Abstract
Background
Non-linear heart rate variability (HRV) indices were hypothesized to correlate with cardiac function, fluid overload and physical performance in hemodialysis patients
Methods
24-hour Holter electrocardiograms were recorded in patients enrolled in the Frequent Hemodialysis Network (FHN) Daily Dialysis Trial. Correlations between nonlinear HRV indices and left-ventricular ejection fraction (LVEF), left-ventricular end-diastolic volume (LVEDV), extracellular volume (ECV)/total-body water (TBW) ratio, the SF-36 Physical Health Composite (PHC) and Physical Functioning scores (PF) were tested.
Results
We studied 210 subjects [average age 49.8±13.5 years, 62% males, 42% diabetics]. In non-diabetic patients, MSE SampEn and MSE ApEn correlated positively with LVEF, PF, PH, and inversely with LVEDV and ECV/TBW. SPS correlated positively with ECV/TBW (r=0.27). Irregularity measures (MSE ApEn, MSE SampEn) correlated positively with LVEDV (r=0.19 and 0.20).
Conclusion
Nonlinear HRV indices indicated an association between a deteriorated heart rate regulatory system and impaired cardiac function, fluid accumulation and poor physical condition.
Keywords: Cardiac function, cardiac MRI, fluid status, hemodialysis, heart rate variability
Introduction
Cardiovascular disease is the leading cause of death in hemodialysis (HD) patients. Left ventricular hypertrophy (LVH) and left ventricular dilatation, both conditions commonly diagnosed by either magnetic resonance imaging (MRI) [1] or echocardiography [2], are present in a substantial fraction of HD patients primarily due to salt and volume overload [3,4]. LVH has been shown to be associated with outcomes in dialysis patients [2]. Chronic and acute inflammation [5], anemia [6], high blood pressure [7], elevated levels of fibroblast growth factor-23 (FGF-23) [8,9] and myocardial hypoperfusion [10,11] during HD have been shown to be associated with left ventricular function and LVH [5,12]. Besides structural cardiac abnormalities, reduced left ventricular ejection fraction (LVEF) has been reported in a substantial percentage of HD patients [13–15].
Disturbances of autonomic control of heart rate are also common among HD patients, with sympathetic overactivity being the principal alteration [16,17]. Cardiac autonomic function can be characterized through the analysis of the variability of the so-called RR time series, i.e. the series of time intervals between consecutive R peaks (heart beats) (R-R intervals) measured by electrocardiograms (ECG).
Non-linear methods were proposed to investigate the heart rate variability (HRV) regulatory system from a more complex perspective. HRV is the result of a complex regulatory system related to the electrical depolarization of cardiac cells, which is primarily regulated by a) the autonomic nervous system b) the mechanical and functional properties of cardiac cells, and c) electrolytes acting on the refractory period of the action potential in the cardiac cells. Non-linear methods were proposed to characterize quantitatively these properties of the cardiac regulatory system from one of its measures, i.e. the heart rate [18].
Certain pathological conditions and aging are defined by the loss of complexity in the dynamics of the heart rate regulatory system [19]. In particular, diabetic patients often suffer from cardiovascular autonomic neuropathy (CAN), a pathology characterized by a generally reduced HRV.
In the general population, nonlinear HRV indices are established to provide additional information to “traditional” HRV indices and improve the ability to identify patients at high risk of cardiovascular death [19–21]. The diagnostic advantages of non-linear HRV indices suggest they capture unique information. Comparisons of nonlinear HRV measures between HD and healthy subjects [22–25] have so far focused on their prognostic value.
In this work, we hypothesized non-linear HRV indices would correlate cross-sectionally with cardiac function, fluid overload and physical performance, and we examined correlations using all subjects and separately by diabetic status. To that end we characterized non-linear HRV measures in a large cohort of HD patients and explored their correlations with left ventricular mass (LVM), left-ventricular end-diastolic volume (LVEDV), left ventricular ejection fraction (LVEF), extracellular volume (ECV)/total body water (TBW) ratio, and self-reported physical performance.
Subjects and Methods
Frequent Hemodialysis Network (FHN) Daily Trial
The FHN Daily Dialysis Trial (ClinicalTrials.gov#NCT00264758) was a multicenter randomized trial comparing conventional thrice weekly with short 6x weekly in-center HD. Study design with inclusion and exclusion criteria was described previously [26]. Patients were enrolled between 3/2006 and 5/2009 and the trial concluded in 5/2010. The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at each participating site. An independent Data Safety Monitoring Board provided oversight of the trial.
Measurements
We determined LVM, LVEDV and LVEF by cardiac MRI at baseline. All MRI images were analyzed centrally in a blinded manner. A detailed explanation of the MRI methodology was recently published [1]. Holter ECG was recorded by the study staff using the KCI X5 digital Holter recorder (KCI Technology and Services, NJ, USA). Systolic and diastolic blood pressure (SBP, DBP) were measured pre HD by an automated oscillometric method.
We determined TBW, ECV, intracellular volume (ICV), phase angle and lean body mass based on anthropometric measures and single frequency (50 kHz) whole body bioimpedance analysis (BIA; RJL Systems, Clinton Township, NJ). TBW and total body potassium content were calculated using polynomial regression modeling of bioimpedance-derived data [27]. Extracellular fluid volume (ECV) was calculated as the difference between TBW and ICV. Accuracy and precision of this BIA method was recently reported [28]. Residual renal urea clearance was determined based on 24 hour urine samples. The Physical Health Component (PHC) and the Physical Functioning (PF) scores were assessed using the RAND short form (SF)-36 survey [29].
HRV analysis
R-R time series were extracted from 24-hour Holter ECG recordings. QRS complexes were detected and classified with the Holter software CubeHolter (Cardioline s.p.a, Italy). Holter recordings shorter than 15 hours were excluded from subsequent analyses. Differences in time length may affect estimation of long term correlation indices, as well as artifacts (noise, detachment of electrodes, etc…) [30].
