Abstract
Background/Aims
As technology continues to advance for our aging population, an increasing number of DBS candidates will have preexisting implanted electrical devices. In this article, we discuss safe and successful DBS in a patient with Parkinson's disease (PD) and bilateral cochlear implants.
Methods
A 70 year-old male with PD and bilateral cochlear implants underwent successful microelectrode-guided DBS implantation into bilateral subthalamic nuclei (STN). The patient's cochlear implant magnets were removed and replaced in outpatient clinic for pre-operative MRI and stereotactic targeting. The cochlear implants were turned off intraoperatively for STN microelectrode recordings.
Results
Precise, MRI-guided stereotactic DBS implantation was possible. Intraoperative high-fidelity microelectrode recordings confirmed STN neurons with the cochlear implants turned off. These recordings were not possible with active cochlear implant devices. Our literature review describes the other approaches/techniques that have been used to manage DBS surgery in the setting of cochlear implants.
Conclusions
Despite the risk of electrical interference between implanted medical devices, DBS and cochlear implants may be safe and compatible in the same patient if necessary precautions are taken.
Keywords: deep brain stimulation, Parkinson's disease, cochlear implant
BACKGROUND & IMPORTANCE
Advancements in technology and an aging population have led to an increasing use of implantable medical devices. An example is the cochlear implant, an electronic device that improves hearing capability in patients with bilateral severe-to-profound sensorineural hearing loss. As of December 2010, approximately 219,000 patients worldwide have cochlear implants. In the United States, roughly 42,600 adults and 28,400 children have received them.1 It is inevitable that clinicians will encounter a PD patient with a cochlear implant. Clinicians must recognize that preexisting cochlear implants may interfere with DBS implantation and performance. In this article, we report the successful microelectrode-guided implantation and performance of bilateral STN DBS in a PD patient with preexisting bilateral cochlear implants. We document electrical interference from the cochlear device on microelectrode recordings during STN DBS implantation and discuss modifications in surgical technique.
CLINCAL PRESENTATION
Patient background
70-year-old male with idiopathic PD for over a decade and bilateral severe-to-profound sensorineural hearing loss presented for DBS surgery. The patient's hearing loss was secondary to viral meningitis in 2006 and managed with bilateral cochlear implants. He reported significant benefit from the cochlear implants and denied side effects such as tinnitus, vertigo, or imbalance.
Despite maximum medical therapy, motor fluctuations with rigidity and gait initiation dysfunction became progressively worse. With demonstrated responsiveness to levodopa (UPDRS III 34 to 11; doubled gait speed) and after neuropsychological evaluation revealed no contraindications, DBS surgery was recommended.
Microelectrode-guided bilateral STN DBS implantation
Two months prior to DBS surgery, the patient's cochlear implant magnets were removed in order to obtain a pre-operative MRI (Siemens Avanto 1.5 Tesla, T2-weighted Turbo Spin-Echo and Fast Gray Matter Acquisition T1 Inversion Recovery sequences) for surgical planning. The magnets were replaced the same day following MRI. In spite of magnet removal, there was significant artifact that made targeting STN more favorable than Globus Pallidus internus (GPi) (Figure 1).
Figure 1. Pre-operative axial MR imaging of deep brain nuclei after cochlear implant magnet removal.
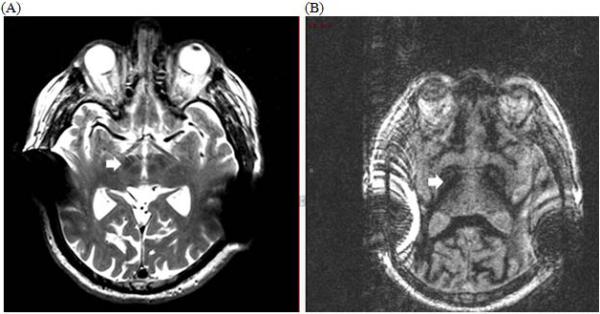
(A) Axial T2-weighted MRI Turbo Spin-Echo sequence depicting subthalamic nuclei (arrow) (B) Axial MRI Fast Gray Matter Acquisition T1 Inversion Recovery sequence depicting globus pallidus internus (arrow), but the image is significantly degraded with artifact from the cochlear implants despite magnet removal.
The patient underwent frame-based, stereotactic microelectrode-guided insertion of DBS leads (Medtronic Inc., 3389S-40) into STN bilaterally. After frame placement with local anesthesia, a volumetric CT was fused to the pre-operative MRI for stereotactic planning using direct targeting of STN from T2-weighted MRI. During the surgery, the patient's right cochlear device was turned off, but the left device remained active in order to communicate intra-operatively. Notably, the active left cochlear implant interfered with microelectrode recordings during STN lead placement. After turning off the left cochlear device, microelectrode-guided technique proceeded (Figure 2), and characteristic subthalamic signals were recorded.
