Abstract
Cortisol output in response to emotion induction procedures was examined at child age 24 months in a prospective longitudinal sample of 1,292 children and families in predominantly low-income and non-urban communities in two regions of high poverty in the US. Multilevel analysis indicated that observed emotional reactivity to a mask presentation but not a toy removal procedure interacted with sensitive parenting to predict cortisol levels in children. For children experiencing high levels of sensitive parenting, cortisol output was high among children exhibiting high emotional reactivity and low among children exhibiting low emotional reactivity. For children experiencing low levels of sensitive parenting, cortisol output was unrelated to emotional reactivity.
Keywords: Cortisol, emotion, parenting, poverty, early childhood
There is growing interest in the physiological response to stress in young children. Largely, this interest stems from the concern that early adversity, particularly that associated with poverty is physiologically stressful (Evans, 2003; Shonkoff, Boyce, & McEwen 2009). Chronic elevations in stress hormones can affect the development of neural circuitry that underlies the regulation of emotional and physiological responses to stimulation as well as that associated with executive functions important for behavior regulation (Liston & Gan, 2011; Radley et al., 2006). Resting elevations in cortisol, the end product of activity in the hypothalamic-pituitary-adrenal (HPA) axis, have been shown in human and animal models to be related to increased behavioral reactivity to stress, poor regulation of the stress response, and difficulty on complex as opposed to simple learning tasks (Davies, Sturge-Apple, Cicchetti, & Cummings, 2007; Holmes & Wellman, 2009; Meaney, 2001).
Given strong interest in stress physiology in children in poverty, it is notable that conditions under which cortisol might be expected to be elevated, such as in response to emotion induction, and whether or not such elevations are a “good” or “bad” thing, remain somewhat unclear. Several studies have used emotion induction procedures to examine cortisol output in typical income samples. Systematic review of these studies suggests that these procedures do not reliably produce meaningful change in cortisol, both for fear inducing and anger inducing manipulations (Gunnar, Talge, & Herrera, 2009). A number of methodological points, however, limit conclusions that can be drawn from the literature. One is the absence of data on observed child behavioral reactivity to emotion induction in most studies. Research with adults indicates that the controllability and social evaluative nature of a stressor are central to the cortisol response (Dickerson & Kemeny, 2004). In children, issues of controllability and social-evaluation can be difficult to determine but observed emotional reactivity serves as an indicator of the extent to which the child is experiencing stimulation that is beyond a tolerable threshold (Sroufe, 1995). Of further relevance is the type of emotional reaction induced. Manipulations that convey threat and induce a fear response, such as mask presentation, are known to activate neural circuitry that potentiate cortisol release (LeDoux, 2012; Loman & Gunnar, 2010). In contrast, manipulations to induce frustration and anger, such as toy removal, would be expected to more directly activate neural circuitry associated with the processing of reward and the autonomic nervous system (Calkins, Graziano, & Keane, 2007).
In addition to observed emotional reactivity, the quality of parental care that children receive is also central to understanding the physiological response to stress. Quality of parenting, defined in terms of warmth, positive regard, and sensitivity to child cues in part shapes the development of children’s ability to regulate emotion, attention, and physiological reactivity to stimulation (Sturge-Apple, Davies, Martin, Cicchetti, & Hentges, 2012). In combination with observed emotional reactivity, parenting quality can define expectations for change in salivary cortisol. Due to a variety of constraints on families, children in poverty are less likely to experience high levels of sensitive parental care (McLoyd, 1998). Therefore, it may be that any effect of parenting sensitivity on cortisol is pronounced for children in low-income homes. We have previously found in this predominantly low-income sample that parenting sensitivity is positively related to salivary cortisol in response to emotion induction at child age 7 months but associated with an overall lower mean level of salivary cortisol eight months later, at age 15 months (Blair et al., 2008). We also found that basal level of salivary cortisol, adjusted for time of day, decreased by one-third of a standard deviation on average in early childhood from age 7 months to age 48 months and that this decline was not evident for children in highly chaotic homes (Blair et al., 2011). We interpret these findings as evidence of the effect of early experience on the development of stress response physiology in the context of early disadvantage.