ECG recordings can be corrupted by noise, which results in a failure of automatic classification system to detect the actual heart beat duration and it labels it as an unreliable measure (artifact). From the RR time series with sufficient length and less than 5% of RR intervals classified as artifacts, we measured the following nonlinear indices: the slope of the detrended fluctuation analysis (DFA) estimated with a linear detrend and with a quadratic detrend, the slope of the power spectrum at very low frequencies (power slope, SPS), the slope from the multiscale entropy (MSE) measured with two different entropy estimators (the approximate entropy ApEn and the sample entropy SampEn), and a global index of regularity estimated as the entropy value of the entire recordings (ApEn and SampEn). Technical details are illustrated in the appendix.
Statistical analyses
Descriptive statistics for continuous variables were summarized using mean ± SD or medians and 10th and 90th percentiles as appropriate. Categorical variables were summarized using frequencies and proportions. Demographic and non-linear HRV parameters were compared between diabetic and non-diabetic subjects using Student’s t-test, exact Wilcoxon signed rank test, Chi-square or Fisher Exact test, as appropriate. The same comparisons were performed also between randomized and non-randomized patient for completeness.
We derived correlations between the non-linear HRV parameters and: a) cardiovascular (LVM, LVEDV), b) fluid status (ECV/TBW), and c) self-reported Physical Health (PCS and PF) measures according to a predefined analysis plan. As demonstrated previously, a correlation with age and pathology progression [31,32], we explored correlations with age and ESRD vintage also in our population. The significant correlation with self-reported Physical Health was further investigated with the correlation analysis with physical condition-related parameters (phase angle, lean body mass and intracellular volume as assessed by BIA) as ancillary analyses. We examined correlations using all subjects (ALL) and separately by diabetic status (ND and D). We depicted associations using scatter plots with separate local regression curves for diabetics and non-diabetics and also provided Pearson’s and Spearman’s correlation coefficients based on subjective assessment of influential data points.
Two-tailed p-values <0.05 were considered statistically significant. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
We studied 210 patients with qualifying Holters, 176 of whom were eventually randomized into the FHN Daily Dialysis Trial. The subjects’ baseline characteristics are illustrated in Table 1. Furthermore, they did not differ significantly between the patients who were eventually randomized (N=176) and those who were not (N=34). Table 2 reports the non-linear HRV parameters in the enrolled patients. The number of artifacts and unreliable RR intervals was 5% and deemed reasonable.
Table 1.
Subject Characteristics at enrollment for FHN Patients with available data for non-linear HRV Indices. Results are shown as mean ± standard deviation or frequency (%), as appropriate.
| Variables | All (N=210) | Diabetic (N=85) | Non-diabetic (N=118) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | N | N | ||||
|
| ||||||
| Age (years) | 210 | 49.8 ± 13.5 | 85 | 55.4 ± 12.3§ | 118 | 45.8 ± 13.1 |
|
| ||||||
| Male | 210 | 130 (62%) | 85 | 50 (59%) | 118 | 76 (64%) |
|
| ||||||
| Diabetes | 203 | 85 (42%) | -- | 73 (41%) | 27 | 12 (44%) |
|
| ||||||
| Pre-dialysis albumin (per 0.1 g/dL) | 206 | 39.3 ± 4.0 | 85 | 38.3 ± 3.6* | 117 | 40.1 ± 4.0 |
|
| ||||||
| Pre-dialysis phosphorus (mg/dL) | 206 | . | 85 | . | 117 | . |
| <5.5 | 98 (48%) | 39 (46%) | 56 (48%) | |||
| >=5.5 and <7.0 | 65 (32%) | 27 (32%) | 37 (32%) | |||
| >=7.0 | 43 (21%) | 19 (22%) | 24 (21%) | |||
|
| ||||||
| Hemoglobin (g/dL) | 198 | 11.9 ± 1.3 | 82 | 11.8 ± 1.3 | 112 | 12.0 ± 1.4 |
|
| ||||||
| Residual Renal Clearance (L) | . | . | . | |||
| 0 | 127 (62%) | 50 (59%) | 77 (66%) | |||
| >0 and <=0.8 | 204 | 22 (11%) | 85 | 10 (12%) | 117 | 12 (10%) |
| >0.8 and <1.4 | 20 (10%) | 9 (11%) | 11 (9%) | |||
| >1.4 | 35 (17%) | 16 (19%) | 17 (15%) | |||
|
| ||||||
| Weekly enPCR (g/kg/d) | 204 | 10.6 ± 2.8 | 85 | 10.8 ± 2.9 | 117 | 10.4 ± 2.7 |
|
| ||||||
| eKt/V | 204 | 1.42 ± 0.25 | 85 | 1.40 ± 0.26 | 117 | 1.45 ± 0.24 |
|
| ||||||
| Left Ventricular Mass (g) | 177 | 146 ± 59 | 74 | 142 ± 49 | 103 | 149 ± 64 |
|
| ||||||
| Left Ventricular End-diastolic Vol. (ml) | 177 | 182 ± 64 | 74 | 174 ± 52 | 103 | 188 ± 71 |
|
| ||||||
| Left Ventricular Ejection Fraction (%) | 177 | 56.7 ± 11.2 | 74 | 54.5 ± 13.0# | 103 | 58.3 ± 9.6 |
|
| ||||||
| Heart Rate as Measured Centrally (bpm) | 177 | 75.9 ± 13.9 | 74 | 75.9 ± 12.6 | 103 | 75.9 ± 14.8 |
|
| ||||||
| Urine Volume (L) | 210 | . | 85 | . | 118 | . |
| 0 | 151 (72%) | 58 (68%) | 86 (73%) | |||
| >0 and <=1 | 57 (27%) | 27 (32%) | 30 (25%) | |||
| >1.0 | 2 (1%) | 0 | 2 (2%) | |||
|
| ||||||
| Extra-cellular Volume (L) | 193 | 22.9 ± 4.7 | 80 | 24.5 ± 4.7* | 112 | 21.9 ± 4.4 |
|
| ||||||
| Extra-cellular Volume/Total Volume | 193 | 0.523 ± 0.067 | 80 | 0.536 ± 0.068# | 112 | 0.513 ± 0.065 |
|
| ||||||
| Lean Body Mass | 193 | 44.6 ± 10.3 | 80 | 46.3 ± 10.3 | 112 | 43.5 ± 10.1 |
|
| ||||||
| Ultrafiltration volume (L) | 206 | 3.33 ± 1.20 | 85 | 3.60 ± 1.23# | 117 | 3.17 ± 1.16 |
|
| ||||||
| Physical Health Composite (RAND SF36) | 203 | 37.4 ± 10.9 | 83 | 34.4 ± 10.6* | 115 | 39.6 ± 10.7 |
|
| ||||||
| Physical Functioning Scale (SF36) | 204 | 55.9 ± 27.4 | 84 | 44.8 ± 26.6§ | 115 | 63.6 ± 25.4 |
|
| ||||||
| Short Physical Performance Battery Score | 206 | 10.9 ± 3.0 | 85 | 9.92 ± 3.08§ | 117 | 11.6 ± 2.8 |
Significant differences between diabetic and non-diabetic groups are shown in bold font:
p-value≤0.0001,
p-value<0.005,
p-value<0.05
Table 2.
