Abstract
Breast milk is the most effective strategy to protect infants against necrotizing enterocolitis (NEC), a devastating disease which is characterized by severe intestinal necrosis. Previous studies have demonstrated that the lipopolysaccharide receptor toll-like receptor 4 (TLR4) plays a critical role in NEC development via deleterious effects on mucosal injury and repair. We now hypothesize that breast milk protects against NEC by inhibiting TLR4 within the intestinal epithelium, and sought to determine the mechanisms involved. Breast milk protected against NEC and reduced TLR4 signaling in wild-type neonatal mice, but not in mice lacking the epidermal growth factor receptor (EGFR), while selective removal of EGF from breast milk reduced its protective properties, indicating that breast milk inhibits NEC and attenuates TLR4 signaling via EGF/EGFR activation. Over-expression of TLR4 in the intestinal epithelium reversed the protective effects of breast milk. The protective effects of breast milk occurred via inhibition of enterocyte apoptosis and restoration of enterocyte proliferation. Importantly, in IEC-6 enterocytes, breast milk inhibited TLR4 signaling via inhibition of GSK3β. Taken together, these findings offer mechanistic insights into the protective role for breast milk in NEC, and support a link between growth factor and innate immune receptors in NEC pathogenesis.
Keywords: necrotizing enterocolitis, innate immunity, epidermal growth factor receptor, breast milk, GSK3β
Introduction
Necrotizing enterocolitis (NEC) is the most common and lethal gastrointestinal emergency in preterm infants1, and despite extensive research investigating the underpinnings of this disease, the molecular mechanisms involved remain incompletely understood. NEC is characterized by the acute onset of patchy intestinal necrosis and systemic sepsis, and is seen most commonly in premature infants, of whom approximately 12% will develop this devastating disease1, 2. Studies from several labs including our own, have shown that the development of NEC reflects the interaction between the bacteria that inhabit the intestinal tract of the premature infant and the underlying intestinal mucosa, leading to a marked pro-inflammatory response that requires activation of the lipopolysaccharide receptor toll-like receptor 4 (TLR4) on the intestinal epithelium3–5. Activation of TLR4 leads to increased enterocyte apoptosis and reduced mucosal healing6, 7, and also contributes to the impaired intestinal perfusion that leads to the intestinal necrosis that is a cardinal feature of NEC8. The importance of intestinal TLR4 in the pathogenesis of NEC is revealed by studies in which mice deficient in TLR4 in the intestinal epithelium are protected from NEC development4, as are wild-type mice that have received a novel TLR4 inhibitor9. It has been long recognized that breast milk is an effective protective agent against NEC10–13, although the components in breast milk that mediate this protection and the signaling pathways involved remain incompletely understood. Epidermal growth factor (EGF) is abundant in breast milk and amniotic fluid and is critical for intestinal development14–18. Our laboratory has shown that amniotic fluid inhibits TLR4 signaling in the neonatal intestinal epithelium via the epidermal growth factor receptor19. In view of these findings and the growing literature on the causative role for intestinal TLR4 in the pathogenesis of NEC from our lab3, 4, 20–22 and others5, 23, 24, we now hypothesize that breast milk prevents NEC via inhibition of TLR4 signaling, and sought to define the mechanisms involved. In support of this hypothesis, we now show that breast milk prevents the exaggerated TLR4 signaling that is required for NEC, via effects on the epidermal growth factor receptor.
Results
Breast milk inhibits TLR4 signaling in enterocytes via activation of the epidermal growth factor receptor (EGFR)
We first sought to determine whether breast milk could inhibit TLR4 signaling in enterocytes in vitro. To do so, we treated IEC-6 enterocytes, which we and others have previously shown to express both TLR43, 21, 25–27 and EGFR21, with LPS, and the degree of TLR4 signaling was measured by assessing the extent of translocation of the p65 subunit of NF-κB from the cytoplasm to the nucleus and the expression of the pro-inflammatory cytokine IL-6. Consistent with our previous observations21, LPS induced TLR4 signaling in IEC-6 cells as shown in Figure 1. Pre-treatment of cells with breast milk significantly reduced the extent of LPS-induced TLR4-mediated nuclear translocation of NF-κB (Fig. 1Aii–iii, Ei), IL-6 induction (Fig. 1Eii), and TLR4 expression (Fig. 1Eiii). The protective effects of breast milk on TLR4 signaling could be reversed after pre-heating the milk to 95 degrees Celsius, suggesting that a heat sensitive factor, likely a protein, was involved in the down-regulatory effect of breast milk on TLR4 (Fig. 1Avi, Ei). Given our previous studies which indicated that EGFR activation by components of amniotic fluid could inhibit TLR4 signaling21, we explored whether EGFR was required for the breast milk mediated inhibition of TLR4. In support of this possibility, breast milk did not prevent LPS-mediated TLR4 signaling in IEC-6 cells that had undergone lentiviral-mediated knock down of EGFR (Fig. 1Bii–iii, D, Fi–ii). Further, immunodepletion of EGF from breast milk reversed the inhibitory effects of breast milk on TLR4 signaling (Fig. 1Aiv vs. iii, Ei), while replenishment of EGF-depleted breast milk with EGF restored its ability to inhibit TLR4 signaling in wild-type enterocytes (Fig. 1Av vs. iv, Ei), but not in EGFR-deficient enterocytes (Fig. 1Biii–v, Fi). Taken together, these findings indicate that breast milk inhibits TLR4 signaling via activation of the EGF receptor by EGF.
Figure 1. Breast milk inhibits TLR4 signaling in enterocytes via activation of the epidermal growth factor receptor (EGFR) by EGF.
A–C: Representative confocal micrographs of either wild-type IEC-6 cells (Ai–vi) or IEC-6 cells that were deficient in EGFR (Bi–v) or control scrambled shRNA (Ci–iii) which were treated with either LPS (50μg/ml, 1 h, Aii, Bii, Cii) and after 1 hour pretreatment with breast milk (25μl/ml media, Aiii, Biii, Ciii), or EGF-immunodepleted breast milk (IBM, Aiv, Biv) or IBM with exogenous EGF (400ng/mL media, Av, Bv). Size bar = 10μm. p65 subunit of NF-κB (green). D: RT-PCR showing EGFR (upper) and loading control GAPDH (lower) in wild-type (left lanes), EGFR knock-down (EGFR-k/d) IEC-6 cells (right lanes). E: Quantification of the extent of NF-κB translocation (Ei). IL-6 mRNA expression (LPS 50μg/ml, 6 h, Eii) or TLR4 mRNA expression (LPS, 50μg/ml, 24 h Eiii) and the above pretreatments with breast milk in wild-type IEC-6 cells. F: NF-κB translocation under the above conditions in EGFR-kd IEC-6 cells (Fi) and IL-6 mRNA expression (Fii). *p<0.05 versus control (white bars), **p<0.05 versus LPS, ***p<0.05 versus LPS+BM by ANOVA. These data are mean ±SD. Results representative of at least three separate experiments with greater than 50 fields each.
