Abstract
Chronic pain is a major complaint for up to 85% of Parkinson Disease patients, however, often not identified as a symptom of Parkinson’s disease. Adequate treatment of motor symptoms often provides analgesic effects in Parkinson’s patients, how this occurs remains unclear. Studies have shown both Parkinson’s patients and 6-hydroxydopamine lesioned rats exhibit decreased sensory thresholds. In humans, some show improvements in these deficits after subthalamic deep brain stimulation, while others report no change. Differing methods of testing and response criteria may explain these varying results. We examined this effect in 6-hydroxydopamine lesioned rats. Sprague dawley rats were unilaterally implanted with subthalamic stimulating electrodes in the lesioned right hemisphere and sensory thresholds were tested using vonFrey, tail flick and hot plate tests. Tests were done during and off subthalamic stimulation at 50 and 150Hz to assess its effects on sensory thresholds. 6-hydroxydopamine lesioned animals exhibited lower mechanical (left paw, p<0.01) and thermal thresholds than shams (hot plate, p<0.05). Both 50 and 150Hz increased mechanical (left paw; p<0.01) and thermal thresholds in 6-hydroxydopamine lesioned rats (HPT; 150Hz, p<0.05, 50Hz, p<0.01). Interestingly, the magnitude of improvement in mechanical thresholds during LFS was similar between animals, whereas during HFS was much more diverse. This study shows that subthalamic deep brain stimulation improves mechanical allodynia and thermal hyperalgesia in 6-hydroxydopamine lesioned animals at both high and low frequencies. Furthermore, we suggest considering using low frequency stimulation when treating Parkinson’s patients where pain remains the predominant complaint.
Keywords: Parkinson’s disease, mechanical allodynia, thermal hyperalgesia, hot plate test, vonFrey test
Graphical abstract
Introduction
Parkinson’s Disease (PD) affects approximately 1 million Americans and is characterized by progressive degeneration of the nigrostriatal pathway (McNamara et al., 2010). Disruption of this pathway leads to both cardinal motor symptoms and non-motor symptoms related to PD. Chronic pain is the most common non-motor symptom occurring in 60–85% of patients, significantly hampering their quality of life (Beiske et al., 2009). Patients present with advanced age, making it difficult to distinguish PD-related pain from aging pain, which affects approximately 49–83% of elderly populations (Brochet, 1998; Fox et al., 1999). Physicians must take careful histories to diagnose PD pain (i.e. stiffness responsive to dopaminergic medication) or other pains (i.e. stiffness from osteoarthritis) (Lee et al., 2006). Chronic pain remains a significant problem in PD patients, and can result in patients undergoing unnecessary surgical procedures and extensive medical treatments without benefit.
PD patients experience a variety of nociceptive and neuropathic pain types including musculoskeletal, dystonic, akathitic, radicular, exacerbated somatic pain and central pain (Drake et al., 2005; Kim et al., 2008; Borsook, 2012; Oshima et al., 2012; Hess et al., 2013). They experience pain fluctuations during “ON” and “OFF” states, and can present with more than one type of pain, which increases the complexity of diagnosis and treatment ((Drake et al., 2005; Kim et al., 2008). Pain can present before or after the diagnosis of motor symptoms and similarly, alteration in sensory threshold testing is noted in both early and late stage rodent models of PD (Lin et al., 1981; Saade et al., 1997; Chudler & Lu, 2008; Marques et al., 2013; Zengin-Toktas et al., 2013). Optimal treatment of motor symptoms with dopaminergic medication or subthalamic (STN) deep brain stimulation (DBS) can provide some analgesic effects earlier in the disease process (Ciampi de Andrade et al., 2012; Fil et al., 2013; Marques et al., 2013), but many become refractory to pain treatment as PD progresses (Benabid et al., 2009; Surucu et al., 2013). The mechanism by which STN DBS improves pain in PD patients remains unclear and represents a significant barrier to optimization of stimulation parameters for pain symptoms in these patients.
As pain is a subjective phenomenon, thermal and mechanical threshold assessments are often used as surrogate markers of how pain is perceived. In both patients and pre-clinical animal models, mechanical and thermal thresholds are lower in PD than healthy controls (Carey, 1986; Saade et al., 1997; Djaldetti et al., 2004; Takeda et al., 2005; Chudler & Lu, 2008; Ciampi de Andrade et al., 2012; Zengin-Toktas et al., 2013). In patients, STN DBS increases mechanical and thermal thresholds in some studies, but not in others (Gierthmuhlen et al., 2010; Maruo et al., 2011; Spielberger et al., 2011), which may be due to differences between patients or testing methodology (Marques et al., 2013). In this study, our goal was to examine the effects of STN DBS on sensory thresholds in 6-hydroxydopamine (6OHDA) lesioned rats. We hypothesized that STN DBS could increase sensory thresholds in our lesioned animal model.
Methods
Animals and surgery
All animal use was conducted after approval from the Institutional Animal Care and Use Committee at Albany Medical College. Sixty two male Sprague dawley rats, 7 weeks old weighing 225–270g (TACONIC), were used in these experiments. 31 rats were made parkinsonian, 27 were made sham and four remained naive. Twenty two parkinsonian and 6 sham animals were treated with stimulation. Rats were anesthetized using 2% isoflurane using an anesthesia inhalant system (Harvard Apparatus, Holliston, MA, USA) attached to a stereotactic frame (David Kopf Instruments, Tajunga, CA, USA). Rats were injected intraperitoneally (IP) with desipramine HCl (25mg/kg) and pargyline (50mg/kg) 20 minutes prior to craniotomy and body temperature was maintained at 37°C during the surgery with a warming blanket with feedback control (Homeothermic monitor, Harvard Apparatus, MA, USA). Animals were shaved and placed into the stereotactic frame and 2% xylocaine (APP Pharmaceuticals, Schaumburg, IL, USA) was injected subcutaneously below the incision site. Lidocaine jelly (Akorn Pharmaceutics, Lake Forest, IL, USA) was applied to ear bars before the animals were placed into the stereotactic frame, and Lubifresh P.M. (Major Pharmaceuticals, Livonia, MI, USA) was applied to the eyes to prevent dryness. A small incision was made down the midline and bregma was visualized. A burr hole was drilled in the skull over the right medial forebrain bundle (MFB) at the following coordinates with respect to bregma: −4.4mm anterior/posterior, 1.5mm medial/lateral. After, an injection needle (10 μl Hamilton syringe, Hamilton Company, Reno, NV, USA) was positioned into the hole and lowered into the brain 7.8mm ventral from the dura to inject 4.5μL 6OHDA (3 μg/μL, made up with 0.1% ascorbic acid, Sigma-Aldrich, MO) at a rate of 0.5μL/min. The needle was left in place for 8 minutes after injection. Sham animals were injected with 4.5μL saline (0.9% NaCl) into the right MFB.
