Abstract
BACKGROUND
The protective effect of colonoscopy against proximal colorectal cancer is variable, and depends upon the detection and complete removal of precancerous polyps.
OBJECTIVE
To estimate the efficacy of colonoscopy in a medical center with open access screening colonoscopy since 1998.
DESIGN
Nested case-control study with incidence density sampling.
SETTING
University affiliated Veterans Affairs Medical Center.
PATIENTS
CRC cases and controls selected from screening age patients matched by age, gender, and date of first primary care visit.
MAIN OUTCOME MEASUREMENT
Colonoscopy preceding the CRC diagnosis date.
RESULTS
20.2% of CRC cases had a colonoscopy in the preceding 10 years compared with 49.0% of controls (aOR, 0.20; 95% CI, 0.11 – 0.34). Colonoscopy was strongly associated with decreased odds of both distal CRC (aOR, 0.16; 95% CI, 0.07 – 0.34) and proximal CRC (aOR, 0.26; 95% CI, 0.11–0.58). The fraction of cases attributed to interval cancers was 10.5%. Missed lesions predominantly localized to the cecum and rectum, and recurrent lesions clustering in the hepatic flexure. Cecal intubation rate was 93% (98% in adequately prepped patients), and the adenoma detection rate was 45.2% in the control group.
LIMITATIONS
Single center, retrospective case-control design.
CONCLUSION
In an open access colonoscopy program characterized by a high cecal intubation rate and adenoma detection rate, colonoscopy was strongly associated with reduced odds of both distal and proximal CRC. Among interval cancers, missed lesions clustered in the cecum and rectum, and recurrent lesions in the hepatic flexure.
Keywords: colonoscopy, colorectal cancer, screening, interval colorectal cancer
INTRODUCTION and BACKGROUND
Despite continued reductions in the rates of colorectal cancer (CRC) incidence and death, CRC remains the third most common non-cutaneous cancer diagnosed in both men and women and the third leading cause of cancer related death.1 The decreasing CRC incidence over time has been attributed to increased patient participation in CRC screening programs,1 which is recommended for average risk adults 50 years of age and older.2 Fecal occult blood testing (FOBT),3, 4 flexible sigmoidoscopy,5–9 and colonoscopy7, 10–14 have all been shown to reduce CRC incidence and mortality; however, the protective effect of colonoscopy against the incidence of CRC in the proximal colon has not been consistently demonstrated.7, 15–17 Cancers arising after a prior colonoscopy, termed “post-colonoscopy” CRC (PCCRC)18 account for 0.6% to 9% of CRCs in the literature.18–22 In addition to proximal colon location, the risk of developing PCCRC has been associated with the quality of the index examination,23 patient factors,21, 23 tumor biology,7 and procedural factors, such as endoscopist subspecialty, cecal intubation rate and adenoma detection rate (ADR).17–20, 22, 24, 25 Recent studies indicate that most PCCRCs are attributable to procedural factors, such as missed lesions, inadequate examinations, or incomplete resection.26, 27
The San Francisco Veterans Administration Medical Center (SFVAMC) instituted an open access screening colonoscopy program in 1998, after participating in the Veterans Affairs Cooperative Study Group 380.28 Providers are able to request a screening colonoscopy by placing an electronic consult through the Veterans Health Administration (VHA) computerized patient record system (CPRS). Patients are scheduled for the procedure and given instruction for pre-procedure preparation over the phone by trained nursing staff. Since 1999, an automated clinical computerized reminder was implemented throughout the VHA to ensure that each individual member would have up to date health maintenance information.29 The SFVMAC required resolution of the computerized reminder for CRC screening in approximately 2001. Although before 1998 a minority of the colonoscopies performed at the SFVAMC were screening colonoscopies (9% in 1996; 17% in 1997), the number steadily increased between1998 and 2006, peaking in 2006 with 49% of procedures performed for a screening indication. Since 2006, screening colonoscopies have comprised 35% to 40% of our colonoscopy examinations.
In this study, we sought to evaluate whether colonoscopy use is associated with a reduced odds of both proximal and distal CRC. In addition, we sought to examine the potential factors contributing to interval cancers in a healthcare system where screening colonoscopy is rigorously applied.
METHODS
Study Design
This was a nested case-control study with incidence density sampling of colorectal cancer cases and controls at the SFVAMC between 1998 and 2011. Fourteen colonoscopists (13 board-certified gastroenterologists, 1 board certified colorectal surgeon) performed colonoscopies at our institution during this time frame. This research was approved by the University of California San Francisco Institutional Review Board and the San Francisco VA Clinical Research Office on 3/24/2006 (11-05427).
Data Sources
Cases were identified using the SFVAMC cancer registry, which collects and reviews all diagnoses of cancer from the catchment area and reports to the Surveillance Epidemiology and End Results Program. Patient-level data on cases and controls were gathered from the Veterans Health Administration (VHA) computerized patient record system (CPRS).