In light of these prior findings, we now examine the cortisol response to emotion induction as a function of both observed child emotional reactivity and parenting sensitivity at child age 24 months. One the one hand, research and theory indicating chronic elevations in cortisol in conditions of poverty suggest that higher parenting sensitivity would be associated with lower cortisol in response to emotion induction. Such a scenario would be indicative of the idea that parenting buffers the child from potentially deleterious effects of high cortisol output that might predispose to poor mental and physical health. In contrast, from a perspective that emphasizes the beneficial effects of glucocorticoids, higher parenting quality would be associated with cortisol increase in response to emotional arousal. Short term increases in cortisol have beneficial effects on neuronal excitability as a function of the relative occupation of glucocorticoid and mineralocorticoid receptors in the brain (de Kloet, 2004). Cortisol increase accompanying emotional reactivity would be expected to promote learning and memory and to lead to effective energy storage and mobilization.
In contrast to competing hypotheses among children exhibiting high emotional reactivity to emotion induction, parenting sensitivity would be expected to be negatively associated with average cortisol among children exhibiting low or no behavioral reactivity to emotion induction. This expectation is consistent with a large body of research indicating relations between caregiving quality and the activity of the HPA axis (Loman & Gunnar, 2010) and with prior longitudinal examinations of cortisol in this (Blair, Granger et al., 2011; Blair, Raver et al., 2011) and other samples (Davies, Sturge-Apple, Cicchetti, & Cummings, 2008; Sturge-Apple, Davies, Cicchetti, & Manning, 2012). In the absence of emotional reactivity, the literature clearly indicates that cortisol will be lower among children receiving sensitive parental care. In the presence of emotional reactivity, however, we test competing hypotheses as to direction of the effect of parenting quality on child cortisol.
Method
The Family Life Project (FLP) was designed to study families living in two of the four major geographical areas of high child rural poverty (Dill, 2001). Complex sampling procedures were used to recruit a representative sample of 1,292 families at the time of the target child’s birth, with oversampling of low-income families and families of African American ethnicity. Further details on the Family Life Project sampling plan and recruitment procedures are available in Vernon-Feagans, Cox, and the Family Life Project Investigators (2013). Seventy percent of families had an average income of less than 200% of poverty. Additionally, forty percent of mothers had a 12 years of schooling or less, while only 16% had 4 or more years of post-secondary education. A little more than half of the sample are White, 57%, and 43% are African American.
Procedures
At child age 24 months, N=1021 families were visited in their homes and the primary caregiver (the mother in almost all instances) answered questions about demographics, including child race, sex, and household income. In addition to a number of other procedures, primary caregivers and children participated in a standardized semi-structured interaction for 10 min (Cox, Paley, Burchinal, & Payne, 1999; NICHD Early Child Care Research Network, 1999). The interaction involved presenting the child with a jigsaw puzzle to complete and asking the parent to assist the child in any way that he or she chose. After one puzzle was completed, another puzzle of increased complexity was presented to the child, for up to a total of three puzzles. All children received identical puzzles.
Children also participated in two emotionally arousing procedures including a fear inducing mask presentation as well as a frustration eliciting toy removal procedure (Goldsmith & Rothbart, 1996). The mask presentation followed the toy removal. In the mask presentation, the experimenter donned four different masks one at a time for 10 seconds each while calming saying the child’s name (Goldsmith & Rothbart, 1996). In the toy removal, the child was presented with an attractive toy for 2mins and encouraged by the mother to play with it. The toy was then removed and placed in a translucent plastic jar and returned to the child for 2mins. The toy was then removed from the jar and returned to the child for 1min.
Three saliva samples were collected from children to assess the cortisol response to the emotion induction procedures. The first sample (time = 0) was collected prior to the administration of the procedures, at which time the researcher had been in the home for one hour. The second sample was collected approximately 20 minutes after the infant reached peak emotional arousal. The third sample was collected about 40 minutes following peak arousal. Unstimulated whole saliva was collected by using either cotton or hydrocellulose absorbent material and expressing the sample into 2-ml cryogenic storage vials using a needleless syringe (cotton) or by centrifugation (hydrocellulose). After collection, samples were immediately placed on ice and stored frozen (−20 °C).
Measures
Cortisol
All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 ml of saliva, had a range of sensitivity from 0.007 to 3.0 mg/dl, and average intra- and interassay coefficients of variation less than 10% and 15%, respectively. All samples were assayed in duplicate and the average of the duplicates was used in all analyses. Natural log transformations were applied to the cortisol values to correct for positive skew.