STN stimulation electrode implantation surgery
Following 6OHDA injection, rats were implanted with stainless steel twisted wire electrodes (125 μm diameter for each of two wires, bundled together for a total diameter approximately 350 μm, 12mm long, Plastics One Inc., Roanoke, VA, USA) to deliver bipolar monophasic stimulation to the right STN (Coordinates with respect to bregma: −4.1mm posterior, 2.5mm lateral, 7.6mm ventral from dura). Electrodes were placed in a capped pedestal, secured with anchor screws (0–80 × 1/8, Plastics one Inc., Roanoke, VA, USA) and dental cemented to the skull (Duralay; Reliance Dental, Worth, IL, USA). Sham animals underwent identical electrode implantation. Antibiotic cream was applied to the wound and animals were given penicillin (80μg/kg) subcutaneously to prevent infection. Post-operative analgesics were not administered to ensure that alteration in pain circuitry did not occur from this treatment, as others have shown (Bjorkman, 1995; Loguinov et al., 2001; Batista et al., 2009). Animals were given 2 weeks to fully recover from surgery and for the manifestation of motor symptoms to occur (Ungerstedt, 1968).
Assessment of 6OHDA phenotype
Two weeks after 6OHDA or saline injection, the limb-use asymmetry test (LAT) was performed as done previously (Sutton et al., 2013), to assess behavioral forelimb impairment in 6OHDA lesioned rats (Schallert et al., 2000). Animals were placed in a transparent cylinder, videotaped for 5 minutes and behavior was analyzed during rearing and landing. The number of exploratory forepaw touches was quantified for each forepaw ((number of right contacts/number of total contacts) x100). Marked degeneration of dopaminergic neurons in the right striatum and substantia nigra pars compacta (SNc) coincides with a touch bias of 80% from the unimpaired right paw (Schallert et al., 2000).
Behavioral tests of mechanical and thermal thresholds
Mechanical thresholds were tested using vonFrey filaments (VF). In this study, fibers with the following weights were used: 1.0g, 1.4g, 2.0g, 4.0g, 6.0g, 8.0g, 10.0g, 15.0g, and 26.0g. Rats were placed in an elevated cylinder with a mesh floor and allowed to acclimatize for 5 min before testing. Filaments of ascending strength (1.0g through 26.0g) were applied to the left impaired hind paws according to Chaplan et al 1994 (Chaplan et al., 1994) and Dixon’s up-down method (Dixon, 1965) to determine the 50% threshold. In brief, ascending fibers were applied to the hind paw until fiber bending is noted and then held for 5 seconds. If the animal did not elicit a response, the experimenter moved on to the next strength fiber and continued this process until a response was observed. When the animal responded, this fiber is denoted was the starting point of the “up-down” method. Six trials were included in this method beginning one fiber strength less than the initial response. For each trial, the fiber strength was marked with an “x” if the animal responded and the experimenter moved down one fiber. If the animal did not respond, the fiber was denoted as an “o” and the experimenter moved up a fiber strength. At the end of the 6 trials, an “xo” pattern was generated (i.e. xooxxo) and used to calculate the 50% threshold. Chaplan’s formula: 50% threshold = 10Xf+kd/10,000), where Xf was the final VF used, and kd was determined by their appendix value for each paw (Dixon, 1965; Chaplan et al., 1994). Animals with no response to any of the VF fiber strengths above, were assigned to a cut off value of 28.84, which was determined using “o” for no response and Xf = 26.0g (the last tested fiber). This cut-off was established as the point where the fiber would lift the paw due to its tensile strength.
Thermal threshold assessments were performed using two different tests; the hot plate test (HPT) and the tail flick test (TFT) (Sandkuhler, 2009). The HPT consists of a metal pan, placed in the water bath and warmed to 52.5±0.5°C. The latency of hind-paw licking, vocalization or foot stomping was measured. The TFT was done at room temperature using radiant heat (300W, 82V EXR Apollo) on the ventral surface of the distal inch of the tail. Animals were manually restrained, and the intensity of the light box was set to elicit a tail withdrawal 2–3s after heat in naïve and sham lesioned animals. The intensity of the box was not changed between animals, and maximum exposure time allowed was 15s to prevent tissue damage (Hoerbelt et al., 2013). Latency of flick response was measured in seconds and each animal was tested three times with 60 seconds between each trial. These scores were averaged over the 3 trials for each animal’s final score.
DBS settings
6OHDA and sham animals were tested during and off stimulation. Animals were stimulated using a Grass 44 Stimulator (Grass Products, Natus Neurology, Warwick, RI, USA), coupled to a PSIU-6 current isolation unit (Grass Products, Natus Neurology, Warwick, RI, USA) under three conditions: OFF stimulation, ON high frequency stimulation (HFS at 150 Hz) and ON low frequency stimulation (LFS at 50 Hz). Once stimulation was turned on at 150 or 50Hz, animals were stimulated for 30 minutes before mechanical or thermal testing began, and remained on stimulation for the duration of the behavioral testing (1–5 minutes). Animals were allowed to rest off stimulation for 30 minutes prior to behavioral testing for OFF stimulation data. The order of testing was generated using an online randomizer to avoid bias (www.researchrandomizer.com). To find the optimal settings for STN stimulation for analgesic efficacy, we applied STN DBS to the lesioned hemisphere at various settings similar to those used before (Aleksandrova et al., 2013; Dorval, 2014; Sutton et al., 2014). In selected animals (n=4), we assessed mechanical thresholds at constant current amplitudes set to 200–400μA on the lesioned side and cathodic pulse widths from 30–300μs. Our final stimulation settings were a 90μs pulse width and 300–400μA. Pulse widths above 90μs were not well tolerated, and some animals displayed left forelimb dyskinetic behavior above 400μA (Dorval, 2014). These settings were then used for both mechanical and thermal threshold assessments.
Immunohistochemistry for confirmation of electrode placement and assessment of dopaminergic cell loss
To assess dopaminergic axonal and cell body degeneration in the right striatum and SNc, respectively, 6OHDA injected rats underwent transcardiac perfusion with 4% paraformaldehyde and heparin and 24-hour postfixed with 4% paraformaldehyde. Brains were cryoprotected with 30% sucrose and free-floating 60μm sections containing the striatum and SNc were collected and placed in phosphate-buffer solution for tyrosine hydroxylase (TH) immuno-reactivity. In brief, sections were processed in primary antiTH antibodies (Santa Cruz Biotechnology, rabbit IgG CAT# sc-14007), treated with peroxidase conjugated goat anti-rabbit secondary antibodies (Jackson Immunoresearch, West Grove, PA) visualized using a 3,3′diaminobenzidine (DAB) kit (Vector labs, Burlingame, CA) and mounted onto slides (Superfrost Gold Plus, Fisher Scientific) for image quantification. TH density was calculated for both hemispheres in the striatum and SNc using ImageJ (ver1.47, NIH, MD, USA). After, values from regions of interest in the lesioned right side were divided by coinciding regions of interest in the contralateral intact left side to quantify percent TH depletion (Rasband, 2014). Brain sections containing the STN were cut at 60 μm and collected on slides for cresyl violet staining to confirm correct electrode placement in this area (Figure 2C) using a rat atlas (Paxinos, 1998).