CPRS, released in 1996, is the graphical user interface for the Veterans Health Information Systems and Technology Architecture (VistA) integrated electronic medical record system and is used throughout the VHA for all aspects of patient care and treatment.29, 30 CPRS allows health care providers to review and update all electronic medical records for patients enrolled in the local facility or community based outpatient clinics (CBOCs), including problem lists, inpatient and outpatient progress notes, medications, orders, consults, lab results, radiology results, procedure and pathology results, operative reports, and discharge summaries. CPRS documentation is also available on the VistA intranet (VistaWeb), which is a read-only intranet web portal that combines patient records from multiple VHA facilities. CPRS supports clinical decision-making and includes a clinical reminders package, which targets patients of a particular age, diagnosis or other site-defined criteria for preventive health care and management of chronic conditions, ensuring that timely clinical interventions are initiated at the point of care.31 Clinical reminders were mandated nationally in the VHA in 1999.29 The requirement to resolve computerized reminders for CRC screening at the SFVAMC began in 2001. Clearance of clinical reminders is audited internally on a yearly basis. Only performance of CRC screening (via colonoscopy, sigmoidoscopy, barium enema, or FOBT) is able to resolve the clinical reminder, except in cases of a current cancer diagnosis or short life expectancy. Therefore, exposure to colonoscopy or other CRC screening could be reliably ascertained by reviewing the medical records.
Identification of Cases
Case subjects included members 50 years of age or older who were found on pathology (by either colonoscopy or on surgical specimen) to have an adenocarcinoma of the colon or rectum between 1998 and 2011 and who had been seen by a primary care provider at least 6 months before diagnosis. Patients were excluded if they were diagnosed with CRC within 6 months of their first primary care visit. These “acute referrals” were not considered cases in this study because they did not have an adequate opportunity to undergo screening colonoscopy through the VHA system, and they were likely to receive examinations prompted by symptoms or signs of CRC. Exclusion criteria included a history of ulcerative or Crohn’s colitis, familial cancer syndromes (i.e., hereditary non-polyposis colorectal cancer or familial adenomatous polyposis), or history of polyps or CRC before age 50.
Cancer-specific data were abstracted including tumor size, stage, location, and histology.. The abstraction of cancer-specific data elements was separated from abstraction of subsequent CRC screening data elements.
Identification of Controls
A list of potential controls was generated from the SFVAMC membership plan, matched by age (+/− 6 months), sex, and date of first PCP visit (+/− 6 months). Controls were matched to cases on the amount of follow-up time using incidence density sampling, where the date of the first PCP visit was the proxy for follow-up time in the VHA system. Up to 4 controls were matched to each case in a nested case-control study design, in which individuals who later developed CRC were eligible as controls for earlier CRC cases. Study staff manually validated each control, starting at the top of the computer generated list and reviewing potential matches until up to four were selected for each case. Cases that did not have at least one control were excluded.
Chart Reviews
Medical records were available via CPRS and the read-only intranet web portal VistAWeb for the study period 1998–2011. All data from the medical record abstraction were directly entered into a Microsoft Access database. Inclusion/exclusion criteria were independently checked for cases and controls by two chart reviewers. Any discrepancy between inclusion/exclusion criteria was resolved by another investigator (A.S.). For all eligible cases and controls, reviewers entered demographic information and dates and results of any screening tests. For all colonoscopies performed at the SFVAMC or other facilities, detailed information was recorded about the referral date, indication, colonic preparation quality, polyps (appearance, location, method of removal, and histology), and follow-up recommendations.
PCCRC cases were reviewed in detail to determine location, stage, interval between exposure to colonoscopy and cancer diagnosis, and the characteristics of the preceding colonoscopy (preparation quality, location of polyps, histology, polypectomy technique, recommended follow-up, and adherence).
Definitions
Durability of colonoscopy protection: to evaluate the durability of colonoscopy protection, we divided the preceding exposure period into segments and calculated the odds ratios for having a screening test during each time segment. The time segments evaluated were 6 months to 3 years, 3 to 5 years and 5 to 10 years preceding the censor date.
Prep quality was directly abstracted from the endoscopy report text, and the lexicon was grouped into four categories: excellent/good, fair/adequate, poor/inadequate, or not mentioned.
“Interval cancers” were defined as cancers that occurred within the standard recommended surveillance interval (+/− 6 month grace period) and within 5 years of the previous colonoscopy.20 Interval cancers were categorized as recurrent lesions if they occurred in a segment of the colon with a prior polypectomy or a likely missed lesions if the prior colonoscopy was negative in this region of the colon.
Statistical Analysis
Baseline study characteristics between cases and controls were compared using chi-square tests and Mann-Whitney U test, as appropriate.
The primary outcome was the odds of colonoscopy among cases and controls using conditional logistic regression. All analyses considered the 10 years before CRC diagnosis, but excluded the exposures within the preceding 6 months of the CRC diagnosis. A similar follow-up period was used to extract exposures to CRC testing for the matched control subjects. Among cases, the censor date was set as the date of CRC diagnosis. Among matching controls, censoring occurred at the same date or earlier if the follow-up time was shorter than that of the matched CRC case.