Parenting sensitivity
Parent-child interactions were video recorded and coded to assess levels of sensitivity, detachment, intrusiveness, stimulation, positive regard, negative regard, and animation. Ratings ranged from 1 (not at all characteristic) to 5 (highly characteristic). Inter-rater reliability was determined by calculating the intra-class correlation coefficients for ratings made by pairs of trained coders. Analyses indicated that parenting codes were subsumed by two based parenting factors, sensitive/responsive and harsh/controlling behaviors. Inter-rater reliability calculated for 30% of cases was high with intraclass correlation of .91 sensitive parenting and .86 for harsh parenting.
Behavioral reactivity to emotion induction
Behavioral reactivity was coded second by second from videotapes of the duration of the mask and toy removal tasks. Low reactivity included behaviors such as fussing, whining, frowning, furrowed brow, crinkled nose, slightly open or pressed lips; medium reactivity including crying, wide squared mouth, and eyes open or partially opened; and high reactivity including screams, wails, eyes partially or completely closed, and wide open mouth. A composite score for negative reactivity was created by summing the seconds of low, medium, and high negative reactivity. The proportion was then calculated by dividing this sum of negative reactivity by the total duration of the task. Coders were trained to achieve .75 (Cohen’s K) reliability. Inter-rater reliability for both tasks was calculated for at least 15% of completed cases. The Kappa at 24 months was .90.
Data Analysis
We used multi-level modeling to examine child cortisol at 24 months of age, using the amount of time between saliva samples as the index variable, thus incorporating small individual variation in the length of time between saliva collections into the model. The first time point is coded as 0, with subsequent time points coded as the exact amount of time (in fractions of hours) elapsed since the first sample. We first ran unconditional growth models and then examined whether positive parenting interacted with behavioral reactivity to the mask presentation and toy removal, respectively, to predict between subjects variation in cortisol. Covariates included time of day, race, resting cortisol at 7 months of age, caregiver education, income-to-needs ratio, and negative parenting. Where appropriate, simple slopes were calculated as equal to the slope of the predictor variable when the predictor variable was centered at its mean and the moderating variable was centered at 1 SD above or 1 SD below its mean (Aiken & West, 1991). All variables were centered prior to analysis and models were run in Mplus 6.12, using full information maximum likelihood estimation to minimize potential bias arising from missing data.
Results
Descriptive statistics
Means, standard deviations, and correlations among study variables are presented in Table 1. Correlations are for the most part small to moderate, with large and increasing stability in cortisol across the three saliva collection time points. Notably, emotional reactivity to the mask presentation is uncorrelated with cortisol at the first time point and positively correlated with cortisol at the second and third time points. Conversely, emotional reactivity to the toy removal is negatively correlated with cortisol at the first time point and uncorrelated with cortisol at the second and third time points. Reactivity to the masks and reactivity to the toy removal are minimally correlated.
Table 1.
| Cort 1 | Cort 2 | Cort 3 | Ed | INR | Time | Mask | Toy | Pos | Neg | M (SD) | N | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (ln) Cort 1 (μg/dL) | --- | −2.08 (.73) | 939 | |||||||||
| (ln) Cort 2 (μg/dL) | .506** | --- | −2.07 (.77) | 942 | ||||||||
| (ln) Cort 3 (μg/dL) | .446** | .774** | --- | −2.13 (.75) | 917 | |||||||
| Caregiver education | −.069* | −.101** | −.076* | --- | 14.78 (2.69) | 1021 | ||||||
| Income to need ratio | −.109** | −.130** | −.142** | .513** | --- | 1.77 (1.62) | 1021 | |||||
| Time of day sample 1 | −.322** | −.274** | −.309** | .201** | .248** | --- | 13.31 (3.19) | 1018 | ||||
| Mask peak | −.013 | .194** | .112** | −.104** | −.026 | −.072* | --- | 0.37 (.34) | 826 | |||
| Toy removal peak | −.112** | −.028 | −.024 | .023 | .043 | .073* | .152** | --- | 0.30 (.28) | 909 | ||
| Positive parenting | −.130** | −.137** | −.099** | .456** | .395** | .145** | −.084* | −.027 | --- | 2.90 (.81) | 963 | |
| Negative parenting | .127** | .149** | .132** | −.336** | −.293** | −.117** | .066 | .017 | −.532** | --- | 2.43 (.86) | 963 |
| (ln) Cort 1 7mos) | .104** | .108** | .086* | −.048 | −.073* | −.119** | −.016 | −.034 | −.127** | .075* | −1.88 (.69) | 935 |
Unconditional means model
The unconditional means model indicated an average initial cortisol level of −2.08 (B= − 2.08, S.E. = 0.02, p < .001) with no average linear (B = 0.01, S.E. = 0.06, p = .892) or quadratic (B = −0.03, S.E. = 0.05, p = .542) change. Effects for random linear slopes (B = 0.27, S.E. = 0.03, p < .001) as well as random intercepts (B =0.41, S.E. = 0.03, p < .001), however, indicated that despite no average cortisol change in the sample as a whole, there was significant variation in both level and change in cortisol responses across children. We thus included terms for random intercepts and random linear slopes in all subsequent models.