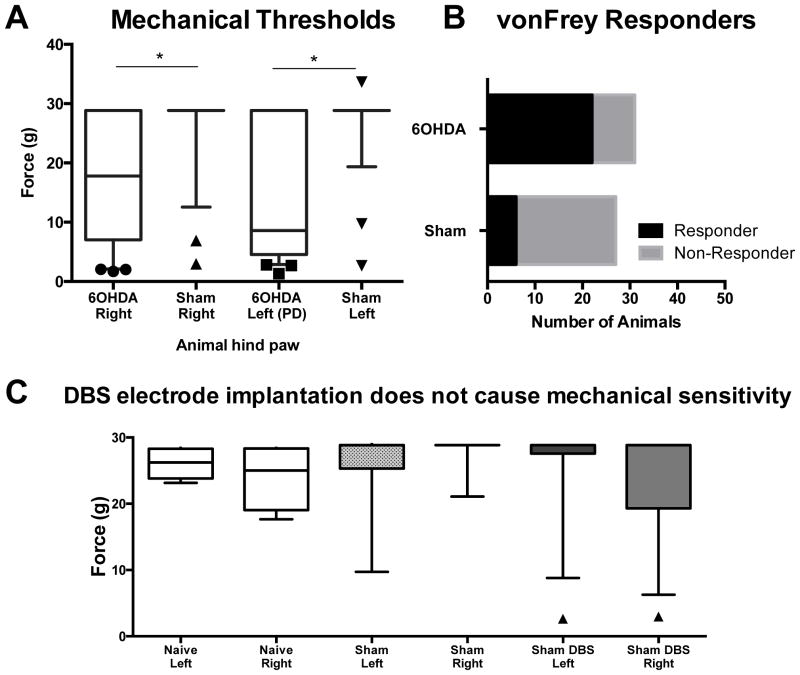
Figure 2. Mechanical thresholds in 6OHDA lesioned and sham rats.
(A) 6OHDA animals (n=31) display reduced mechanical thresholds in their left and right paws as compared with sham controls (n=27) (p<0.05). (B) The proportion of 6OHDA lesioned rats that responded to vonFrey filaments was 0.72 (22 responders, 9 non responders) whereas the proportion of the sham control group was 0.22 (6 responders, 21 non responders). The difference of the proportions was significantly different, χ2= 13.73, p=0.0002, C) STN DBS electrode implantation had no effect on mechanical thresholds in sham (n=9) or naïve (n=4) animals (p>0.05). Sham DBS left and right paw groups denote animals with STN DBS was implanted but without stimulation (n=12). Significance as noted in (A) and (C), Kruskal-Wallis One way ANOVA with Dunns multiple comparisons. Boxes represent median with IQR, whiskers represent 10–90th percentile. *represents significance of p<0.05.
Statistics
Statistical analyses were performed in SigmaPlot (ver11 Systat Software, San Jose, CA, USA) using both parametric and non-parametric tests. Non-parametric statics were employed for data with non-normal distribution. Non-parametric statistics included Kruskal-Wallis one-way ANOVAs with Dunn’s method multiple comparisons procedure. Box plots display the median with upper and lower edges representing 25th and 75th percentile. Whiskers denote the 10th percentiles. In text, data are represented by mean and interquartile range (IQR) from 25th to 75th percentile; median (IQR 25th–75th). Parametric analyses were done for datasets with normal distribution and were one-way ANOVAs (repeated where appropriate) followed by SNK post hoc comparisons, with data represented as mean ± SEM. Correlation analyses were also performed and reported with r2 and p values. Graphs were compiled using GraphPad prism (ver6, CA, USA).
Results
Confirmation of 6OHDA lesioned phenotype and electrode placement
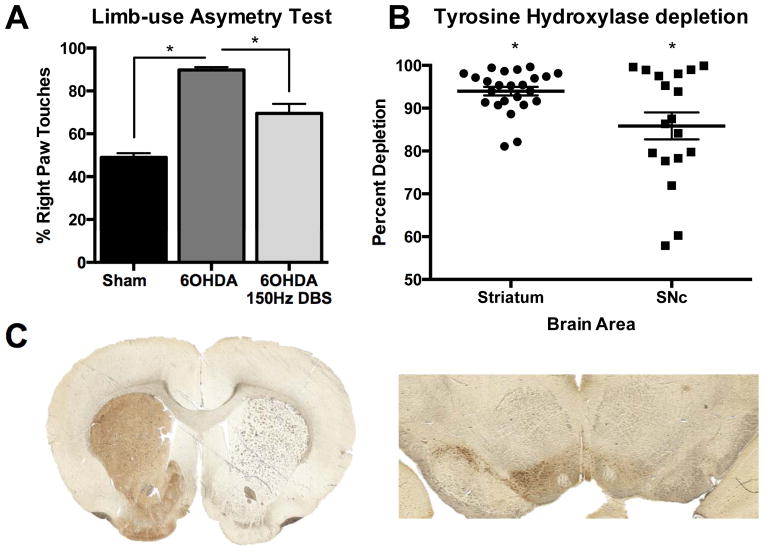
Parkinsonian phenotype was confirmed in 6OHDA lesioned rats using behavioral testing and TH quantification. These rats displayed significantly greater right paw touches than sham animals (90.21% ± 1.34 and 50.02% ± 1.94, respectively, n=31), indicating left forelimb akinesia (p<0.001, Figure 1A). Notably, STN DBS at 150 Hz, 90 μs pulse, and 400 μA improved motor function in 6OHDA lesioned rats since they touched 63.10% ± 4.13 of the time with their right forepaw during stimulation (p<0.001) (One way ANOVA, F2,62 = 65.78, p<0.001, Figure 1A). Pixel quantification in ImageJ revealed 6OHDA lesioned hemispheres displayed a >80% reduction in TH immuno-reactivity in the striatum (n=24, t23=93.70, p<0.001) and >50% in SNc on the lesioned side (n=18, t17=27.40, p<0.001, Figure 1B). A representative image of 6OHDA lesioned and intact striatum and SNc is displayed in Figure 1C.
Figure 1. Hemiparkinsonian confirmation.
(A) 6OHDA lesioned animals (n=31) exhibit greater than 80% right paw touches on the forelimb use asymmetry test, which is significantly higher than sham animals (n=16, p<0.001). Furthermore, HFS used to treat motor symptoms of PD significantly reduces behavioral asymmetry in 6OHDA animals (p<0.001, n=18). One way ANOVA, SNK post hoc, mean ± SEM, * represents significance level p<0.05. (B,C) Tyrosine hydroxylase quantification in the striatum (left) and SNc (right) reveals dopaminergic cell loss in 6OHDA lesioned hemisphere, as compared to the contralateral hemisphere. Dots represent values from individual animals. Two tailed one sample t-test (p<0.001).
Baseline mechanical and thermal threshold assessment
To determine whether placing stimulating electrodes in the STN was sufficient to cause changes in mechanical thresholds, we examined naïve animals, sham animals without an electrode and sham animals with STN implanted stimulating electrodes. For this experiment, sham animals with implanted electrodes did not have stimulation turned on during testing. We did not observe any difference in VF median force sensitivity between any animal groups in either hind paw (Figure 2C, H=6.20, d.f.=5, p=0.288).
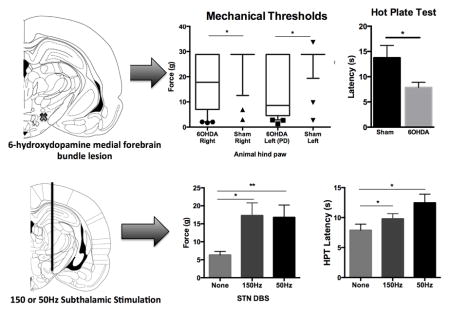
With VF testing, 6OHDA lesioned rats exhibited left impaired hind paw mechanical allodynia since they responded to a median force value of 8.610g (4.54–28.84), which contrasts with sham animals with a force median value of 28.84g (28.84–28.84) (p<0.05). Interestingly, the median score in the right (unimpaired) paw of 6OHDA lesioned rats differed from sham rats with the former having a median force of 17.79g (7.03–28.84) and latter with 28.84g (28.84–28.84) (p<0.05) (Kruskal-Wallis One-way ANOVA (H=31.41, d.f. = 3, p<0.001) with Dunns Method, Figure 2A). Furthermore, the proportion of 6OHDA lesioned rats and sham rats that reached cut off values was also significantly different. 71% of 6OHDA lesioned rats and 22% of sham rats responded to VF filaments lower than 26.0g in their left (impaired) paw (χ2=13.73, p=0.0002, Figure 2B).