Conditional logistic regression was performed to calculate the odds ratio for any exposure to colonoscopy as compared with no colonoscopy test, and to calculate the odds ratio for exposure to colonoscopy between patients with CRC located in the proximal or distal colon and controls. Adjustment was made for confounding factors including a family history of CRC, exposure to other screening tests, smoking, BMI, and race. All statistical analyses were calculated using Stata 11.0 (Stata Corp, College Station, Tex).
CRC diagnosed during screening or surveillance colonoscopies were compared with those for other indications using a chi-square and Mann-Whitney U tests, as appropriate. Characteristics tested included age, size of tumor, location, and TNM stage.
Finally, descriptives for quality indicators were run on colonoscopies performed at the VA among control subjects including the preparation quality (excellent, good, fair, adequate, poor), cecal intubation rate and its relationship to preparation quality, and the findings on examination (ADR, prevalence of advanced neoplasia, hyperplastic polyp, and serrated polyp). The case subjects were evaluated separately.
RESULTS
Patient Demographics
In 1998, 3,467 screen eligible (50–80 year old) patients were enrolled at the SFVAMC and its community based outpatient clinics; this number increased to 15,987 by 2011. Compliance with CRC screening reminder clearance ranged from 45% in 1998, to 77% in 2011 (supplemental Figure A). A total of 438 cases of colon and rectal cancer included in the SFVAMC Cancer Registry between 1998–2011 were reviewed. Of these 438 cases, the majority (314) were cases presenting to the SFVAMC as new patients with a known or suspected diagnosis of CRC, or ascolorectal malignancy other than adenocarcinoma (carcinoid, lymphoma, squamous cell carcinoma).
One hundred twenty four cases (28.3%) met inclusion criteria, and were matched with 488 controls. The cases and controls were adequately matched for age and gender (Table 1), but one woman was dropped from the final analysis due to inadequate matching (unable to find controls who were of similar age and who had been seen for a first visit with a San Francisco VA PCP within a six month timeframe of the case patient’s first PCP visit). Although controls and cases were matched for the first date of PMD visit, follow-up time was not matched, which led to a slightly longer follow-up time for cases versus controls that was not significant. (4.32 vs. 3.87 years, p=0.11). The proportion of cases that were of Black race was higher than in controls, which is consistent with national statistics and published reports.32, 33
Table 1.
Baseline characteristics of the study subjects
| Characteristic | Cases (n=124) | Controls (n=488) | p-value |
|---|---|---|---|
| Age, median | 69.5 | 69.6 | 0.84 |
| Male sex - % | 100 | 100 | 1.0 |
| Race, % | |||
| Black | 16.9 | 10.0 | 0.03 |
| White | 61.3 | 71.3 | 0.03 |
| Hispanic | 4.0 | 4.1 | 0.97 |
| Asian | 5.7 | 2.5 | 0.07 |
| Other/unknown | 12.1 | 12.1 | 0.95 |
| Time with VA PCP before case diagnosis, years | 4.3 | 3.9 | 0.11 |
VA PCP: Veterans Affairs primary care provider; CRC: Colorectal cancer
CRC was diagnosed during a screening or surveillance colonoscopy in 29.8% of cases; the remainder was diagnosed during colonoscopies performed for other indications, including positive FOBT, abnormal imaging, rectal bleeding, and anemia.. Cases diagnosed during a screening or surveillance examination were significantly younger (66.4 vs. 71.9 years, p=0.003), had CRC that was smaller in size (31.0 mm vs. 41.6 mm, p=0.008), and were more likely to have non-metastatic disease (p=0.01) than those in whom colonoscopy was performed for other indications.
Exposure to Colonoscopy among Cases and Controls
A significantly smaller proportion of cancer case subjects (20.2%) than controls (49.0%) underwent a colonoscopy in the period preceding the CRC diagnosis, representing a 75% overall reduction in odds of CRC (OR, 0.25; 95% CI, 0.15–0.40). After adjustment for race, BMI, exposure to other screening tests, smoking and family history, this difference remained significant (adjusted OR [aOR], 0.20; 95% CI, 0.11–0.34) (Table 2).
Table 2.
Exposure to colonoscopy and its estimated efficacy
| Exposure to Colonoscopy (%) | Odds Ratio (95% CI) | |||
|---|---|---|---|---|
| Cases (n=124) | Controls (n=488) | Unadjusted | Adjusted a | |
| Location | ||||
| Overall | 20.2 | 49.0 | 0.25 (0.15–0.40) | 0.20 (0.11–0.34) |
| Distal CRC (n=76)c | 17.1 | 48.7 | 0.21 (0.11–0.39) | 0.16 (0.07–0.34)b |
| Proximal CRC (n=48)c | 25.0 | 49.5 | 0.33 (0.16–0.67) | 0.26 (0.11–0.58)b |
| Durability | ||||
| < 3 | 6.5 | 20.3 | 0.28 (0.13–0.59) | |
| 3–5 | 9.7 | 17.4 | 0.45 (0.23–0.88) | |
| 5–10 | 11.3 | 20.1 | 0.48 (0.26–0.88) | |
Conditional logistic model was adjusted for race, family history, smoking, BMI, and exposure to other screening tests.