Breast milk attenuates TLR4 signaling in vivo via EGFR
We next sought to determine whether breast milk could also inhibit TLR4 signaling in vivo, and thus potentially explain its protective effects in NEC. To determine the degree that breast milk inhibits LPS-mediated TLR4 signaling, we administered breast milk by oral gavage to transgenic neonatal mice that express NF-κB on the luciferase promoter28. One hour after oral gavage with either saline (Figure 2Ai, v) or breast milk (Figure 2Aiii, v), the NF-κB-luciferase expressing mice were injected with LPS in order to induce TLR4 activation. After luciferin injection, whole animal imaging was performed to assess NF-κB activity as determined by the extent of luciferase activity9. As shown in Figure 2, injection of mice with LPS caused a significant increase in whole animal luciferase activity, while animals that were orally administered breast milk 1 hour prior to LPS injection demonstrated significantly decreased luciferase (i.e. NF-κB) activity along with decreased expression of the pro-inflammatory cytokine IL-6 and TLR4 within the intestine (Fig. 2Aii–iii, v, Bi–ii). In order to evaluate whether EGFR activation was required for the inhibition of TLR4 signaling in the intestine in vivo, we performed experiments in the presence of the selective EGFR inhibitor cetuximab. As shown in Figure 2Aiv, pretreatment with cetuximab prevented the protection of breast milk on TLR4 signaling, as shown by a lack of inhibitory effect of breast milk on NF-κB luciferase activity (Fig. 2Aiv vs. iii, v, Bi–ii). Taken together, these findings support the conclusion that breast milk inhibits TLR4 in vivo via EGFR activation.
Figure 2. Breast milk attenuates TLR4 signaling in vivo via EGFR.
A: Pseudocolor images demonstrating whole animal NF-κB luciferase activity treated with either saline (i), LPS (5 mg/kg, 6 hr, ii), gavage pretreatment with breast milk (50 μL/gram body weight, iii) and/or cetuximab (Cetux), an EGFR inhibitor (100 μg/day for 3 days, iv) 1 h prior to LPS administration, quantification of images in Total Flux (photons/sec x 104, v). B: IL-6 (i) and TLR4 mRNA expression (ii) for above groups. *p<0.05 versus saline (white bar), **p<0.05 versus LPS, ***p<0.05 versus LPS + breast milk (BM). Data are mean ±SD. Representative of at least three separate experiments with at least three mice per group.
Breast milk reversed TLR4-mediated induction of apoptosis in the intestinal epithelium in an EGFR dependent manner
We and others have shown that TLR4 activation induces intestinal epithelial injury in NEC in part through induction of enterocyte apoptosis6, 7, 20–22, 29, 30. Having shown that breast milk inhibits TLR4 signaling in vivo, we next sought to evaluate the effects of breast milk on TLR4-induced inhibition of proliferation and induction of apoptosis, and whether EGFR signaling could be involved. As shown in Figure 3, the injection of LPS into newborn pups caused a marked increase in apoptosis of enterocytes as revealed by increased TUNEL positive cells in the intestinal epithelium (Fig. 3Ai–ii, arrows, Di), as well as increased 3-nitrotyrosine (3-NT) staining, which is a marker of gut inflammation seen in NEC31, 32, known to be TLR4 dependent33, and is a byproduct of nitric oxide signaling derived from peroxynitrite31, which were reversed by gavage pretreatment with breast milk (Fig. 3Aii–iii, Di). Three lines of evidence suggest that EGFR activation by EGF is responsible for the protective effects of breast milk on enterocyte apoptosis and proliferation. First, pre-treatment of mice with the EGFR inhibitor cetuximab reversed the protective effects of breast milk on TLR4-mediated inhibition of proliferation and induction of apoptosis (Fig. 3Aiv vs. iii, Di). Second, immuno-depletion of EGF from breast milk reversed the protective effects of breast milk on apoptosis (Fig. 3Av vs. iii, Di), while reconstitution of breast milk with EGF restored the protective properties of breast milk (Fig. 3Avi vs. v, Di). Third, mice lacking EGFR in the intestinal epithelium (EGFRΔIEC mice) were found to demonstrate susceptibility to LPS-induced apoptosis which was not protected by the presence of breast milk (Fig. 3Bi–iv, Dii). It is also noteworthy that TLR4ΔIEC-OVER mice, in which TLR4 was overexpressed in the intestinal epithelium on a global TLR4 KO background, demonstrated increased enterocyte apoptosis that was not protected by breast milk (Fig. 3Ci–iv, Diii), indicating that TLR4 signaling when maintained at high levels overcomes the protective effects of breast milk. Taken together, these findings illustrate that LPS-mediated TLR4 signaling in the intestinal mucosa is attenuated by breast milk through activation of EGFR. We next sought to determine the mechanisms involved.
Figure 3. Breast milk reversed TLR4-mediated induction of apoptosis in the intestinal epithelium in an EGFR dependent manner.
A–C: Representative confocal micrographs of the terminal ileum of either wild-type mice (Ai–vi), EGFRΔIEC mice (Bi–iv), or TLR4ΔIEC-OVER mice (Ci–iv) stained for TUNEL (green, arrows), 3-nitrotyrosine (3NT, red), and DAPI (blue) as indicated after treatments with saline (Control, Ai, Bi, Ci), LPS (5 mg/kg, 16 h, Aii, Bii; 2.5mg/kg, 6 h, Cii), LPS + breast milk (LPS+BM, Aiii, Biii, Ciii), LPS + breast milk + cetuximab (LPS+BM+Cetux, Aiv), LPS + EGF-depleted breast milk (LPS+IBM, Av), LPS + IBM + EGF (Avi). Size bars = 100μm. RT-PCR showing expression of EGFR (Biv, upper) or TLR4 (Civ, upper) within the small intestine of the indicated strains, along with the housekeeping gene RPLO (Biv, Civ, lower). D: Quantification of the TUNEL positive cells per villus in wild-type mice (i), EGFRΔIEC mice (ii) or TLR4ΔIEC-OVER mice (iii). *p<0.05 versus saline (Ctrl, white bar), **p<0.05 versus LPS, ***p<0.05 versus LPS + breast milk (BM). ****p<0.05 versus LPS + IBM. Data are mean±SEM. Representative of at least three separate experiments with at least three mice per group. Arrows delineate apoptotic cells.