Along with changes in mechanical sensitivity, 6OHDA lesioned animals exhibited a significantly reduced latency on the HPT at 7.88 ± 1.01 when compared with sham controls at 13.68 ±1.14 (t22=2.17, p=0.041, Figure 3A). Conversely, there was no difference between 6OHDA lesioned animals response latency of 2.14 ± 0.22 and sham latency 2.51 ± 0.14 on the TFT (t14=−1.42, p=0.178, Figure 3B).
Figure 3. Thermal thresholds in 6OHDA lesioned and sham rats.
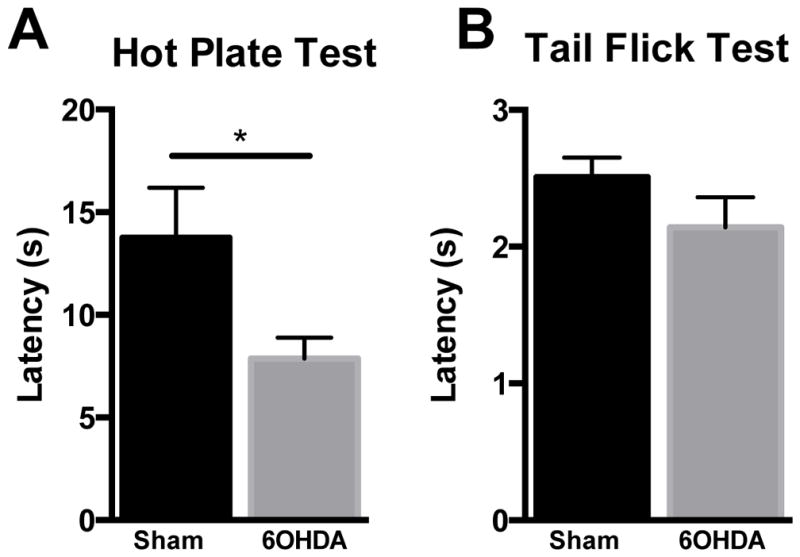
(A) 6OHDA lesioned animals have reduced latency on the HPT (n=11 PD and 13 sham, *p<0.05). B) No difference was found between 6OHDA animals (n=8) and shams (n=8) on the TFT. Two tailed t test, data represented as mean ± SEM.
Effect of STN DBS on mechanical and thermal thresholds
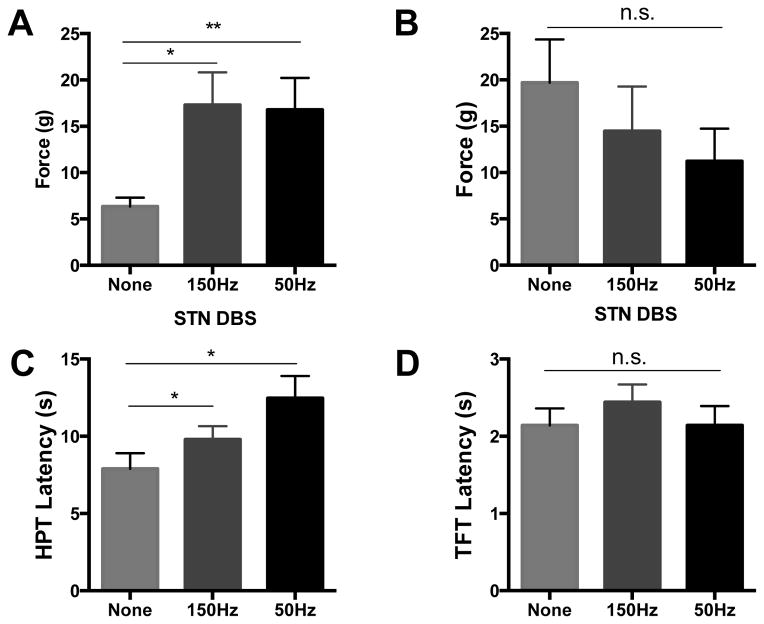
For mechanical threshold testing ON stimulation, sham (n=6) and 6OHDA lesioned animals (n=12) exhibiting a response to VF fibers below the cut off were used as it is not possible to identify improvements in mechanical thresholds already at ceiling level. During HFS, 6OHDA lesioned animals significantly improved mean force threshold from 6.10g ± 0.92g to 16.33g ± 3.38g (p=0.004, Figure 4A). On stimulation at LFS, mechanical thresholds in 6OHDA lesioned rats improved from 6.10g ± 0.92g to 16.26g ± 3.19g (p=0.005) (RM-ANOVA, F2,21= 9.53, p=0.001, Figure 4A). As a control group, we evaluated mechanical thresholds ON HFS and LFS in sham animals and noted that both remained unchanged from OFF stimulation values with mean values of 19.69g ± 4.68 OFF vs. 14.48g ± 4.81 ON HFS and 19.69g ± 4.68 OFF vs. 11.22g ± 3.53g ON LFS (F2,10 = 1.863, p=0.21, Figure 4B, n=6).
Figure 4. STN DBS improves mechanical and thermal thresholds in 6OHDA lesioned rats.
Mechanical thresholds were increased in 6OHDA lesioned animals (n=12) during high frequency (150Hz) (p=0.004) and low frequency (50Hz) (p=0.005) subthalamic deep brain stimulation (A). Similar changes were not seen in sham animals (n=6) on either high or low frequency stimulation (B, p=0.205). (C) 6OHDA lesioned rats on 150Hz stimulation tolerated the hot plate longer than without stimulation (p=0.02, n=11). LFS also improved 6OHDA latency on the HPT (p=0.002, n=5–8). No increase in latency was found on the TFT at either frequency. One way ANOVA, SNK post hoc tests, data represented as mean ± SEM.
In the HPT and TFT, thermal thresholds in 6OHDA lesioned rats improved with STN DBS in the former, but not the latter. More specifically, ON stimulation at HFS improved HPT latency from 7.88 ± 1.01 to 9.79 ± 0.87 (p=0.02, Figure 4C), and ON LFS, latency was improved from 7.88 ± 1.01 to 12.47±1.44 (p=0.002)(RM-ANOVA, F2,17=8.70, p=0.003, Figure 4C). No significant increases in latency were noted on the TFT with HFS or LFS (RM-ANOVA F2,11=1.25, p=0.33, Figure 4D, n=8).
