Abstract
Allogeneic hematopoietic stem-cell transplantation (alloHSCT) survivors treated with total body irradiation (TBI) exhibit bone deficits and excess adiposity, potentially related to altered mesenchymal stem cell differentiation into osteoblasts or adipocytes. We examined associations among fat distribution, bone microarchitecture, and insulin resistance in alloHSCT survivors after TBI. This was a cross-sectional observational study of 25 alloHSCT survivors (aged 12–25 years) a median of 9.7 (4.3–19.3) years after alloHSCT compared to 25 age-, race-, and sex-matched healthy controls. Vertebral MR spectroscopic imaging and tibia micro-MRI were used to quantify marrow adipose tissue (MAT) and trabecular microarchitecture. Additional measures included DXA whole-body fat mass (WB-FM), leg lean mass (Leg-LM), trunk visceral adipose tissue (VAT), and CT calf muscle density. Insulin resistance in alloHSCT survivors was estimated by HOMA-IR. AlloHSCT survivors had lower Leg-LM (p<0.001), and greater VAT (p<0.01), MAT (p<0.001) and fat infiltration of muscle (p=0.04) independent of WB-FM, vs. matched-controls; BMI did not differ. Survivors had lower bone volume fraction and abnormal microarchitecture including greater erosion and more rod-like structure vs. controls (all p=0.04); 14 had vertebral deformities and two had compression fractures. Greater WB-FM, VAT, MAT and muscle fat infiltration were associated with abnormal trabecular microarchitecture (p<0.04 for all). AlloHSCT HOMA-IR was elevated, associated with younger age at transplantation (p<0.01), and positively correlated with WB-FM and VAT (both p<0.01). In conclusion, the markedly increased marrow adiposity, abnormal bone microarchitecture, and abnormal fat distribution highlight the risks of long-term treatment-related morbidity and mortality in alloHSCT recipients after TBI. Trabecular deterioration was associated with marrow and visceral adiposity. Furthermore, long-term survivors demonstrated sarcopenic obesity, insulin resistance, and vertebral deformities. Future studies are needed to identify strategies to prevent and treat metabolic and skeletal complications in this growing population of childhood alloHSCT survivors.
Keywords: Marrow adiposity, visceral adipose tissue, hematopoietic stem cell transplantation, trabecular microarchitecture, vertebral fracture
Introduction
Allogeneic hematopoietic stem-cell transplantation (alloHSCT) is an established treatment for benign and malignant hematologic disorders.(1) With emerging indications for transplantation and improved outcomes, the number of survivors is increasing. Unfortunately, chemotherapy, total body irradiation, glucocorticoid therapy, immune dysregulation, graft vs. host disease, and treatment-related endocrine disorders result in significant late effects. Consequently, medical attention has shifted to the prevention and treatment of long-term alloHSCT-related morbidities.(2)
Impaired glucose metabolism and increased risk of insulin resistance is a growing threat to survivors of childhood alloHSCT,(3) with higher risks of diabetes in survivors compared to healthy siblings.(4) Recent studies reported an increased incidence of premature arterial vascular disease, hypertension,(4) and dyslipidemia with a prevalence of metabolic syndrome approaching 50%.(5) However, a clear picture of predisposing factors has not emerged, with total body irradiation, graft vs. host disease, endocrine abnormalities, obesity, and genetic predisposition implicated as potential risk factors.(6)
We recently reported dual energy x-ray absorptiometry (DXA) measures of total body fat and lean mass in 55 long-term survivors of childhood alloHSCT.(7) Although body mass index (BMI) Z-scores did not differ between alloHSCT and > 650 reference participants, alloHSCT recipients had significant sarcopenic obesity. AlloHSCT survivors also had substantial deficits in trabecular volumetric bone mineral density and cortical geometry by peripheral quantitative CT (pQCT), compared with reference participants.(8) These abnormalities were more pronounced in survivors with a history of total body irradiation and growth hormone deficiency.
Bone and fat cells share a common mesenchymal stem cell (MSC) within the bone marrow, and hormones and transcription factors such as growth hormone, insulin-like growth factor-1 (IGF-1), leptin, and peroxisomal proliferator-activated receptor γ influence MSC differentiation into osteoblasts or adipocytes.(9) Human cell culture studies suggest that MSC osteogenic potential is more vulnerable to radiation than adipogenic potential.(10) These findings highlight the risk for an abnormal fat-bone axis in alloHSCT survivors.
Adipose tissue is a dynamic secretory organ, capable of producing adipokines, markers of inflammation, and cytokines.(11) Adiponectin, produced by adipocytes, plays a crucial role in the regulation of energy homeostasis and insulin sensitivity.(12) The location of fat deposition is implicated in adverse cardiovascular and bone outcomes.(13,14) Importantly, central obesity or visceral adipose tissue (VAT), and fat infiltration of muscle have been linked to insulin resistance, atherosclerosis, lower levels of vitamin 25(OH)D,(15) greater inflammation, and low bone mineral density.(16,17) In a recent study in healthy young women, greater VAT was associated with inferior trabecular microarchitecture.(18) Our previous study of long-term alloHSCT survivors did not assess fat distribution, including VAT, marrow adipose tissue, or muscle density (lower muscle density represents greater fat infiltration), trabecular microarchitecture, or measures of insulin resistance. Therefore, the aim of this study was to assess these parameters in 25 alloHSCT long-term survivors with a history of total body irradiation, compared with matched controls, and to examine associations among fat distribution, bone outcomes and measures of insulin resistance in alloHSCT survivors. We hypothesized that greater visceral adiposity in long-term alloHSCT survivors is associated with higher markers of inflammation, and lower adiponectin, and vitamin 25(OH)D levels.
Materials and methods
Subjects
The study population included 25 children and young adults treated with alloHSCT for hematologic malignancy or bone marrow failure syndrome and followed at the Children’s Hospital of Philadelphia. Twenty-two participated in our prior study approximately three to four years earlier,(7,8) and three newly enrolled. Inclusion criteria included total body irradiation exposure, age > 12 years at the time of study (for non-sedated MRI scans), and ≥3 years since alloHSCT with confirmed remission status. Exclusion criteria included alloHSCT for mucopolysaccharides given musculoskeletal complications, pregnancy, active malignancy or relapse after alloHSCT, estimated glomerular filtration rate < 60 mL/min/1.73 m2, and ferromagnetic implants.