Figure 2. Microelectrode recording.
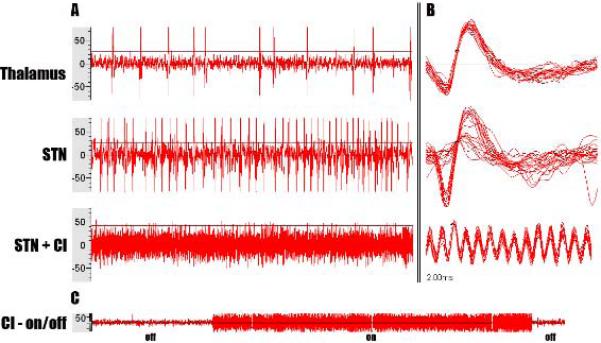
This image demonstrates microelectrode recordings along the right DBS trajectory. (A) Microelectrode recordings from thalamus, STN, and STN + active cochlear implant (CI). (B) Microelectrode recordings with time up-scaled (2 ms) showing an action potential from individual neurons in thalamus, STN, and STN + active CI. Activation of the cochlear implant distorts STN microelectrode recordings at a frequency equal to the cochlear implant's total stimulation rate (7.2 KHz). The total stimulation rate is the frequency of the biphasic current pulse that the cochlear implant delivers. It is calculated by multiplying the per channel rate (900 Hz) by the number of maxima (8).11 (C) Microelectrode recording from deep nuclei showing a transition between true nuclei recording (off) and the distorted cochlear implant recording (on).
While undergoing intra-operative test macrostimulation, the patient's left cochlear implant was reactivated so the patient could hear and interact with the surgical team. Right STN intraoperative test stimulation yielded transient paresthesias in the left hand and forearm without corticospinal activation. Left STN intraoperative test stimulation suppressed the patient's right leg tremor. After satisfactory macrostimulation testing, the electrodes were anchored and their position was confirmed with fluoroscopy. On post-operative day 1, the patient was discharged home after CT imaging. DBS electrodes positions were verified by fusing the post-operative CT images with the pre-operative MRI (Figure 3). The implanted pulse generator was placed during a second stage operation three weeks later. Care was taken to tunnel the lead extensions posterior to the cochlear implant internal receiver.
Figure 3. Post-operative images.
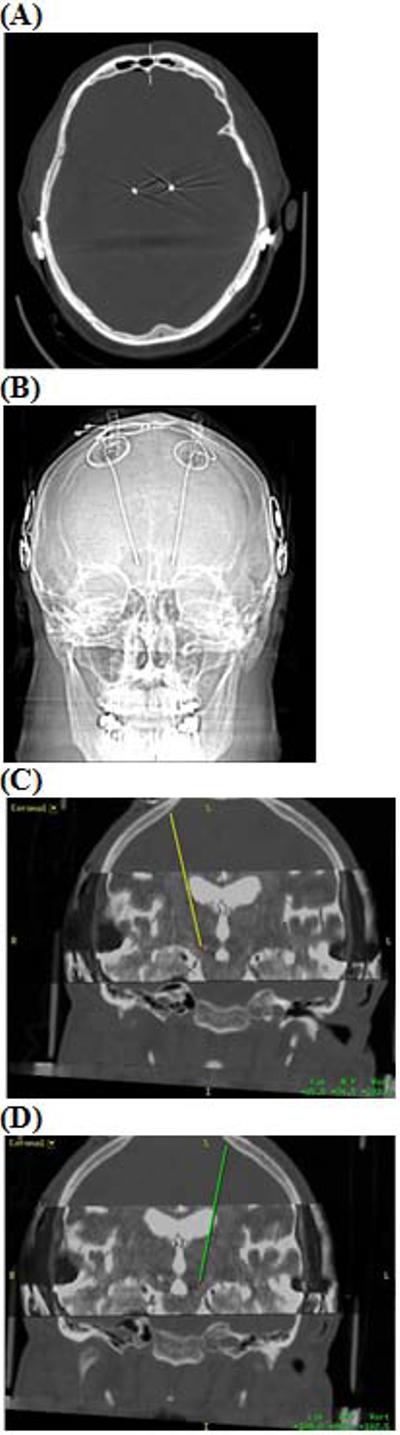
Post-operative CT Head (A) bone window and (B) scout view depict preexisting bilateral cochlear implants after successful microelectrode-guided STN DBS implantation. Using Medtronic StealthStation, we fused the post-operative CT Head to the pre-operative MRI Brain. Reconstructed coronal images reveal satisfactory placement of DBS electrodes into the (C) right STN and (D) left STN.
