Abstract
Transforming growth factor-β (TGF-β) family signaling pathways have roles in both neuronal development and the regulation of synaptic function. Here we identify a novel role for the C. elegans DAF-7/TGF-β signaling pathway in the regulation of the AMPA-type glutamate receptor GLR-1. We found that the abundance of GLR-1 increases at synapses in the ventral nerve cord (VNC) of animals with loss-of-function mutations in multiple DAF-7/TGF-β pathway components including the TGF-β ligand DAF-7, the type I receptor DAF-1, and the Smads DAF-8 and DAF-14. The GLR-1 defect can be rescued by expression of daf-8 specifically in glr-1-expressing interneurons. The effect on GLR-1 was specific for the DAF-7 pathway because mutations in the DBL-1/TGF-β family pathway did not increase GLR-1 levels in the VNC. Immunoblot analysis indicates that total levels of GLR-1 protein are increased in neurons of DAF-7/TGF-β pathway mutants. The increased abundance of GLR-1 in the VNC of daf-7 pathway mutants is dependent on the transcriptional regulator DAF-3/Smad suggesting that DAF-3-dependent transcription controls GLR-1 levels. Furthermore, we found that glr-1 transcription is increased in daf-7 mutants based on a glr-1 transcriptional reporter. Together these results suggest that the DAF-7/TGF-β signaling pathway functions in neurons and negatively regulates the abundance of GLR-1, in part, by controlling transcription of the receptor itself. Finally, DAF-7/TGF-β pathway mutants exhibit changes in spontaneous locomotion that are dependent on endogenous GLR-1 and consistent with increased glutamatergic signaling. These results reveal a novel mechanism by which TGF-β signaling functions in the nervous system to regulate behavior.
Keywords: Transforming growth factor-β, glutamate, synapse, GLR-1, AMPA, C. elegans
Introduction
The transforming growth factor-β (TGF-β) family is a well-known regulator of development in many tissues including the nervous system, where it regulates processes such as axon outgrowth and synaptogenesis (Liu and Niswander, 2005; Packard et al., 2003). However, TGF-β can also regulate synaptic function in the mature nervous system. Initial studies using cultured Aplysia neurons showed that TGF-β can enhance long-term facilitation at sensory to motor neuron synapses (Zhang et al., 1997). Work from several groups have since established that multiple TGF-β family members can function in the mammalian nervous system to regulate synaptic transmission and plasticity (reviewed in Krieglstein et al., 2011 and Poon et al., 2013). While there is growing evidence that TGF-β family members play important non-developmental roles in the nervous system the molecular mechanisms that mediate this control are largely undefined.
In this study we have investigated how a C. elegans TGF-β signaling pathway affects the nervous system. In C. elegans, there are three TGF-β family members: UNC-129, DBL-1 and DAF-7 (Patterson and Padgett, 2000), all of which function in the nervous system. UNC-129 is a BMP-like ligand that likely signals in an unconventional manner to regulate axon outgrowth (Colavita et al., 1998; Patterson and Padgett, 2000). DBL-1/TGF-β and its downstream signaling components regulate neuronal function by controlling GABA signaling at the neuromuscular junction (Vashlishan et al., 2008). DAF-7/TGF-β, which is most homologous to mammalian BMP and Drosophila DPP, is a neuroendocrine signal that is secreted from ASI chemosensory neurons in response to changes in environmental conditions (Bargmann, 2006; Ren et al., 1996). During development, DAF-7/TGF-β signaling responds to changes in the environment to control whether larvae develop normally into reproductive adults, or enter an alternative developmental stage known as dauer (Ren et al., 1996). In adult worms that have passed the dauer decision point, DAF-7/TGF-β signaling functions to maintain chemoreceptor gene expression in sensory neurons (Nolan et al., 2002), and regulates a number of behaviors including egg laying (Trent et al., 1983), feeding rate (Greer et al., 2008), food leaving (Milward et al., 2011), quiescence following feeding (Gallagher et al., 2013; You et al., 2008), and avoidance of pathogenic bacteria (Meisel 2014). DAF-7/TGF-β control of multiple behaviors implicates this pathway in the modulation of neuronal signaling.
In this study, we identify a novel role for the DAF-7/TGF-β signaling pathway in regulating the glutamate receptor GLR-1 in C. elegans. GLR-1 is an AMPA-type glutamate receptor expressed in interneurons where it is localized to sensory-interneuron and interneuron-interneuron synapses (Burbea et al., 2002; Hart et al., 1995; Maricq et al., 1995; Rongo et al., 1998). GLR-1 signaling controls several behaviors in C. elegans including a simple mechanosensory response and spontaneous locomotion (Hart et al., 1995; Maricq et al., 1995; Zheng et al., 1999). This study shows that the DAF-7/TGF-β signaling pathway negatively regulates glr-1 transcription to control the abundance of the glutamate receptor GLR-1 and impact behavior.
Materials and Methods
Strains and Maintenance
The following strains were used in this study: N2 (Bristol) wild-type, nuIs24 (Pglr-1::GLR-1::GFP), daf-7 (e1376), daf-1 (m40), daf-8 (e1393), daf-14 (m77), daf-3 (e1376), sma-6 (wk7), pzEx155 (Pglr-1::DAF-8), nuIs125 (Pglr-1::SNB-1::GFP), nuEx993 (Pglr-1::LIN-10::GFP), nuEx1004 (Pglr-1::MAGI-1::YFP), pzIs29 (Pglr-1::NLS::LAC-Z::GFP), nuIs89 (Pglr-1::myc-Ub), nuIs108 (Pglr-1::GLR-1(4KR)::GFP), glr-1(n2461). Strains were maintained at 15°C, and L4 animals were shifted to the non-permissive temperature, 25°C, for 16–20 hours prior to each experiment. To generate strain pzEx155 daf-8 cDNA was PCR amplified from a daf-8 clone (R05D11.1, OpenBiosystems) (primers 5′-AGCTGCTAGCAAAAATGGACGATTTTCCTTCACC-3′ and 5′-AGCTGGTACCCTAAGTTCTGGATGAACATATACGTGG-3′) and inserted under the control of the glr-1 promoter in pV6 (Rongo et al., 1998) to generate plasmid FJ#118.
Fluorescence imaging
Fluorescence imaging was performed as described previously (Kowalski et al., 2011). Briefly, animals were immobilized using 30 mg/ml 2,3-butanedione monoxamine (Sigma-Aldrich) and the anterior ventral nerve cord was imaged. 1μm (total depth) Z-series stacks were collected using a Carl Zeiss Axiovert M1 microscope with a 100× Plan Apochromat (1.4 numerical aperture) objective equipped with GFP and red fluorescent protein filters. Images were collected with an Orca-ER CCD camera (Hamamatsu) and MetaMorph (version 7.1) software (Molecular Devices). Maximum intensity projections of Z-series stacks were used for quantitative analyses of fluorescent puncta. Exposure settings and gain were adjusted to fill the 12-bit dynamic range without saturation and were identical for all images taken of each fluorescent marker. Line scans of ventral cord puncta were generated using Meta-Morph (version 6.0) and were analyzed with Igor Pro (version 5) (Wavemetrics) (Burbea et al., 2002). Arc lamp output was monitored by measuring the fluorescence intensity of 0.5 μm FluoSphere beads (Invitrogen).
qPCR
Total RNA was isolated from mixed staged wild type, daf-7 and daf-8 animals using an RNeasy Fibrous Tissue Mini Kit (Qiagen). All strains were maintained at 15°C and shifted to the non-permissive temperature of 25°C for 16–20h prior to RNA isolation. Four independent RNA preparations were made for each genotype. cDNA was synthesized from this RNA using Superscript III Reverse Transcriptase (Invitrogen). qPCR was performed using the SYBR Green QPCR Master Mix. Reactions were run on a Stratagene MX3000P real-time PCR machine (Tufts Center for Neuroscience Research). The ddCt method was used to calculate relative fold change using act-1 as a reference gene. Significant differences were determined using the Student’s t test on the dCt values.