Values of HRV indices at enrollment for FHN Patients with available data for nonlinear HRV Indices. Results are shown as mean ± standard deviation.
| Variables | All (N=210) | Diabetic (N=85) | Non-diabetic (N=118) | |||
|---|---|---|---|---|---|---|
| N | N | N | ||||
| Detrended Fluctuation Analysis | 210 | 1.07 ± 0.07 | 85 | 1.08 ± 0.07 | 118 | 1.07 ± 0.07 |
| Quadratic Detrended Fluctuation Analysis | 210 | 1.05 ± 0.07 | 85 | 1.05 ± 0.08 | 118 | 1.05 ± 0.07 |
| Spectral Power Slope | 210 | 1.32 ± 0.22 | 85 | 1.40 ± 0.22§ | 118 | 1.26 ± 0.21 |
| Sample Entropy (2,0.15) | 210 | 0.638 ± 0.344 | 85 | 0.644 ± 0.324 | 118 | 0.642 ± 0.364 |
| Multiscale Entropy Slope Sample Entropy | 210 | 0.0088 ± 0.0614 | 85 | −0.014 ± 0.060# | 118 | 0.025 ± 0.058 |
| Multiscale Entropy Slope Approximate Entropy | 210 | 0.0099 ± 0.0691 | 85 | −0.019 ± 0.065 | 118 | 0.030 ± 0.066 |
| Approximate Entropy (2,0.15) | 210 | 0.909 ± 0.372 | 85 | 0.913 ± 0.364 | 118 | 0.916 ± 0.384 |
| Percent Deleted Heart Beats | 210 | 4.47 ± 5.87 | 85 | 5.06 ± 7.29 | 118 | 4.15 ± 4.76 |
Significant differences between diabetic and non-diabetic groups are shown in bold font:
p-value≤0.0001,
p-value<0.05.
Cardiac MRI parameters and HRV indices
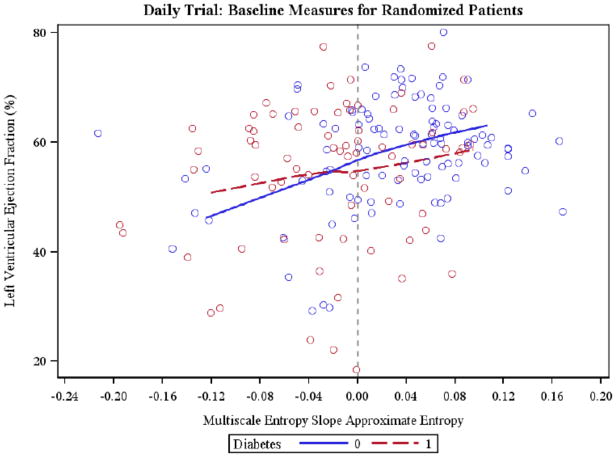
The two MSE slopes correlated significantly with LVEF by considering all patients and only non-diabetic patients. In non-diabetic patients LVEDV correlated significantly with the two MSE slopes as well (Table 3), and correlated positively with ApEn and SampEn. Figure 1 depicts the relationship between LVEF and the MSE ApEn (ALL r=0.27 p-val<0.01, ND r=0.26 p-val<0.01, D r=0.19, n.s.). In diabetic subjects no significant correlations were found (Table 3).
Table 3.
Pearson and Spearman correlation coefficients for associations between HRV indices and measures related to heart functioning, fluid overload and physical condition for all subjects (All), and for non diabetic (ND) and diabetic (D) subjects.