Test of effect modification by cancer location did not reach significance (p=0.36).
76 cases of distal CRC were matched with 302 controls. 48 cases of proximal CRC were matched with 186 controls.
Proximal versus Distal CRC
In the unadjusted analysis, prior exposure to colonoscopy was associated with a 67% reduction in odds of proximal CRC (OR, 0.33; 95% CI, 0.16–0.67) and a 79% reduction in odds of distal CRC (OR, 0.21; 95% CI, 0.11–0.39) (Table 2). After adjustment for race, BMI, exposure to other screening tests, smoking and family history, the adjusted odds ratios for proximal and distal CRC were 0.26 (95% CI, 0.11–0.58) and 0.16 (95% CI, 0.07–0.34), respectively. Although the estimated efficacy of colonoscopy for distal CRC (OR 0.21) was stronger compared with proximal CRC (OR 0.33), this difference was not significant (p=0.36).
Durability of Colonoscopy
When stratified by the time interval of the previous colonoscopy, more recent exposure to colonoscopy tended to be more protective (OR, 0.28; 95% CI, 0.13–0.59 for exposure within 3 years; OR, 0.45; 95% CI, 0.23–0.88 for exposure between 3 and 5 years; and OR, 0.48; 95% CI, 0.26–0.88 for exposure between 5 and 10 years, Table 2).
Colonoscopy Characteristics
Of colonoscopies performed in the control group, 58% were direct referrals; this increased to 80% (138/171) after implementation of the automated clinical computerized reminder (January 2002). Among the control subjects, 322 colonoscopy procedures were performed in 239 control subjects, of which 290 colonoscopies were available for examination of colonoscopy quality (Table 3). The distribution of the preparation quality is shown in Table 3. A majority of colonoscopy bowel preps were at least fair or adequate (88%), 6% did not have preparation quality mentioned, and 5.5% were poor or inadequate. The overall cecal intubation rate was 93%, but in the 256 colonoscopies with at least fair or adequate preparation, the cecal intubation rate was 98%. By contrast, if the preparation quality was poor or inadequate, the cecum was intubated in only half (8/16) of the procedures. Colonic neoplasms were common. Overall, the ADR was 45% and advanced neoplasia was present in approximately 14% of all procedures. When restricted to screening colonoscopy procedures, the ADR was 41% and the rate of advanced neoplasia was nearly 19%. A snare polypectomy was performed in 44% of colonoscopies (128/290).
Table 3.
Characteristics of colonoscopy exam among control subjects performed at the VA
| Characteristics | Overall | Screening | Surveillance | Other a |
|---|---|---|---|---|
| VA Procedure, % | 290 (100) | 97 (33.4) | 104 (35.9) | 89 (30.7) |
| Prep Quality, % | ||||
| Excellent/Good | 205 (70.7) | 73 (75.3) | 72(69.2) | 60(67.4) |
| Fair/Adequate | 51 (17.6) | 15 (15.5) | 21 (20.2) | 15 (16.8) |
| Poor/Inadequate | 16 (5.5) | 5 (5.2) | 6 (5.8) | 5 (5.6) |
| Not mentioned | 18 (6.2) | 4 (4.1) | 5 (4.8) | 9 (10.1) |
| Cecum Reached, % | ||||
| Overall | 271 (93.4) | 92 (94.8) | 99 (95.2) | 78 (87.6) |
| Prep quality ≥ fair | 251/256 (98.0) | 85/88 (96.6) | 93/93 (100) | 70/75 (93.3) |
| Prep quality was poor or inadequate | 8/16 (50.0) | 4/5 (80.0) | 1/6 (16.7) | 4/5 (80.0) |
| Prep not mentioned | 12/18 (66.7) | 3/4 (75.0) | 5/5 (100) | 4/9 (44.4) |
| Polypectomy rate, % | ||||
| Any adenoma b | 131 (45.2) | 40 (41.2) | 58 (55.8) | 33 (37.1) |
| Hyperplastic polyp c | 86 (29.7) | 25 (25.8) | 35 (33.6) | 26 (29.2) |
| Advanced histology d | 8 (2.8) | 1 (1.0) | 2 (1.9) | 5 (5.6) |
| Advanced neoplasia e | 42 (14.4) | 18 (18.6) | 15 (14.4) | 9 (10.1) |
Other included anemia, hematochezia, FOBT, and multiple other indications (melena, constipation, diarrhea, weight loss).
Any adenoma includes any size polyp with adenomatous features or more advanced histological features. This excluded hyperplastic polyps.
