649. [DOI] [PubMed] [Google Scholar]
- 14.Aburahma A F, Campbell J E, Mousa A Y. et al. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J Vasc Surg. 2011;54(1):13–21. doi: 10.1016/j.jvs.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Liaw J V, Clark M, Gibbs R, Jenkins M, Cheshire N, Hamady M. Update: Complications and management of infrarenal EVAR. Eur J Radiol. 2009;71(3):541–551. doi: 10.1016/j.ejrad.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Ueda T, Takaoka H, Petrovitch I, Rubin G D. Detection of broken sutures and metal-ring fractures in AneuRx stent-grafts by using three-dimensional CT angiography after endovascular abdominal aortic aneurysm repair: association with late endoleak development and device migration. Radiology. 2014;272(1):275–283. doi: 10.1148/radiol.14130920. [DOI] [PubMed] [Google Scholar]
- 17.Veith F J, Baum R A, Ohki T. et al. Nature and significance of endoleaks and endotension: summary of opinions expressed at an international conference. J Vasc Surg. 2002;35(5):1029–1035. doi: 10.1067/mva.2002.123095. [DOI] [PubMed] [Google Scholar]
- 18.Nolz R, Teufelsbauer H, Asenbaum U. et al. Type II endoleaks after endovascular repair of abdominal aortic aneurysms: fate of the aneurysm sac and neck changes during long-term follow-up. J Endovasc Ther. 2012;19(2):193–199. doi: 10.1583/11-3803.1. [DOI] [PubMed] [Google Scholar]
- 19.Güntner O, Zeman F, Wohlgemuth W A. et al. Inferior mesenteric arterial type II endoleaks after endovascular repair of abdominal aortic aneurysm: are they predictable? Radiology. 2014;270(3):910–919. doi: 10.1148/radiol.13130489. [DOI] [PubMed] [Google Scholar]
- 20.Filippi F, Tirotti C, Stella N, Rizzo L, Taurino M. Endotension-related aortic sac rupture treated by endograft relining. Vascular. 2013;21(2):113–115. doi: 10.1177/1708538113478725. [DOI] [PubMed] [Google Scholar]
- 21.Ricotta J J II Endoleak management and postoperative surveillance following endovascular repair of thoracic aortic aneurysms J Vasc Surg 201052(4, Suppl):91S–99S. [DOI] [PubMed] [Google Scholar]
- 22.Carpenter J P, Anderson W N, Brewster D C. et al. Multicenter pivotal trial results of the Lifepath System for endovascular aortic aneurysm repair. J Vasc Surg. 2004;39(1):34–43. doi: 10.1016/j.jvs.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 23.Sampaio S M, Panneton J M, Mozes G. et al. AneuRx device migration: incidence, risk factors, and consequences. Ann Vasc Surg. 2005;19(2):178–185. doi: 10.1007/s10016-004-0166-7. [DOI] [PubMed] [Google Scholar]
- 24.Carroccio A, Faries P L, Morrissey N J. et al. Predicting iliac limb occlusions after bifurcated aortic stent grafting: anatomic and device-related causes. J Vasc Surg. 2002;36(4):679–684. [PubMed] [Google Scholar]
- 25.Maleux G, Koolen M, Heye S, Nevelsteen A. Limb occlusion after endovascular repair of abdominal aortic aneurysms with supported endografts. J Vasc Interv Radiol. 2008;19(10):1409–1412. doi: 10.1016/j.jvir.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Hellinger J C. Endovascular repair of thoracic and abdominal aortic aneurysms: pre- and postprocedural imaging. Tech Vasc Interv Radiol. 2005;8(1):2–15. doi: 10.1053/j.tvir.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Sharif M A, Lee B, Lau L L. et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2007;46(3):442–448. doi: 10.1016/j.jvs.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Ducasse E, Calisti A, Speziale F, Rizzo L, Misuraca M, Fiorani P. Aortoiliac stent graft infection: current problems and management. Ann Vasc Surg. 2004;18(5):521–526. doi: 10.1007/s10016-004-0075-9. [DOI] [PubMed] [Google Scholar]