Immunoblotting
Lysates were prepared by lysing 100 animals of each genotype in SDS lysis buffer (2% SDS, 10% glycerol, 100mM dithiothreitol, 0.1% bromophenol blue, 50mM Tris pH=6.8) using vortexing and boiling. The entire sample was analyzed by SDS-PAGE, transferred to nitrocellulose and analyzed by immunoblotting using antibodies against GFP (JL-8, Covance) and β-tubulin (Abcam).
Transcriptional reporter assays
The glr-1 promoter (5.3 kb upstream of the glr-1 transcription start site) was cloned into pPD96.04 (Addgene – Fire Lab C. elegans Vector Kit) containing NLS-GFP::LacZ to generate plasmid FJ#119, and injected at 50 ng/μl to make pzEx260. pzEx260 was integrated to make pzIs29. To measure fluorescence, roughly 40 L4 worms for each genotype were immobilized with 30 mg/mL 2,3-butanedione monoxamine (Sigma-Aldrich) on a 2% agarose pad. To quantitate GFP fluorescence, maximum intensity projections from Z-series stacks of 1 μm depth were taken from the PVC nucleus using MetaMorph software. Maximum pixel intensity from the nucleus was used for quantification.
Behavioral assays
Locomotion assays were performed as previously described (Juo et al., 2007; Kowalski et al., 2011). Briefly, animals were placed on a plate with no food and allowed to acclimate for two minutes. Animals were subsequently observed for five minutes and reversals were counted manually. All behavioral assays were performed by an experimenter who was unaware of the genotypes being observed.
Results
DAF-7/TGF-β signaling negatively regulates GLR-1 abundance in the VNC
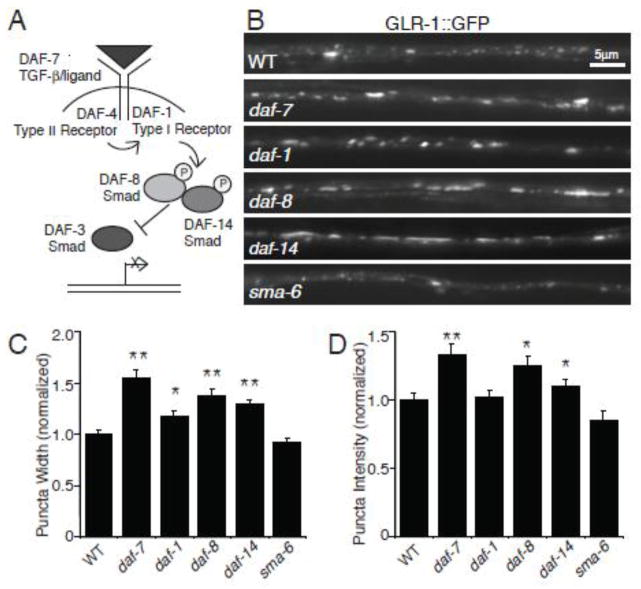
We investigated the effects of the DAF-7/TGF-β signaling pathway (Figure 1 A) on the AMPA-type glutamate receptor GLR-1 by examining levels of GFP-tagged GLR-1 (GLR-1::GFP) at puncta in the ventral nerve cord (VNC). Greater than 80% of these GLR-1::GFP puncta are closely apposed by presynaptic markers, indicating that the majority of these GLR-1 puncta represent postsynaptic sites (Burbea et al., 2002; Rongo et al., 1998). Expression of GLR-1::GFP under the control of its own promoter can rescue the behavioral defects observed in glr-1 null mutant animals, indicating that GLR-1::GFP is functional (Rongo et al., 1998). Because DAF-7/TGF-β inhibits entry into the dauer developmental stage, loss-of-function of daf-7 results in constitutive dauer entry (Ren et al., 1996). Thus, we took advantage of temperature-sensitive daf-7 pathway mutants to avoid any potential effects of dauer development on GLR-1 (Swanson and Riddle, 1981). We maintained both wild type and the daf-7 pathway mutant animals at the permissive temperature of 15° C until the fourth larval (L4) stage, well after the decision point to enter dauer, and then shifted the animals to the restrictive temperature of 25° C for 16–20 hours prior to imaging. We used quantitative fluorescence imaging to analyze the distribution of GLR-1::GFP in the VNC of animals with mutations in the DAF-7/TGF-β ligand (Figure 1 B–D). We estimated levels of GLR-1::GFP in the VNC puncta by measuring peak fluorescence intensity and width of each punctum using custom written software, as described previously (see Materials and Methods) (Burbea et al., 2002; Kowalski et al., 2011). We found that the abundance of GLR-1::GFP was increased in animals that contained a mutation in the TGF-β-like ligand DAF-7 compared to wild type animals. daf-7 mutants had increased fluorescence intensity of GLR-1::GFP and increased punctal width compared with wild type animals (Figure 1 C–D). The density of GLR-1-containing synapses was estimated by measuring the number of GLR-1::GFP puncta per unit length of VNC. The density of GLR-1::GFP puncta did not change in daf-7 mutants as compared to wild type (Average density/10μm ± SEM: WT: 2.59±0.04 (n=154); daf-7: 2.34±0.07 (n=48), p>0.05), suggesting that the DAF-7 pathway does not affect the number of GLR-1-containing synapses.
Figure 1. Multiple components of the DAF-7/TGF-β signaling pathway are required to maintain levels of GLR-1::GFP in the VNC.
(A) Schematic of the DAF-7/TGF-β signaling pathway. (B) Representative images of GLR-1::GFP in the VNC of wild type (WT), daf-7(e1372), daf-1(m40), daf-8(e1393), daf-14(m77), and sma-6(wk7) worms. (C–D) Quantification of puncta width (B) and intensity (C) for WT n=154, daf-7(e1372) n=48, daf-1(m40) n=38, daf-8(e1393) n=36, daf-14(m77) n=32, and sma-6(wk7) n=24. Error bars denote SEM. Values that differ significantly from wild type are indicated by * p<0.05, ** p<0.01 (Tukey-Kramer test). For this experiment, and all subsequent experiments, strains were maintained at 15°C. L4 animals were shifted to the non-permissive temperature, 25°C, for 16–20 hours prior to analysis.