Of the 86 patients with any hyperplastic polyps, there were a total of 111 hyperplastic polyps. Location included the cecum (5), ascending (7), hepatic flexure (1), transverse (12), splenic (1), descending (8), sigmoid (29), rectum (37), rectosigmoid (14). Six polyps were 10 mm or larger. Half were located proximal to the sigmoid colon.
Advanced histology includes tubulovillous adenoma or high grade dysplasia
Advanced neoplasia includes polyps greater than 10 mm in size with adenomatous features or polyps of any size with advanced histological features. This excluded hyperplastic polyps.
Of the cases who had previously undergone a colonoscopy, 86% of colonoscopy bowel preps were at least fair or adequate (data not shown). The cecal intubation rate was 97% in colonoscopies with at least a fair or adequate prep. The ADR was 67%, and the advanced neoplasia rate was 39% in the case population.
Post-Colonoscopy Colorectal Cancer and Interval Cancers
Twenty-five PCCRC cases were identified (see Table 4). Three of these PCCRC cases were prior negative colonoscopies recommended for a ten year follow-up examination, but presented >5 years after the index colonoscopy. Nine of the PCCRC cases presented outside their recommended surveillance intervals, the majority with a history of high-risk adenomas. Several patients developed colorectal cancer despite multiple (≥2) colonoscopies.
Table 4.
Characteristics of colonoscopy prior to diagnosis of colorectal cancer
| Sub- ject |
Age | Location of CRC |
Stage | Colonoscopy Number before Dx |
Interval before Dx (mo) |
Prep Quality |
Cecum Reached (Y/N) |
Classification | Recommended Surveillance Interval (years) |
CRC in segment of colon with prior polypectomy |
Suspected Etiology of Interval CRC |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Within Surveillance Interval | Interval CRC = Within 5 Years of Colonoscopy | 1 | 63 | HF/TV | IV | 7* | 14 | Fair | Y | HRA | 1 | Y | Inc/Bio |
| 2 | 69 | HF | I | 2* | 17 | Good | Y | HRA | 1 | Y | Inc | ||
| 3 | 65 | TV | - | 2 | 27 | - | - | HRA | 2 | - | Miss/Bio? | ||
| 4 | 78 | Rectum | IIIB | 2* | 7 | Adequate | Y | HRA | 3 | N | Miss | ||
| 5 | 67 | HF/Asc | IV | 2* | 35 | Good | Y | HRA | 3 | Y | Inc | ||
| 6 | 69 | Rectum | IIIB | 1* | 38 | Fair | Y | HRA | 3 | N | Miss | ||
| 7 | 81 | Rectosig | IIA | 1* | 30 | - | N | LRA | 5 | N | Miss | ||
| 8 | 63 | Rectum | IIIB | 1* | 47 | Good | Y | LRA | 5 | Y | Inc | ||
| 9 | 74 | Cecum | IIA | 2* | 64 | Good | Y | h/o HRA | 5 | N | Miss | ||
| 10 | 84 | Cecum | IIIB | 1 | 54 | - | - | NORM | 10 | Miss | |||
| 11 | 83 | Rectum | I | 1 | 54 | - | - | NORM | 10 | Miss | |||
| 12 | 60 | Asc | IV | 1 | 54 | - | Y | NORM | 10 | Miss | |||
| 13 | 87 | Cecum | IIA | 3* | 46 | Excellent | Y | h/o HRA | None (given age) | N | Miss | ||
| >5Y | 14 | 56 | Rectum | IIIA | 1* | 69 | Good | Y | NORM | 10 | |||
| 15 | 61 | Sigmoid | I | 1 | 101 | - | - | NORM | 10 | ||||
| 16 | 83 | Restosig | I | 1 | 106 | - | - | NORM | 10 | ||||
| Outside Surveillance | ≤5 | 17 | 74 | Desc | I | 2* | 44 | Good | Y | HRA | 1 | Y | |
| 18 | 80 | Sigmoid | IIA | 2* | 51 | Good | Y | h/o HRA | 1 | Y | |||
| >5 years | 19 | 77 | Rectum | III | 2* | 83 | Adequate | Y | HRA | 1 | N | ||
| 20 | 74 | Rectum | IIA | 2 | 68 | - | - | HRA | 3 | Y | |||
| 21 | 79 | TV | IIA | 1* | 78 | Good | Y | HRA | 3 | N | |||
| 22 | 81 | TV | II | 2* | 120 | - | - | LRA | 5 | N | |||
| 23 | 80 | Cecum | IIA | 1* | 126 | - | - | HRA | 5 | N | |||
| Unk | 24 | 83 | Rectosig | IIA | 3 | 55 | - | - | - | - | - | ||
| 25 | 67 | Asc | IIA | 2 | 33 | - | - | - | - | - |
one or more colonoscopy performed at SFVA
- indicates missing data
Abbreviations: Unk = Unknown surveillance recommendation, Rectosig=rectosigmoid colon; Desc= descending colon, TV= transverse colon, HF= hepatic flexure, Asc=ascending colon; h/o = history of, HRA = high risk adenoma, LRA=low risk adnemoa, NORM=normal colonoscopy, Inc = possible incomplete polypectomy, Bio = possible biological factors, Miss = possible missed lesion
Thirteen cases (10.5%) were determined to be interval cancers (Table 4). Eight of these cancers occurred in the proximal colon, and five occurred in the distal colon. A larger proportion of interval cancers were located in the proximal colon compared with sporadic cancers (61.5% vs. 36.4%, p=0.07); this difference was not statistically significant, although the study was not powered to examine this. All distal interval cancers occurred within the rectosigmoid region (see Figure 1).