Electrode placement and the effects of high frequency stimulation
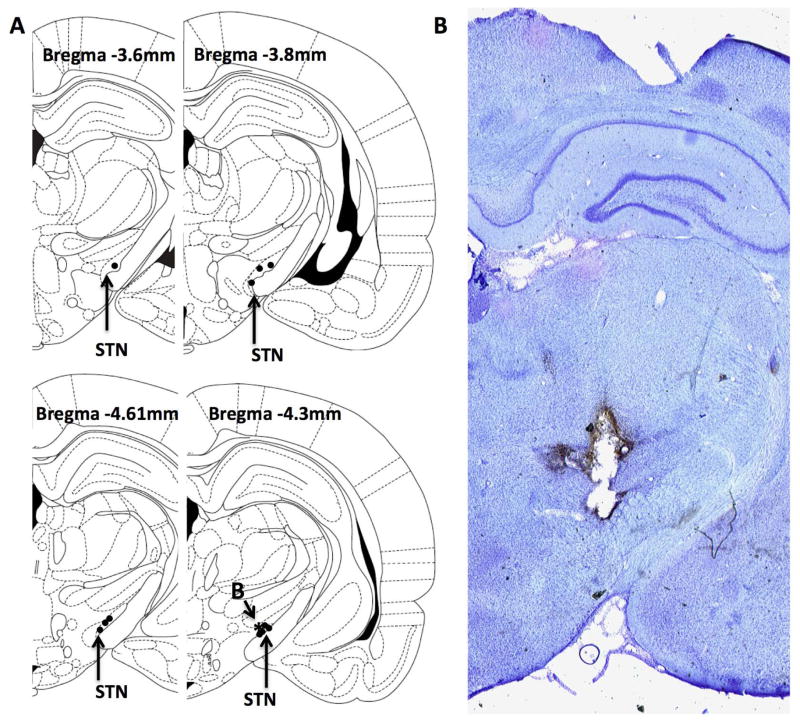
While 6OHDA lesioned animals improved on high frequency stimulation, we noted that some animals had a more robust improvement than others. We then examined whether there was a correlation between the placement of electrodes in the STN (Figure 5), and the amount of improvement a given animal exhibited (n=12). We found no correlation between improvement on HFS and posterior distance from bregma (r2=0.14, p=0.24), medial/lateral distance within the STN (r2=0.02, p=0.70), on stimulation LAT scores (r2=0.07, p=0.46) or dopaminergic depletion in the striatum (r2=3.14, p=0.75) or SNc (r2=10.54, p=0.92).
Figure 5.
Placement of electrodes in the STN. (A) Representative marking of electrode placements for animals used in mechanical stimulation experiments and correlation analysis. Star (*) indicates animal placement for representative section in (B) showing electrode placement in STN.
Discussion
Our study is the first to demonstrate that STN DBS increases mechanical and thermal thresholds in 6OHDA lesioned rats. Specifically, a 6OHDA MFB lesion caused unilateral mechanical allodynia and thermal hyperalgesia when compared to sham rats with saline MFB injection. Furthermore, we show that HFS at 150Hz and LFS at 50 Hz in the STN increased sensory thresholds in 6OHDA animals.
Our results OFF stimulation in 6OHDA lesioned rats are in line with several other studies in the field (Saade et al., 1997; Takeda et al., 2005; Chudler & Lu, 2008; Zengin-Toktas et al., 2013). In 1997, using 6OHDA lesions in the substantia nigra and ventral tegmental area, Saade et al found reduced latency in nociceptive responses (tail flick, hot plate and paw pressure tests) 1 week after surgery (Saade et al., 1997). Furthermore, others studying only mechanical thresholds, have shown reduced thresholds or latency to response using 6OHDA lesions of bilateral and unilateral medial forebrain bundles respectively, when compared to control animals (Takeda et al., 2005; Zengin-Toktas et al., 2013). In our study, we found mechanical thresholds were reduced on both hind paws. Moreover, bilaterally reduced mechanical thresholds have been reported in one other study, showing a reduction in 6OHDA intrastriatially lesioned animals 3 weeks after surgery (Chudler & Lu, 2008), while others argue that only the contralateral (Saade et al., 1997) or ipsilateral side was affected (Takeda et al., 2005). This discrepancy warrants further investigation, but we speculate that this may be due to compensation in the non-lesioned hemisphere following dopamine depletion (Henning et al., 2008). We did not find a significant difference between sham rats and 6OHDA lesioned rats on the TFT as Saade did, and we hypothesize this may be due to slight differences in methodology. Originally, the TFT was thought to be a response organized at the spinal level (King et al., 1997), which may not be affected by central 6OHDA lesions. Recent work however has shown that under certain circumstances (for example being manually held or in a cool heat environment) supraspinal circuits are activated during this response (King et al., 1997). Although we did use manual restraint, our experiments were not done in a cool heat environment, which potentially activated only spinal reflexes in our rats. Further studies must be done to tease apart the relationship between different thermal tests in 6OHDA lesioned rats.
PD patients demonstrate both increased thresholds (Shin et al., 2005; Conte et al., 2010; Lee et al., 2010; Lyoo et al., 2012) and abnormal somatosensory perception, specifically, tactile and proprioceptive deficits (Conte et al., 2013). Various two point discrimination tests are altered in PD, such as grating orientation, object discrimination, and the somatosensory temporal discrimination threshold (STDT) (Weder et al., 1999). Levodopa improves grating orientation (Shin et al., 2005) and the STDT (Conte et al., 2010). DBS has similar effects on STDT (Conte et al., 2010). Additionally, PD patients have decreased conscious awareness of limb position as measured by accuracy in reaching movements (Mongeon et al., 2009) and arm proprioception (O’Suilleabhain et al., 2001), which again improved with levodopa and DBS (O’Suilleabhain et al., 2001; Mongeon et al., 2009) It is likely that the reduction in sensory thresholds impairs more complex sensory pathways as described above.
With the mounting evidence that STN DBS improves pain and reduces elevated sensory thresholds in PD patients (Drake et al., 2005; Kim et al., 2008; Gierthmuhlen et al., 2010; Maruo et al., 2011; Ciampi de Andrade et al., 2012; Oshima et al., 2012; Pellaprat et al., 2014), we expected that HFS would increase thresholds in 6OHDA lesioned rats. We found a significant improvement, in mechanical and thermal thresholds using VF and HPT. Interestingly, looking at each animal individually, we noted that some animals improved mechanical thresholds to the cut-off point, whereas others only improved slightly. We further studied the location of the electrode in the STN and found no correlation with either medial(limbic/associative)/lateral placement or anterior/posterior placement within this nucleus. Similarly, in our animal cohort we found no significant correlation between the degree of dopaminergic denervation and the percent change on HFS. The cause of this separation is of great interest, as it may also explain the different reported effects of STN DBS on thresholds in PD patients. Specifically, one study revealed no changes in sensitivity to innocuous and painful mechanical stimuli while ON stimulation (Gierthmuhlen et al., 2010), while another showed no change in innocuous mechanical sensitivity with a decreased sensitivity to painful mechanical stimuli while ON stimulation (Ciampi de Andrade et al., 2012). Thermal thresholds have also shown variable improvement in patient populations. For example, in one of these studies STN DBS resulted in increased heat pain thresholds in PD patients (Ciampi de Andrade et al., 2012), while in the other, patients remained unchanged (Gierthmuhlen et al., 2010).
Interestingly, the dichotomy of response was not seen in 6OHDA lesioned rats ON stimulation at LFS. Instead, these animals all responded to a similar degree. This finding is clinically relevant because LFS may be a plausible option for PD patients with pain as a predominant complaint. Recent studies demonstrate successful treatment of motor symptoms following low frequency STN DBS. Specifically, a randomized, double blind, crossover study showed significant reduction in UPDRS total motor score and axial motor signs in patients treated with bilateral LFS at 60Hz (Khoo et al., 2014). These results were equally as effective as HFS and provided better control of axial symptoms and akinesia in PD patients (Khoo et al., 2014). Ventral contacts were used more frequently for LFS in these patients (Moreau et al., 2008; Khoo et al., 2014). Unfortunately, none of these studies address sensory threshold changes in PD patients during LFS.