Multilevel model: Effects on the intercept
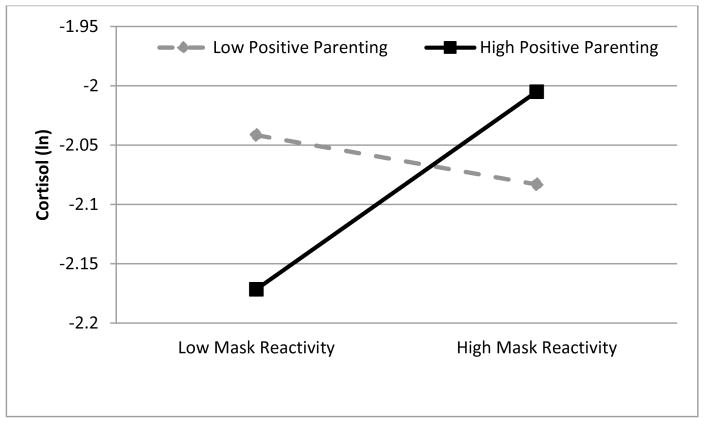
Our next model, Model B, included predictors of variation in the intercept for cortisol but did not include any predictors of variation in linear slope. Results in Table 2 indicated that parenting sensitivity and observed child emotional reactivity to the masks interacted to predict variation in the intercept for cortisol (B = 0.19, S.E. = 0.09, p < .05). As shown in Figure 1 and as indicated by tests of simple slopes, observed emotional reactivity to the masks was positively related to cortisol for children experiencing higher parenting sensitivity in the parent child interaction (B = .21, S.E. = .09, p < .05). Among children experiencing less sensitive parenting, emotional reactivity was unrelated to cortisol (B = −.06, S.E. = .09, p = .514). Notably, this effect is on the intercept for cortisol and is observed prior to the mask presentation. The finding indicates that among children experiencing sensitive care, cortisol was high among those who exhibited a higher level of emotional reactivity to the masks.
Table 2.
Multilevel Models predicting cortisol
| Model A | Model B | Model C | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Est. | S.E. | p | Est. | S.E. | p | Est. | S.E. | p | |
| Intercept | −2.08 | 0.02 | < .001 | −2.14 | 0.03 | < .001 | −2.14 | 0.03 | < .001 |
| Time | 0.01 | 0.06 | .892 | −0.02 | .02 | .285 | −0.02 | .02 | .307 |
| Effects on Intercept | |||||||||
| Time of day | −0.07 | 0.01 | < .001 | −0.07 | 0.01 | < .001 | |||
| Baseline Cort 7m | 0.05 | 0.03 | .061 | 0.06 | 0.03 | <.05 | |||
| Caregiver Education | 0.01 | 0.01 | .223 | 0.01 | 0.01 | .209 | |||
| Black | 0.15 | 0.05 | <.001 | 0.13 | 0.05 | <.01 | |||
| Income to needs ratio | .002 | 0.01 | .991 | −.001 | 0.01 | .968 | |||
| Negative Parenting | 0.06 | 0.03 | .048 | 0.05 | 0.03 | <.05 | |||
| Positive Parenting | −0.02 | 0.03 | .630 | −0.02 | 0.03 | .632 | |||
| Toy Removal Reactivity | −0.11 | 0.08 | .059 | −0.19 | 0.09 | <.05 | |||
| Mask Reactivity | 0.12 | 0.07 | .151 | −0.09 | 0.08 | .231 | |||
| Pos. Parent * Mask Reactivity | 0.18 | 0.09 | <.05 | 0.19 | 0.09 | <.05 | |||
| Effects on Linear Slope | |||||||||
| Mask Reactivity | 0.31 | 0.08 | < .001 | ||||||
| Toy Removal Reactivity | 0.15 | 0.09 | .077 | ||||||
| Variances | |||||||||
| Intercept | 0.41 | 0.03 | < .001 | 0.35 | 0.03 | < .001 | 0.33 | 0.03 | < .001 |
| Linear Slope | 0.27 | 0.03 | < .001 | 0.26 | 0.03 | < .001 | 0.25 | 0.03 | < .001 |
| Int. with Lin. | −0.10 | 0.02 | <.001 | −0.11 | 0.02 | < .001 | −0.10 | 0.02 | <.001 |
Figure 1.