| Measure Characterization | Specific Measures | Cohort | Long term correlations | Complexity over different time scales | Irregularity | ||||
|---|---|---|---|---|---|---|---|---|---|
| Detrended Fluctuation Analysis | Quadratic Detrended Fluctuation Analysis | Spectral Power Slope | Multiscale Entropy Slope Approximate Entropy | Multiscale Entropy Slope Sample Entropy | Approximate Entropy (2,0.15) † | Sample Entropy (2,0.15) † | |||
| heart functioning | LVEF (%) | All | 0.12 | 0.17* | 0.05 | 0.27§ | 0.25§ | −0.13 | −0.09 |
| ND | 0.17 | 0.18 | 0.10 | 0.26§ | 0.23* | −0.11* | −0.02 | ||
| D | 0.11 | 0.18 | 0.11 | 0.19 | 0.19 | −0.15 | −0.14 | ||
| Left Ventricular End-diastolic Vol. (ml) | All | −0.10 | −0.12 | −0.12 | −0.06 | −0.06 | 0.12 | 0.11 | |
| ND | −0.12 | −0.15 | −0.09 | −0.20* | −0.20* | 0.23* | 0.22* | ||
| D | −0.05 | −0.07 | −0.09 | 0.08 | 0.09 | −0.04 | −0.04 | ||
| fluid overload | ECV/TBW | All | 0.13 | 0.08 | 0.23§ | −0.30§ | −0.29§ | −0.03 | −0.03 |
| ND | 0.16 | 0.13 | 0.25* | −0.33§ | −0.30§ | −0.06 | −0.06 | ||
| D | 0.07 | 0.02 | 0.12 | −0.18 | −0.20 | 0.02 | 0.00 | ||
| physical condition | Physical Functioning Scale (RAND SF-36) | All | −0.08 | −0.04 | −0.19* | 0.30§ | 0.30§ | 0.00 | 0.05 |
| ND | −0.12 | −0.10 | −0.18 | 0.30§ | 0.32§ | 0.04 | 0.08 | ||
| D | −0.02 | 0.01 | 0.03 | 0.05 | 0.07 | −0.01 | 0.03 | ||
| Physical Health Composite (RAND SF-36) | All | 0.01 | 0.01 | −0.13 | 0.22§ | 0.23§ | 0.04 | 0.09 | |
| ND | 0.05 | 0.01 | −0.09 | 0.22* | 0.24* | 0.00 | 0.03 | ||
| D | −0.04 | −0.01 | −0.01 | 0.03 | 0.06 | 0.13 | 0.16 | ||
p-value<0.01 (dark grey);
p-value ≤0.05 (light grey)
Associations for these HRV parameters summarized with Spearman correlation coefficients. All other associations summarized with Pearson correlation coefficients.
Figure 1.
Scatter plot relating Multiscale Entropy Slope (MSE ApEn) and left ventricular ejection fraction (LVEF) in diabetic (r=0.19; n.s.) and non-diabetic (r=0.26; P<0.01) subjects is shown. Local regression curve are displayed.
Fluid status and HRV indices
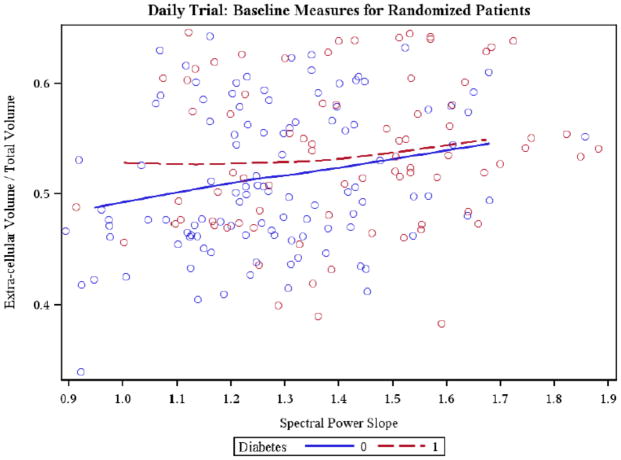
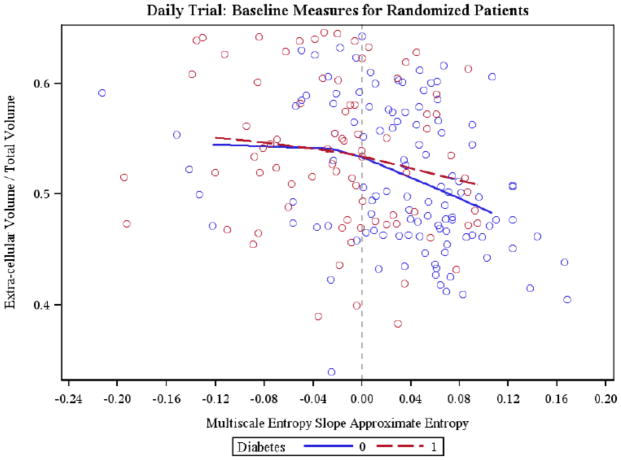
ECW/TBW showed a positive correlation with the SPS (Figure 2), while it inversely correlated with MSE ApEn and MSE SampEn both considering all patients and only non-diabetic patients. An inverse relationship between ECV/TBW and MSE SampEn was also present in diabetic subjects (Table 3). Figure 3 depicts the relationship between ECV/TBW and MSE ApEn (ALL r=−0.30 p-val<0.01, ND r=−0.33; p-val<0.01, D r=−0.18, n.s.). In diabetic subjects no significant correlations were found.
Figure 2.
Scatter plot relating Spectral Power Slope (SPS) and Extracellular Volume/Total Body Water (ECV/TBW) in diabetic (r=0.12; n.s.) and non-diabetic (r=0.25; P<0.05) subjects is shown. Local regression curve are displayed.
Figure 3.
Scatter plot relating Multiscale Entropy Slope (MSE ApEn) and Extracellular Volume/Total Body Water (ECV/TBW) in diabetic (r=−0.18 n.s.) and non-diabetic (r=−0.33; P<0.01) subjects is shown. Local regression curve are displayed.
Physical condition and HRV indices
Physical function and physical health showed positive correlations with MSE ApEn and MSE SampEn in the entire population and in non-diabetic subjects, but not in diabetic subjects (Table 3). In particular, higher physical function scores were associated with higher MSE slopes.
Non-linear HRV indices were also correlated with phase angle, lean body mass and intracellular volume assessed by bioimpedance analysis (Table 4). Lean body mass showed significant inverse correlations with DFA and quadratic DFA in the entire population and in non-diabetic subjects, and positive correlations with MSE Slope ApEn and MSE Slope SampEn in diabetic subjects only. (Table 4). Intracellular volume associated inversely with DFA and quadratic DFA in the entire population and in non-diabetic subjects. Positive correlations were found between intracellular volume and MSE Slope ApEn and MSE Slope SampEn for both diabetic and non-diabetic subjects (Table 4). Finally, phase angle showed positive correlations with MSE Slope ApEn and MSE Slope SampEn (Table 4).
Table 4.