Current thinking posits the difference between HFS and LFS lies in inhibition and excitation of STN neurons respectively (Drake et al., 2005; Bourne et al., 2012; Lavian et al., 2013). However, Lavian et al 2013 demonstrated that both HFS and LFS of the STN in brain slices produced reductions in firing frequency in STN neurons. They also reported that globus pallidus can undergo either long lasting depression or excitation with STN HFS and LFS (Lavian et al., 2013). Yamawaki et al emphasized the importance of cortico-subthalamic connections, and believe that the weight of this pathway determines stimulation effects. Their studies show that HFS causes synaptic depression of STN neurons in dopamine-depleted tissue, but does not show significant effects in intact tissue. Conversely, 40Hz LFS potentiated cortico-subthalamic inputs from STN neurons in dopamine intact tissue, but did not do so in dopamine deplete tissue (Yamawaki et al., 2012). Thus, LFS may have a greater effect on cortical pathways and nuclei, such as the anterior cingulate cortex and prefrontal cortex, which are known to be involved in pain processing (Uylings et al., 2003; Fil et al., 2013).
Other brain areas have been targeted for DBS specifically to treat chronic pain syndromes in non-PD individuals. These include the periaqueductal grey, ventroposterior lateral thalamus and anterior cingulate cortex. Reported efficacy is 36–71% depending on the etiology and type of pain syndrome (Boccard et al., 2013; Parmar et al., 2014). The most successful analgesic responses were with LFS in the periaqueductal grey (5–50Hz) and LFS or HFS in the sensory thalamus (20–100Hz) (Bittar et al., 2005). These settings were chosen by the physician during trial stimulation phases where the optimal reduction in pain was noted (Levy et al., 1987; Kumar et al., 1997; Bittar et al., 2005). Other studies found significant improvements in pain reduction after LFS of the periaqueductal grey between 5–35Hz, whereas higher frequencies (50–100Hz) exacerbated the pain (Nandi et al., 2003). Pulse widths were similar in these studies and voltages were between 1–8V (Hosobuchi, 1986; Levy et al., 1987; Kumar et al., 1997; Bittar et al., 2005). In the STN, our results suggest that 150 and 50Hz stimulation may provide some symptom relief. However, it still remains unclear why some rodents exhibited stronger responsiveness to either frequency. Variability in analgesic efficacy could be caused by the central location of DBS targets, within or interacting with a potentially damaged nucleus. Supporting this, studies have shown centrally located DBS targets are more successful at treating peripheral pain etiologies than central pain etiologies (Papuc & Rejdak, 2013; Parmar et al., 2014). In STN DBS, as with chronic pain DBS, variability in sensory testing may be caused by the central location of the target, and the significant changes occurring in the basal ganglia during dopaminergic neurodegeneration. Taken together, these results suggest that stimulation delivery, target location and pain syndrome play a significant role in efficacy of DBS. Moreover, investigating how STN DBS alters pain circuits in the 6OHDA lesioned rat may reveal other ways to increase management of this non-motor symptom in PD.
We acknowledge several limitations in our study. First, while vonFrey tests accurately assess innocuous stimuli (Chaplan et al., 1994), we were unable to specifically test painful mechanical stimuli in our animal model. Second, LFS STN DBS in PD patients significantly improved motor function at 60Hz, not 50 Hz (Moreau et al., 2008; Khoo et al., 2014). Therefore, future experiments can investigate efficacy of this stimulation frequency on sensory testing in 6OHDA lesioned rats. Lastly, current spread from STN DBS to surrounding sensory fiber systems is a consideration when interpreting our findings. Of note, the Zona Incerta (ZI) is situated dorsal to the STN and has been shown to regulate posterior thalamic activity. Removing tonic inhibitory input from the ZI to the thalamus is hypothesized to cause central pain syndrome (Masri et al., 2009), and it’s plausible that altering this input via STN DBS could contribute to analgesic effects.
In conclusion, although HFS STN DBS is a well validated approach to manage motor symptoms in patients with PD (Benabid et al., 2009), these individuals often present with debilitating chronic pain (Toda & Harada, 2010). Our data suggests that both HFS and LFS improves mechanical and thermal thresholds in 6OHDA lesion rats, and it may be pragmatic to consider using LFS when treating PD patients where pain remains a predominant complaint and motor symptoms can still be controlled. Individualizing waveforms and DBS settings to address specific complaints is critical to the advancement of DBS.
Acknowledgments
Many thanks to Dr. Paul Feustel for his expertise in statistical analysis. Dr. Pilitsis is a consultant for Medtronic, Boston Scientific and St. Jude and receives grant support from Medtronic, Boston Scientific, St. Jude and NIH 1R01CA166379. Dr. Adolfo Ramirez-Zamora is a consultant for TEVA neuroscience.
Abbreviations
- DBS
deep brain stimulation
- HFS
high frequency stimulation
- HPT
hot plate test
- IQR
interquartile range
- LAT
limb-use asymmetry test
- LFS
low frequency stimulation
- MFB
medial forebrain bundle
- PD
Parkinson’s disease
- SNc
substantia nigra pars compacta
- STN
subthalamic nucleus
- TFT
Tail flick test
- TH
tyrosine hydroxylase
- VF
vonFrey filaments
- 6OHDA
6-hydroxydopamine
Footnotes
All other authors have no conflict of interest or financial disclosures related directly to this manuscript.