The 25 alloHSCT participants were compared to 25 age (± 1 year)-, sex-, and race-matched healthy controls recruited from practices in the Philadelphia area. The matched controls underwent all of the same imaging procedures as the alloHSCT participants. These controls were excluded for a history of illnesses or medications that may affect growth, nutritional status, or bone health. In addition, the DXA measures of lean and fat mass in the alloHSCT participants and matched controls were compared with reference data previously generated in 1,001 healthy participants (59% Caucasian), 5–30 years of age, as previously described.(19) That study did not include the novel outcomes included here. Therefore, in order to examine marrow adipose tissue, trabecular microarchitecture and measures of fat distribution (e.g. visceral adiposity) relative to total adiposity on specific outcomes of interest including bone and insulin resistance in alloHSCT, we recruited 25 age (±1 year)-, sex-, and race-matched healthy controls from practices in the Philadelphia area. Reference participants and matched-controls were excluded for a history of illnesses or medications that may affect growth, nutritional status, or bone health. The study protocol was approved by the Institutional Review Board at each institution. Informed consent was obtained from participants ≥18 years of age, and assent with parental consent from those < 18 years.
Anthropometry and physical maturity
Height and sitting height were measured with a stadiometer and weight with a digital scale. Tanner pubertal stage was determined by a pediatric endocrinologist (S.M.M.) in alloHSCT participants and by validated self-assessment questionnaire in the reference participants and controls.(20)
Blood pressure
Blood pressure was measured in the alloHSCT participants and matched controls using Carescape V100 Non-Invasive Blood Pressure Device (GE Healthcare, Milwaukee, WI). Elevated blood pressure was defined as systolic blood pressure > 90th percentile for age, sex and height Z-score.(21)
Disease and treatment characteristics
Myeloablative conditioning regimens consisted of cyclophosphamide, thiotepa, and fractional total body irradiation (range 1200–1320 cGy). For subjects with graft vs. host disease, details of prior glucocorticoid exposure (cumulative mg/kg and interval since last dose) were recorded.(7,8) Endocrinopathy characteristics and hormone therapy were documented by participant interviews and medical chart review.
Magnetic resonance measures of tibia trabecular microarchitecture and vertebral marrow adiposity
High-resolution distal tibia images in alloHSCT and matched controls were acquired using a 1.5 Tesla whole-body scanner (Siemens Sonata, Erlangen, Germany), as described.(22) Digital topological analysis parameters were surface-to-curve ratio (an index of a plate- vs. rodlike structure) and erosion index;(23) a lower surface-to-curve ratio and greater erosion index have been associated with vertebral deformities.(24)
Lumbar spine magnetic resonance spectroscopic imaging (MRSI) was performed on the same scanner. A 2D interleaved multiple-gradient-echo chemical-shift imaging sequence was implemented to quantify lipid content in marrow using the manufacturer’s spine and body matrix coils.(25) The sequence parameters were: Repetition Time/Echo Time (spin echo) 500/8ms, Field of View 30×60cm2, Matrix 60×120, Slice Thickness 10mm, Scan Time 4.3 mins, Spectral Bandwidth 1 kHz, and Spectral Resolution 15.625 Hz. The raw data were Fourier transformed to yield spectra, which were integrated from 0–3 ppm (fat region, F) and from 3.5–6 ppm (water region, W). The marrow fat volume fraction f =F/(F +W) was calculated for the five vertebral levels across a manually-selected, central 3×3-voxel region of interest at each vertebra, and averaged across the five sites to generate MAT (%). The voxel size was 2.5×2.5 mm2.(25)
DXA measures of fat mass and lean mass
Whole body DXA scans were obtained in alloHSCT, matched controls and healthy reference participants with a Hologic Delphi densitometer (Bedford, Massachusetts).(7) Outcomes included whole body fat mass (WB-FM, kg) and lean mass (WB-LM, kg) excluding the head, and Leg-LM (kg) as a measure of skeletal muscle. DXA trunk visceral (VAT) and subcutaneous adipose tissue (SAT) area (cm2) in alloHSCT and matched controls were quantified in a 5 cm region at the L4 level (Hologic APEX 3.1 software) with VAT coefficient of variation reported at 2.3%.(26,27)
Measures of muscle density
Calf muscle density (mg/cm3) in alloHSCT participants and matched controls was measured by peripheral quantitative CT at a site 66% of tibia length proximal to the medial malleolus, as previously described.(28) Muscle density serves as a composite measure of inter- and intra-muscular adipose tissues.(29)
Physical activity
Physical activity was assessed in the alloHSCT and matched-control participants using a questionnaire that captured over 30 sports and play activities, summarized as hours/week.(30)
Vertebral fractures and deformities
Vertebral deformity fractures of the thoracolumbar spine was assessed on the basis of MR images obtained using a fast spin-echo sequence (TR/TE of 4000/13.6 ms, echo train length 8, bandwith 31.25 kHz, NEX 2, field of view 40×30 cm, 0.78 mm×0.78 mm pixel size, and 5 mm sagittal slices. A pediatric radiologist (K.S.) reviewed all sagittal MRI images to identify vertebral fractures and deformities. Vertebral (wedge, compression or biconcave) fractures were identified based on Eastell’s criterion.(31) Thoracolumbar vertebral height and endplate changes were used to qualify deformities as mild, moderate or severe.(32,33)
Laboratory studies
Fasting laboratory studies were performed in alloHSCT participants only. Fasting glucose and insulin were measured using colorimetric assay. Insulin resistance was assessed by Homeostasis Model Assessment of Insulin Resistance (HOMA-IR).(34) Serum 25 hydroxyvitamin D [25(OH)D] concentration was analyzed by tandem mass-spectrometry,(35) total adiponectin by ELISA (R&D Systems) and high sensitivity C-Reactive Protein (hsCRP) by Fixed Point Immuno Rate Assay. IGF-1 levels were measured by ELISA (R&D Systems), and categorized as low, normal, or high based on the manufacturer’s normal ranges for age and Tanner stage.