We next tested whether DAF-7-regulation of GLR-1 was mediated by the classical TGF-β signaling pathway known to function downstream of the ligand by analyzing the abundance of GLR-1::GFP in the VNC of animals with temperature-sensitive mutations in several signaling components. The ligand DAF-7/TGF-β signals via DAF-1 and DAF-4, which are type I and type II TGF-β receptors, respectively. Activation of these tyrosine kinase receptors results in phosphorylation of the R-Smad DAF-8, which together with the Smad DAF-14 antagonizes the Smad transcriptional regulator, DAF-3 (Patterson and Padgett, 2000; Patterson et al., 1997; Thatcher et al., 1999) (Figure 1 A). We found that GLR-1::GFP puncta intensity and widths increased in daf-8 and daf-14 loss-of-function Smad mutants (Figure 1 B–D). We also observed increased GLR-1::GFP puncta width in daf-1 TGF-β type I receptor mutants (Figure 1). Although we did not observe increased GLR-1::GFP puncta intensity in daf-1 mutants, this could be due to incomplete loss of function of this conditional allele. We did not analyze daf-4 mutants because this type II receptor is shared by both DAF-7/TGF-β and DBL-1/TGF-β signaling pathways. These results suggest that DAF-7/TGF-β signals through the canonical Smad-dependent pathway in order to affect GLR-1::GFP abundance in the VNC.
Like DAF-7/TGF-β, the TGF-β family ligand DBL-1 also signals via a Smad-dependent pathway in C. elegans (Gumienny, 2013). In order to determine whether both of these TGF-β signaling pathways can similarly affect GLR-1, we measured the abundance of GLR-1::GFP in the VNC of animals containing mutations in sma-6, a type I receptor specific for the DBL-1/TGF-β ligand (Krishna et al., 1999). We found that in contrast to DAF-7/TGF-β pathway mutants, sma-6 mutant worms did not have increased abundance of GLR-1::GFP in the VNC (Figure 1 B–D). This result suggests that specifically the DAF-7/TGF-β signaling pathway, and not the DBL-1/TGF-β pathway, affects the abundance of GLR-1::GFP in the VNC.
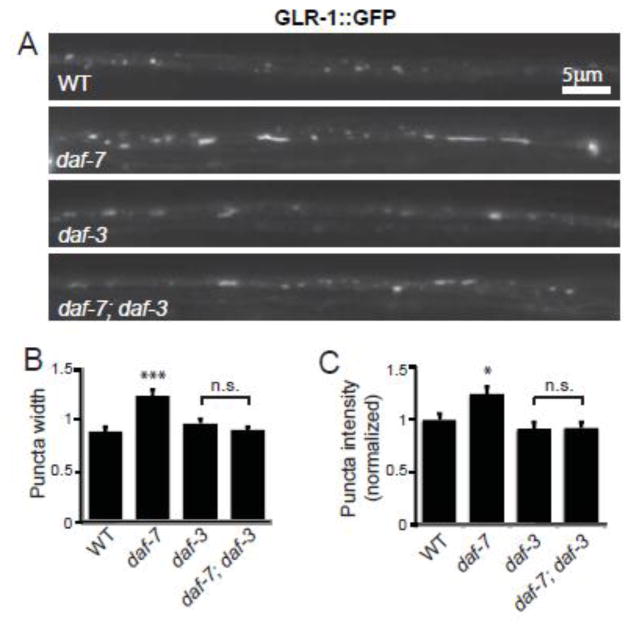
The DAF-7/TGF-β signaling pathway can act either by opposing the action of the transcriptional regulator DAF-3/Smad, or through a DAF-3-independent mechanism (Park et al., 2010; Patterson et al., 1997). In order to distinguish between these two possibilities we looked at whether DAF-3/Smad was required for the increase in GLR-1::GFP in the VNC of daf-7 mutants. Consistent with our previous results, both the fluorescence intensity and width of GLR-1::GFP puncta increased in the VNC of daf-7 mutants compared to wild type animals (Figure 2). However, in daf-7; daf-3 double mutant animals the intensity and width of GLR-1::GFP puncta were indistinguishable from that of daf-3 single mutant animals (Figure 2). Taken together, these results suggest that the DAF-7/TGF-β signaling pathway acts to negatively regulate the abundance of GLR-1::GFP in the VNC through the transcriptional regulator DAF-3/Smad.
Figure 2. DAF-3/Smad is required for the increase in GLR-1::GFP abundance in the VNC of daf-7 mutants.
(A) Representative images of GLR-1::GFP in the VNC of wild type (WT), daf-7(e1372), daf-3(e1376) and daf-7(e1372);daf-3(e1376) worms. (B–C) Quantification of the puncta width (B) and intensity (C) for WT n=30, daf-7(e1372) n=22, daf-3(e1376) n=25 and daf-7(e1372); daf-3(e1376) n=32. Error bars denote SEM. * p<0.05, *** p<0.001, n.s. p>0.05 (Tukey-Kramer test).
The DAF-7/TGF-β signaling pathway acts cell autonomously and specifically to affect GLR-1
We next wanted to identify the cells in which the DAF-7/TGF-β signaling pathway acts to control levels of GLR-1::GFP in the VNC. DAF-7/TGF-β signaling could act either cell autonomously in cells that express GLR-1, or could exert non-cell autonomous effects through signaling in other cell types. Since DAF-7/TGF-β is a secreted factor it can have effects in cells other than those in which it is expressed, therefore we chose to use the DAF-8/Smad protein in order to investigate where pathway signaling is required. We expressed daf-8 cDNA in glr-1-expressing interneurons using the glr-1 promoter (Pglr-1::daf-8). Expression of DAF-8/Smad under the control of the glr-1 promoter (rescue) was sufficient to rescue the increased intensity and width of GLR-1::GFP puncta observed in daf-8 mutant worms (Figure 3). These results suggest that the DAF-7/TGF-β signaling pathway functions in GLR-1-expressing neurons to control the abundance of GLR-1::GFP in the VNC.
Figure 3. DAF-8/Smad acts in glr-1-expressing interneurons to control GLR-1::GFP abundance in the VNC.
(A) Representative images of GLR-1::GFP in the VNC of wild type (WT), daf-8(e1393) and rescue (daf-8(e1393); Pglr-1::DAF-8) animals. (B–C) Quantification of puncta width (B) and intensity (C) for WT n=27, daf-8(e1393) n=25 and rescue n=32. Error bars denote SEM. * p<0.05, ** p<0.01 (Tukey-Kramer test).