- 29.Fatima J, Duncan A A, de Grandis E. et al. Treatment strategies and outcomes in patients with infected aortic endografts. J Vasc Surg. 2013;58(2):371–379. doi: 10.1016/j.jvs.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 30.van den Berg H R, Leijdekkers V J, Vahl A. Aortic stent-graft infection following septic complications of a kidney stone. Cardiovasc Intervent Radiol. 2006;29(3):443–445. doi: 10.1007/s00270-005-0028-x. [DOI] [PubMed] [Google Scholar]
- 31.Saratzis N, Saratzis A, Melas N, Ktenidis K, Kiskinis D. Aortoduodenal fistulas after endovascular stent-graft repair of abdominal aortic aneurysms: single-center experience and review of the literature. J Endovasc Ther. 2008;15(4):441–448. doi: 10.1583/08-2377.1. [DOI] [PubMed] [Google Scholar]
- 32.Röntgen W C. On a New Kind of Rays: translation of a paper read before the Würzburg Physical and Medical Society. Nature. 1895;53:274–276. [Google Scholar]
- 33.Fearn S, Lawrence-Brown M MD, Semmens J B, Hartley D. Follow-up after endovascular aortic aneurysm repair: the plain radiograph has an essential role in surveillance. J Endovasc Ther. 2003;10(5):894–901. doi: 10.1177/152660280301000508. [DOI] [PubMed] [Google Scholar]
- 34.Harrison G J, Oshin O A, Vallabhaneni S R, Brennan J A, Fisher R K, McWilliams R G. Surveillance after EVAR based on duplex ultrasound and abdominal radiography. Eur J Vasc Endovasc Surg. 2011;42(2):187–192. doi: 10.1016/j.ejvs.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 35.Hodgson R, McWilliams R G, Simpson A. et al. Migration versus apparent migration: importance of errors due to positioning variation in plain radiographic follow-up of aortic stent-grafts. J Endovasc Ther. 2003;10(5):902–910. doi: 10.1177/152660280301000509. [DOI] [PubMed] [Google Scholar]
- 36.Viano A M, Gronemeyer S A, Haliloglu M, Hoffer F A. Improved MR imaging for patients with metallic implants. Magn Reson Imaging. 2000;18(3):287–295. doi: 10.1016/s0730-725x(99)00135-6. [DOI] [PubMed] [Google Scholar]
- 37.van der Vliet J A, Kool L J, van Hoek F. Simplifying post-EVAR surveillance. Eur J Vasc Endovasc Surg. 2011;42(2):193–194. doi: 10.1016/j.ejvs.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Bobadilla J L, Suwanabol P A, Reeder S B, Pozniak M A, Bley T A, Tefera G. Clinical implications of non-contrast-enhanced computed tomography for follow-up after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2013;27(8):1042–1048. doi: 10.1016/j.avsg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Armerding M D, Rubin G D, Beaulieu C F. et al. Aortic aneurysmal disease: assessment of stent-graft treatment-CT versus conventional angiography. Radiology. 2000;215(1):138–146. doi: 10.1148/radiology.215.1.r00ap28138. [DOI] [PubMed] [Google Scholar]
- 40.Iezzi R, Cotroneo A R, Filippone A. et al. Multidetector CT in abdominal aortic aneurysm treated with endovascular repair: are unenhanced and delayed phase enhanced images effective for endoleak detection? Radiology. 2006;241(3):915–921. doi: 10.1148/radiol.2413050959. [DOI] [PubMed] [Google Scholar]
- 41.Shah A, Stavropoulos S W. Imaging surveillance following endovascular aneurysm repair. Semin Intervent Radiol. 2009;26(1):10–16. doi: 10.1055/s-0029-1208378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picel A C, Kansal N. Essentials of endovascular abdominal aortic aneurysm repair imaging: postprocedure surveillance and complications. AJR Am J Roentgenol. 2014;203(4):358–372. doi: 10.2214/AJR.13.11736. [DOI] [PubMed] [Google Scholar]
- 43.Macari M, Chandarana H, Schmidt B, Lee J, Lamparello P, Babb J. Abdominal aortic aneurysm: can the arterial phase at CT evaluation after endovascular repair be eliminated to reduce radiation dose? Radiology. 2006;241(3):908–914. doi: 10.1148/radiol.2413051571. [DOI] [PubMed] [Google Scholar]