Breast milk restores enterocyte proliferation via EGF and the EGFR
Previously we have shown that TLR4 activation inhibits enterocyte proliferation in experimental models of endotoxemia and NEC6, 7, 20–22, 29, 30. As shown in Figure 4, in wild-type mice LPS inhibited enterocyte proliferation as revealed by a reduction in PCNA staining that was reversed by pre-treatment with breast milk (Fig. 4Ai–iii, Ci). Pre-treatment of mice with the EGFR inhibitor cetuximab (Fig. 4Aiv) or immunodepletion of EGF from breast milk (Fig. 4Av) reversed the protective effects of breast milk on enterocyte proliferation, which was restored in the presence of EGF-supplemented breast milk (Fig. 4Av–vi, Ci). As shown in Figure 4, EGFRΔIEC mice were also found to demonstrate a LPS-mediated inhibition of proliferation, which was not restored in the presence of breast milk treatment (Fig. 4Bi–iii, Cii). These findings provide additional support for the role of EGF and EGFR in the protection against TLR4-mediated signaling by breast milk in the neonatal gut.
Figure 4. Breast milk restores enterocyte proliferation via EGF and the EGFR.
A–B: Representative confocal micrographs of the small intestinal crypts of either wild-type mice (Ai–vi) or EGFRΔIEC mice (Bi–iii) stained for PCNA (green, arrows) and DAPI (blue) as indicated after treatments with saline (Control, Ai, Bi), LPS (5 mg/kg, 6 h, Aii, Bii), LPS + breast milk (LPS+BM, Aiii, Biii), LPS + breast milk + cetuximab (LPS+BM+Cetux, Aiv), LPS + EGF-depleted breast milk (LPS+IBM, Av), LPS + IBM + EGF (Avi). Size bars = 50μm. C: Quantification of the PCNA positive cells per crypt in wild-type mice (i) or EGFRΔIEC mice (ii). *p<0.05 versus saline (Ctrl, white bar), **p<0.05 versus LPS, ***p<0.05 versus LPS + breast milk (BM), ****p<0.05 versus LPS + IBM. Data are mean±SEM. Representative of at least three separate experiments with at least three mice per group. Arrows indicate proliferative cells.
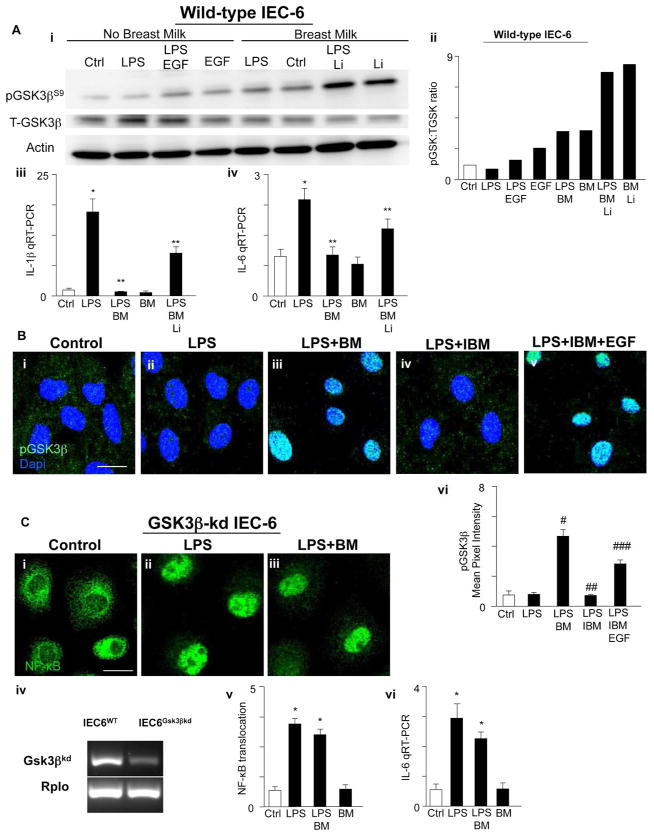
Breast milk attenuates TLR4-mediated NF-κB activation by inhibiting GSK3β in vitro
The GSK3β/β-catenin signaling pathway plays a key role in determining the extent of enterocyte proliferation that occurs in response to EGFR ligands and is situated canonically downstream of both EGFR and TLR434–37. To assess the potential mechanisms by which EGFR activation by breast milk inhibits TLR4 in the intestinal epithelium, we next explored whether impaired GSK3β signaling may be involved. As shown in Figure 5, in wild-type IEC-6 cells, pre-treatment with either EGF or breast milk prior to LPS administration significantly increased the degree of phosphorylation of GSK3β at serine 9 as determined by SDS-PAGE (Fig. 5Ai–ii) and confocal microscopy (Fig. 5B). Given that phosphorylation of GSK3β is associated with its inactivation36–38, these findings suggest that GSK3β inhibition may underlie the effects of breast milk on TLR4. To evaluate this possibility directly, we inhibited or inactivated GSK3β in enterocytes and assessed whether breast milk was still capable of exerting its negative effects on TLR4 signaling. To do so, we first treated cells with the GSK3β inhibitor lithium chloride (LiCl), which blocks GSK3β signaling via phosphorylation of serine 939, 40 and by phosphorylating the N-terminal serine residues of GSK3β41, 42. As shown in Figure 5Ai–ii, pretreatment with LiCl increased the phosphorylation of GSK3β and also decreased the expression of TLR4-mediated IL-1β (Fig. 5Aiii) and IL-6 (Fig. 5Aiv) in IEC-6 cells. In parallel, we knocked-down GSK3β via lentiviral transduction of GSK3β shRNA (Fig. 5Civ). As shown in Figure 5C, treatment of GSK3β-kd cells with LPS significantly increased NF-κB translocation and the pro-inflammatory cytokine IL-6 (Fig. 5Ci–ii, v–vi) similar to the situation in wild-type cells (Fig. 1Ai–ii, Ei–ii), which was not reversed by breast milk (Fig. 5Ciii, v–vi). Taken together, these findings strongly imply that breast milk inhibits TLR4 activation in enterocytes through the effects of GSK3β signaling.
Figure 5. Breast milk attenuates TLR4-mediated NF-κB activation by inhibiting GSK3β in vitro.
A: SDS-PAGE showing the phosphorylation of GSK3β at serine 9 (pGSK3βS9, upper), total GSK3β (T-GSK3β, middle) and actin (loading control, lower) in serum and insulin starved wild-type IEC-6 cells, with treatment groups as indicated (LPS 50 μg/mL, 15 min, after 1 h pretreatment with EGF 400 ng/mL or breast milk 50 μL/ml media or Lithium chloride, Li, 100μM, i); blots were stripped and reprobed for T-GSK3β and Actin. Quantification of pGSK3β to total GSK3β expression ratio (Image J Software, NIH, ii). IL-1β (iii) and IL-6 mRNA expression (iv) in the indicated treatment groups (BM=breast milk, Li=Lithium chloride). B: Representative confocal micrographs of serum/insulin starved wild-type IEC-6 cells, with treatment groups as indicated, stained for pGSK3βS9 (green) and DAPI (blue) (i–v). Quantification of pGSK3βS9 staining (vi, Mean Pixel Intensity, Image J Software, NIH). C: Representative confocal micrographs of GSK3β-kd IEC-6 cells stained for the p65 subunit of NF-κB (green) with indicated treatment groups (i–iii). RT-PCR showing GSK3β (upper) and loading control RPLO (lower) in wild-type IEC-6 cells (left lanes), GSK3β knock-down (GSK3β-kd) IEC-6 cells (right lanes) (iv). Quantification of the extent of NF-κB translocation (v) or IL-6 mRNA expression (vi) under the indicated conditions in GSK3β-kd IEC-6 cells. *p<0.05 versus control (Ctrl, white bar), **p<0.05 versus LPS; #p<0.05 versus LPS, ##p<0.05 versus LPS + breast milk (LPS+BM), ###p<0.05 versus LPS + EGF-depleted breast milk (IBM). Results representative of three separate experiments with over 50 high power fields per group. Size bar = 10μm. Data are mean±SEM.