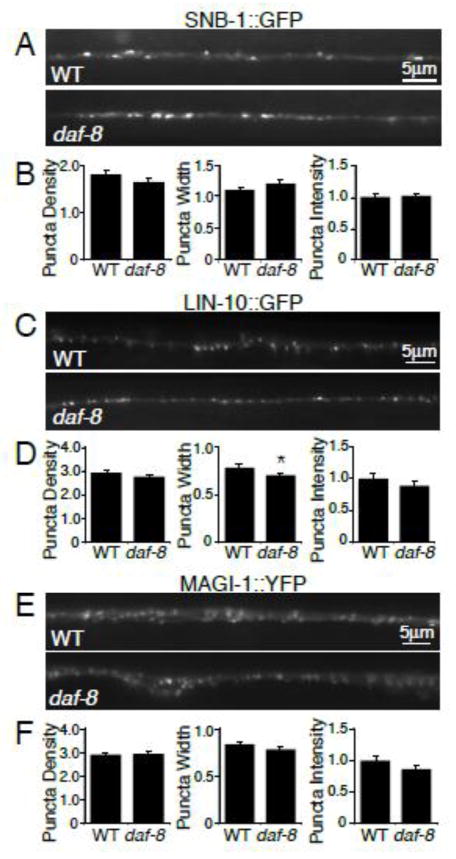
The effect on GLR-1::GFP abundance could be a secondary effect of altered synapse number or architecture. In order to investigate this possibility, we imaged several synaptic markers in daf-8 mutant worms. We analyzed the distribution of the presynaptic vesicle protein synaptobrevin (SNB-1), and the synaptic PDZ scaffolding proteins LIN-10/Mint and MAGI-1/S-SCAM (Emtage et al., 2009; Rongo et al., 1998) in the VNC of wild type and daf-8 mutant worms. We found no increases in puncta density, width or intensity of SNB-1::GFP (Figure 4 A–B), LIN-10::GFP (Figure 4 C–D) or MAGI-1::YFP (Figure 4 E–F) in daf-8 mutants compared to wild type controls. Since we observed no alterations in the abundance or localization of these three synaptic markers in daf-8 mutant animals, the altered GLR-1::GFP levels detected in daf-8 mutants are unlikely to be due to general defects in synapse development or number. Instead, these GLR-1::GFP alterations are likely to be the consequence of a more specific effect of DAF-7/TGF-β signaling on GLR-1.
Figure 4. Synaptic markers are unaltered in the VNC of daf-8 mutants.
(A, C, E) Representative images of synaptic proteins synaptobrevin (SNB-1::GFP) (A), Lin-10/Mint (LIN-10::GFP) (C), and Magi-1/S-SCAM (MAGI-1::YFP) (E) in the VNC of wild type (WT) and daf-8(e1393) worms. (B, D, F) Quantification of puncta density (per 10μm), width, and intensity (normalized) for (B) WT n=25 and daf-8(e1393) n=29, (D) WT n= 31 and daf-8(e1393) n=29, (F) WT n=33 and daf-8(e1393) n=30. Error bars denote SEM. * p<0.05 (Student’s t-test).
GLR-1 protein levels are affected by DAF-7/TGF-β signaling
We next wanted to investigate the mechanism by which the DAF-7/TGF-β pathway regulates GLR-1::GFP abundance in the VNC. We tested whether the increased GLR-1::GFP observed in the VNC of DAF-7/TGF-β pathway mutants was due to an increase in the total amount of GLR-1 protein in neurons. We used immunoblotting to assess total levels of GLR-1::GFP in whole worm lysates from wild type or daf-8 mutant animals. GLR-1::GFP protein levels were normalized to levels of tubulin, which was used as a loading control. We found that GLR-1::GFP protein levels were consistently higher in lysates from daf-8 mutants compared to wild type animals (Figure 5 A). When normalized to tubulin, levels of GLR-1::GFP were ~50% higher in daf-8 mutants compared to wild type controls (Figure 5 B). This suggests that the DAF-7/TGF-β pathway exerts its effect on GLR-1 via alterations in GLR-1 protein abundance.
Figure 5. GLR-1::GFP protein levels and promoter activity are increased in DAF-7/TGF-β pathway mutants.
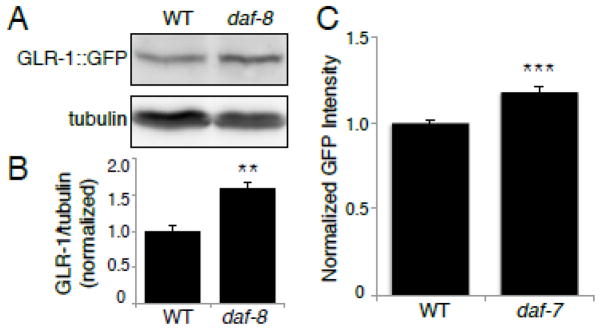
(A) Immunoblot analysis of protein levels in wild type (WT) and daf-8(e1393) worms. The proteins analyzed are GLR-1::GFP (anti-GFP antibody) and tubulin (anti-β-tubulin antibody). (B) Protein levels were quantified using densitometry, shown is the quantification of GLR-1::GFP levels normalized to tubulin, n=6. (C) Maximum GFP fluorescence intensity was measured in the nucleus of PVC neurons of WT and daf-7(e1372) worms expressing Pglr-1::NLS-LacZ-GFP. (WT: 1.00±0.02 SEM, n=78) (daf-7: 1.18±0.02 SEM, n=77). Error bars denote SEM. ** p<0.01, *** p<0.001 (Student’s t-test).
Increased abundance of GLR-1 protein could be explained by an increase in synthesis of GLR-1, a reduced rate of its degradation, or a combination of these two mechanisms. Since we showed that the DAF-7/TGF-β signaling pathway acts through the transcriptional regulator DAF-3/Smad to affect GLR-1::GFP abundance, we hypothesized that the DAF-7/TGF-β pathway directly regulates glr-1 transcription. In order to investigate glr-1 transcription, we first measured glr-1 transcript levels using quantitative real-time PCR (qPCR) on total RNA isolated from whole worms. We found a 31% increase (p < 0.05) in glr-1 transcript in daf-7 mutants compared to wild type controls (see Materials and Methods). We also found a small 15% increase in glr-1 transcript in daf-8 mutants, however this effect did not reach statistical significance (p = 0.06). Secondly, we used a fluorescent glr-1 transcriptional reporter that allowed us to measure the effects of daf-7 on glr-1 transcription at single cell resolution. glr-1 is expressed in about 30 neurons in the animal and the DAF-7 pathway may be active in only a subset of these neurons. We generated an integrated, transgenic line expressing a glr-1 transcriptional reporter to allow us to measure transcription from the glr-1 promoter. In this reporter, the glr-1 promoter drives expression of nuclear localized GFP-tagged LacZ (Pglr-1::NLS::LacZ::GFP). Wild type and daf-7 mutant animals expressing this reporter were imaged and the maximum GFP fluorescence intensity was measured in the nucleus of PVC interneurons (Figure 5 C). We found a small but significant increase in the fluorescence intensity of GFP in daf-7 mutants compared to wild type controls (Figure 5 C). Together, these results suggest that the DAF-7/TGF-β signaling pathway negatively regulates transcription of glr-1.