- 44.Wintersperger B, Jakobs T, Herzog P. et al. Aorto-iliac multidetector-row CT angiography with low kV settings: improved vessel enhancement and simultaneous reduction of radiation dose. Eur Radiol. 2005;15(2):334–341. doi: 10.1007/s00330-004-2575-y. [DOI] [PubMed] [Google Scholar]
- 45.Back M R. Surveillance after endovascular repair. Perspect Vasc Surg Endovasc Ther. 2007;19:395–400. doi: 10.1177/1531003507312612. [DOI] [PubMed] [Google Scholar]
- 46.Hansen N J, Kaza R K, Maturen K E, Liu P S, Platt J F. Evaluation of low-dose CT angiography with model-based iterative reconstruction after endovascular aneurysm repair of a thoracic or abdominal aortic aneurysm. AJR Am J Roentgenol. 2014;202(3):648–655. doi: 10.2214/AJR.13.11286. [DOI] [PubMed] [Google Scholar]
- 47.Flors L, Leiva-Salinas C, Norton P T, Patrie J T, Hagspiel K D. Endoleak detection after endovascular repair of thoracic aortic aneurysm using dual-source dual-energy CT: suitable scanning protocols and potential radiation dose reduction. AJR Am J Roentgenol. 2013;200(2):451–460. doi: 10.2214/AJR.11.8033. [DOI] [PubMed] [Google Scholar]
- 48.Schindera S T, Graca P, Patak M A. et al. Thoracoabdominal-aortoiliac multidetector-row CT angiography at 80 and 100 kVp: assessment of image quality and radiation dose. Invest Radiol. 2009;44(10):650–655. doi: 10.1097/RLI.0b013e3181acaf8a. [DOI] [PubMed] [Google Scholar]
- 49.Utsunomiya D, Oda S, Funama Y. et al. Comparison of standard- and low-tube voltage MDCT angiography in patients with peripheral arterial disease. Eur Radiol. 2010;20(11):2758–2765. doi: 10.1007/s00330-010-1841-4. [DOI] [PubMed] [Google Scholar]
- 50.Haulon S, Lions C, McFadden E P. et al. Prospective evaluation of magnetic resonance imaging after endovascular treatment of infrarenal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;22(1):62–69. doi: 10.1053/ejvs.2001.1405. [DOI] [PubMed] [Google Scholar]
- 51.Ayuso J R, de Caralt T M, Pages M. et al. MRA is useful as a follow-up technique after endovascular repair of aortic aneurysms with nitinol endoprostheses. J Magn Reson Imaging. 2004;20(5):803–810. doi: 10.1002/jmri.20170. [DOI] [PubMed] [Google Scholar]
- 52.Pandey N Cook T Stavropoulos S W et al. Imaging surveillance for recurrent endoleak after prior embolization Paper presented at the Annual Meeting for the North American Society for Cardiovascular Imaging; October 1, 2013; Atlanta, GA
- 53.Habets J, Zandvoort H J, Reitsma J B. et al. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg. 2013;45(4):340–350. doi: 10.1016/j.ejvs.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 54.Lookstein R A, Goldman J, Pukin L, Marin M L. Time-resolved magnetic resonance angiography as a noninvasive method to characterize endoleaks: initial results compared with conventional angiography. J Vasc Surg. 2004;39(1):27–33. doi: 10.1016/j.jvs.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 55.Alerci M, Oberson M, Fogliata A, Gallino A, Vock P, Wyttenbach R. Prospective, intraindividual comparison of MRI versus MDCT for endoleak detection after endovascular repair of abdominal aortic aneurysms. Eur Radiol. 2009;19(5):1223–1231. doi: 10.1007/s00330-008-1253-x. [DOI] [PubMed] [Google Scholar]
- 56.Cohen E I, Weinreb D B, Siegelbaum R H. et al. Time-resolved MR angiography for the classification of endoleaks after endovascular aneurysm repair. J Magn Reson Imaging. 2008;27(3):500–503. doi: 10.1002/jmri.21257. [DOI] [PubMed] [Google Scholar]