Statistical analysis
Analyses were performed using Stata 13.0 (StataCorp, College Station, TX). A p value <0.05 was considered statistically significant, and two-sided tests were used throughout. A Bonferroni correction for multiple comparisons was not performed as the outcomes were highly correlated. Group differences in alloHSCT recipients versus matched controls or reference participants were tested using t tests, with adjustment for unequal variance, or Wilcoxon rank sum test if indicated. Differences in proportions were assessed using chi-square test. Correlations between continuous variables were assessed by Pearson or Spearman’s rank correlations, where appropriate. Skewed variables were natural log-transformed.
Age- and sex-specific Z-scores were calculated for height and BMI using national data.(36) Height Z-score was calculated relative to age 20 years in the 14 participants > 20 years and BMI Z-scores limited to those ≤20 years. The DXA results in the 1,001 reference participants were used to generate sex- and race-specific curves for WB-FM, WB-LM and Leg-LM relative to age (LMS Chartmaker Program version 2.3). WB-LM and WB-FM were highly correlated with height (R=0.95 and 0.56, respectively) in reference participants (both p<0.0001), and alloHSCT was associated with marked growth impairment.(7,37) Therefore, WB-FM and WB-LM Z-scores were adjusted for height Z-score, and Leg-LM Z-scores adjusted for leg length Z-score, as described.(38)
The correlations between some absolute body composition measures were confounded by height; therefore, their results are reported as the partial correlation adjusted for height. Linear regression analyses were used to assess associations of bone volume fraction with MAT, VAT, SAT, and muscle density in alloHSCT compared to matched-controls, adjusted for sex (age was not significant). Models for MAT, VAT, SAT, and muscle density outcomes were adjusted for WB-FM to determine if group differences were explained by greater WB-FM. VAT models were further adjusted for sitting height since shorter sitting height relative to height in alloHSCT (due to radiation effects on spine growth) could mask greater VAT area, vs. controls. Analyses within alloHSCT participants included associations of bone, marrow adiposity, and body composition with glucocorticoid exposure (cumulative mg/kg and date since last exposure), growth hormone deficiency, age at alloHSCT, laboratory studies and physical activity scores. We examined the associations of IGF-1 levels with bone, MAT and body composition outcomes, with IGF-1 as a continuous (adjusted for age and sex) and categorical (low vs. normal/high) variable.
Results
Participant characteristics
Thirty-eight of the alloHSCT participants from our previous study had a history of TBI and 31 were eligible for this study based on the age criteria. Twenty-two (71%) participated in this study; the nine that did not were not different in age, sex or alloHSCT characteristics. The three eligible black participants from the previous study were lost to follow up. Three new alloHSCT recipients were enrolled. We approached five eligible alloHSCT recipients to enroll the three new alloHSCT participants. HSCT and matched-control participants were enrolled over a 15-month period. The alloHSCT and matched-control characteristics are summarized in Table 1. All participants were Caucasian. AlloHSCT was associated with delayed maturation. Height Z-scores were markedly lower in alloHSCT participants, while BMI Z-scores did not differ significantly. AlloHSCT sitting height relative to height was significantly lower compared to controls (p<0.01). Height and BMI Z-scores did not differ according to sex in alloHSCT participants or controls.
Table 1.
AlloHSCT and matched-control participant characteristics
| AlloHSCT n=25 |
Matched Controls* n=25 |
P value | |
|---|---|---|---|
| Age, years | 17.3 (12.2 to 25.1) | 17.2 (13.0 to 25.1) | -- |
| Sex, n (%) male | 17 (68%) | 17 (68%) | -- |
| Pubertal status, n (%) | 0.05 | ||
| Tanner stage 1 | 1 (4%) | 0 (0%) | |
| Tanner stage 2–3 | 8 (32%) | 2 (8%) | |
| Tanner stage 4–5 | 16 (64%) | 23 (92%) | |
| Blood pressure (mmHg) | |||
| Systolic Z-score | −0.19 (−2.33 to 3.50) | 0.18 (−1.24 to 1.54) | NS |
| Diastolic Z-score | −0.22 (−1.37 to 2.41) | −0.47 (−1.16 to 1.21) | NS |
| Sitting height (cm) | 126.5 (120.5 to 139.3) | 134.2 (121.2 to 146.2) | <0.01 |
| Height (cm) | 158.3 (141.4 to 179.5) | 169.7 (147.8 to 192.6) | <0.01 |
| Height Z-score | −1.41 (−3.15 to 0.41) | −0.02 (−1.62 to 2.27) | <0.001 |
| BMI (kg/m2) | 19.8 (15.3 to 34.4) | 22.4 (18.0 to 28.0) | NS |
| BMI Z-score** | −0.28 (−2.94 to 2.22) | 0.44 (−1.42 to 1.72) | NS |
Controls were sex-, race-, and age-matched (± one yr) to alloHSCT recipients
BMI Z-scores restricted to the 36 participants ≤20 years
Results are reported as n (%) or median (range)
Disease and treatment characteristics
AlloHSCT characteristics are summarized in Table 2. All 13 with a history of graft vs. host disease completed glucocorticoid treatment within two years of alloHSCT and were off glucocorticoids for at least 5 years. Disease and treatment characteristics did not differ according to sex. Twenty (80%) were diagnosed with an endocrinopathy and all received appropriate hormone replacement therapy. Ten (40%) were diagnosed with two hormonal deficits (growth hormone deficiency and hypothyroidism), and three (12%) with multiple abnormalities (growth hormone deficiency, hypothyroidism, and gonadal failure). None had adrenal insufficiency requiring steroid replacement.
Table 2.