Figure 1.
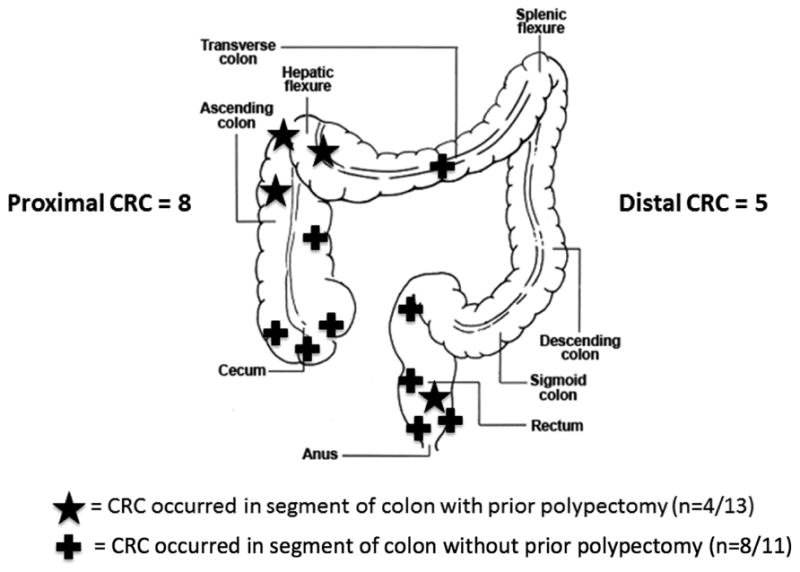
Location of interval cancers.
Available endoscopy reports were reviewed in detail for all 13 patients who developed interval cancers. Four interval cancers (31%) were believed to be recurrent lesions, three in the hepatic flexure and one in the rectum (Figure 1). Two cases of interval hepatic flexure adenocarcinomas had endoscopic evidence of residual serrated adenoma before their diagnosis of CRC, one at the index resection requiring piecemeal endoscopic mucosal resection (EMR) (Table 5, subject 2), and the second at three month surveillance of a piecemeal snare polypectomy site (Table 5, subject 5). In all cases, adenocarcinoma was detected at the time of recommended surveillance. The remaining nine interval cancer cases were classified as likely missed lesions. These included three interval cancer cases reported to have prior normal colonoscopies, however the index procedures were performed at outside facilities, and the endoscopy reports were unavailable for review. Three other interval cancers presented as advanced lesions in the rectum/rectosigmoid region shortly after examinations at the SFVAMC that revealed only small adenomas. Two cecal interval cancers occurred in patients with history of HRAs but a normal colonoscopy at the most recent examination. A transverse colon interval cancer was detected during a scheduled surveillance examination, at which time multiple other advanced lesions also were detected, indicating biological factors may have also played a role.
DISCUSSION
In this nested case-control study, colonoscopy was associated with an 80% reduced odds of developing CRC. This reduction was most pronounced for distal CRC (aOR, 0.16) but was also evident for proximal CRC (aOR, 0.26). Durability of colonoscopy protection persisted over the 10-year analysis period. Our results are consistent with prior studies demonstrating a 55–85% reduction in the odds of distal CRC in subjects previously exposed to colonoscopy.14, 34, 35 In these studies, the protective effect of colonoscopy against proximal CRC has been variable, ranging from no protection to as much as a 57% reduction in the odds of developing CRC.
Several of the previously published studies relied on administrative claims data, in which indication and quality of examination were not known. A strength of our study was the ability to conduct labor-intensive and detailed chart review using the integrated clinical data from the VHA CPRS with clinical decision support functionality. By requiring cases and controls to have a primary care provider within the VHA system, colonoscopy use could be accurately assessed. The indication and findings for each colonoscopy were known, and quality measures were directly abstracted from the endoscopy and pathology reports. Within this practice setting, we report one of the highest exposure rates to colonoscopy (49% in the control group vs. 4.4% to 41% in prior studies).10, 15, 24, 34, 35 Screening or surveillance colonoscopy accounted for 69% of the colonoscopy examinations in controls. In 30% of the 124 cases, the CRC was detected during a screening or surveillance examination. Unlike other studies, we did not find a statistically significant difference in detection of earlier stage of screen or surveillance detected cancers,36 but we did find that patients had smaller lesions and were more likely to have non-metastatic disease.