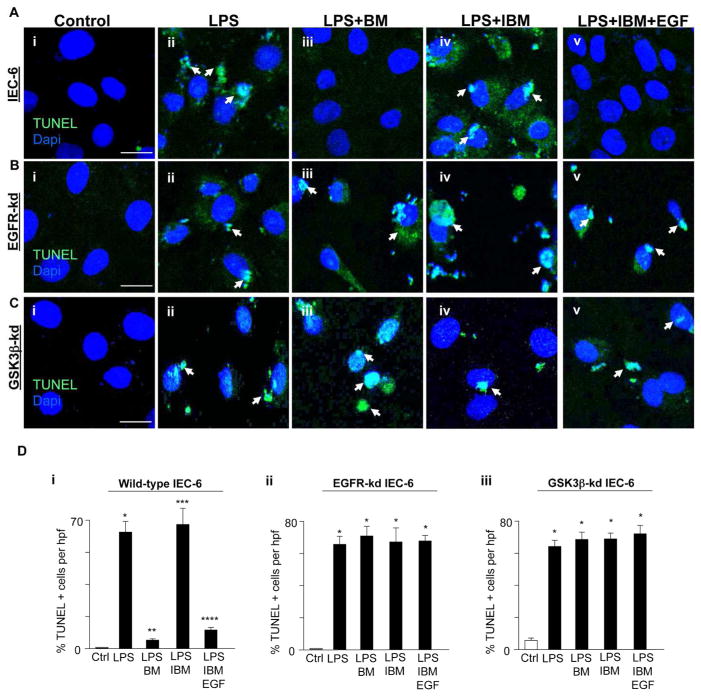
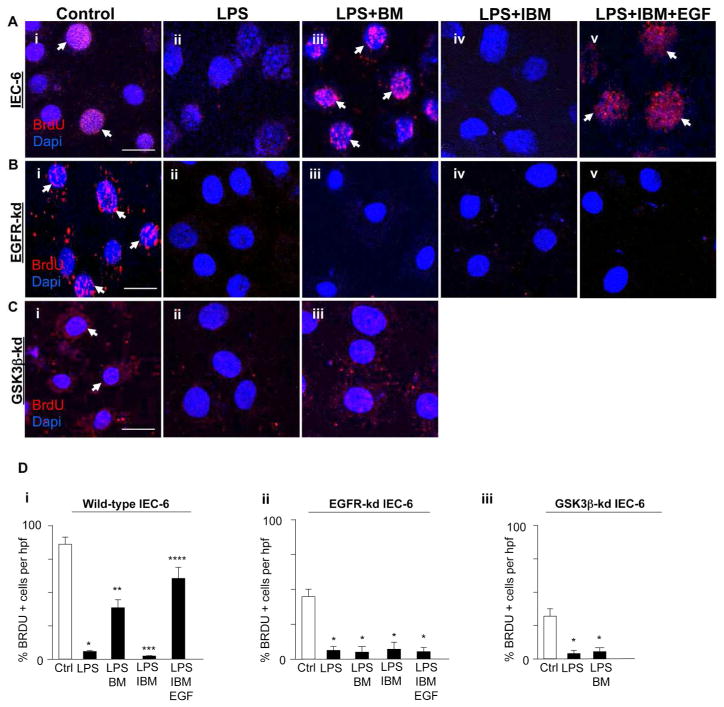
Breast milk inhibits TLR4-mediated apoptosis and proliferation in enterocytes via EGFR and GSK3β
Shown in Figures 6 and 7 is evidence that breast milk reversed the effects of TLR4 on apoptosis and proliferation via EGFR activation by EGF in a GSK3β-dependent manner. In wild-type cells, breast milk protected against TLR4-mediated enterocyte apoptosis (Fig. 6Aiii vs. ii, Di) and increased enterocyte proliferation (Fig. 7Aiii vs. ii, Di), while immuno-depletion of EGF from breast milk blocked the protective effects on TLR4 signaling (Fig. 6Aiv vs. iii, 6Di and Fig. 7Aiv vs. iii, 7Di) which was restored by replacement with exogenous EGF (Fig. 6Cv vs. iv, 6Di and Fig. 7Av vs. iv, 7Di). In parallel, breast milk treatment of IEC-6 cells in which EGFR had been knocked down were not protected from TLR4-mediated apoptosis (Fig. 6Bi–v, Dii) or proliferation (Fig. 7Bi–v, Dii). Likewise, treatment with breast milk in GSK3β-deficient cells did not protect against apoptosis (Fig. 6Ci–iii, Diii) or proliferation (Fig. 7Ci–iii, Diii). Taken together, these findings suggest that breast milk reverses the effects of TLR4 on enterocyte apoptosis and proliferation via EGFR and its inhibition of GSK3β.
Figure 6. Breast milk inhibits TLR4-mediated apoptosis via EGFR and GSK3β.
A–C: Representative confocal micrographs of either wild-type IEC-6 cells (Ai–v) or EGFR-kd IEC-6 (Bi–v) or GSK3β-kd IEC-6 cells (Ci–v) which were serum starved 6 h, treated as indicated, then stained for TUNEL (green, arrows) and DAPI (blue). D: Quantification of the TUNEL positive cells per high power field (hpf) in wild-type IEC-6 cells (i) or EGFR-kd IEC-6 (ii) or GSK3β-kd IEC-6 cells (iii). *p<0.05 versus control (Ctrl, white bar), **p<0.05 versus LPS, ***p<0.05 versus LPS + breast milk (BM), ****p<0.05 versus LPS + IBM. Results representative of three separate experiments with over 50 high power fields per group. Size bar = 10μm. Data are mean±SEM. Arrows delineate apoptotic cells.
Figure 7. Breast milk restores enterocyte proliferation via EGFR and GSK3β.