AlloHSCT disease and treatment characteristics
| Characteristics | |
|---|---|
| Age at study enrollment, years | 17.3 (12.2 to 25.1) |
| Age at diagnosis, years | 5.9 (0.2 to 17.9) |
| Age at HSCT, years | 8.5 (0.4 to 18.3) |
| Time since HSCT, years | 9.7 (4.3 to 19.3) |
| Diagnosis, n (%) | |
| Acute Lymphoblastic Leukemia | 13 (52%) |
| Acute Myelogenous Leukemia | 7 (28%) |
| Juvenile Myelomonocytic Leukemia | 2 (8%) |
| Aplastic Anemia | 2 (8%) |
| Chronic Myelogenous Leukemia | 1 (4%) |
| Donor source | |
| Related | 12 (48%) |
| Unrelated | 12 (48%) |
| Cord | 1 (4%) |
| Graft versus host disease | |
| Acute or chronic | 13 (52%) |
| None | 12 (48%) |
| Glucocorticoid Therapy since HSCT | |
| None | 12 (48%) |
| Interval since last steroid dose, years | 9 (5 to 18) |
| Endocrine Disorders | |
| Hypothyroidism on treatment | 12 (48%) |
| Growth Hormone Deficiency | 15 (60%) |
| Treatment with Growth Hormone* | 14 (93%) |
| On Growth Hormone therapy at visit | 8 (53%) |
| Testosterone replacement (males)** | 3 (18%) |
| Hormone replacement therapy (females)*** | 5 (83%) |
Data are presented as n (%) or median (range)
One patient with Growth Hormone deficiency was not treated due to limited growth potential at the time of diagnosis based on bone age; five had a previous diagnosis of precocious puberty requiring treatment with Lupron during Growth Hormone therapy.
Based on n=3 males with treatment-related primary gonadal failure, all initiated treatment at > 16 years of age.
Based on n=6 post-menarchal alloHSCT females at the time of study visit.
Trabecular microarchitecture
Among controls and alloHSCT participants combined, bone volume fraction (BVF) was higher, the structure was more plate-like, and the erosion index was lower (all indicating greater strength) in males, compared with females. AlloHSCT participants demonstrated significantly lower BVF and abnormal bone microarchitecture, compared to matched controls (Table 3).
Table 3.
Bone and body composition parameters in alloHSCT and matched controls
| AlloHSCT n=25 |
Matched Controls n=25 |
P value vs. Matched Controls | P value vs. Reference Participants | |
|---|---|---|---|---|
| Trabecular Architecture | ||||
| Bone volume fraction (%) | 11.0 (8.6 to 13.7) | 11.7 (9.4 to 14.0) | 0.04* | -- |
| Surface to curve | 6.68 (4.53 to 9.49) | 7.60 (5.35 to 10.03) | 0.04* | -- |
| Erosion index | 0.68 (0.45 to 0.94) | 0.57 (0.05 to 0.81) | 0.04* | -- |
| Adipose | ||||
| Whole Body Fat Mass (kg) | 14.8 ± 8.6 | 12.7 ± 3.6 | 0.26 | |
| Whole Body Fat Mass Z-score | 0.72 ± 1.06 | 0.37 ± 0.75 | 0.19 | <0.001 |
| Visceral Fat Area (cm2) | 55.6 (4.6 to 166.7) | 43.8 (15.9 to 75.1) | <0.01* | -- |
| Subcutaneous Fat Area (cm2) | 177.4 (62.6 to 490.5) | 142.4 (48.2 to 321.7) | 0.04 | |
| Marrow Adipose Tissue (%) | 57.4 (36.3 to 76.0) | 27.3 (13.5 to 57.1) | <0.001 | -- |
| Muscle density (g/cm3) | 75.8 (72.2 to 77.9) | 76.4 (75.0 to 77.6) | 0.04 | -- |
| DXA Lean Body Mass | ||||
| Whole Body Lean Mass (kg) | 35.6 ± 11.3 | 46.0 ± 10.8 | <0.001 | |
| Whole Body Lean Mass Z-score | −0.88 ± 1.28 | −0.18 ± 0.75 | 0.04 | <0.001 |
| Leg Lean Mass (kg) | 12.4 ± 4.1 | 16.7 ± 4.1 | <0.001 | |
| Leg Lean Mass Z-score | −1.44 ± 1.49 | 0.00 ± 0.85 | <0.001 | <0.001 |
| Laboratory Parameters | ||||
| Vitamin D 25 (OH) (ng/mL) | 31.5 (13.1 to 41.6) | -- | -- | -- |
| Adiponectin (ng/mL) | 8,407 (2,091 to 17,056) | -- | -- | -- |
| C-Reactive Protein (mg/mL) | 1.5 (0.8 to 11.1) | -- | -- | -- |
| HOMA-IR | 2.7 (0.7 to 35.1) | -- | -- | -- |
Data are presented as median (range) or mean ± SD
Significantly lower in females in alloHSCT and controls; the p-value represents the group difference adjusted for sex.
AlloHSCT participants were compared to two sets of healthy children and young adults. DXA measures of fat and lean mass were compared to CHOP sex- and race-specific reference curves in 1,001 healthy participants, limited to Caucasians since the entire alloHSCT cohort was Caucasian; however, given differences in age and sex distributions, the absolute DXA whole body fat mass, lean mass, and leg lean mass results are not compared between alloHSCT and reference participants. Marrow adipose tissue, trabecular microarchitecture, and measures of fat distribution in alloHSCT were compared to age-, race- and sex-matched healthy controls.
Adiposity and lean body mass
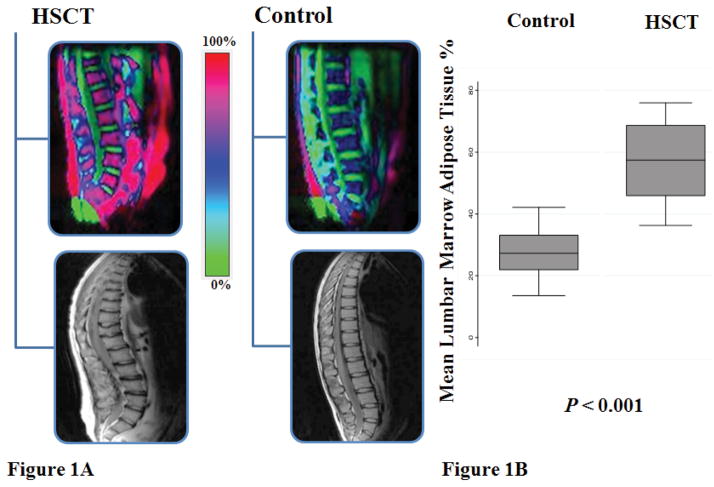
Body composition results are summarized in Table 3. AlloHSCT participants had significantly greater WB-FM Z-scores compared with reference participants (p<0.001). DXA VAT, SAT, and MAT were significantly higher in alloHSCT, and muscle density was significantly lower (indicative of greater fat infiltration of muscle); none of the group differences was attenuated with adjustment for greater WB-FM in alloHSCT participants. The magnitude of VAT excess in alloHSCT was amplified when adjusted for the shorter sitting height; the group difference in natural log (VAT) variable increased from 0.28 to 0.39. Age at and interval since alloHSCT was not associated with measures of adiposity. The markedly greater MAT in alloHSCT, vs. controls, is shown in Figure 1B. The MR specroscopy images demonstrated greater marrow and visceral adiposity (Figure 1A).