Post-operative outcome
At 4 months, the patient reported improvement in PD symptoms with the following DBS settings: both left and right DBS were set to unipolar configuration with amplitude 1.2 V, pulse width 60 microseconds, and frequency 140 Hz. The patientdecreased his levodopa requirement by almost 50%. .
Since the DBS surgery, the patient continued to report benefit from his Nucleus Freedom cochlear implants (Cochlear Corp., Sydney, Australia). He denied change in his hearing. No distorted sounds were heard in either ear. His device settings have remained unchanged. Even though the manufacturer (Cochlear Corp.) warns of neurostimulation in the Physician Packet Insert “Do not use neurostimulation directly over the cochlear implant. High currents induced into the electrode lead can cause tissue damage to the cochlea or permanent damage to the implant”2, the patient did not report adverse effects from the device.
DISCUSSION AND LITERATURE REVIEW
As technology advances, an increasing number of PD patients considering DBS will also have preexisting implanted electrical devices. Before implanting DBS electrodes, clinicians should be aware of the risk for electromagnetic interference between implanted electrical devices. Medtronic Inc. (Minneapolis, Minnesota) reports possible device interactions between DBS systems and cardiac pacemakers, implantable defibrillators, cochlear implants, and other active implanted devices in their prescriber manual.3
Serious complications have occurred after exposure of DBS systems to high-energy electromagnetic fields. Nutt et al report the case of a PD patient who suffered permanent diencephalic and brainstem lesions after receiving microwave diathermy near his STN DBS lead during a dental procedure. The authors concluded that the diathermy induced a radiofrequency current, which heated the electrodes and destroyed nearby CNS tissue. The patient was left in a vegetative state.4 In another case report, Yamamoto et al describe a patient who suffered an unexpected thalamotomy near his implanted DBS after cardioversion. The patient had a radiofrequency-coupled DBS system. During cardioversion, the patient's radiofrequency receiver transmitted the external cardioversion current, causing the thalamotomy.5
In spite of these risks, DBS and other electromagnetic devices have been successfully implanted into the same patient.6-7 In our patient, there were no adverse effects that would suggest electrical interference between his cochlear implants and STN DBS electrodes. This may be because of the distance between the devices and their relatively low, localized amount of electrical stimulation. Other cases of patients having successful implantation of both DBS and cochlear implants have been reported using different techniques (Table 1).
Table 1.
Previous case reports of patients with both DBS and a cochlear implant
| Case Report | Patient Background | DBS | Cochlear Implant | Surgical Modification | Outcome |
|---|---|---|---|---|---|
| Martin et al [8] | 57 y/o male with Parkinson's disease status post DBS after unsuccessful gamma knife pallidotomy. Developed severe sensorineural hearing loss. | Medtronic Activa, bilateral thalamus | Nucleus Contour, unilateral (left ear) | Placement of superior pinna incision and internal receiver had to avoid the DBS extension wires | Successful cochlear device implantation. No device interference. |
| De Los Reyes et al* [9] | 69 y/o male with sensorineural hearing loss status post cochlear implant. Essential tremor with difficulty writing, brushing teeth, and feeding. | Medtronic Lead Model 3387, left ventrolateral thalamic nucleus | Unilateral (right ear) | Removal of cochlear magnet for pre-op imaging & surgery. MRI with significant artifact. CT required for DBS targeting. Magnet replaced after surgery. | Post-op CT Head well-positioned DBS lead. No device interference. |
| Cif et al [10] | 8 y/o boy with Mohr-Tranebjaerg Syndrome (deafness-dystonia-optic neuropathy) status post cochlear implant now with worsening dystonia. | Medtronic Model 3389, bilateral Globus Pallidus internus | Nucleus 24 ST, unilateral | Cochlear implant removed pre-operatively and re-implanted 8 months after DBS surgery. | Good relief of dystonia. No device interference. |
Microelectode-guided technique without documented interference in recordings from the cochlear implant because the device was turned off and the magnet replaced after DBS surgery.