Prior studies have shown that physician ADR is inversely correlated with interval cancer development.20, 25 Colonoscopies in the SFVAMC system were associated with high ADR, which suggest that the interval cancer incidence rate should be low. Even though the fraction of CRC cases that are interval cancers is higher than previously reported, it likely is a reflection of the high penetration of colonoscopy in the overall study population.18, 19, 21, 37 For example, in a longitudinal cohort in which everyone has been exposed to a colonoscopy, all incident cancers will have had exposure to colonoscopy. Although colonoscopy is highly effective against CRC, there are limitations to the protective effect of colonoscopy..
Our study results suggest that in addition to lesion pathology, incorporation of patient age and lesion location and resection technique may lead to further refinement and individualization of surveillance guidelines. Consistent with other studies documenting older age as an independent risk factor for interval CRC,20, 38 the average age in our interval cancer group was 73 years. Although biological risk factors are difficult to quantify, 62% of patients with interval cancer had a history of HRAs on prior colonoscopies, portending a higher risk for recurrent neoplasia. Interval cancers ascribed to missed lesions clustered in the cecum and rectum, areas that are potential ‘blind spots” for colonoscopy. This emphasizes the need for meticulous inspection behind folds, especially the ileocecal valve and rectal valves of Houston. Other studies also report that the majority of missed distal lesions are within 10 cm of the anorectal verge. 39, 40 Consistent with prior studies, approximately one third of our interval cancers are ascribed to incomplete resection of detected lesions.37, 41, 42 Piecemeal resections are associated with a higher rate of residual neoplasia as compared with en bloc resection, and sessile serrated adenomas are more likely to be incompletely resected.43,44 Techniques to ensure complete adenoma resection deserve further study, such as the use of adjunctive imaging technologies (surface, digital or injection chromoendoscopy) to better delineate polyp edges.44, 45 Our findings are consistent with recent studies implicating procedural factors as the main etiology of interval cancer development.26, 27 Going forward, quality measures that include measures of technical competence, such as adenoma resection technique and incomplete resection rate44, may add an additional dimension to quality assessment independent of ADR and cecal intubation.
A limitation of this study is that it was a single center retrospective study that included primarily male Veterans within a system that strongly promoted CRC screening. Although each record was individually reviewed, comorbidity data was neither collected nor included in the adjusted model. It is possible that case subjects may have been more ill and less likely to have received colonoscopy. However, other studies have suggested that screening rates among those with and without multiple comorbidities are not significantly different.46 As with any retrospective study, despite attempts in the study design and analytic phase to reduce confounding, there may be unrecognized confounding factors in this case-control study for which we have not adjusted.
In conclusion, this study lends further evidence that high quality colonoscopy is strongly associated with reduction of both proximal and distal CRC. Nonetheless, interval cancers occurred that are attributable to incomplete polypectomy and missed lesions, highlighting the emphasis upon continued quality improvement efforts to optimize the detection and complete resection of lesions during colonoscopy.
Supplementary Material
Acknowledgments
Financial support: MS is supported by the NIH/NCI K23 CA157929
Ann Hayes was instrumental to making open access colonoscopy possible, in conjunction with the San Francisco Gastrointestinal Interventional and Diagnostic Center (GIDC) team; Ellen Zufall and Linda Clark provided the investigators with administrative data on cases and controls; Barbara Miller provided the investigators with VISTA reminder clearance data.
Abbreviations and Acronyms
- CRC
colorectal cancer
- PCCRC
“post-colonoscopy” colorectal cancer
- ADR
adenoma detection rate
- SFVAMC
San Francisco Veterans Administration Medical Center
- VHA
Veterans Health Administration
- CPRS
computerized patient record system
- PCP
primary care provider
- HRA
high risk adenoma
- LRA
low risk adenoma
Footnotes
- Amandeep K. Shergill, MD, MS- Contributed to project conception and study design; supervision of data collection; collected and inputted data; analysis and interpretation of data; drafted article; critical revision; final approval
- Erin E. Conners, MPH- Assisted in the study design; collected and inputted data; contributed to analysis and interpretation of data; drafted article; critical revision; final approval.
- Kenneth R. McQuaid, MD- Contributed to conception and study design; collected and interpreted the data; contributed to critical revision; final approval.
- Sara Epstein, MD- Collected and inputted data; contributed to critical revision; final approval.
- James C. Ryan, MD- Contributed to project conception and study design; contributed to critical revision; final approval.
- Janak N. Shah, MD - Contributed to project conception and study design; contributed to critical revision; final approval.
- John Inadomi, MD - Contributed to project conception and study design; contributed to critical revision; final approval.