Results from Model B demonstrate that positive parenting and emotional reactivity interact to predict cortisol at baseline.
In contrast to the effect for the interaction of reactivity to the masks with parenting sensitivity, the effect for observed reactivity to the toy removal, on its own or in interaction with parenting sensitivity, was not significant. Consistent with the correlational analysis, however, the coefficient for the toy removal on the intercept was negative (B = −0.11, SE = 0.08, p = .06), indicating lower cortisol among children exhibiting higher behavioral reactivity to the toy removal.
Harsh parenting was positively related to the intercept for cortisol (B = 0.06, S.E. = 0.03, p < .05) but did not interact with either mask or toy removal reactivity. Baseline cortisol at 7 months of age was also positively related to the intercept for cortisol at 24 months (B = 0.06, S.E. = 0.03, p < .05). African American children exhibited higher cortisol relative to White participants (B = 0.15, S.E. = 0.05, p < .001). Consistent with the diurnal rhythm of cortisol, time of day was significantly related to cortisol (B = −0.07, S.E. = 0.01, p < .001) such that cortisol was lower later in the day. Child sex, caregiver education, and income-to-need ratio were not significantly related to cortisol.
Multilevel model: Effects on linear slope
In Model C, observed emotional reactivity to the masks was the only variable associated with linear slopes (B = 0.97, S.E. = 0.20, p < .001). Children exhibiting higher emotional reactivity to the masks exhibited greater increase in cortisol. The interaction of reactivity to the masks and parenting sensitivity did not predict variation in linear slopes. Observed reactivity to the toy removal, on its own or in interaction with parenting sensitivity, was also not significantly related to the linear slope for cortisol. The coefficient for behavioral reactivity to toy removal, however, is positive (B = 0.15, SE = 0.09, p = .08), indicating an increase in cortisol with reactivity to this procedure. The addition of predictors of variation in slopes did not alter the between subjects interaction between parenting sensitivity and observed child reactivity to the masks. The pattern of results for the covariates was also no different from that seen in Model B.
Concordance between emotional reactivity and cortisol reactivity
To further examine relations among parenting sensitivity, reactivity to the mask presentation, and cortisol, we reran the multilevel model, placing the intercept at the second and third time points, respectively. In both of these subsequent model specifications, the interaction of observed reactivity to the mask presentation and parenting sensitivity continued to predict variation in the intercept. Reactivity to the mask presentation continued to be the only predictor of the slope. To illustrate, the second of these model specifications, we examined the correlation between observed reactivity to the masks and cortisol in groups characterized by high as opposed to low sensitive parenting as defined by the upper and lower quartiles of the distribution of parenting sensitivity in the sample. Results indicated that observed emotional reactivity to the masks was positively related to cortisol at 20mins post peak arousal (adjusted for baseline cortisol and time of day of saliva collection) among children in the upper quartile of parenting quality, r(200) = .27, p < .001, but uncorrelated in children in the lower quartile of parenting quality, r(159) = .06, p = .44. Importantly, observed emotional reactivity to the masks was similar in these two groups, M=.38 SD=.34 for the lower parenting quartile vs. M=.32 SD=.33 for the upper quartile of parenting quality.
Discussion
Overall, results confirm that emotional reactivity is related to change in salivary cortisol in very young children but also demonstrate the role that parenting sensitivity plays in this association. For children experiencing sensitive parenting, cortisol is high among children exhibiting high emotional reactivity to a fear inducing procedure, a mask presentation, and low among children exhibiting low observed reactivity to this procedure. Notably, this association is present at baseline, prior to the mask presentation as well as following this emotion inducing procedure. This association between emotional reactivity and salivary cortisol was not observed for children experiencing less sensitive parenting. For children experiencing less sensitive care, behavior and physiology were unrelated despite the fact that these children were as emotionally reactive to the mask presentation as children experiencing more sensitive parenting. Associations among cortisol, emotional reactivity, and parenting were not observed for an emotion induction procedure designed to induce anger. Observed emotional reactivity to this procedure, a toy removal, was minimally correlated with observed emotional reactivity to the mask presentation and tended to be inversely related to cortisol at baseline and positively related to cortisol change.