A–C: Representative confocal micrographs of either wild-type IEC-6 cells (Ai–v) or EGFR-kd IEC-6 (Bi–v) or GSK3β-kd IEC-6 cells (Ci–iii), treated as indicated, then stained for the proliferation marker bromodeoxyuridine (BrdU, red, arrows) and DAPI (blue). D: Quantification of the BrdU positive cells per high power field (hpf) in wild-type IEC-6 cells (i) or EGFR-kd IEC-6 (ii) or GSK3β-kd IEC-6 cells (iii). *p<0.05 versus control (Ctrl, white bar), **p<0.05 versus LPS, ***p<0.05 versus LPS + breast milk (BM), ****p<0.05 versus LPS + IBM. Results representative of three separate experiments with over 50 high power fields per group. Size bar = 10μm. Data are mean±SEM. Arrows delineate proliferative cells.
Breast milk attenuates NEC severity via EGFR activation
In the next series of studies, we sought to investigate whether breast milk could protect against the development of NEC through EGFR activation. As shown in Figures 8–9, experimental NEC was induced in newborn pups using an established model that involves a combination of formula gavage and hypoxia treatment4, 21, 43, 44. The administration of breast milk significantly attenuated experimental NEC, as manifest by a reduction in NEC severity (Fig. 8Ai–ii, Cii), the pro-inflammatory gene expression inducible nitric oxide synthase (iNOS) (Fig. 8Ci), as well as a reduction in enterocyte apoptosis and 3-NT (Fig. 9Ai–iii, Ei), and enhanced proliferation (Fig. 9Bi–iii, Fi). Importantly, mice lacking intestinal EGFR were found to exhibit increased NEC severity compared to wild-type mice that was not reduced by the administration of breast milk (Fig. 8Bi–iii, Di–ii). Breast milk did not protect against NEC-mediated enterocyte apoptosis (Fig. 9Ci–iii, Eii) nor enhance enterocyte proliferation (Fig. 9Di–iii, Fii) in EGFRΔIEC mice, indicating that breast milk activation of EGFR is required for these protective effects. Additionally, pretreatment of animals with cetuximab in conjunction with breast milk blocked the protective effects of breast milk on NEC severity (Fig. 8Aiv vs. iii, Ci–ii), enterocyte apoptosis (Fig. 9Aiv vs. iii, Ei) and proliferation (Fig. 9Biv vs. iii, Fi). Wild-type animals that were treated with the EGF-depleted milk resulted in a loss of the protective effects of breast milk on NEC severity (Fig. 8Av, Cii), concomitant with expression of iNOS (Fig. 8Ci), enterocyte apoptosis (Fig. 9Av, Ei) and reduced proliferation (Fig. 9Bv, Fi), all of which are restored when recombinant EGF is supplemented (Figs. 8Avi, Ci–ii and Fig. 9Avi, Ei, Fi). Taken together, these findings indicate that breast milk protects against experimental NEC in part through inhibition of TLR4 and that this protective effect requires EGF/EGFR signaling.
Figure 8. Breast milk attenuates NEC severity via EGFR activation.
A–B: Representative H&E micrographs of the terminal ileum of either wild-type mice (Ai–vi) or EGFRΔIEC mice (Bi–iii) for the indicated experimental NEC treatment groups. C–D: iNOS mRNA expression for indicated groups in wild-type mice (Ci) or EGFRΔIEC mice (Di) and NEC severity score assigned by a pathologist blinded to the treatment groups in wild-type mice (Cii) or EGFRΔIEC mice (Dii). *p<0.05 versus breast fed control animals (Ctrl, white bar), **p<0.05 versus NEC, ***p<0.05 versus NEC + breast milk (BM), ****p<0.05 versus NEC + EGF-immunodepleted breast milk (IBM). Data are mean ±SEM. Representative of at least three separate experiments with at least ten mice per group.
Figure 9. Breast milk inhibits NEC-mediated apoptosis and enhances crypt proliferation via EGF/EGFR.
A: Representative confocal micrographs of the terminal ileum of wild-type mice stained for TUNEL (green, arrows), 3-nitrotyrosine (3NT, red), and DAPI (blue) from the indicated experimental NEC treatment groups (i–vi). B: Representative confocal micrographs of the small intestinal crypts of wild-type mice stained for PCNA (green, arrows) and DAPI (blue) from the indicated experimental NEC treatment groups (i–vi). C: Representative confocal micrographs of the terminal ileum of EGFRΔIEC mice stained for TUNEL (green, arrows), 3-nitrotyrosine (3NT, red), and DAPI (blue) from the indicated experimental NEC treatment groups (i–iii). D: Representative confocal micrographs of the small intestinal crypts of wild-type mice stained for PCNA (green, arrows) and DAPI (blue) from the indicated experimental NEC treatment groups (i–iii). E: Quantification of the TUNEL positive cells per villus in wild-type mice (i) or EGFRΔIEC mice (ii). F: Quantification of the PCNA positive cells per crypt in wild-type mice (i) or EGFRΔIEC mice (ii). *p<0.05 versus breast fed control animals (Ctrl, white bar), **p<0.05 versus NEC, ***p<0.05 versus NEC + breast milk (BM), ****p<0.05 versus NEC + EGF-immunodepleted breast milk (IBM). Data are mean ±SEM. Representative of at least three separate experiments with at least ten mice per group.
Discussion
In the current study, we demonstrate a mechanism by which breast milk inhibits the LPS-mediated activation of TLR4 and the ensuing inflammatory cascade leading to protection against NEC. We show that breast milk inhibits TLR4-mediated inflammatory signaling in vitro and that these effects are dependent on EGFR signaling, via the downstream target GSK3β. Furthermore, we discovered that breast milk attenuates experimental NEC via inhibition of TLR4 signaling through EGF, as treatment with EGF-deficient breast milk abrogated the protective effects, which were restored with exogenous EGF added back to the formula, thus confirming the role of EGF on the prevention of NEC. Further support of the mechanism that EGF and its receptor EGFR are critical components mediating the protection against NEC are demonstrated by the current observation that mice in which EGFR had been deleted specifically from the intestinal epithelium were not protected from experimental NEC when breast milk was added to their formula. Taken together, these findings present a new paradigm to explain the protective effects of breast milk on TLR4 signaling, via effects on a growth factor receptor.