References
- Aleksandrova LR, Creed MC, Fletcher PJ, Lobo DS, Hamani C, Nobrega JN. Deep brain stimulation of the subthalamic nucleus increases premature responding in a rat gambling task. Behavioural brain research. 2013;245:76–82. doi: 10.1016/j.bbr.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Batista LM, Batista IM, Almeida JP, Carvalho CH, Castro-Costa SB, Castro-Costa CM. Preemptive analgesic effect of lidocaine in a chronic neuropathic pain model. Arquivos de neuro-psiquiatria. 2009;67:1088–1092. doi: 10.1590/s0004-282x2009000600024. [DOI] [PubMed] [Google Scholar]
- Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson’s disease: Prevalence and characteristics. Pain. 2009;141:173–177. doi: 10.1016/j.pain.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Chabardes S, Mitrofanis J, Pollak P. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. The Lancet. Neurology. 2009;8:67–81. doi: 10.1016/S1474-4422(08)70291-6. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, Bear RE, Green A, Wang S, Aziz TZ. Deep brain stimulation for pain relief: a meta-analysis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bjorkman R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta anaesthesiologica Scandinavica Supplementum. 1995;103:1–44. [PubMed] [Google Scholar]
- Boccard SG, Pereira EA, Moir L, Aziz TZ, Green AL. Long-term outcomes of deep brain stimulation for neuropathic pain. Neurosurgery. 2013;72:221–230. doi: 10.1227/NEU.0b013e31827b97d6. discussion 231. [DOI] [PubMed] [Google Scholar]
- Borsook D. Neurological diseases and pain. Brain : a journal of neurology. 2012;135:320–344. doi: 10.1093/brain/awr271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne SK, Eckhardt CA, Sheth SA, Eskandar EN. Mechanisms of deep brain stimulation for obsessive compulsive disorder: effects upon cells and circuits. Frontiers in integrative neuroscience. 2012;6:29. doi: 10.3389/fnint.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet BMP, Barberger-Gateau P, Dartigues J. Population-based study of pain in elderly people: a descriptive survey. Age Ageing. 1998;27:279–284. [Google Scholar]
- Carey RJ. Acute ipsilateral hyperalgesia and chronic contralateral hypoalgesia after unilateral 6-hydroxydopamine lesions of the substantia nigra. Experimental neurology. 1986;91:277–284. doi: 10.1016/0014-4886(86)90068-3. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chudler EH, Lu Y. Nociceptive behavioral responses to chemical, thermal and mechanical stimulation after unilateral, intrastriatal administration of 6-hydroxydopamine. Brain research. 2008;1213:41–47. doi: 10.1016/j.brainres.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampi de Andrade D, Lefaucheur JP, Galhardoni R, Ferreira KS, Brandao Paiva AR, Bor-Seng-Shu E, Alvarenga L, Myczkowski ML, Marcolin MA, de Siqueira SR, Fonoff E, Barbosa ER, Teixeira MJ. Subthalamic deep brain stimulation modulates small fiber-dependent sensory thresholds in Parkinson’s disease. Pain. 2012;153:1107–1113. doi: 10.1016/j.pain.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Conte A, Khan N, Defazio G, Rothwell JC, Berardelli A. Pathophysiology of somatosensory abnormalities in Parkinson disease. Nature reviews. Neurology. 2013;9:687–697. doi: 10.1038/nrneurol.2013.224. [DOI] [PubMed] [Google Scholar]
- Conte A, Modugno N, Lena F, Dispenza S, Gandolfi B, Iezzi E, Fabbrini G, Berardelli A. Subthalamic nucleus stimulation and somatosensory temporal discrimination in Parkinson’s disease. Brain : a journal of neurology. 2010;133:2656–2663. doi: 10.1093/brain/awq191. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. The up-and-down method for small samples. Journal of the American Statistical Association. 1965;60:967–978. [Google Scholar]
- Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology. 2004;62:2171–2175. doi: 10.1212/01.wnl.0000130455.38550.9d. [DOI] [PubMed] [Google Scholar]
- Dorval ADG, WM Deep brain stimulation of the subthalamic nucleus reestablishes neuronal information transmission in the 6-OHDA rat model of parkinsonism. Journal of neurophysiology. 2014;111:1949–1959. doi: 10.1152/jn.00713.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake DF, Harkins S, Qutubuddin A. Pain in Parkinson’s disease: pathology to treatment, medication to deep brain stimulation. NeuroRehabilitation. 2005;20:335–341. [PubMed] [Google Scholar]
- Fil A, Cano-de-la-Cuerda R, Munoz-Hellin E, Vela L, Ramiro-Gonzalez M, Fernandez-de-Las-Penas C. Pain in Parkinson disease: a review of the literature. Parkinsonism & related disorders. 2013;19:285–294. doi: 10.1016/j.parkreldis.2012.11.009. discussion 285. [DOI] [PubMed] [Google Scholar]
- Fox PL, Raina P, Jadad AR. Prevalence and treatment of pain in older adults in nursing homes and other long-term care institutions: a systematic review. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 1999;160:329–333. [PMC free article] [PubMed] [Google Scholar]
- Gierthmuhlen J, Arning P, Binder A, Herzog J, Deuschl G, Wasner G, Baron R. Influence of deep brain stimulation and levodopa on sensory signs in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25:1195–1202. doi: 10.1002/mds.23128. [DOI] [PubMed] [Google Scholar]
- Henning J, Strauss U, Wree A, Gimsa J, Rolfs A, Benecke R, Gimsa U. Differential astroglial activation in 6-hydroxydopamine models of Parkinson’s disease. Neuroscience research. 2008;62:246–253. doi: 10.1016/j.neures.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Hess CW, Vaillancourt DE, Okun MS. The temporal pattern of stimulation may be important to the mechanism of deep brain stimulation. Experimental neurology. 2013;247:296–302. doi: 10.1016/j.expneurol.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerbelt P, Nalwalk JW, Phillips JG, Wentland MP, Shan Z, Hough LB. Antinociceptive activity of CC44, a biotinylated improgan congener. Eur J Pharmacol. 2013;714:464–471. doi: 10.1016/j.ejphar.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi Y. Subcortical electrical stimulation for control of intractable pain in humans. Report of 122 cases (1970–1984) Journal of neurosurgery. 1986;64:543–553. doi: 10.3171/jns.1986.64.4.0543. [DOI] [PubMed] [Google Scholar]
- Khoo HM, Kishima H, Hosomi K, Maruo T, Tani N, Oshino S, Shimokawa T, Yokoe M, Mochizuki H, Saitoh Y, Yoshimine T. Low-frequency subthalamic nucleus stimulation in Parkinson’s disease: a randomized clinical trial. Movement disorders : official journal of the Movement Disorder Society. 2014;29:270–274. doi: 10.1002/mds.25810. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Paek SH, Kim JY, Lee JY, Lim YH, Kim MR, Kim DG, Jeon BS. Chronic subthalamic deep brain stimulation improves pain in Parkinson disease. Journal of neurology. 2008;255:1889–1894. doi: 10.1007/s00415-009-0908-0. [DOI] [PubMed] [Google Scholar]
- King TE, Joynes RL, Grau JW. Tail-flick test: II. The role of supraspinal systems and avoidance learning. Behavioral neuroscience. 1997;111:754–767. doi: 10.1037//0735-7044.111.4.754. [DOI] [PubMed] [Google Scholar]
- Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736–746. doi: 10.1097/00006123-199704000-00015. discussion 746-737. [DOI] [PubMed] [Google Scholar]
- Lavian H, Ben-Porat H, Korngreen A. High and low frequency stimulation of the subthalamic nucleus induce prolonged changes in subthalamic and globus pallidus neurons. Frontiers in systems neuroscience. 2013;7:73. doi: 10.3389/fnsys.2013.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MA, Walker RW, Hildreth TJ, Prentice WM. A survey of pain in idiopathic Parkinson’s disease. Journal of pain and symptom management. 2006;32:462–469. doi: 10.1016/j.jpainsymman.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Lee MS, Lyoo CH, Lee MJ, Sim J, Cho H, Choi YH. Impaired finger dexterity in patients with parkinson’s disease correlates with discriminative cutaneous sensory dysfunction. Movement disorders : official journal of the Movement Disorder Society. 2010;25:2531–2535. doi: 10.1002/mds.23304. [DOI] [PubMed] [Google Scholar]