Figure 1. Vertebral marrow adipose tissue in alloHSCT and matched controls.
(A) Magnetic Resonance Spectroscopic Imaging (MRSI) of vertebral marrow adipose tissue in alloHSCT versus matched-control. Red color (100%) represents greater adiposity and green (0%) greater water content. An occult lower lumbar vertebral compression fracture is noted in the MR lateral spine image of the alloHSCT participant. (B) AlloHSCT participants demonstrate significantly higher mean lumbar vertebral marrow adipose tissue compared to matched-controls.
WB- and Leg-LM Z-scores were significantly lower in alloHSCT compared to reference population (p<0.001 for both) and matched controls (Table 3). The magnitude of lean mass deficits was more pronounced for Leg-LM, compared with WB-LM Z-scores. Correlations among all body composition measures are summarized in Table 4.
Table 4.
Correlations among adipose and lean mass measures in alloHSCT and matched controls
| Whole Body Fat Mass | Visceral Adipose Tissue | Subcutaneous Adipose Tissue | Marrow Adipose Tissue | Muscle Density | Whole Body Lean Mass | |
|---|---|---|---|---|---|---|
| Visceral Adipose Tissue | 0.65 | |||||
| <0.001 | ||||||
| Subcutaneous Adipose Tissue | 0.92* | 0.55* | ||||
| <0.001 | <0.001 | |||||
| Marrow Adipose Tissue | 0.21 | 0.34 | 0.42 | |||
| 0.18 | 0.02 | <0.01 | ||||
| Muscle Density | −0.46 | −0.37 | −0.40 | −0.47 | ||
| 0.001 | 0.01 | <0.01 | <0.01 | |||
| Whole Body Lean Mass | 0.41* | 0.31 | −0.08 | −0.40 | 0.03 | |
| 0.02 | 0.03 | 0.60 | <0.01 | 0.82 | ||
| Leg Lean Mass | 0.28 | 0.23 | −0.14 | −0.48 | 0.06 | 0.96 |
| 0.05 | 0.11 | 0.32 | <0.01 | 0.70 | <0.001 |
All variables were natural log transformed.
Results represent the correlation (R) and p-value.
Partial correlations adjusted for height.
Adiposity, lean mass, and trabecular bone volume fraction
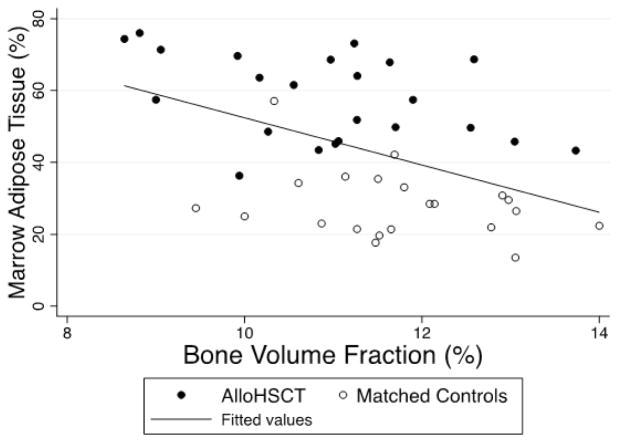
Higher WB-FM (p=0.03), VAT (p=0.02), SAT (p=0.02), and MAT (p<0.01), as well as lower muscle density (p<0.01) and Leg-LM (p=0.04) were associated with lower bone volume fraction, adjusted for sex. The association between MAT and bone volume fraction is shown in Figure 2. AlloHSCT participants with growth hormone deficiency had marginally higher MAT (p=0.05); however, growth hormone deficiency was not associated with any bone parameters. Prior glucocorticoid treatment (cumulative mg/kg and interval since last dose) or younger age at HSCT were not associated with bone volume fraction, MAT, any measures of adiposity or lean mass.
Figure 2. Vertebral marrow adipose tissue and tibia bone micro-architecture.
Vertebral marrow adipose tissue measured by Magnetic Resonance Spectroscopic Imaging (MRSI) is inversely and significantly associated with tibia micro-Magnetic Resonance Imaging (MRI) measures of bone volume fraction in alloHSCT and matched-control participants.
Adiposity, insulin sensitivity and inflammation in alloHSCT
AlloHSCT participants demonstrated significant insulin resistance (Table 3) based on the widely adopted HOMA-IR cutoff value of 2.60.(39) Younger age at HSCT was associated with a greater HOMA-IR (p=0.002); this was not explained by interval since HSCT or age at study visit. HOMA-IR was positively associated with WB-FM (p=0.01), SAT (p<0.01), and VAT (p<0.01), adjusted for age at HSCT, but not MAT (p=0.27) or muscle density (p=0.29). Adiponectin levels were within assay’s normative published results [6,641±3,665 (range 865–21,424) ng/mL], and inversely associated with muscle density (R=−0.62, p<0.01) and HOMA-IR (R=−0.43, p=0.03). HOMA-IR was not associated with vitamin D, or hsCRP levels, adjusted for age at transplantation, or with growth hormone deficiency.
Vitamin D and IGF-1 levels
25(OH)D was < 20 ng/mL in four alloHSCT participants. Greater WB-FM (R=−0.49, p=0.01), WB-FM Z-score (−0.47, p=0.02), SAT (p=0.02), and VAT (−0.44, p=0.03) were associated with lower 25(OH)D concentrations.(18) None of the other body composition or laboratory measures were associated with 25(OH)D. IGF-1 (ng/mL) levels were low for age and pubertal status in eight (32%) alloHSCT participants. Of these, two males were growth hormone deficient and previously treated with growth hormone and the remaining six participants did not have prior diagnosis of growth hormone deficiency and were not treated with growth hormone. IGF-1 levels were not associated with bone or body composition measures.