Inhibition of TLR4 activation within the intestinal epithelium has been shown to enhance enterocyte proliferation7, 21 and to inhibit enterocyte apoptosis in the small intestine of the premature host22, 29. We have previously shown an important link between TLR4 and the GSK3β/β-catenin signaling pathway in NEC pathogenesis7. TLR4 activation was found to increase GSK3β expression, resulting in enhanced phosphorylation of β-catenin and its targeted degradation, culminating in reduced enterocyte proliferation7. We now demonstrate that in the pathogenesis of NEC, breast milk and EGF have protective roles on the intestinal mucosa, via inhibition of enterocyte apoptosis and enhanced intestinal mucosal healing, providing a potential link to our prior findings. Specifically, the GSK3β/β-catenin signaling pathway plays a critical role in determining the extent of enterocyte proliferation that occurs in response to EGFR activation34. Furthermore, growth factor receptor/ligand interaction initiates a signaling cascade that affects GSK3β, which modulates TLR4-mediated NF-κB activity to transition between the generation of pro- and anti-inflammatory signals45–47. Inactivation of GSK3β by phosphorylation at serine 9, negatively affects NF-κB activation45–47 thus decreasing NF-κB-dependent pro-inflammatory cytokine production46. Moreover, inactivation of GSK3β also leads to the stabilization of β-catenin, a critical factor responsible for growth and proliferation34, 48. Moreover, although PPARγ has been linked to EGFR receptor signaling, we have previously demonstrated that deletion of PPARγ in intestinal epithelial cells had no effect on EGFR expression, raising the possibility that other effector mechanisms are involved19. By extension, given that breast milk is rich in EGF, it is possible that the EGF in breast milk could play similar roles via GSK3β/β-catenin signaling and inactivation of GSK3β19, although it appears now that effects of EGFR activation on TLR4 expression predominate.
Our data is consistent with previous studies that have demonstrated an important role for EGF in reducing the incidence of NEC development16, 17. In addition, a recent study has shown that a low concentration of epidermal growth factor in the cord blood of extremely premature infants is associated with the development of NEC49. We now extend these findings by providing a novel mechanistic link associated with breast milk mediated protection in NEC via EGF. Other compounds are known to be present in breast milk and may also be important in the regulation of NEC pathogenesis. These include human milk oligosaccharides, which are non-digestible carbohydrates thought to influence the gastrointestinal microbiome50 and possibly protect against NEC51. Besner and colleagues have evaluated the effects of heparin-binding epidermal growth factor-like growth factor (HB-EGF) on experimental NEC and have shown that HB-EGF is protective by accelerating enterocyte migration and proliferation52, along with increasing the microvascular blood flow of the intestine53. These studies demonstrate the clinical importance of identifying the components of breast milk that are protective against NEC, so as to provide potential therapeutic targets for this devastating disease. We readily acknowledge that although we provide here a mechanism by which breast milk attenuates experimental NEC, there may be other signaling pathways involved in the protection against NEC, such as autophagy, the mTOR pathway or inhibition of ER stress. Further studies will be required to explore the potential roles – if any – of these varying signaling pathways in mediating the observed protection of breast milk in infants with NEC.
In summary, we have now described a mechanistic pathway to explain how breast milk mediates protection against the development of necrotizing enterocolitis in mice. Further studies into how breast milk offers such profound protection against the development of this disease – with a focus on the anti-inflammatory properties of breast milk – may open doors towards novel approaches for the rational design of new therapies of this devastating condition.
Materials and Methods
Reagents
Antibodies were obtained from the following sources: p65 subunit of NF-κB, phospho and total GSK3β (Cell Signaling); PCNA (Santa Cruz); 3-nitrotyrosine (Abcam), actin (Genscript), bromodeoxyuridine (BrdU, Novus Biosciences); DAPI (Invitrogen). TUNEL (Roche Applied Science) was performed according to the manufacturer’s instructions as in 22. Appropriate secondary antibodies for immunohistochemistry and SDS-PAGE were obtained from Molecular Probes and Jackson ImmunoResearch Laboratories. Cetuximab (Dose: 100 μg/day i.p. for 3 days prior to experiments) was a generous gift of Jennifer Grandis (University of Pittsburgh, Pittsburgh, PA). Human Recombinant EGF was from EMD Millipore (400 ng/mL media in vitro, 0.5ng per μL of NEC formula in vivo). Lithium chloride (LiCl) from J.T. Baker (100μM). Lipopolysaccharide (LPS) (Escherichia coli 0111:B4 purified by gel-filtration chromatography, >99% pure) was obtained from Sigma-Aldrich and concentrations used were those that we have demonstrated to be present in mice and humans with NEC (50 μg/mL for cells, 5mg/kg for all mice3 with the exception of the TLR4ΔIEC-OVER mice who are sensitive to LPS and required a decreased dosage (2.5 mg/kg).
Cell culture and lentiviral knock down
The small intestinal cell line, IEC-6 was obtained from the American Type Culture Collection (ATCC, Manassas, VA), maintained as in 54 and rendered deficient in EGFR or GSK3β using lentiviral particles containing EGFR or GSK3β shRNA (Open Biosystems) from the four-plasmid lentiviral packaging system (Invitrogen) with permissive HEK 293 cells. In parallel, IEC-6 cells were treated with lentiviruses expressing scrambled shRNA. Stable integration of lentivirus was obtained by selection of cells in medium containing puromycin (5 μg/mL), and the extent of knockdown of EGFR or GSK3β was verified by RT-PCR or SDS-PAGE as in 21. Serum and/or insulin starvation of the cells for 6 h was performed where indicated in the figure legends.
Quantitative real-time polymerase chain reaction
Quantitative real-time PCR with the Bio-Rad CFX96 Real-Time System (Biorad, Hercules, CA) was performed as in 22 using the following primers using either RPLO or GAPDH as a housekeeping gene as shown in Table 1.
Table 1.
List of primers.
| Gene | Species | Forward sequence | Reverse sequence | Amplicon Size (bP) |
|---|---|---|---|---|
| RPLO | Mouse/Rat/Human | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 143 |
| IL-6 | Mouse/Rat | GGCTAAGGACCAAGACCATCCAA | TCTGACCACAGTGAGGAATGTCCA | 138 |
| TLR4 | Mouse | TTTATTCAGAGCCGTTGGTG | CAGAGGATTGTCCTCCCATT | 186 |
| IL-1β | Mouse/Rat | AGTGTGGATCCCAAGCAATACCCA | TGTCCTGACCACTGTTGTTTCCCA | 175 |
| iNOS | Mouse/Rat | CTGCTGGTGGTGACAAGCACATTT | ATGTCATGAGCAAAGGCGCAGAAC | 167 |
| Tg-TLR4 | Mouse | AGAAAATGCCAGGATGATGC | TGTCATCAGGGACTTTGCTG | 164 |
| GAPDH | Mouse/rat | TGAAGCAGGCATCTGAGGG | CGAAGGTGGAAGAGTGGGAG | 102 |
| EGFR k/d | Mouse/rat | ATGGTGTCACTGTGTGGGAACTGA | ACTTTGGGCGGCTATCAGCATCTA | 183 |
| EGFR K/O gt | Mouse | CTCAGCCAGATGATGTTGAC | CCTCGTCTGTGGAAGAACTA | 129 |
| GSK3β k/d | Rat | GACACACCTGCCCTCTTCAA | AGAAGCGGCGTTATTGGTCT | 177 |
Statement of ethics
The animal experiments described in these studies were approved by the University of Pittsburgh Animal Care and Use Committee (Protocol Number: 12040382) and were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Collection of mouse breast milk
We obtained breast milk from isoflurane anesthesized lactating mice on postpartum days 8–12 as in 8 that had been injected with oxytocin (0.15IU SQ per kg of body weight for 3 minutes prior to milk being collected via an electronic breast pump (Lansinoh Laboratories). We typically obtain approximately 1 ml per mouse, which was immediately aliquoted and stored at −80 degrees Celsius and frozen just once prior to use.