We interpret findings indicating interaction between parenting sensitivity and fear reactivity as evidence of the role of sensitive caregiving in the coherence and organization of development (Sroufe, 1979). A central principle of developmental science concerns the integration of diverse influences on behavior as they are “fused in ontogeny” and shaped by the context in which development is occurring (Magnusson & Cairns, 1996). Early parenting has long been hypothesized to influence the organization of development, as seen in the taxonomy of attachment classifications and in the characterization of attachment as an organizational construct (Sroufe & Waters, 1977). Here, sensitive caregiving is understood to provide a context for development that enables flexible and organized regulation of behavior as needed in response to specific contingencies. No single variable in isolation can be taken on its own as an indicator of healthy development. Rather, it is the expected pattern of relations among variables in ways that are appropriate for a given context that best indicates the integrity of development.
The organizational approach helps to clarify the idea central to healthy psychological development that just as behavior is flexibly deployed in response to context, so is its underlying physiology. In the context of sensitive parenting, high cortisol output is understood to be indicative of healthy development, i.e., a ‘good thing’ when it accompanies emotional reactivity to stimulation. The finding that synchrony between emotion and physiology is seen in the context of sensitive parenting is consistent with the hypothesis that this type of care promotes behavior that is well organized; that is, a pattern of physiological response to stimulation that is consistent with an accompanying behavioral response. An emotional response that is accompanied by a cortisol response can be considered beneficial in terms of the facilitative effects of glucocorticoids. In contrast, when care is less sensitive, relations between cortisol and emotional reactivity are more likely to be uncoordinated and as a result less likely to rise when needed in response to a stressor and to be elevated in the absence of a behavioral response (Sturge-Apple, Davies, Cicchetti, & Manning, 2012).
One implication of this analysis is the light it potentially sheds on expectations about the relation of stress response physiology as indicated by cortisol to development in early childhood. Chronically elevated basal or resting cortisol is associated with disadvantageous home environments (Blair et al., 2011; Davies et al., 2007) and extended out of home care (Watamura, Donzella, Alwin, & Gunnar, 2003). Chronically low basal cortisol is associated with specific types of maltreatment early in life (Cicchetti & Rogosch, 2009) and highly disadvantageous early rearing environments. From an organizational perspective, however, it is also important to focus on the coordination of physiological and behavioral responses as much as on each in isolation (Davies et al., 2008). From the perspective of psychobiological models of development (Blair & Raver, 2012; Gatzke-Kopp, 2011; Gottlieb, 1983), information about the quality of the environment in which development will occur is communicated to the organism through experiential influences acting on stress hormones. In higher quality caregiving environments, the coordination of physiological and behavioral responses is presumed to support sustained attention and engagement with caregivers in learning activities. In contrast, in more disadvantaged environments, lack of coordination between physiological and behavioral responses is understood to allow for systems to operate independently, promoting a vigilant state and heightened responsiveness to unexpected contingencies.
Although speculative, the foregoing provides expectations for the analysis of cortisol in children that are grounded in neurobiology. Regulation of stress response systems in ways that allow for a moderate increase in hormones is known to facilitate activity in neural systems that subserve the regulation of thought and behavior (Arnsten, 2009; Holmes & Wellman, 2009). Aspects of experience that promote this flexible regulation assist the individual in meeting stressful contingencies in the future (Lyons & Parker, 2007). In evaluations of exemplary intervention programs focusing on parenting in high risk contexts, beneficial effects on child physiology as well as behavior have been demonstrated (Brotman et al., 2007; Fisher, Gunnar, Dozier, Bruce, & Pears, 2006).