Physical activity
The physical activity scores did not differ in alloHSCT participants and the matched-controls (2.2 ± 0.8 versus 2.4 ± 0.5; p=0.48). None of the bone or body composition results were associated with physical activity scores.
Vertebral fractures and deformities
Two (8%) alloHSCT participants had occult vertebral compression fractures (Figure 1A) and 14 (58%) had a total of 51vertebral deformities vs. none in the matched controls (p<0.01). Three alloHSCT participants had a single deformity, six had two to four, and five had five to nine vertebral deformities. Only three deformities included mild loss of vertebral height, while the remainder involved the endplate and were classified as mild, with 37% from T11-L2 and 35% from L3–L5. Two alloHSCT participants demonstrated moderate, and one exhibited severe endplate deformities of the thoracolumbar region, respectively, separate from the two identified occult vertebral fractures. Bone volume fraction, trabecular architecture, and MAT were not significantly different in those with and without vertebral deformities.
Discussion
Survivors of childhood alloHSCT after total body irradiation demonstrate a pattern of excess adiposity and muscle deficits consistent with sarcopenic obesity.(40) AlloHSCT was associated with insulin resistance and an adverse fat distribution characterized by greater visceral adiposity and fat infiltration of muscle, independent of WB-FM. BMI did not reflect these abnormalities. Marrow adipose tissue was two-fold greater, compared with controls, was associated with greater visceral adiposity and fat infiltration of muscle, and was associated with reduced bone volume fraction as well as abnormal bone microarchitecture. Importantly, younger age at the time of transplantation was associated with greater insulin resistance, independent of interval since transplantation. Together with our prior report of significantly smaller cortical dimensions in alloHSCT recipients treated with total body irradiation,(8) these findings typify alterations in the fat-bone axis and herald deleterious metabolic and skeletal abnormalities in a growing survivor population after successful childhood HSCT.
The bone marrow microenvironment houses MSCs and hematopoietic stem cells. Multi-potent MSCs differentiate into multiple cell lineages including osteoblasts and adipocytes,(41) with stress-induced conditions such as chemotherapy or radiation altering the differentiation of both hematopoietic and stromal precursors.(42,43) As adipocytes and osteoblasts share a common MSC precursor, preferential differentiation of adipogenic over osteogenic lineage following bone marrow injury due to cancer therapy may result in bone loss and increased marrow adiposity.(44)
Previous studies reported that greater marrow adipose tissue was associated with lower trabecular bone mineral density or smaller cortical dimensions in healthy children and young adults.(45,46) Women with anorexia nervosa and reduced estrogen secretion exhibited increased vertebral marrow adiposity, associated with lower bone mineral density at multiple skeletal sites.(47,48) Furthermore, in pre-diabetic overweight children, VAT, but not WB-FM, was an independent predictor of lower bone mass.(49) Therefore, our findings are congruent with previous cross-sectional studies demonstrating that marrow adipose tissue and VAT were associated with poor bone mass in the absence of chemotherapy or total body irradiation.(18,50)
AlloHSCT participants in our study were exposed to total body irradiation as part of HSCT treatment regimen. Radiation has damaging effects on the bone marrow microenvironment.(51) Mice treated with total body irradiation demonstrate depletion of total femur bone marrow cellularity and increased marrow adiposity.(52) Adult patients receiving pelvic radiation therapy in combination with chemotherapy experience significant bone marrow cell depletion, bone loss with increased fracture risks, and enhanced MAT.(43) Radiation, even at low doses, predominantly affects MSC’s osteogenic differentiation potential with dose-dependent reduction in cell viability.(10,53) While the molecular mechanisms by which radiation affects MSC cell survival, commitment, differentiation, and recovery are not clearly elucidated, the involvement of Wnt/β-catenin signaling has been suggested.(54) To date, no previous studies have examined marrow adiposity in long-term cancer survivors after total body irradiation to address the fat-bone axis. Our findings of occult vertebral compression fractures and frequent and widespread vertebral deformities highlight the fracture implications in long-term HSCT survivors. Additional studies are needed to examine the molecular effects on the marrow microenvironment and to identify potential therapeutic targets.
Previous studies reported increased obesity, insulin resistance, and dyslipidemia in adult survivors of pediatric leukemia.(55) Pediatric and adult HSCT recipients are at increased risk for cardiometabolic abnormalities,(4) with total body irradiation identified as a major risk factor for metabolic derangements.(56) Similar to previous reports, this study identified insulin resistance in long-term alloHSCT survivors.(4) Total body and cranial radiation, particularly at a young age, is a risk factor for subsequent growth hormone deficiency.(57) AlloHSCT participants with growth hormone deficiency had higher measures of insulin resistance, a recognized risk factor for metabolic syndrome in this population.(58) In addition, younger age at time of alloHSCT and total body irradiation exposure was inversely associated with measures of insulin sensitivity, with higher HOMA-IR levels noted in alloHSCT survivors treated with total body irradiation at a young age. Moreover, alloHSCT participants with greater fat mass demonstrated lower 25(OH)D levels likely attributable to sequestration of fat soluble vitamin D in adipocytes;(59) however, lower 25(OH)D levels in alloHSCT survivors showed no association with measures of insulin resistance or lean mass.
The lean mass deficits in alloHSCT recipients were more pronounced when assessed as Leg-LM rather than WB-LM Z-scores (mean of -1.44 and -0.88, respectively). The explanation may be two-fold. First, the clinical significance of cachexia lies in muscle deficits and Leg-LM is a better indicator of skeletal muscle.(37,60) Second, the alloHSCT recipients had significantly shorter trunk height, and hence longer leg length relative to height, compared with controls. The majority of lean mass is in the limbs. Therefore, when comparing an alloHSCT recipient and control of comparable age and height, the relatively longer leg in the alloHSCT recipient may mask some lean mass deficits. Similarly, short trunk height may mask excess visceral adipose tissue, as shown here. Thus, future studies of body composition in alloHSCT survivors should consider aberrations in body proportions, particularly as cachexia is a recognized independent risk factor for mortality, and predicts toxicity in cancer patients undergoing therapy.(61) Furthermore, as sarcopenic obesity is associated with cardiometabolic consequences including increased mortality,(62,63) future studies are imperative to further elucidate treatment-related and hormonal causes contributing to persistent muscle deficits in the face of increased adiposity in alloHSCT survivors.