Induction of experimental murine endotoxemia and whole animal NF-κB signaling
Wild-type mice were obtained from either Jackson Laboratory or Charles River Laboratories. After being placed nil per os for 6 hours, endotoxemia was induced in 10-day-old mice with intraperitoneal injection of LPS (5 mg/kg, 6–16 hr). In parallel, mice were administered saline as vehicle control. Where indicated, mice were gavaged expressed murine breast milk or saline as a control (50 μl per gram of mouse body weight) as a pretreatment 1 hr prior to LPS injection. Mice lacking EGFR specifically in the intestinal epithelium were generated by breeding Egfr loxP/loxP (obtained from David W. Threadgill, University of North Carolina, Chapel Hill, NC) with transgenic mice expressing Villin-cre (termed EGFRΔIEC mice). Mice were generated to selectively express TLR4 within in the intestinal epithelium (TLR4ΔIEC-OVER) as in 22. Experiments with the NF-κB-luciferase reporter mice, in which NF-κB is upstream of the luciferase gene (strain NF-κB-RE-luc, Taconic Farms Inc, Hudson, NY) were performed as in 9 and imaged using the IVIS Lumina 3D Optimal in vivo imaging system 10 minutes after an intraperitoneal injection of luciferin (150 μg/kg, Caliper Life Sciences). Samples of the terminal ileum were obtained 1 cm proximal to the ileocecal valve and prepared as described in 3.
Induction of experimental murine necrotizing enterocolitis
Experimental NEC was induced in 5–10 day old mice as in 21, 22, 44. Briefly, NEC formula [Similac Advance infant formula (Abbott Nutrition):Esbilac (PetAg) canine milk replacer, 2:1] was supplemented with enteric bacteria made from a stock created from a specimen obtained from an infant with surgical NEC (12.5 μl original stool slurry in 1ml formula) and was orally gavage fed five times/day (50 μl per gram of mouse body weight) as in 44. The mice also received hypoxia for 10 minutes twice daily (5%O2, 95%N2) in a hypoxic chamber (Billups-Rothenberg) for a total of 4 days. We have shown that this experimental NEC protocol induces intestinal inflammation and the release of pro-inflammatory cytokines which closely resembles human NEC3, 4, 22, 44. Control (i.e., non-NEC) animals remained with their mothers and were breast fed on demand. Where indicated, formula fed mice were enterally administered expressed murine breast milk which was obtained as in 8 or EGF-deficient breast milk once per day with or without recombinant EGF (0.5 ng/μL of formula) added back to that feeding only. The expression of mucosal cytokines was assessed by qRT-PCR as in 44. Immediately after sacrifice, the terminal ileum was harvested 1 cm proximal to the ileocecal valve and fixed in 4% paraformaldehyde. The severity of disease was determined on histological sections of the terminal ileum by a pediatric pathologist who was blinded to the study conditions according to our previously published scoring system from 0 (normal) to 3 (severe)3.
Immunohistochemistry, immunofluorescence, and SDS-PAGE
Immunohistochemistry, immunofluorescence and confocal microscopy was performed as in 21 except for in vitro BrdU proliferation assay, which is described below. Images were assembled using Adobe Photoshop CS6 software (Adobe Systems Inc., San Jose, CA). SDS-PAGE was performed as in 21 and the densitometry quantified with Image J Software (NIH).
In Vitro BrdU Proliferation Assay
To assess proliferative S-phase cells, wild-type IEC-6, EGFR-k/d IEC-6 or GSK3β-k/d IEC-6 cells were plated on cover slips in 12-well cell culture dishes overnight, treatment groups as indicated in Results along with BrdU labeling reagent (10uL/ml media, Invitrogen) for 6 hrs. To detect BrdU incorporation, cells were fixed with 4% PFA on ice for 10 min., processed for antigen retrieval with 2M Hydrochloric acid for 30 min, blocked with 5% donkey serum in 0.5% bovine serum albumin (BSA), incubated overnight at 4 degrees Celsius with rat-anti-BrdU antibodies (Novus Biosciences) and detected with cy3-labelled secondary anti-rat antibodies using a confocal microscope (Zeiss).
Assessment of NF-κB activation in enterocytes
Cultured wild-type IEC-6 cells or cells in which knockdown of EGFR or GSK3β IEC-6 cells were treated with LPS (50 μg/mL, 1 h) after pretreatments of murine breast milk (25 μL/well, 1h) or EGF (400 ng/mL, 1 h). The extent of NF-κB translocation was determined as in 21 and the average integrated pixel intensity which pertained to the corresponding NF-κB staining within the cytoplasmic and nuclear regions was determined for >200 cells per treatment group in at least three experiments per group using MetaMorph software version 6.1 (Molecular Devices Corporation, Downingtown, PA).
Where indicated, the extent of apoptosis was quantified in vitro and in vivo as we have done previously22 using the TUNEL assay and enumerating the number of TUNEL positive cells as a percentage of the total number of cells present. At least 100 fields were assessed for each experimental group where indicated.
Immunodepletion of EGF from breast milk
Immunodepletion of EGF from breast milk was performed as in 21. Briefly, 500ul aliquots of freshly harvested murine breast milk were filtered via Amicon Ultra 10K Centrifugal Filters (Millipore) at 13,000 RPM for 15 minutes. Filtered aliquots were then incubated at 4 degrees Celsius for 30 minutes with control goat IgG antibody (10 μl) and protein A/G-agarose (20 μl, Santa Cruz Biotechnology), and centrifuged at 3000RPM for 30 seconds. The supernatant was incubated for 1 h at 4 degrees Celsius with 2μg of anti-EGF antibody (Santa Cruz Biotechnology) followed by overnight incubation on a rotator with an additional 20 μl of protein A/G-agarose. Supernatants were collected after centrifugation at 3000RPM for 30 seconds and an EGF ELISA (Abcam) was performed as per manufacturers instructions using a 1:500 dilution (wild-type murine breast milk [22907±976 pg/ml]; EGF-depleted murine breast milk 468.5±87.1 pg/ml].
Statistical analysis
Statistical analysis was performed using PRISM 6 (GraphPad) software and ANOVA, Chi-squared or Two-tailed student’s t-test were used for comparisons where indicated. Data are mean +/− standard deviation or standard error of the mean as indicated, and comparisons are by two-tailed Student t test or analysis of variance with statistical significance at a P value of less than 0.05. All experiments were repeated at least in triplicate, with more than 50–200 cells/high-power field for in vitro experiments. For in vivo experiments, at least 3 mice per group for endotoxemia and at least 10 pups per group for experimental NEC were assessed.