Martin et al reported a PD patient with DBS who later underwent cochlear device implantation for severe sensorineural hearing loss.8
De Los Reyes et al reported a patient with a unilateral cochlear implant and essential tremor that required contralateral thalamic DBS. Even though the cochlear implant magnet was removed to obtain pre-operative MRI, the study was contaminated with artifact and required CT targeting. Details of microelectrode recording were not provided.9
Cif et al reported an 8 year-old boy with Mohr-Tranebjaerg Syndrome (deafness-dystonia-optic neuropathy) and a unilateral cochlear implant. The cochlear device was removed pre-operatively and re-implanted 8 months after DBS surgery. There was no device interference and the patient's dystonia dramatically improved with DBS.10
Based on our experience and after literature review, we found that there are several necessary technique modifications in order to perform successful DBS surgery in patients with preexisting cochlear implants. First, it is imperative that the DBS clinicians work together with the patient's otologist in order to coordinate magnet removal/replacement for a pre-operative MRI. Despite magnet removal, there still may be significant artifact from the cochlear device and thus CT may be used for targeting purposes. Postoperatively, MRI may not be possible for lead localization if the magnets have been replaced, and so instead, CT must suffice.
Second, avoid damaging the preexisting cochlear implants when tunneling DBS lead extensions past the cochlear device's internal receiver. We used fluoroscopy to safely identify and avoid the internal receiver.
Finally, intra-operative patient communication may require a functional cochlear implant, so we recommend replacing the magnets prior to DBS surgery. Even thoughan activated cochlear implant will interfere with microelectrode recordings, it can be temporarily deactivated during surgery. After satisfactory DBS lead placement, the cochlear implant can be reactivated to facilitate patient-to-surgeon communication for intra-operative stimulation testing.
CONCLUSIONS
Subthalamic DBS can be successfully performed in PD patients with preexisting cochlear implants. Preexisting cochlear implants should not be regarded as a contraindication to DBS as the magnet can be easily removed and replaced in the outpatient setting for MR imaging, and electrical interference during microelectrode recordings can be mitigated by turning off the implant.
REFERENCES
- 1.National Institute on Deafness and Other Communication Disorders . Cochlear Implants. [November 3, 2013]. Available at: http://www.nidcd.nih.gov/health/hearing/pages/coch.aspx. [Google Scholar]
- 2.Cochlear [November 4, 2013];Nucleus cochlear Implants: Physician’s Packet Insert. http://products.cochlearamericas.com/sites/default/files/Nucleus_Insert_web.pdf.
- 3.Medtronic Corporation [November 2, 2013];Medtronic DBS Therapy Implanted neurostimulators – Information for prescribers. Available at: http://professional.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@neuro/documents/documents/dbsifp.pdf.
- 4.Nutt JG, Anderson VC, Peacock JH, et al. DBS and diathermy interaction induces severe CNS damage. Neurology. 2001;56:1384–1386. doi: 10.1212/wnl.56.10.1384. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto T, Katayama Y, Fukaya C, et al. Thalamotomy caused by cardioversion in a patient treated with deep brain stimulation. Stereotact Funct Neurosurg. 2000;74:73–82. doi: 10.1159/000056466. [DOI] [PubMed] [Google Scholar]
- 6.Senatus PB, McClelland S, 3rd, Ferris AD, et al. Implantation of bilateral deep brain stimulators in patients with Parkinson disease and preexisting cardiac pacemakers. Report of two cases. J Neurosurg. 2004;101(6):1073–1077. doi: 10.3171/jns.2004.101.6.1073. [DOI] [PubMed] [Google Scholar]
- 7.Capelle HH, Simpson RK, Jr, Kronenbuerger M, Michaelsen J, Tronnier V, Krauss JK. Long-term deep brain stimulation in elderly patients with cardiac pacemakers. J Neurosurg. 2005 Jan;102(1):53–9. doi: 10.3171/jns.2005.102.1.0053. [DOI] [PubMed] [Google Scholar]
- 8.St Martin MB, Hirsch BE. Cochlear implantation in a patient with bilateral deep brain stimulators. Laryngoscope. 2007 Jan;117(1):183–5. doi: 10.1097/01.mlg.0000245943.95695.65. [DOI] [PubMed] [Google Scholar]
- 9.De Los Reyes K, Chandrasekhar SS, Tagliati M, Alterman R. Successful implantation of a deep brain stimulator for essential tremor in a patient with a preexisting cochlear implant: surgical technique: technical case report. Neurosurgery. 2010 Jun;66372(6 Suppl Operative) doi: 10.1227/01.NEU.0000369646.01287.42. discussion 372. [DOI] [PubMed] [Google Scholar]
- 10.Cif L, Gonzalez V, Garcia-Ptacek S, James S, Boetto J, Seychelles A, Roujeau T, Moura De Ribeiro AM, Sillon M, Mondain M, Coubes P. Progressive dystonia in Mohr-Tranebjaerg syndrome with cochlear implant and deep brain stimulation. Mov Disord. 2013 Jun;28(6):737–8. doi: 10.1002/mds.25519. [DOI] [PubMed] [Google Scholar]
- 11.Cochlear [December 24, 2013];Clinical Guidance Document. :12–13. [Google Scholar]