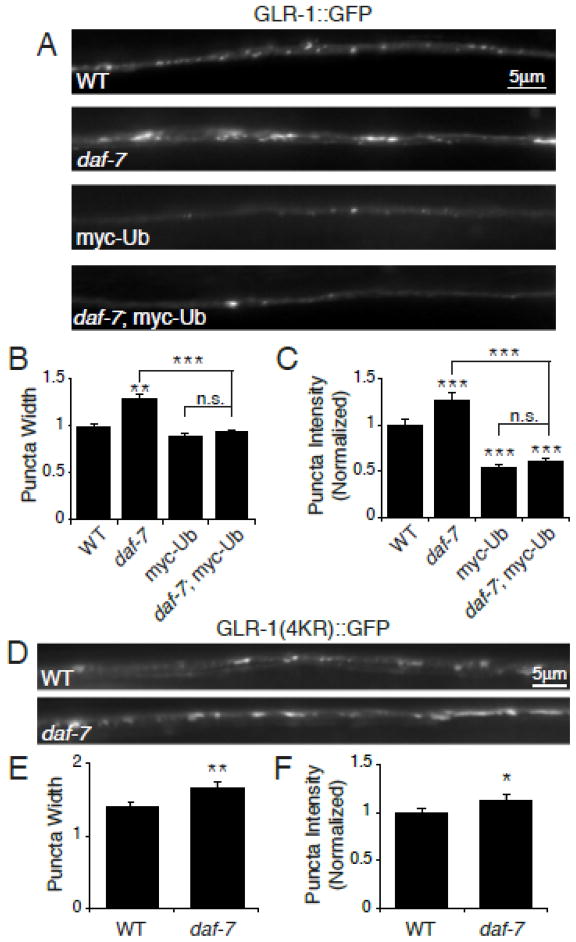
The smaller magnitude effect of the DAF-7/TGF-β signaling pathway mutants on glr-1 transcriptional reporter activity (~20% increase) versus GLR-1::GFP protein levels (~50% increase) prompted us to test whether degradation of GLR-1::GFP was also altered in daf-7 pathway mutants. Ubiquitination of GLR-1 promotes receptor internalization and degradation in the lysosome (Burbea et al., 2002; Chun et al., 2008, Kowalski et al., 2011). We conducted two experiments to test whether ubiquitin-mediated degradation of GLR-1 was reduced in daf-7 pathway mutants. First, we overexpressed myc-tagged ubiquitin (myc-Ub) in the glr-1-expressing interneurons and analyzed its effects on GLR-1::GFP. Overexpression of myc-Ub results in decreased levels of GLR-1::GFP due to increased ubiquitination and degradation of the receptor (Burbea et al., 2002; Dreier et al., 2005; Juo and Kaplan, 2004). Similarly, we found that overexpression of myc-Ub resulted in decreased levels of GLR-1::GFP in the VNC of wild type animals (Figure 6 A–C). If Ub-mediated degradation were blocked in daf-7 mutants we would expect that the overexpression of myc-Ub would not be able to drive degradation in these mutants, and levels of GLR-1::GFP would not decrease with myc-Ub overexpression. In contrast, we found that overexpression of myc-Ub in daf-7 mutants was still able to reduce GLR-1::GFP fluorescence in the VNC, and this effect was indistinguishable from myc-Ub overexpression alone (Figure 6 A–C). This result suggests that in daf-7 mutants the machinery required for ubiquitin-mediated degradation of GLR-1 is still functional. Second, we tested whether a non-ubiquitinatable version of GLR-1(expressed under control of the glr-1 promoter) in which all the cytoplasmic lysine residues are mutated (GLR-1(4KR)::GFP) (Burbea et al., 2002), was resistant to the effects of daf-7 mutation. If daf-7 pathway mutations increase GLR-1::GFP levels by inhibiting ubiquitin-mediated degradation of the receptor, then the levels of GLR-1(4KR)::GFP in the VNC should not increase in daf-7 mutants. In contrast, we found that GLR-1(4KR)::GFP puncta fluorescence intensities and widths were increased in the VNC of daf-7 mutants relative to controls (Figure 6 D–F). Taken together, these data suggest that changes in ubiquitin-mediated degradation of GLR-1::GFP alone are unlikely to be responsible for the increased levels of GLR-1::GFP observed in daf-7 pathway mutants. Because GLR-1(4KR)::GFP expression is under control of the glr-1 promoter, the increase in GLR-1(4KR)::GFP fluorescence observed in daf-7 mutants could simply be due to increased expression from the glr-1 promoter, which is consistent with our glr-1 transcriptional reporter results (Figure 5 C). Together, these data suggest that glr-1 transcription is increased in daf-7 pathway mutants.
Figure 6. Ubiquitin-dependent degradation of GLR-1 in daf-7 mutants.
(A) Representative images of GLR-1::GFP in the VNC of wild type (WT), daf-7(e1372), worms overexpressing myc-Ub (Pglr-1::myc-Ub), and daf-7(e1372); Pglr-1::myc-Ub. (B–C) Quantification of puncta width (B) and normalized fluorescence intensity (C) of WT n=38, daf-7(e1372) n=28, myc-Ub n=29, and daf-7(e1372); myc-Ub n=38. (D) Representative images of GLR-1(4KR)::GFP in the VNC of WT and daf-7(e1372) worms. (E–F) Quantification of puncta width (E) and normalized fluorescence intensity (F) of WT n=28 and daf-7(e1372) n=21. Error bars denote SEM. * p<0.05, ** p<0.01, *** p<0.001 (Tukey-Kramer test).
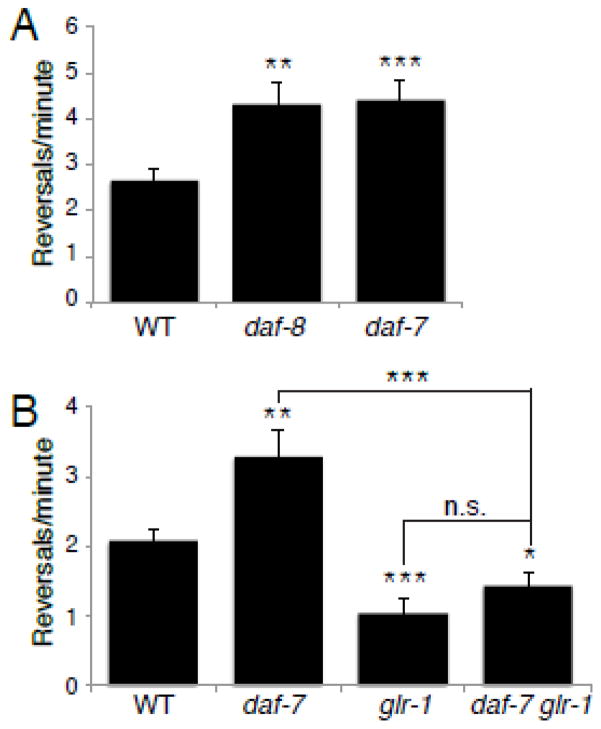
Locomotion is regulated by the DAF-7/TGF-β signaling pathway
We next tested whether the increased GLR-1 in the VNC of daf-7 pathway mutants had consequences for glutamate-dependent locomotion behavior. Signaling through GLR-1 controls the rate at which animals reverse during spontaneous locomotion (Zheng et al., 1999). Mutants with reduced glutamate signaling, such as glr-1 null mutants or animals lacking the vesicular glutamate transporter eat-4/VGLUT, exhibit decreased reversal frequencies (Brockie et al., 2001; Burbea et al., 2002; Zheng et al., 1999), whereas mutants with increased GLR-1 signaling exhibit a higher rate of reversals (Burbea et al., 2002; Juo and Kaplan, 2004; Juo et al., 2007; Schaefer and Rongo, 2006; Zheng et al., 1999, Monteiro et al., 2012). We found that the spontaneous reversal frequency of daf-8 and daf-7 mutant animals is significantly higher than in wild type animals (Figure 7 A), consistent with increased signaling through GLR-1 receptors. In order to test whether this change in reversal frequency is dependent on endogenous GLR-1, we measured the rate of spontaneous reversals in daf-7;glr-1 double mutants. We found that the increased reversal frequency observed in daf-7 mutants was blocked in the glr-1 mutant background (Figure 7 B). In addition, daf-7;glr-1 double mutants exhibited decreased reversal frequencies that were indistinguishable from glr-1 single mutants (Figure 7 B). Together, these data suggest that the DAF-7/TGF-β signaling pathway acts to limit the rate of spontaneous reversals by controlling levels of the glutamate receptor GLR-1.