- Levy RM, Lamb S, Adams JE. Treatment of chronic pain by deep brain stimulation: long term follow-up and review of the literature. Neurosurgery. 1987;21:885–893. doi: 10.1227/00006123-198712000-00017. [DOI] [PubMed] [Google Scholar]
- Lin MT, Wu JJ, Chandra A, Tsay BL. Activation of striatal dopamine receptors induces pain inhibition in rats. Journal of neural transmission. 1981;51:213–222. doi: 10.1007/BF01248953. [DOI] [PubMed] [Google Scholar]
- Loguinov AV, Anderson LM, Crosby GJ, Yukhananov RY. Gene expression following acute morphine administration. Physiological genomics. 2001;6:169–181. doi: 10.1152/physiolgenomics.2001.6.3.169. [DOI] [PubMed] [Google Scholar]
- Lyoo CH, Ryu YH, Lee MJ, Lee MS. Striatal dopamine loss and discriminative sensory dysfunction in Parkinson’s disease. Acta neurologica Scandinavica. 2012;126:344–349. doi: 10.1111/j.1600-0404.2012.01657.x. [DOI] [PubMed] [Google Scholar]
- Marques A, Chassin O, Morand D, Pereira B, Debilly B, Derost P, Ulla M, Lemaire JJ, Durif F. Central pain modulation after subthalamic nucleus stimulation: A crossover randomized trial. Neurology. 2013;81:633–640. doi: 10.1212/WNL.0b013e3182a08d00. [DOI] [PubMed] [Google Scholar]
- Maruo T, Saitoh Y, Hosomi K, Kishima H, Shimokawa T, Hirata M, Goto T, Morris S, Harada Y, Yanagisawa T, Aly MM, Yoshimine T. Deep brain stimulation of the subthalamic nucleus improves temperature sensation in patients with Parkinson’s disease. Pain. 2011;152:860–865. doi: 10.1016/j.pain.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Masri R, Quiton RL, Lucas JM, Murray PD, Thompson SM, Keller A. Zona incerta: a role in central pain. Journal of neurophysiology. 2009;102:181–191. doi: 10.1152/jn.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara P, Stavitsky K, Harris E, Szent-Imrey O, Durso R. Mood, side of motor symptom onset and pain complaints in Parkinson’s disease. International journal of geriatric psychiatry. 2010;25:519–524. doi: 10.1002/gps.2374. [DOI] [PubMed] [Google Scholar]
- Mongeon D, Blanchet P, Messier J. Impact of Parkinson’s disease and dopaminergic medication on proprioceptive processing. Neuroscience. 2009;158:426–440. doi: 10.1016/j.neuroscience.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Moreau C, Defebvre L, Destee A, Bleuse S, Clement F, Blatt JL, Krystkowiak P, Devos D. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology. 2008;71:80–84. doi: 10.1212/01.wnl.0000303972.16279.46. [DOI] [PubMed] [Google Scholar]
- Nandi D, Aziz T, Carter H, Stein J. Thalamic field potentials in chronic central pain treated by periventricular gray stimulation -- a series of eight cases. Pain. 2003;101:97–107. doi: 10.1016/s0304-3959(02)00277-4. [DOI] [PubMed] [Google Scholar]
- O’Suilleabhain P, Bullard J, Dewey RB. Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. Journal of neurology, neurosurgery, and psychiatry. 2001;71:607–610. doi: 10.1136/jnnp.71.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima H, Katayama Y, Morishita T, Sumi K, Otaka T, Kobayashi K, Suzuki Y, Fukaya C, Yamamoto T. Subthalamic nucleus stimulation for attenuation of pain related to Parkinson disease. Journal of neurosurgery. 2012;116:99–106. doi: 10.3171/2011.7.JNS11158. [DOI] [PubMed] [Google Scholar]
- Papuc E, Rejdak K. The role of neurostimulation in the treatment of neuropathic pain. Annals of agricultural and environmental medicine : AAEM. 2013;(Spec no. 1):14–17. [PubMed] [Google Scholar]
- Parmar VK, Gee L, Smith H, Pilitsis JG. Supraspinal stimulation for treatment of refractory pain. Clinical neurology and neurosurgery. 2014;123:155–163. doi: 10.1016/j.clineuro.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Paxinos GWC. The Rat Brain in stereotaxic coordinates. 1998. [Google Scholar]
- Pellaprat J, Ory-Magne F, Canivet C, Simonetta-Moreau M, Lotterie JA, Radji F, Arbus C, Gerdelat A, Chaynes P, Brefel-Courbon C. Deep brain stimulation of the subthalamic nucleus improves pain in Parkinson’s disease. Parkinsonism & related disorders. 2014;20:662–664. doi: 10.1016/j.parkreldis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. US National Institutes of Health; Bethesda, Maryland, USA: 2014. [Google Scholar]
- Saade NE, Atweh SF, Bahuth NB, Jabbur SJ. Augmentation of nociceptive reflexes and chronic deafferentation pain by chemical lesions of either dopaminergic terminals or midbrain dopaminergic neurons. Brain research. 1997;751:1–12. doi: 10.1016/s0006-8993(96)01164-x. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological reviews. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Shin HW, Kang SY, Sohn YH. Dopaminergic influence on disturbed spatial discrimination in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2005;20:1640–1643. doi: 10.1002/mds.20642. [DOI] [PubMed] [Google Scholar]
- Spielberger S, Wolf E, Kress M, Seppi K, Poewe W. The influence of deep brain stimulation on pain perception in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2011;26:1367–1368. doi: 10.1002/mds.23570. author reply 1368–1369. [DOI] [PubMed] [Google Scholar]
- Surucu O, Baumann-Vogel H, Uhl M, Imbach LL, Baumann CR. Subthalamic deep brain stimulation versus best medical therapy for L-dopa responsive pain in Parkinson’s disease. Pain. 2013;154:1477–1479. doi: 10.1016/j.pain.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Sutton AC, O’Connor KA, Pilitsis JG, Shin DS. Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain structure & function. 2014 doi: 10.1007/s00429-014-0876-8. [DOI] [PubMed] [Google Scholar]
- Sutton AC, Yu W, Calos ME, Smith AB, Ramirez-Zamora A, Molho ES, Pilitsis JG, Brotchie JM, Shin DS. Deep brain stimulation of the substantia nigra pars reticulata improves forelimb akinesia in the hemiparkinsonian rat. Journal of neurophysiology. 2013;109:363–374. doi: 10.1152/jn.00311.2012. [DOI] [PubMed] [Google Scholar]
- Takeda R, Ikeda T, Tsuda F, Abe H, Hashiguchi H, Ishida Y, Nishimori T. Unilateral lesions of mesostriatal dopaminergic pathway alters the withdrawal response of the rat hindpaw to mechanical stimulation. Neuroscience research. 2005;52:31–36. doi: 10.1016/j.neures.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Toda K, Harada T. Prevalence, classification, and etiology of pain in Parkinson’s disease: association between Parkinson’s disease and fibromyalgia or chronic widespread pain. The Tohoku journal of experimental medicine. 2010;222:1–5. doi: 10.1620/tjem.222.1. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. European Journal of Pharmacology. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behavioural brain research. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Weder BJ, Leenders KL, Vontobel P, Nienhusmeier M, Keel A, Zaunbauer W, Vonesch T, Ludin HP. Impaired somatosensory discrimination of shape in Parkinson’s disease: association with caudate nucleus dopaminergic function. Human brain mapping. 1999;8:1–12. doi: 10.1002/(SICI)1097-0193(1999)8:1<1::AID-HBM1>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki N, Magill PJ, Woodhall GL, Hall SD, Stanford IM. Frequency selectivity and dopamine-dependence of plasticity at glutamatergic synapses in the subthalamic nucleus. Neuroscience. 2012;203:1–11. doi: 10.1016/j.neuroscience.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zengin-Toktas Y, Ferrier J, Durif F, Llorca PM, Authier N. Bilateral lesions of the nigrostriatal pathways are associated with chronic mechanical pain hypersensitivity in rats. Neuroscience research. 2013;76:261–264. doi: 10.1016/j.neures.2013.05.003. [DOI] [PubMed] [Google Scholar]