Pearson and Spearman correlation coefficients for associations between non-linear HRV indices and age and ESRD vintage for all subjects (All), and for non diabetic (ND) and diabetic (D) subjects.
| Measure Characterization | Specific Measures | Age | ESRD Vintage | ||||
|---|---|---|---|---|---|---|---|
| All | ND | D | All | ND | D | ||
| Long term correlations | Detrended Fluctuation Analysis | 0.12 | 0.16 | 0.02 | 0.15 | 0.25* | 0.03 |
| Quadratic Detrended Fluctuation Analysis | 0.04 | 0.10 | −0.05 | 0.13 | .23* | −0.01 | |
| Spectral Power Slope | 0.37§ | 0.39§ | 0.13 | −0.01 | 0.10 | −0.07 | |
| Complexity over different time scales | Multiscale Entropy Slope Approximate Entropy | −0.34§ | −0.34§ | −0.10 | 0.11 | −0.03 | 0.24* |
| Multiscale Entropy Slope Sample Entropy | −0.29§ | −0.34§ | 0.00 | 0.09 | −0.05 | 0.22* | |
| Irregularity | Approximate Entropy (2,0.15) † | 0.16* | 0.18* | 0.19 | −0.07 | −0.04 | −0.13 |
| Sample Entropy (2,0.15) † | 0.09 | 0.13 | 0.09 | −0.09 | −0.07 | −0.13 | |
p-value<0.01 (dark grey);
p-value ≤0.05 (light grey)
Associations for these HRV parameters summarized with Spearman correlation coefficients. All other associations summarized with Pearson correlation coefficients.
Additional correlations
Additional analyses of the relationships between non-linear HRV parameters and age and ESRD vintage, respectively, showed significant correlations (Table 5). In non-diabetic subjects and for the entire population, age positively correlated SPS and ApEn, and inversely correlated with MSE Slope ApEn and MSE Slope SampEn (Table 5). In non-diabetic subjects, DFA and quadratic DFA correlated positively with ESRD vintage, while in diabetic subjects only MSE Slope ApEn and MSE Slope SampEn correlated significantly with ESRD vintage (Table 5).
Table 5.
Pearson and Spearman correlation coefficients for associations between non-linear HRV indices and parameters reflecting physical condition for all subjects (All), and for non diabetic (ND) and diabetic (D) subjects.
| Measure Characterization | Specific Measures | Parameters associated with physical condition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase Angle | Lean Body Mass | Intracellular Volume | ||||||||
| All | ND | D | All | ND | D | All | ND | D | ||
| Long term correlations | Detrended Fluctuation Analysis | −0.17* | −0.18 | −0.15 | −0.21§ | −0.25* | −0.20 | −0.21§ | −0.25* | −0.19 |
| Quadratic Detrended Fluctuation Analysis | −0.10 | −0.13 | −0.09 | −0.18* | −0.22* | −0.15 | −0.17* | −0.21* | −0.13 | |
| Spectral Power Slope | −0.12 | −0.18* | −0.10 | −0.04 | −0.13 | −0.07 | −0.12 | −0.18* | −0.10 | |
| Complexity over different time scales | Multiscale Entropy Slope Approximate Entropy | 0.25§ | 0.31§ | 0.16 | 0.12 | 0.15 | 0.26* | 0.21§ | 0.24* | 0.26* |
| Multiscale Entropy Slope Sample | 0.23§ | 0.29§ | 0.15 | 0.17* | 0.18 | 0.32§ | 0.24§ | 0.25* | 0.31§ | |
| Irregularity | Approximate Entropy (2,0.15) † | 0.02 | −0.06 | 0.09 | 0.09 | 0.11 | 0.07 | 0.08 | 0.11 | 0.07 |
| Sample Entropy (2,0.15) † | 0.02 | −0.06 | 0.10 | 0.04 | 0.06 | 0.02 | 0.05 | 0.07 | 0.03 | |
p-value<0.01 (dark grey);
p-value ≤0.05 (light grey)
Associations for these HRV parameters summarized with Spearman correlation coefficients. All other associations summarized with Pearson correlation coefficients.
Discussion
In this, to date, largest assessment of non-linear HRV parameters in HD patients, we characterized 210 of the FHN Daily Trial subjects. This allowed direct comparison to general population data and to individuals suffering with cardiac disease. We found significant correlations between non-linear HRV parameters and a) cardiac parameters, b) biomarkers of fluid overload and c) indicators of self-reported physical functioning.
Cross-sectional evaluation
The 210 HD patients showed an ApEn of 0.91±0.37, a DFA of 1.07±0.07 and a power slope of 1.32±0.22 (Table 2). These values are comparable with values reported in the literature for other cardiovascular populations. Bigger et al. reported a lower power slope 1.06±0.12 in 274 healthy subjects in the same age group [20]. Pikkujämsä et al. reported an ApEn of 1.01±0.16, a DFA long-term scaling exponent (α2) of 1.07±0.07 and a power slope of 1.32±0.14 in twenty-nine healthy 40 to 60 year old subjects [33].
The trend of reduced HRV among those with more severe comorbid conditions is corroborated by increased power slope among patients with a myocardial infarction (power slope= 1.15±0.19 [n=715]) and those monitored after a cardiac transplant (power slope= 2.08±0.22 [n=19]) [20]. Earlier work in 54 HD patients by Fukuta et al. showed a lower ApEn (0.93±0.18) and a larger of DFA (α2) 1.14±0.07 [22] with respect to healthy control subject.
The comparison with literature suggests that the complexity loss of heart rate regulatory system is present also in end-stage renal disease and it is associated to the worsening of cardiac function/pathology, this reinforces our hypothesis that nonlinear HRV indices should have a similar relationship also in HD population.