In conclusion, while this analysis sheds potentially valuable light on relations of cortisol to children’s development, it is limited in specific ways. For one, although the analysis is predicated on the idea that cortisol response to emotional reactivity is potentially beneficial to the organism, the analysis provides no specific data with which to validate this expectation. Secondly, the sample is predominantly low-income and residing in non-urban settings. This might restrict the generalizability of findings, particularly if the child and parent characteristics that are the focus of the analysis are unique to these settings. Here, it is important to note the finding that African American ethnicity in this sample is persistently associated with higher levels of cortisol at child age 24 months, as it was at child ages 7 and 15 months (Blair et al., 2008). The African American sample participating in the study is generally at much higher risk than White participants (Vernon-Feagans et al., 2013) and this finding is robust despite the inclusion of several indicators of risk in the analysis predicting cortisol. Further research with this and other samples on stress physiology in African American infants and toddlers is needed. As well, although results are statistically significant, demonstrating theoretically expected relations with parenting behavior and child emotional reactivity, the extent to which they are practically meaningful in terms of effects on child cognitive and behavioral development remains to be seen. Further analyses with this and other data sets can help to address these important questions. It is useful to note, however, that the findings reported here are consistent with the idea that preventive interventions such as those referenced above, and other similar programs to enhance caregiving quality (Landry, Smith & Swank, 2006) are likely to yield benefits to child physiology in ways that would be expected to contribute to positive longer-term physical and mental health and development.
Acknowledgments
We thank the many families and research assistants that made this study possible. Support was provided by the National Institute of Child Health and Human Development grants R01 HD51502 and P01 HD39667 with co-funding from the National Institute on Drug Abuse. DAG is founder and Chief Scientific and Strategy Advisor at Salimetrics LLC and SalivaBio LLC. These relationships are managed by the policies of the Committee on Conflict of Interest at the Johns Hopkins School of Medicine and the Office of Research Adherence and Integrity at Arizona State University.
The Family Life Project Key Investigators include Lynne Vernon-Feagans, Martha Cox, Clancy Blair, Peg Burchinal, Linda Burton, Keith Crnic, Ann Crouter, Patricia Garrett-Peters, Mark Greenberg, Maureen Ittig, Stephanie Lanza, Roger Mills-Koonce, Debra Skinner, Cynthia Stifter and Michael Willoughby.
References
- Aiken LS, West SG. Multiple Regression: Testing and interpreting interactions. Thousand Oaks: Sage; 1991. [Google Scholar]
- Arnsten AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Kivlighan KT, Mills-Koonce R, Willoughby M, Greenberg MT, Hibel L, Fortunato C the Family Life Project Investigators. Maternal and child contributions to cortisol response to emotional arousal in young children from low-income, rural communities. Developmental Psychology. 2008;44:1095–1109. doi: 10.1037/0012-1649.44.4.1095. [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT the FLP Investigators. Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development. 2011;82(6):1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger D, Razza RP. Cortisol reactivity is positively related to executive function in preschool children attending Head Start. Child Development. 2005;76(3):554–567. doi: 10.1111/j.1467-8624.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012;67:309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC, Granger D, Mills-Koonce R, Hibel L the FLP Key Investigators. Allostasis and allostatic load in the context of poverty in early childhood. Development and Psychopathology. 2011;23(03):845–857. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman LM, Gouley KK, Huang KY, Kamboukos D, Fratto C, Pine DS. Effects of a psychosocial family-based preventive intervention on cortisol response to a social challenge in preschoolers at high risk for antisocial behavior. Archives of General Psychiatry. 2007;64(10):1172–1179. doi: 10.1001/archpsyc.64.10.1172. [DOI] [PubMed] [Google Scholar]
- Buss KA, Davidson RJ, Kalin NH, Goldsmith HH. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Developmental Psychology. 2004;40(4):583–594. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- Calkins S, Graziano P, Keane S. Cardiac vagal regulation differentiates among children at risk for behavior problems. Biological Psychology. 2007;74:144–153. doi: 10.1016/j.biopsycho.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neuroscience and Biobehavioral Reviews. 2005;29(4–5):843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Adaptive coping under conditions of extreme stress: Multilevel influences on the determinants of resilience in maltreated children. New Directions for Child and Adolescent Development. 2009;2009(124):47–59. doi: 10.1002/cd.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M, Paley B, Burchinal M, Payne C. Marital perceptions and interactions across the transition to parenthood. Journal of Marriage and the Family. 1999;61:611–625. [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. Adrenocortical underpinnings of children’s psychological reactivity to interparental conflict. Child Development. 2008;79:1693–1706. doi: 10.1111/j.1467-8624.2008.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, Cummings EM. The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Developmental Psychology. 2007;43:918–930. doi: 10.1037/0012-1649.43.4.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER. Hormones and the stressed brain. Annals of the New York Academy of Sciences. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dill BT. Rediscovering rural America. In: Blau J, editor. The Blackwell Companion to Sociology. Malden MA: Blackwell; 2001. pp. 196–210. [Google Scholar]