- 57.Cornelissen S A, Prokop M, Verhagen H J, Adriaensen M E, Moll F L, Bartels L W. Detection of occult endoleaks after endovascular treatment of abdominal aortic aneurysm using magnetic resonance imaging with a blood pool contrast agent: preliminary observations. Invest Radiol. 2010;45(9):548–553. doi: 10.1097/RLI.0b013e3181e992ac. [DOI] [PubMed] [Google Scholar]
- 58.Wieners G, Meyer F, Halloul Z. et al. Detection of type II endoleak after endovascular aortic repair: comparison between magnetic resonance angiography and blood-pool contrast agent and dual-phase computed tomography angiography. Cardiovasc Intervent Radiol. 2010;33(6):1135–1142. doi: 10.1007/s00270-010-9984-x. [DOI] [PubMed] [Google Scholar]
- 59.van Bogerijen G H, van Herwaarden J A, Conti M. et al. Importance of dynamic aortic evaluation in planning TEVAR. Ann Cardiothorac Surg. 2014;3(3):300–306. doi: 10.3978/j.issn.2225-319X.2014.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21(4):1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 61.Resta E C, Secchi F, Giardino A. et al. Non-contrast MR imaging for detecting endoleak after abdominal endovascular aortic repair. Int J Cardiovasc Imaging. 2013;29(1):229–235. doi: 10.1007/s10554-012-0060-2. [DOI] [PubMed] [Google Scholar]
- 62.Nyheim T, Staxrud L E, Rosen L, Slagsvold C E, Sandbaek G, Jørgensen J J. Review of postoperative CT and ultrasound for endovascular aneurysm repair using Talent stent graft: can we simplify the surveillance protocol and reduce the number of CT scans? Acta Radiol. 2013;54(1):54–58. doi: 10.1258/ar.2012.110291. [DOI] [PubMed] [Google Scholar]
- 63.Millen A, Canavati R, Harrison G. et al. Defining a role for contrast-enhanced ultrasound in endovascular aneurysm repair surveillance. J Vasc Surg. 2013;58(1):18–23. doi: 10.1016/j.jvs.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 64.Chung Y E, Kim K W. Contrast-enhanced ultrasonography: advance and current status in abdominal imaging. Ultrasonography. 2014;34(1):3–18. doi: 10.14366/usg.14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kranokpiraksa P Kaufman J A Follow-up of endovascular aneurysm repair: plain radiography, ultrasound, CT/CT angiography, MR imaging/MR angiography, or what? J Vasc Interv Radiol 200819(6, Suppl):S27–S36. [DOI] [PubMed] [Google Scholar]
- 66.Ashoke R, Brown L C, Rodway A. et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther. 2005;12(3):297–305. doi: 10.1583/04-1479R.1. [DOI] [PubMed] [Google Scholar]
- 67.Raman K G, Missig-Carroll N, Richardson T, Muluk S C, Makaroun M S. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg. 2003;38(4):645–651. doi: 10.1016/s0741-5214(03)00909-1. [DOI] [PubMed] [Google Scholar]
- 68.Iezzi R, Cotroneo A R, Basilico R, Simeone A, Storto M L, Bonomo L. Endoleaks after endovascular repair of abdominal aortic aneurysm: value of CEUS. Abdom Imaging. 2010;35(1):106–114. doi: 10.1007/s00261-009-9526-7. [DOI] [PubMed] [Google Scholar]
- 69.Bangalore S, Bhatt D L. Femoral arterial access and closure. Circulation. 2011;124(5):e147–e156. doi: 10.1161/CIRCULATIONAHA.111.032235. [DOI] [PubMed] [Google Scholar]
- 70.Baum R A, Carpenter J P, Golden M A. et al. Treatment of type 2 endoleaks after endovascular repair of abdominal aortic aneurysms: comparison of transarterial and translumbar techniques. J Vasc Surg. 2002;35(1):23–29. doi: 10.1067/mva.2002.121068. [DOI] [PubMed] [Google Scholar]
- 71.Choi S Y, Won J Y, Lee Y, Choi D, Shim W H, Lee K H. Percutaneous transabdominal approach for the treatment of endoleaks after endovascular repair of infrarenal abdominal aortic aneurysm. Korean J Radiol. 2010;11(1):107–114. doi: 10.3348/kjr.2010.11.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]