Correlational analysis
HD patients showed significant correlations between nonlinear indices and cardiac MRI measures, fluid status and physical condition. MSE ApEn and MSE SampEn correlate positively with LVEF (r=0.26 and r=0.23, respectively) while both parameters correlated inversely with LVEDV. The directions of the trends are in agreement with the trend obtained in [34], where the majority of congestive heart failure patients had negative slopes on short scales. A reduced LVEF and an increased LVEDV are both signs of a compromised cardiac functionality. For example, a greater end diastolic volume results in a greater distention of the ventricle, i.e. an increase in preload. If such abnormal condition prolongs for a long time, it can cause or contribute to the clinical syndrome of heart failure with a normal LV ejection fraction, namely diastolic heart failure [35]. Lombardi et al. reported associations between linear and non-linear dynamics with LVEF [36].
We also found positive correlations between ApEn and SampEn with LVEDV, suggesting increased entropy with increased LVEDV. Similarly, fluid overload, quantified as EC/TBW, showed an inverse relationship with MSE ApEn and MSE SampEn, and a positive relationship to SPS (Table 2). This is consistent with Ferrario et al. who showed decreased HRV entropy in fluid-overloaded subjects [37] and with earlier work among congestive heart failure subjects whose condition may have resembled those affected by fluid overload [20,38].
Age and HD vintage correlate with various HRV parameters (Table 4). This coincides with earlier reports [20,33,39,40] and reflects a progressive loss of entropy at older age (Table 5). To the best of our knowledge no data on the relationship between ESRD vintage and HRV has yet been reported. The reason for this loss of HRV remains speculative, but progressing uremic neuropathy [41] or consequences of longstanding repeated myocardial stunning [10] may be involved and could represent an exciting new field of study in the context of myocardial compromise during dialysis treatment.
To the best of our knowledge, relationships between non-linear HRV and self-reported physical functioning and indicators of body composition have not been explored previously. The positive relationships between MSE slope ApEn and MSE slope SampEn, respectively, and PF and PH, have the same trends as for LVEF, and clearly underline the relevance of these parameters (Table 4). Accordingly, the correlations between phase angle, lean body mass and intracellular volume (a correlate of muscle mass) with the HRV parameters, suggest the benefits of a good nutritional status in this population where chronic inflammation and malnourishment is highly prevalent. Our analysis of nonlinear HRV indices supports an association between the worsening of the heart rate regulatory system and the worsening of cardiac function, fluid accumulation and physical condition. Furthermore our data suggest these indices as useful parameters for decision support systems to identify worsening conditions of heart funztionality and patients at elevated risk of death. We believe this will be a promising field of research in dialysis patients.
Acknowledgments
The authors wish to acknowledge and thank the entire Frequent Hemodialysis Network (FHN) Trial Group. The data presented in this manuscript was not published in whole or in part.
Appendix
Nonlinear HRV indices
Detrended Fluctuation Analysis (DFA)
DFA can be interpreted as a modified root mean square analysis of a random walk [42]. The time series of the RR time interval is integrated and then divided into boxes of equal length, n. In each box, a least squares line is fit to the data or, in case of quadratic DFA, a least square quadratic trend. Next, the integrated time series is detrended by subtracting the local trend in each box and a root-mean-square fluctuation F(n) is calculated over all time scales (box sizes) in order to characterize the relationship between the average fluctuation F(n) and the box size n. A linear relationship on a log-log plot indicates the presence of power law (fractal) scaling. Under such conditions, the fluctuations can be characterized by a scaling exponent ν, the slope of the line relating log F(n) to log n. In this work, two scaling exponents representing the long-term fluctuations (n>4) are presented: the DFA and the quadratic DFA.
Spectral Power Slope (SPS)
The R-R series was resampled at 2Hz. The power spectrum was computed by Fourier Transform and a regression line was fitted on the very low frequencies between 0.0001 and 0.01 Hz. SPS is strongly correlated to the DFA indices when a scaling law is present [38].
Entropy indices and Multiscale Entropy
Approximate entropy (ApEn), ApEn(m, r), quantify the unpredictability of fluctuations in a time series, measuring the logarithmic probability that patterns of m observations will repeat themselves within predetermined tolerance thresholds r on the next incremental comparisons (m+1). A time series containing many repetitive patterns has smaller entropy values than a time series which does not present such patterns. Sample entropy (SampEn), SampEn(m, r), is closely related to ApEn [43].
Costa et al. [34] introduced the so-called multiscale entropy (MSE). The procedure considers a time series of N points [9] and constructs consecutive coarse-grained time series [y(τ)], as a function of the factor τ:
| (1) |
[y(1)] is the original time series, N/τ is the length of each coarse-grained time series.
An entropy measure is calculated for each sequence [y(τ)] and plotted as a function of the scale factor τ. The MSE curves were estimated by means of ApEn(2,0.15) and SampEn(2,0.15). In this work, the slope of MSE curve with scale factors between 1 and 5 were estimated [31] and the values at scale factor 1 were considered the reference entropy values (ApEn and SampEn) in the statistical analyses.
The multiscale procedure distinguishes repetitive patterns as functions of different scales. Differences in the MSE index at different time scales reflect the regularity and structure of the time series (i.e. short vs. long range).
Footnotes
Daily Trial Clinical Sites – University of California San Francisco (UCSF)/Stanford Consortium: Chertow G (PI); UCSF and San Francisco Bay Area: James S, Chertow G, Tamura M, Hall Y, McCulloch C, Painter P, Gorodetskaya I, Tichy M, Humphreys M, Luan J, Escalada R, Rodriquez R; UC Davis and Sacramento Area: Depner T, Kaysen G, Suter M, Sonico J, Anderson S; El Camino Hospital and Satellite Health Care: Ting G, Schiller B, Coplon N, Doss S, Rogers J, Dominguez A, Atwal J, Lemus D; UCLA and Los Angeles Area: Rastogi A, Nissenson A, Goodman W, Salusky I, Schweitzer S, Rivas M, Smith M, Gayda P, Hernandez A, Rashid M; UCSD and San Diego Area: Mehta R, Pepas J, Bharti B, Nabali A, Manaster R, Mathew R, Shah S, Sanz G, Wei J; University of Texas, San Antonio: Ayus J, Achinger S, Gutierrez M; Renal Research Institute (RRI) New York Consortium: Levin N (PI); Bay W, Carter M, Geronemus R, Kuhlmann M, Handelman G, Gotch F, Finkelstein F, Kimmel P, Lacson E, Ornt D, Greenwood R, Vassalotti J, Burrowes J; RRI New York City: Levin N, Kotanko P, Kaufman A, Winchester J, Meisels I, Radbill B, Chang J, Fofie Y, Ramos R, Sergeyeva O, Callegari J, Arthur B, Tarallo M, Ulloa D, Apruzzese R; University of Western Ontario: Lindsay R, Suri R, Garg A, Bullas R Mazzorato A; Wake Forest University School of Medicine: Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Coppley A; Vanderbilt University Medical Center: Schulman G, McLeroy S, Sika M, Leavell E; Barnes Jewish/Washington University: Miller B, Schussler R, Bardsley J, Skelton R.