- Evans GW. A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology. 2003;39(5):924–933. doi: 10.1037/0012-1649.39.5.924. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Dozier M, Bruce J, Pears KC. Effects of therapeutic interventions for foster children on behavioral problems, caregiver attachment, and stress regulatory neural systems. Annals of the New York Academy of Sciences. 2006;1094:215–225. doi: 10.1196/annals.1376.023. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp LM. The canary in the coalmine: The sensitivity of mesolimbic dopamine to environmental adversity during development. Neuroscience & Biobehavioral Reviews. 2011;35(3):794–803. doi: 10.1016/j.neubiorev.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery, locomotor version (manual) 1996 [Google Scholar]
- Gottlieb G. The psychobiological approach to developmental issues. In: Mussen PM, editor. Handbook of child psychology. 4. Vol. 1. New York, NY: John Wiley & Sons; 1983. [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiology & Behavior. 2007;92(4):583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Talge NM, Herrera A. Stressor paradigms in developmental studies: What does and does not work to produce mean increases in salivary cortisol. Psychoneuroendocrinology. 2009;34(7):953–967. doi: 10.1016/j.psyneuen.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neuroscience and Biobehavioral Reviews. 2009;33(6):773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry SH, Smith KE, Swank PR. Responsive parenting: Establishing early foundations for social, communication, and independent problem-solving skills. Developmental Psychology. 2006;42(4):627–642. doi: 10.1037/0012-1649.42.4.627. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73(4):653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Gan W. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011 doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience and Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Parker KJ. Stress inoculation-induced indications of resilience in monkeys. Journal of Traumatic Stress. 2007;20:423–433. doi: 10.1002/jts.20265. [DOI] [PubMed] [Google Scholar]
- Magnusson D, Cairns RB. Developmental science: Toward a unified framework. In: Cairns RB, Elder GH, Costello J, editors. Developmental Science. New York: Cambridge University Press; 1996. pp. 2–30. [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037/0003-066X.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24(1):1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- National Institute of Child Health and Human Development (NICHD) Early Child Care Research Network. Chronicity of maternal depressive symptoms, maternal sensitivity, and child functioning at 36 months. Developmental Psychology. 1999;35:1297–1310. doi: 10.1037/0012-1649.35.5.1297. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16(3):313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Sroufe LA. The coherence of individual development: Early care, attachment, and subsequent developmental issues. American Psychologist. 1979;34:834–841. [Google Scholar]
- Sroufe LA. Emotional development: The organization of emotional life in the early years. New York, NY: Cambridge University Press; 1995. [Google Scholar]
- Sroufe LA, Waters E. Attachment as an organizational construct. Child Development. 1977;48:1184–1199. [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Manning LG. Interparental violence, maternal emotional unavailability and children’s cortisol functioning in family contexts. Developmental Psychology. 2012;48:237–249. doi: 10.1037/a0025419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Martin MJ, Cicchetti D, Hentges RF. An examination of the impact of harsh parenting contexts on children’s adaptation within an evolutionary framework. Developmental Psychology. 2012;48:791–805. doi: 10.1037/a0026908. [DOI] [PubMed] [Google Scholar]
- Trickett P, Gordis E, Peckins M, Susman E. Stress reactivity in maltreated and comparison male and female young adolescents. Child Maltreatment. 2014;19:27–37. doi: 10.1177/1077559513520466. [DOI] [PubMed] [Google Scholar]
- Ursache A, Blair C, Granger DA, Stifter C, Voegtline K the Family Life Project Investigators. Behavioral reactivity to emotion challenge is associated with cortisol reactivity and regulation at 7, 15, and 24 months of age. Developmental Psychobiology. 2013 doi: 10.1002/dev.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel HJA, Riksen-Walraven JM. Stress reactivity in 15-month-old infants: links with infant temperament, cognitive competence, and attachment security. Developmental Psychobiology. 2004;44(3):157–167. doi: 10.1002/dev.20001. [DOI] [PubMed] [Google Scholar]
- Vernon-Feagans L, Cox M The Family Life Project Key Investigators. The Family Life Project: An Epidemiological and Developmental Study of Young Children Living in Poor Rural Communities. Society for Research in Child Development, Monographs. 2013 doi: 10.1111/mono.12046. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Alwin J, Gunnar MR. Morning-to-afternoon increases in cortisol concentrations for infants and toddlers at child care: Age differences and behavioral correlates. Child Development. 2003;74(4):1006–1020. doi: 10.1111/1467-8624.00583. [DOI] [PubMed] [Google Scholar]