The primary limitations of our study are the cross-sectional design, modest sample size, and the lack of laboratory measurements in the matched controls. Additional limitations include the extended duration since treatment, which prohibits assessment of the impact of chemotherapy on bone and body composition and the small subgroups treated with hormone replacement. Additional limitations included the use of DXA estimates of VAT. In a study in adults, the correlation (R) between CT and DXA measures of VAT and SAT were 0.73 and 0.97, respectively, in normal weight women, and 0.88 and 0.95 in overweight and obese women.(64) Similarly, quantitative CT measures of muscle density do not distinguish between intra- and inter-muscle adipose tissue, or intra- and inter-cellular lipids. However, skeletal muscle attenuation by CT has been associated with skeletal muscle lipid content,(29) and prior studies reported associations of quantitative CT calf muscle density with insulin resistance, inflammation, and bone deficits.(16,65,66) Finally, the study did not include dietary intake or objective measures of physical activity, such as use of accelerometers.
The study has many, important strengths. This is the first study to examine MAT and the associations among fat distribution, bone outcomes and measures of insulin resistance in alloHSCT survivors. While, vertebral compression fractures have been reported in children and adolescents with acute lymphoblastic leukemia during chemotherapy,(33) this is the first study to report occult vertebral compression fractures in long-term alloHSCT survivors. In conclusion, the markedly increased marrow adiposity, abnormal bone microarchitecture, and abnormal fat distribution highlight the risks of long-term treatment-related morbidity and mortality in HSCT recipients after total body irradiation. These findings underline the substantial burden of long-term, life-threatening morbidity, with the need for life-long specialized health care to institute appropriate and timely intervention strategies. Future longitudinal studies are essential to determine the mechanism of enhanced marrow adiposity (altered hematopoietic stem cell homing vs. cancer treatment effect) and abnormal bone microarchitecture and to identify strategies that not only promote normal bone accrual but also improve metabolic outcomes and premature cardiovascular risks in the growing number of childhood alloHSCT recipients.
Acknowledgments
Grant Support: This study was supported by NIH grants K07 CA166177 (S.M.M), K24 DK076808 (M.B.L), Alex Lemonade Stand Foundation (S.M.M), the National Centre for Research Resources (UL1-RR-024134), and National Center for Advancing Translational Sciences (UL1TR000003).
The authors thank the Bone Marrow Transplant and Survivorship teams at the Children’s Hospital of Philadelphia for their support.
Footnotes
Data Presentation: This study was a platform oral presentation at the June 2014 Endocrine Society meeting.
Disclosure Statement: All authors disclose no potential conflicts of interest.
Authorship: S.M.M, M.B.L, F.W, J.M, and B.Z designed the study; S.M.M, E.J.I, W.S and C.R collected and assembled the data; S.M.M, M.B.L, and J.L analyzed data and performed statistical analysis; S.M.M, M.B.L, B.Z, F.W, J.M, W.S, C.R, J.B, and K.S interpreted the results; S.M.M and M.B.L wrote the paper; S.M.M, J.M, E.J.I, W.S, C.R, B.Z, F.W, K.S, J.B, J.L, and M.B.L reviewed and critiqued the manuscript and contributed to revisions. All authors approved the final manuscript.
References
- 1.Ballard C, Krance R, Heslop H. Hematopoietic Stem Cell Transplantation in Pediatric Oncology. In: Pizzo P, Poplack D, editors. Principles and Practice of Pediatric Oncology. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 476–500. [Google Scholar]
- 2.Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS) Blood. 2011;118(5):1413–20. doi: 10.1182/blood-2011-01-331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91(11):4401–7. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 4.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–72. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone marrow transplantation. 2009;43(1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356(9234):993–7. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 7.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160(1):122–8. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27(4):760–9. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bredella MA, Torriani M, Ghomi RH, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Kwong DL, Chan GC. The effects of various irradiation doses on the growth and differentiation of marrow-derived human mesenchymal stromal cells. Pediatr Transplant. 2007;11(4):379–87. doi: 10.1111/j.1399-3046.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 11.Sam S, Haffner S, Davidson MH, et al. Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes care. 2009;32(5):932–7. doi: 10.2337/dc08-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zitsman JL, Hou J, et al. Fat cell size and adipokine expression in relation to gender, depot, and metabolic risk factors in morbidly obese adolescents. Obesity (Silver Spring) 2014;22(3):691–7. doi: 10.1002/oby.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165(7):777–83. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi T, Kanazawa I, Yamamoto M, et al. Associations between components of the metabolic syndrome versus bone mineral density and vertebral fractures in patients with type 2 diabetes. Bone. 2009;45(2):174–9. doi: 10.1016/j.bone.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Young KA, Engelman CD, Langefeld CD, et al. Association of plasma vitamin D levels with adiposity in Hispanic and African Americans. J Clin Endocrinol Metab. 2009;94(9):3306–13. doi: 10.1210/jc.2009-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laddu DR, Farr JN, Lee VR, et al. Muscle density predicts changes in bone density and strength: a prospective study in girls. Journal of musculoskeletal & neuronal interactions. 2014;14(2):195–204. [PMC free article] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71(4):885–92. doi: 10.1093/ajcn/71.4.885. [DOI] [PubMed] [Google Scholar]
- 18.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98(6):2562–72. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95(4):1681–9. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris NN, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980:271–80. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 21.Moyer VA. Screening for primary hypertension in children and adolescents: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(9):613–9. doi: 10.7326/0003-4819-159-9-201311050-00725. [DOI] [PubMed] [Google Scholar]