Disclosure
This work was supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), the Center for Medicare and Medical Services, and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter, and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics, Fresenius Medical Care, Renal Advantage, Renal Research Institute, and Satellite Healthcare.
Peter Kotanko holds stock in Fresenius Medical Care. All other authors have no conflict of interest to disclose.
References
- 1.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Schiller BM, Yang PC, Rajagopalan S. Determinants of left ventricular mass in patients on hemodialysis: Frequent hemodialysis network (fhn) trials. Circ Cardiovasc Imaging. 5:251–261. doi: 10.1161/CIRCIMAGING.111.969923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: Follow-up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767. doi: 10.1681/ASN.V12122759. [DOI] [PubMed] [Google Scholar]
- 3.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24:956–962. doi: 10.1093/ndt/gfn599. [DOI] [PubMed] [Google Scholar]
- 4.Ozkahya M, Ok E, Cirit M, Aydin S, Akcicek F, Basci A, Dorhout Mees EJ. Regression of left ventricular hypertrophy in haemodialysis patients by ultrafiltration and reduced salt intake without antihypertensive drugs. Nephrol Dial Transplant. 1998;13:1489–1493. doi: 10.1093/ndt/13.6.1489. [DOI] [PubMed] [Google Scholar]
- 5.Reyes-Bahamonde J, Raimann JG, Thijssen S, Levin NW, Kotanko P. Fluid overload and inflammation--a vicious cycle. Seminars in dialysis. 2013;26:31–35. doi: 10.1111/sdi.12024. [DOI] [PubMed] [Google Scholar]
- 6.Akaishi M, Hiroe M, Hada Y, Suzuki M, Tsubakihara Y, Akizawa T, Group KRNS. Effect of anemia correction on left ventricular hypertrophy in patients with modestly high hemoglobin level and chronic kidney disease. Journal of cardiology. 2013;62:249–256. doi: 10.1016/j.jjcc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Mancusi C, Gerdts E, De Simone G, Abdelhai YM, Lonnebakken MT, Boman K, Wachtell K, Dahlof B, Devereux RB. Impact of isolated systolic hypertension on normalization of left ventricular structure during antihypertensive treatment (the life study) Blood pressure. 2014;23:206–212. doi: 10.3109/08037051.2013.858482. [DOI] [PubMed] [Google Scholar]
- 8.Sany D, Elsawy AE, Aziz A, Elshahawy Y, Ahmed H, Aref H, El Rahman MA. The value of serum fgf-23 as a cardiovascular marker in hd patients. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2014;25:44–52. doi: 10.4103/1319-2442.124483. [DOI] [PubMed] [Google Scholar]
- 9.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M Chronic Renal Insufficiency Cohort Study I. Fibroblast growth factor-23 and cardiovascular events in ckd. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–1931. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–1332. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raimann J, Liu L, Ulloa D, Kotanko P, Levin NW. Consequences of overhydration and the need for dry weight assessment. Contributions to nephrology. 2008;161:99–107. doi: 10.1159/000130414. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Joseph J, Chonchol M, Kaufman JS, Cheung AK, Rafeq Z, Smits G, Kendrick J, Investigators H. Higher fibroblast growth factor-23 concentrations associate with left ventricular systolic dysfunction in dialysis patients. Clinical nephrology. 2013;80:313–321. doi: 10.5414/CN107991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knap B, Veceric-Haler Z, Benedik M, Buturovic-Ponikvar J, Ponikvar R, Bren AF. Fibroblast growth factor 23 and left ventricular mass index in maintenance hemodialysis patients: Standard versus long nocturnal hemodialysis. Therapeutic apheresis and dialysis: official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2013;17:407–411. doi: 10.1111/1744-9987.12087. [DOI] [PubMed] [Google Scholar]
- 16.Nishimura M, Tokoro T, Nishida M, Hashimoto T, Kobayashi H, Yamazaki S, Imai R, Okino K, Iwamoto N, Takahashi H, Ono T. Sympathetic overactivity and sudden cardiac death among hemodialysis patients with left ventricular hypertrophy. Int J Cardiol. 2010;142:80–86. doi: 10.1016/j.ijcard.2008.12.104. [DOI] [PubMed] [Google Scholar]
- 17.Park J. Cardiovascular risk in chronic kidney disease: Role of the sympathetic nervous system. Cardiology research and practice. 2012;2012:319432. doi: 10.1155/2012/319432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberger AL. Non-linear dynamics for clinicians: Chaos theory, fractals, and complexity at the bedside. Lancet. 1996;347:1312–1314. doi: 10.1016/s0140-6736(96)90948-4. [DOI] [PubMed] [Google Scholar]
- 19.Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol. 1996;271:R1078–1084. doi: 10.1152/ajpregu.1996.271.4.R1078. [DOI] [PubMed] [Google Scholar]
- 20.Bigger JT, Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ. Power law behavior of rr-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation. 1996;93:2142–2151. doi: 10.1161/01.cir.93.12.2142. [DOI] [PubMed] [Google Scholar]
- 21.Ho KK, Moody GB, Peng CK, Mietus JE, Larson MG, Levy D, Goldberger AL. Predicting survival in heart failure case and control subjects by use of fully automated methods for deriving nonlinear and conventional indices of heart rate dynamics. Circulation. 1997;96:842–848. doi: 10.1161/01.cir.96.3.842. [DOI] [PubMed] [Google Scholar]