Figure 7. Spontaneous reversal rates are increased in daf-7 and daf-8 mutants.
Graphs showing the average number of spontaneous reversals per minute of the following genotypes: (A) wild type (WT) n=20, daf-8(e1393) n=16, and daf-7(e1372) n=13. (B) wild type (WT) n=21, daf-7(e1372) n=20, glr-1(n2461) n=21, and daf-7(e1372) glr-1(n2461) n=19. Error bars denote SEM. * p<0.05, ** p<0.01, *** p<0.001, n.s. p>0.05, (Student’s t-test).
Discussion
In addition to performing critical roles during development, a growing body of evidence indicates that TGF-β family signaling pathways are involved in regulating synaptic function in the adult nervous system (Krieglstein et al., 2011; Poon et al., 2013). However, the molecular mechanisms that mediate this regulation are not well characterized. In this study, we evaluated the role of the C. elegans DAF-7/TGF-β signaling pathway in the regulation of the AMPA-type glutamate receptor GLR-1. We show that multiple components of the DAF-7/TGF-β signaling pathway are required to maintain proper levels of GLR-1 in the VNC (Figure 1). Expression of DAF-8/Smad in glr-1-expressing interneurons was sufficient to rescue the defect in GLR-1 levels observed in daf-8 mutants suggesting that the downstream DAF-7/TGF-β signaling components function in glr-1-expressing interneurons (Figure 3). The changes in GLR-1 levels in the DAF-7/TGF-β signaling pathway mutants appear to be specific for GLR-1 and not due to global effects on neuronal proteins because several other synaptic markers are unaffected in DAF-7/TGF-β pathway mutants (Figure 4). The DAF-7/TGF-β signaling pathway likely regulates GLR-1 levels through transcriptional regulation because mutations in the transcriptional regulator daf-3/Smad block the effects of daf-7/TGF-β mutants on GLR-1 (Figure 2). Furthermore, qPCR analysis of glr-1 transcript levels, and the analysis of a glr-1 transcriptional reporter suggest that DAF-7/TGF-β regulation of GLR-1 occurs, in part, through direct regulation of glr-1 transcription (Figure 5). Finally, DAF-7/TGF-β pathway mutants affect GLR-1-dependent locomotion behavior in a manner consistent with increased glutamatergic signaling (Figure 7). Thus, DAF-7/TGF-β-regulation of GLR-1 levels has important consequences for behavior (Figure 7). Together, these findings show that the DAF-7/TGF-β pathway negatively regulates the abundance of AMPA-type glutamate receptors via transcription, identifying a novel mechanism by which TGF-β signaling can modulate neuronal function.
Canonical TGF-β signaling occurs through phosphorylation and activation of Smad proteins, which can directly influence transcriptional activity (Shi and Massagué, 2003). However in the mammalian nervous system, although TGF-β can modulate synaptic plasticity, the signaling appears to occur through a non-canonical, Smad-independent mechanism (Poon et al., 2013; Sun et al., 2010; Zhou et al., 2003). Additional signaling pathways downstream of TGF-β receptors are important in controlling synaptic properties of neurons including the phosphorylation of CREB in hippocampal neurons (Fukushima et al., 2007) and the activation of the MAPK pathway in sensory neurons of Aplysia (Chin et al., 2006). We found that the effect of DAF-7/TGF-β signaling on GLR-1 occurs via downstream Smad proteins because mutations in Smads daf-8 or daf-14 caused increased GLR-1::GFP levels similar to those seen in the daf-7 ligand mutants (Figure 1). Additionally we showed that the effect of DAF-7/TGF-β signaling on GLR-1 depends on the transcriptional regulator DAF-3/Smad because introducing a daf-3 mutation into the daf-7 mutant background eliminates the increase in GLR-1::GFP levels observed in daf-7 single mutants (Figure 2). Thus in C. elegans, DAF-7/TGF-β regulation of the glutamate receptor GLR-1 occurs via a Smad and transcription-dependent signaling pathway.
TGF-β signaling can function both pre- and postsynaptically to regulate synapse development and function. At the fly NMJ, the TGF-β/BMP pathway signals in a retrograde manner from the muscle to the presynaptic motor neuron to regulate synapse development and function (Aberle et al., 2002; Haghighi et al., 2003; Marqués et al., 2002; McCabe et al., 2003). In mammals, both pre- and postsynaptic roles for TGF-β signaling pathways have been identified. Disruption of signaling by TGF-β family receptors in mice results in decreased glutamate signaling and defects in LTP (Ageta et al., 2010; Heupel et al., 2008; Müller et al., 2006; Xiao et al., 2013). A presynaptic function for TGF-β signaling is suggested by a recent study showing that mice lacking both BMPR1a/1b have decreased numbers of docked synaptic vesicles at calyx of Held synapses resulting in reduced glutamatergic signaling (Xiao et al., 2013). While increased BMP signaling in mutant mice lacking the BMP antagonist Chordin results in increased presynaptic transmission and enhanced short- and long-term plasticity (Sun et al., 2007). Postsynaptically, TGF-β family signaling can increase NMDA receptor function in rodent neurons in culture (Kurisaki 2008) and reduce short-term synaptic depression (Fukushima 2007). Thus, TGF-β family members have diverse effects on neuronal signaling and are likely to act both pre- and postsynaptically to regulate synaptic function.