- 22.Rajapakse CS, Leonard MB, Bhagat YA, Sun W, Magland JF, Wehrli FW. Micro-MR imaging-based computational biomechanics demonstrates reduction in cortical and trabecular bone strength after renal transplantation. Radiology. 2012;262(3):912–20. doi: 10.1148/radiol.11111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magland JF, Wehrli FW. Trabecular bone structure analysis in the limited spatial resolution regime of in vivo MRI. Acad Radiol. 2008;15(12):1482–93. doi: 10.1016/j.acra.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajapakse CS, Phillips EA, Sun W, et al. Vertebral deformities and fractures are associated with MRI and pQCT measures obtained at the distal tibia and radius of postmenopausal women. Osteoporos Int. 2014;25(3):973–82. doi: 10.1007/s00198-013-2569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilaire L, Wehrli FW, Song HK. High-speed spectroscopic imaging for cancellous bone marrow R(2)* mapping and lipid quantification. Magn Reson Imaging. 2000;18(7):777–86. doi: 10.1016/s0730-725x(00)00165-x. [DOI] [PubMed] [Google Scholar]
- 26.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20(5):1109–14. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Body Composition User Guide, Document No. MAN-02354 Revision 001. Hologic, Inc; 2010. [Google Scholar]
- 28.Baker JF, Von Feldt J, Mostoufi-Moab S, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(11):1612–8. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol (1985) 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 30.Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the Physical Activity Questionnaire for Older Children. Med Sci Sports Exerc. 1997;29(10):1344–9. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ., 3rd Classification of vertebral fractures. J Bone Miner Res. 1991;6(3):207–15. doi: 10.1002/jbmr.5650060302. [DOI] [PubMed] [Google Scholar]
- 32.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 33.Alos N, Grant RM, Ramsay T, et al. High incidence of vertebral fractures in children with acute lymphoblastic leukemia 12 months after the initiation of therapy. J Clin Oncol. 2012;30(22):2760–7. doi: 10.1200/JCO.2011.40.4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 35.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125(6):914–20. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 36.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 37.Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status and exercise capacity in children and young adults after Fontan palliation. Heart. 2014 doi: 10.1136/heartjnl-2014-305723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95(3):1265–73. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes care. 2003;26(12):3320–5. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- 40.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Current opinion in clinical nutrition and metabolic care. 2008;11(6):693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 42.Georgiou KR, Foster BK, Xian CJ. Damage and recovery of the bone marrow microenvironment induced by cancer chemotherapy - potential regulatory role of chemokine CXCL12/receptor CXCR4 signalling. Curr Mol Med. 2010;10(5):440–53. doi: 10.2174/156652410791608243. [DOI] [PubMed] [Google Scholar]
- 43.Baxter NN, Habermann EB, Tepper JE, Durham SB, Virnig BA. Risk of pelvic fractures in older women following pelvic irradiation. Journal of the American Medical Association. 2005;294(20):2587–93. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 44.Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1(3):205–24. [PMC free article] [PubMed] [Google Scholar]
- 45.Bredella MA, Torriani M, Ghomi RH, et al. Determinants of bone mineral density in obese premenopausal women. Bone. 2011;48(4):748–54. doi: 10.1016/j.bone.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J Clin Endocrinol Metab. 2008;93(6):2281–6. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ecklund K, Vajapeyam S, Feldman HA, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25(2):298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bredella MA, Fazeli PK, Miller KK, et al. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94(6):2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollock NK, Bernard PJ, Wenger K, et al. Lower bone mass in prepubertal overweight children with prediabetes. J Bone Miner Res. 2010;25(12):2760–9. doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 2008;32(12):1854–60. doi: 10.1038/ijo.2008.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura-Ishizu A, Okuno Y, Omatsu Y, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119(23):5429–37. doi: 10.1182/blood-2011-11-393645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hui SK, Sharkey L, Kidder LS, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 2012;7(8):e42668. doi: 10.1371/journal.pone.0042668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mussano F, Lee KJ, Zuk P, et al. Differential effect of ionizing radiation exposure on multipotent and differentiation-restricted bone marrow mesenchymal stem cells. J Cell Biochem. 2010;111(2):322–32. doi: 10.1002/jcb.22699. [DOI] [PubMed] [Google Scholar]
- 54.Su W, Chen Y, Zeng W, Liu W, Sun H. Involvement of Wnt signaling in the injury of murine mesenchymal stem cells exposed to X-radiation. Int J Radiat Biol. 2012;88(9):635–41. doi: 10.3109/09553002.2012.703362. [DOI] [PubMed] [Google Scholar]
- 55.Oeffinger KC, Adams-Huet B, Victor RG, et al. Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;27(22):3698–704. doi: 10.1200/JCO.2008.19.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91(11):4401–7. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 57.Shalet SM, Brennan BM. Growth and growth hormone status after a bone marrow transplant. Horm Res. 2002;58(Suppl 1):86–90. doi: 10.1159/000064768. [DOI] [PubMed] [Google Scholar]
- 58.Taskinen M, Lipsanen-Nyman M, Tiitinen A, Hovi L, Saarinen-Pihkala UM. Insufficient growth hormone secretion is associated with metabolic syndrome after allogeneic stem cell transplantation in childhood. J Pediatr Hematol Oncol. 2007;29(8):529–34. doi: 10.1097/MPH.0b013e3180f61b67. [DOI] [PubMed] [Google Scholar]
- 59.Liel Y, Ulmer E, Shary J, Hollis BW, Bell NH. Low circulating vitamin D in obesity. Calcif Tissue Int. 1988;43(4):199–201. doi: 10.1007/BF02555135. [DOI] [PubMed] [Google Scholar]
- 60.McLean RR, Kiel DP. Developing Consensus Criteria for Sarcopenia: An Update. J Bone Miner Res. 2015 doi: 10.1002/jbmr.2492. [DOI] [PubMed] [Google Scholar]
- 61.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–47. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 62.Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. Journal of the cardiometabolic syndrome. 2007;2(3):183–9. doi: 10.1111/j.1559-4564.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- 63.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55(3):M168–73. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 64.Bredella MA, Gill CM, Keating LK, et al. Assessment of abdominal fat compartments using DXA in premenopausal women from anorexia nervosa to morbid obesity. Obesity (Silver Spring) 2013;21(12):2458–64. doi: 10.1002/oby.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farr JN, Funk JL, Chen Z, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26(9):2217–25. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miljkovic I, Kuipers AL, Kammerer CM, et al. Markers of inflammation are heritable and associated with subcutaneous and ectopic skeletal muscle adiposity in African ancestry families. Metabolic syndrome and related disorders. 2011;9(4):319–26. doi: 10.1089/met.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]