- Ma Somsouk, MD, MAS- Contributed to project conception and study design; supervised data collection; contributed to analysis and interpretation of data; drafted article; critical revision; final approval
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Force USPST. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 3.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 4.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–14. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 5.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 6.Doria-Rose VP, Levin TR, Selby JV, et al. The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology. 2004;127:714–22. doi: 10.1053/j.gastro.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 7.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selby JV, Friedman GD, Quesenberry CP, Jr, et al. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326:653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 10.Doubeni CA, Weinmann S, Adams K, et al. Screening colonoscopy and risk for incident late-stage colorectal cancer diagnosis in average-risk adults: a nested case-control study. Ann Intern Med. 2013;158:312–20. doi: 10.7326/0003-4819-158-5-201303050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller AD, Sonnenberg A. Prevention of colorectal cancer by flexible endoscopy and polypectomy. A case-control study of 32,702 veterans. Ann Intern Med. 1995;123:904–10. doi: 10.7326/0003-4819-123-12-199512150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Muller AD, Sonnenberg A. Protection by endoscopy against death from colorectal cancer. A case-control study among veterans. Arch Intern Med. 1995;155:1741–8. doi: 10.1001/archinte.1995.00430160065007. [DOI] [PubMed] [Google Scholar]
- 13.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014;146:718–725. e3. doi: 10.1053/j.gastro.2013.11.050. [DOI] [PubMed] [Google Scholar]
- 15.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 16.Lakoff J, Paszat LF, Saskin R, et al. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117–21. doi: 10.1016/j.cgh.2008.05.016. quiz 1064. [DOI] [PubMed] [Google Scholar]
- 17.Singh H, Nugent Z, Demers AA, et al. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 18.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Cooper GS, Xu F, Barnholtz Sloan JS, et al. Prevalence and predictors of interval colorectal cancers in medicare beneficiaries. Cancer. 2012;118:3044–52. doi: 10.1002/cncr.26602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 21.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh A, Kuo YF, Riall TS, et al. Predictors of colorectal cancer following a negative colonoscopy in the Medicare population. Dig Dis Sci. 2011;56:3122–8. doi: 10.1007/s10620-011-1788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case-control study. Ann Intern Med. 2012;157:225–32. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 24.Baxter NN, Warren JL, Barrett MJ, et al. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664–9. doi: 10.1200/JCO.2011.40.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298–306. doi: 10.1056/NEJMoa1309086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.le Clercq CM, Bouwens MW, Rondagh EJ, et al. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957–63. doi: 10.1136/gutjnl-2013-304880. [DOI] [PubMed] [Google Scholar]
- 27.Robertson DJ, Lieberman DA, Winawer SJ, et al. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949–56. doi: 10.1136/gutjnl-2012-303796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–8. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 29.Patterson ES, Doebbeling BN, Fung CH, et al. Identifying barriers to the effective use of clinical reminders: bootstrapping multiple methods. J Biomed Inform. 2005;38:189–99. doi: 10.1016/j.jbi.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Department of Veterans Affairs OoITOTPDP. Computerized Patient Record System (CPRS) User Guide: GUI Version. 2014 Jul; [Google Scholar]
- 31.Department of Veterans Affairs; Product Development OoIaT. Clinical Reminders, Manager’s Manual. Aug, 2014. [Google Scholar]
- 32.Howlader NNA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute. National Cancer Institute; Bethesda, MD: [Google Scholar]
- 33.Thornton JG, Morris AM, Thornton JD, et al. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 35.Mulder SA, van Soest EM, Dieleman JP, et al. Exposure to colorectal examinations before a colorectal cancer diagnosis: a case-control study. Eur J Gastroenterol Hepatol. 2010;22:437–43. doi: 10.1097/MEG.0b013e328333fc6a. [DOI] [PubMed] [Google Scholar]
- 36.Pox CP, Altenhofen L, Brenner H, et al. Efficacy of a nationwide screening colonoscopy program for colorectal cancer. Gastroenterology. 2012;142:1460–7. e2. doi: 10.1053/j.gastro.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Farrar WD, Sawhney MS, Nelson DB, et al. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259–64. doi: 10.1016/j.cgh.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Samadder NJ, Curtin K, Tuohy TM, et al. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950–60. doi: 10.1053/j.gastro.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Pickhardt PJ, Nugent PA, Mysliwiec PA, et al. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 40.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am J Gastroenterol. 2006;101:2866–77. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 41.Pabby A, Schoen RE, Weissfeld JL, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385–91. doi: 10.1016/s0016-5107(04)02765-8. [DOI] [PubMed] [Google Scholar]
- 42.Robertson DJ, Greenberg ER, Beach M, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Woodward TA, Heckman MG, Cleveland P, et al. Predictors of complete endoscopic mucosal resection of flat and depressed gastrointestinal neoplasia of the colon. Am J Gastroenterol. 2012;107:650–4. doi: 10.1038/ajg.2011.473. [DOI] [PubMed] [Google Scholar]
- 44.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80. e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 45.Monkemuller K, Wilcox CM. Interventional chromoendoscopy. Gastrointest Endosc. 2013;78:346–50. doi: 10.1016/j.gie.2013.04.181. [DOI] [PubMed] [Google Scholar]
- 46.Walter LC, Lindquist K, Nugent S, et al. Impact of age and comorbidity on colorectal cancer screening among older veterans. Ann Intern Med. 2009;150:465–73. doi: 10.7326/0003-4819-150-7-200904070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