- 22.Fukuta H, Hayano J, Ishihara S, Sakata S, Ohte N, Takahashi H, Yokoya M, Toriyama T, Kawahara H, Yajima K, Kobayashi K, Kimura G. Prognostic value of nonlinear heart rate dynamics in hemodialysis patients with coronary artery disease. Kidney Int. 2003;64:641–648. doi: 10.1046/j.1523-1755.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Celik A, Melek M, Yuksel S, Onrat E, Avsar A. Cardiac autonomic dysfunction in hemodialysis patients: The value of heart rate turbulence. Hemodialysis international International Symposium on Home Hemodialysis. 2011;15:193–199. doi: 10.1111/j.1542-4758.2011.00529.x. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Hiroshi T, Aoyama T, Tanaka M, Ishii H, Kisohara M, Iizuka N, Murohara T, Hayano J. Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:1454–1460. doi: 10.2215/CJN.09430911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 26.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, Beck GJ, Gassman JJ, Eggers PW, Star RA, Ornt DB, Kliger AS. Frequent hemodialysis network (fhn) randomized trials: Study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, St-Onge MP, Lecumberri B, Pi-Sunyer FX, Heshka S, Wang J, Kotler DP, Gallagher D, Wielopolski L, Pierson RN, Jr, Heymsfield SB. Body cell mass: Model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004;286:E123–128. doi: 10.1152/ajpendo.00227.2003. [DOI] [PubMed] [Google Scholar]
- 28.Raimann JG, Zhu F, Wang J, Thijssen S, Kuhlmann MK, Kotanko P, Levin NW, Kaysen GA. Comparison of fluid volume estimates in chronic hemodialysis patients by bioimpedance, direct isotopic, and dilution methods. Kidney Int. 2014;85:898–908. doi: 10.1038/ki.2013.358. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The mos 36-item short-form health survey (sf-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 30.Chen Z, Ivanov PC, Hu K, Stanley HE. Effect of nonstationarities on detrended fluctuation analysis. Physical Review E. 2002;65:041107. doi: 10.1103/PhysRevE.65.041107. [DOI] [PubMed] [Google Scholar]
- 31.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of biological signals. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:021906. doi: 10.1103/PhysRevE.71.021906. [DOI] [PubMed] [Google Scholar]
- 32.Bianchi A, Bontempi B, Cerutti S, Gianoglio P, Comi G, Natali Sora MG. Spectral analysis of heart rate variability signal and respiration in diabetic subjects. Med Biol Eng Comput. 1990;28:205–211. doi: 10.1007/BF02442668. [DOI] [PubMed] [Google Scholar]
- 33.Pikkujämsä SM, Mäkikallio TH, Sourander LB, Räihä IJ, Puukka P, Skyttä J, Peng C-K, Goldberger AL, Huikuri HV. Cardiac interbeat interval dynamics from childhood to senescence: Comparison of conventional and new measures based on fractals and chaos theory. Circulation. 1999;100:393–399. doi: 10.1161/01.cir.100.4.393. [DOI] [PubMed] [Google Scholar]
- 34.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett. 2002;89:068102. doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- 35.Gaasch WH, Little WC. Assessment of left ventricular diastolic function and recognition of diastolic heart failure. Circulation. 2007;116:591–593. doi: 10.1161/CIRCULATIONAHA.107.716647. [DOI] [PubMed] [Google Scholar]
- 36.Lombardi F, Sandrone G, Mortara A, Torzillo D, La Rovere MT, Signorini MG, Cerutti S, Malliani A. Linear and nonlinear dynamics of heart rate variability after acute myocardial infarction with normal and reduced left ventricular ejection fraction. Am J Cardiol. 1996;77:1283–1288. doi: 10.1016/s0002-9149(96)00193-2. [DOI] [PubMed] [Google Scholar]
- 37.Ferrario M, Moissl U, Garzotto F, Cruz DN, Clementi A, Brendolan A, Tetta C, Gatti E, Signorini MG, Cerutti S, Ronco C. Effects of fluid overload on heart rate variability in chronic kidney disease patients on hemodialysis. BMC nephrology. 2014;15:26. doi: 10.1186/1471-2369-15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerutti S, Esposti F, Ferrario M, Sassi R, Signorini MG. Long-term invariant parameters obtained from 24-h holter recordings: A comparison between different analysis techniques. Chaos: An Interdisciplinary Journal of Nonlinear Science. 2007;17 doi: 10.1063/1.2437155. [DOI] [PubMed] [Google Scholar]
- 39.Huikuri HV, Makikallio TH, Airaksinen KE, Seppanen T, Puukka P, Raiha IJ, Sourander LB. Power-law relationship of heart rate variability as a predictor of mortality in the elderly. Circulation. 1998;97:2031–2036. doi: 10.1161/01.cir.97.20.2031. [DOI] [PubMed] [Google Scholar]
- 40.Lipsitz LA, Mietus J, Moody GB, Goldberger AL. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81:1803–1810. doi: 10.1161/01.cir.81.6.1803. [DOI] [PubMed] [Google Scholar]
- 41.Kurata C, Uehara A, Sugi T, Ishikawa A, Fujita K, Yonemura K, Hishida A, Ishikawa K, Tawarahara K, Shouda S, Mikami T. Cardiac autonomic neuropathy in patients with chronic renal failure on hemodialysis. Nephron. 2000;84:312–319. doi: 10.1159/000045605. [DOI] [PubMed] [Google Scholar]
- 42.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5:82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 43.Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. American journal of physiology Heart and circulatory physiology. 2000;278:H2039–2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]