In this study, we show that the DAF-7/TGF-β signaling pathway regulates postsynaptic glutamate receptor levels in ventral cord interneurons in C. elegans. The signaling pathway likely functions in glr-1-expressing interneurons because expression of DAF-8/Smad exclusively in these interneurons is sufficient to rescue the GLR-1 defect observed in daf-8 mutants (Figure 3). Additionally, we did not observe any changes in the distribution or fluorescence intensity of the synaptic vesicle marker synaptobrevin in DAF-7/TGF-β pathway mutants (Figure 4). While this data suggests that daf-7 pathway mutants do not have gross defects in synapse number or development, this data also suggests that the rates of SV exocytosis and endocytosis are relatively normal because changes in the distribution of SNB-1::GFP have previously been shown to correlate with changes in SV release (Dittman and Kaplan, 2006; Sieburth et al., 2005). For example, mutations in genes required for SV exocytosis such as unc-13/MUNC13 or unc-18/MUNC18 exhibit increased SNB-1::GFP puncta fluorescence and decreased diffuse interpunctal cord fluorescence (Dittman and Kaplan, 2006; Sieburth et al., 2005). The fluorescence of this diffuse SNB-1::GFP correlates with the levels of SNB-1 on the plasma membrane (Dittman and Kaplan, 2006). Conversely, mutations in genes required for SV endocytosis such as unc-11/AP180 or unc-57/endophilin exhibit decreased SNB-1::GFP puncta intensity and increased interpunctal cord fluorescence (Dittman and Kaplan, 2006; Sieburth et al., 2005). We found no change in SNB-1::GFP puncta intensity (Figure 2) or interpunctal cord fluorescence (Avg. cord fluorescence (Norm.): WT: 1.0±0.05; daf-8: 1.03±0.04, p=0.7) in DAF-7/TGF-β pathway mutants, which is consistent with the idea that DAF-7/TGF-β signaling does not affect the relative rates of SV exo- and endocytosis. Additionally, DAF-7/TGF-β pathway mutants exhibit increased reversal frequency in the locomotion assay, which is consistent with increased glutamatergic signaling and correlates with the increased levels of GLR-1 in the VNC. Together, our data suggest a model whereby the DAF-7/TGF-β pathway functions in interneurons to negatively regulate glr-1 transcription, resulting in decreased GLR-1 at synapses in the VNC and effects on GLR-1-mediated locomotion.
Our work implicates DAF-7-regulation of GLR-1 as a potential molecular mechanism underlying the ability of C. elegans to modify its behavior in response to environmental conditions. The DAF-7/TGF-β signaling pathway is responsive to environmental conditions such as low food, crowding, and high temperature. Under these conditions, which are not favorable for growth, DAF-7/TGF-β signaling is turned off. Thus, our results predict that under unfavorable growth conditions, when DAF-7/TGF-β signaling is turned off, that the levels of GLR-1 would increase. By changing the levels of GLR-1 in response to environmental stimuli, the animal would be able to finely tune the responsiveness of its nervous system and increase the response to glutamatergic inputs. This change in the responsiveness of the nervous system would allow the animal to alter its behavioral responses appropriately. Indeed, the DAF-7/TGF-β signaling pathway plays a role in helping to fine tune several behaviors including the likelihood an animal will leave a depleting source of food to search for new food sources (Milward et al., 2011) and the quiescence response of an animal to feeding following starvation, a satiety-like behavior (Gallagher et al., 2013; You et al., 2008). It will be interesting in the future to investigate whether DAF-7/TGF-β signaling alters synaptic GLR-1 levels in response to changes in the environment, in order to elicit the appropriate behavioral response.
Acknowledgments
This work was supported in part by a National Institutes of Health (NIH) Grant (NS059953) to P.J., the Tufts Center for Neuroscience Research (NIH Grant P30NS047243), the Training in Education and Critical Research Skills Postdoctoral Fellowship Program (NIH Grant 5K12GM074869) and an American Heart Association Postdoctoral Fellowship to A.M.M. We thank Josh Kaplan, Gary Ruvkun, and the Caenorhabditis Genetics Center (NIH Office of Research Infrastructure Programs P40 OD010440) for strains. We thank the Juo lab for helpful discussions and critical reading of this manuscript.
Abbreviations
- TGF-β
transforming growth factor-β
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- GLR-1
glutamate receptor-1
- VNC
ventral nerve cord
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Annette M. McGehee, Email: amcgehee@suffolk.edu.
Benjamin J. Moss, Email: benjamin.moss@tufts.edu.
Peter Juo, Email: peter.juo@tufts.edu.
References
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. doi: 10.1016/s0896-6273(02)00589-5. [DOI] [PubMed] [Google Scholar]
- Ageta H, Ikegami S, Miura M, Masuda M, Migishima R, Hino T, Takashima N, Murayama A, Sugino H, Setou M, et al. Activin plays a key role in the maintenance of long-term memory and late-LTP. Learn Mem Cold Spring Harb N. 2010;17:176–185. doi: 10.1101/lm.16659010. [DOI] [PubMed] [Google Scholar]
- Bargmann C. Chemosensation in C. elegans. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV. The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron. 2001;31:617–630. doi: 10.1016/s0896-6273(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron. 2002;35:107–120. doi: 10.1016/s0896-6273(02)00749-3. [DOI] [PubMed] [Google Scholar]
- Chin J, Liu RY, Cleary LJ, Eskin A, Byrne JH. TGF-β1-Induced Long-Term Changes in Neuronal Excitability in Aplysia Sensory Neurons Depend on MAPK. J Neurophysiol. 2006;95:3286–3290. doi: 10.1152/jn.00770.2005. [DOI] [PubMed] [Google Scholar]
- Chun DK, McEwen JM, Burbea M, Kaplan JM. UNC-108/Rab2 regulates postendocytic trafficking in Caenorhabditis elegans. Mol Biol Cell. 2008;19:2682–2695. doi: 10.1091/mbc.E07-11-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita A, Krishna S, Zheng H, Padgett RW, Culotti JG. Pioneer axon guidance by UNC-129, a C. elegans TGF-beta. Science. 1998;281:706–709. doi: 10.1126/science.281.5377.706. [DOI] [PubMed] [Google Scholar]
- Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci U S A. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Emtage L, Chang H, Tiver R, Rongo C. MAGI-1 modulates AMPA receptor synaptic localization and behavioral plasticity in response to prior experience. PloS One. 2009;4:e4613. doi: 10.1371/journal.pone.0004613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima T, Liu RY, Byrne JH. Transforming growth factor-β2 modulates synaptic efficacy and plasticity and induces phosphorylation of CREB in hippocampal neurons. Hippocampus. 2007;17:5–9. doi: 10.1002/hipo.20243. [DOI] [PubMed] [Google Scholar]
- Gallagher T, Kim J, Oldenbroek M, Kerr R, You YJ. ASI regulates satiety quiescence in C. elegans. J Neurosci Off J Soc Neurosci. 2013;33:9716–9724. doi: 10.1523/JNEUROSCI.4493-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer ER, Pérez CL, Van Gilst MR, Lee BH, Ashrafi K. Neural and molecular dissection of a C. elegans sensory circuit that regulates fat and feeding. Cell Metab. 2008;8:118–131. doi: 10.1016/j.cmet.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumienny TL. TGF-β signaling in C. elegans. WormBook. 2013:1–34. doi: 10.1895/wormbook.1.22.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378:82–85. doi: 10.1038/378082a0. [DOI] [PubMed] [Google Scholar]
- Heupel K, Sargsyan V, Plomp JJ, Rickmann M, Varoqueaux F, Zhang W, Krieglstein K. Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Develop. 2008;3:25. doi: 10.1186/1749-8104-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerndli FJ, Walser M, Fröhli Hoier E, de Quervain D, Papassotiropoulos A, Hajnal A. A conserved function of C. elegans CASY-1 calsyntenin in associative learning. PloS One. 2009;4:e4880. doi: 10.1371/journal.pone.0004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juo P, Kaplan JM. The anaphase-promoting complex regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Curr Biol CB. 2004;14:2057–2062. doi: 10.1016/j.cub.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Juo P, Harbaugh T, Garriga G, Kaplan JM. CDK-5 regulates the abundance of GLR-1 glutamate receptors in the ventral cord of Caenorhabditis elegans. Mol Biol Cell. 2007;18:3883–3893. doi: 10.1091/mbc.E06-09-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski JR, Dahlberg CL, Juo P. The deubiquitinating enzyme USP-46 negatively regulates the degradation of glutamate receptors to control their abundance in the ventral nerve cord of Caenorhabditis elegans. J Neurosci Off J Soc Neurosci. 2011;31:1341–1354. doi: 10.1523/JNEUROSCI.4765-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Zheng F, Unsicker K, Alzheimer C. More than being protective: functional roles for TGF-β/activin signaling pathways at central synapses. Trends Neurosci. 2011;34:421–429. doi: 10.1016/j.tins.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Krishna S, Maduzia LL, Padgett RW. Specificity of TGFbeta signaling is conferred by distinct type I receptors and their associated SMAD proteins in Caenorhabditis elegans. Dev Camb Engl. 1999;126:251–260. doi: 10.1242/dev.126.2.251. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Maricq AV, Peckol E, Driscoll M, Bargmann CI. Mechanosensory signalling in C. elegans mediated by the GLR-1 glutamate receptor. Nature. 1995;378:78–81. doi: 10.1038/378078a0. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O’Connor MB. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron. 2002;33:529–543. doi: 10.1016/s0896-6273(02)00595-0. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marqués G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Meisel JD, Pando O, Mahanti P, Schroeder FC, Kim DH. Chemosensation of bacterial secondary metabolites modulates neuroendocrine signaling and behavior of C. elegans. Cell. 2014;159:267–280. doi: 10.1016/j.cell.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milward K, Busch KE, Murphy RJ, de Bono M, Olofsson B. Neuronal and molecular substrates for optimal foraging in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108:20672–20677. doi: 10.1073/pnas.1106134109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro MI, Ahlawat S, Kowalski JR, Malkin E, Koushika SP, Juo P. The kinesin-3 family motor KLP-4 regulates anterograde trafficking of GLR-1 glutamate receptors in the ventral nerve cord of Caenorhabditis elegans. Mol Biol Cell. 2012;23:3647–3662. doi: 10.1091/mbc.E12-04-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MR, Zheng F, Werner S, Alzheimer C. Transgenic Mice Expressing Dominant-negative Activin Receptor IB in Forebrain Neurons Reveal Novel Functions of Activin at Glutamatergic Synapses. J Biol Chem. 2006;281:29076–29084. doi: 10.1074/jbc.M604959200. [DOI] [PubMed] [Google Scholar]
- Nolan KM, Sarafi-Reinach TR, Horne JG, Saffer AM, Sengupta P. The DAF-7 TGF-beta signaling pathway regulates chemosensory receptor gene expression in C. elegans. Genes Dev. 2002;16:3061–3073. doi: 10.1101/gad.1027702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard M, Mathew D, Budnik V. Wnts and TGF beta in synaptogenesis: old friends signalling at new places. Nat Rev Neurosci. 2003;4:113–120. doi: 10.1038/nrn1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Estevez A, Riddle DL. Antagonistic Smad transcription factors control the dauer/non-dauer switch in C. elegans. Dev Camb Engl. 2010;137:477–485. doi: 10.1242/dev.043752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson GI, Padgett RW. TGF beta-related pathways. Roles in Caenorhabditis elegans development. Trends Genet TIG. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- Patterson GI, Koweek A, Wong A, Liu Y, Ruvkun G. The DAF-3 Smad protein antagonizes TGF-beta-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev. 1997;11:2679–2690. doi: 10.1101/gad.11.20.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon VY, Choi S, Park M. Growth factors in synaptic function. Front Synaptic Neurosci. 2013;5 doi: 10.3389/fnsyn.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren P, Lim CS, Johnsen R, Albert PS, Pilgrim D, Riddle DL. Control of C. elegans larval development by neuronal expression of a TGF-beta homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Schaefer H, Rongo C. KEL-8 is a substrate receptor for CUL3-dependent ubiquitin ligase that regulates synaptic glutamate receptor turnover. Mol Biol Cell. 2006;17:1250–1260. doi: 10.1091/mbc.E05-08-0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Ch’ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, et al. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- Stetak A, Hörndli F, Maricq AV, van den Heuvel S, Hajnal A. Neuron-specific regulation of associative learning and memory by MAGI-1 in C. elegans. PloS One. 2009;4:e6019. doi: 10.1371/journal.pone.0006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Thomas MJ, Herder R, Bofenkamp ML, Selleck SB, O’Connor MB. Presynaptic contributions of chordin to hippocampal plasticity and spatial learning. J Neurosci Off J Soc Neurosci. 2007;27:7740–7750. doi: 10.1523/JNEUROSCI.1604-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Gewirtz JC, Bofenkamp L, Wickham RJ, Ge H, O’Connor MB. Canonical TGF-beta signaling is required for the balance of excitatory/inhibitory transmission within the hippocampus and prepulse inhibition of acoustic startle. J Neurosci Off J Soc Neurosci. 2010;30:6025–6035. doi: 10.1523/JNEUROSCI.0789-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MM, Riddle DL. Critical periods in the development of the Caenorhabditis elegans dauer larva. Dev Biol. 1981;84:27–40. doi: 10.1016/0012-1606(81)90367-5. [DOI] [PubMed] [Google Scholar]
- Thatcher JD, Haun C, Okkema PG. The DAF-3 Smad binds DNA and represses gene expression in the Caenorhabditis elegans pharynx. Dev Camb Engl. 1999;126:97–107. doi: 10.1242/dev.126.1.97. [DOI] [PubMed] [Google Scholar]
- Trent C, Tsung N, Horvitz HR. Egg-Laying Defective Mutants of the Nematode Caenorhabditis Elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashlishan AB, Madison JM, Dybbs M, Bai J, Sieburth D, Ch’ng Q, Tavazoie M, Kaplan JM. An RNAi screen identifies genes that regulate GABA synapses. Neuron. 2008;58:346–361. doi: 10.1016/j.neuron.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, Schneggenburger R. BMP signaling specifies the development of a large and fast CNS synapse. Nat Neurosci. 2013;16:856–864. doi: 10.1038/nn.3414. [DOI] [PubMed] [Google Scholar]
- You Y, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Endo S, Cleary LJ, Eskin A, Byrne JH. Role of transforming growth factor-beta in long-term synaptic facilitation in Aplysia. Science. 1997;275:1318–1320. doi: 10.1126/science.275.5304.1318. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV. Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron. 1999;24:347–361. doi: 10.1016/s0896-6273(00)80849-1. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Zhao M, Li D, Shimazu K, Sakata K, Deng CX, Lu B. Cerebellar deficits and hyperactivity in mice lacking Smad4. J Biol Chem. 2003;278:42313–42320. doi: 10.1074/jbc.M308287200. [DOI] [PubMed] [Google Scholar]