Acknowledgments
Funding sources: MG is supported by K08DK101608 from the National Institutes of Health and the Children’s Hospital of Pittsburgh of the UPMC Health System. DJH is supported by R01GM078238 and R01DK083752 from the National Institutes of Health.
Footnotes
Conflict of interest: None.
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364(3):255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry MC, Moss RL. Necrotizing enterocolitis. Annual review of medicine. 2009;60:111–124. doi: 10.1146/annurev.med.60.050207.092824. [DOI] [PubMed] [Google Scholar]
- 3.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. Journal of immunology. 2007;179(7):4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 4.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012;143(3):708–718. e701–705. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jilling T, Simon D, Lu J, Meng FJ, Li D, Schy R, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. Journal of immunology. 2006;177(5):3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson WM, Sodhi CP, Russo A, Siggers RH, Afrazi A, Gribar SC, et al. Nucleotide-binding oligomerization domain-2 inhibits toll-like receptor-4 signaling in the intestinal epithelium. Gastroenterology. 2010;139(3):904–917. 917 e901–906. doi: 10.1053/j.gastro.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodhi CP, Shi XH, Richardson WM, Grant ZS, Shapiro RA, Prindle T, Jr, et al. Toll-like receptor-4 inhibits enterocyte proliferation via impaired beta-catenin signaling in necrotizing enterocolitis. Gastroenterology. 2010;138(1):185–196. doi: 10.1053/j.gastro.2009.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarbiou J, Tjabringa GS, Verhoosel RM, Ninaber DK, White SR, Peltenburg LT, et al. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm Res. 2006;55(3):119–127. doi: 10.1007/s00011-005-0062-9. [DOI] [PubMed] [Google Scholar]
- 9.Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, et al. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PloS one. 2013;8(6):e65779. doi: 10.1371/journal.pone.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gephart SM, McGrath JM, Effken JA, Halpern MD. Necrotizing enterocolitis risk: state of the science. Advances in neonatal care: official journal of the National Association of Neonatal Nurses. 2012;12(2):77–87. doi: 10.1097/ANC.0b013e31824cee94. quiz 88–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 12.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatric research. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 13.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. Journal of perinatology: official journal of the California Perinatal Association. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollack PF, Goda T, Colony PC, Edmond J, Thornburg W, Korc M, et al. Effects of enterally fed epidermal growth factor on the small and large intestine of the suckling rat. Regulatory peptides. 1987;17(3):121–132. doi: 10.1016/0167-0115(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 15.Dvorak B, Williams CS, McWilliam DL, Shinohara H, Dominguez JA, McCuskey RS, et al. Milk-borne epidermal growth factor modulates intestinal transforming growth factor-alpha levels in neonatal rats. Pediatric research. 2000;47(2):194–200. doi: 10.1203/00006450-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Dvorak B, Halpern MD, Holubec H, Williams CS, McWilliam DL, Dominguez JA, et al. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. American journal of physiology Gastrointestinal and liver physiology. 2002;282(1):G156–164. doi: 10.1152/ajpgi.00196.2001. [DOI] [PubMed] [Google Scholar]
- 17.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. American journal of physiology Gastrointestinal and liver physiology. 2006;291(5):G938–949. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hirai C, Ichiba H, Saito M, Shintaku H, Yamano T, Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. Journal of pediatric gastroenterology and nutrition. 2002;34(5):524–528. doi: 10.1097/00005176-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences. 2012;109(28):11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, et al. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. The Journal of biological chemistry. 2012;287(44):37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Good M, Siggers RH, Sodhi CP, Afrazi A, Alkhudari F, Egan CE, et al. Amniotic fluid inhibits Toll-like receptor 4 signaling in the fetal and neonatal intestinal epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11330–11335. doi: 10.1073/pnas.1200856109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afrazi A, Branca MF, Sodhi CP, Good M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. The Journal of biological chemistry. 2014;289(14):9584–9599. doi: 10.1074/jbc.M113.526517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan KL, Wong KF, Luk JM. Role of LPS/CD14/TLR4-mediated inflammation in necrotizing enterocolitis: pathogenesis and therapeutic implications. World journal of gastroenterology: WJG. 2009;15(38):4745–4752. doi: 10.3748/wjg.15.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, et al. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. American journal of physiology Gastrointestinal and liver physiology. 2009;297(3):G442–450. doi: 10.1152/ajpgi.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neal MD, Leaphart C, Levy R, Prince J, Billiar TR, Watkins S, et al. Enterocyte TLR4 mediates phagocytosis and translocation of bacteria across the intestinal barrier. Journal of immunology. 2006;176(5):3070–3079. doi: 10.4049/jimmunol.176.5.3070. [DOI] [PubMed] [Google Scholar]
- 26.Ruemmele FM, Beaulieu JF, Dionne S, Levy E, Seidman EG, Cerf-Bensussan N, et al. Lipopolysaccharide modulation of normal enterocyte turnover by toll-like receptors is mediated by endogenously produced tumour necrosis factor alpha. Gut. 2002;51(6):842–848. doi: 10.1136/gut.51.6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summers ST, Bass BL. Protein kinase C inhibits epidermal growth factor receptor phosphorylation in enterocytes. The Journal of surgical research. 1997;69(1):208–211. doi: 10.1006/jsre.1997.5054. [DOI] [PubMed] [Google Scholar]
- 28.Connolly JM, Rose DP. Epidermal growth factor-like proteins in breast fluid and human milk. Life sciences. 1988;42(18):1751–1756. doi: 10.1016/0024-3205(88)90041-0. [DOI] [PubMed] [Google Scholar]
- 29.Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. Journal of immunology. 2013;190(7):3541–3551. doi: 10.4049/jimmunol.1202264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatric research. 2004;55(4):622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 31.Abraham SN, Beachey EH, Simpson WA. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infection and immunity. 1983;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cetin S, Leaphart CL, Li J, Ischenko I, Hayman M, Upperman J, et al. Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner. American journal of physiology Gastrointestinal and liver physiology. 2007;292(5):G1347–1358. doi: 10.1152/ajpgi.00375.2006. [DOI] [PubMed] [Google Scholar]
- 33.Adachi K, Yamauchi K, Bernaudin JF, Fouret P, Ferrans VJ, Crystal RG. Evaluation of fibronectin gene expression by in situ hybridization. Differential expression of the fibronectin gene among populations of human alveolar macrophages The American journal of pathology. 1988;133(2):193–203. [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Saito H, Masuda S, Yang X, Takano Y. Phosphorylated GSK3beta-ser9 and EGFR are good prognostic factors for lung carcinomas. Anticancer research. 2007;27(5B):3561–3569. [PubMed] [Google Scholar]
- 35.Shaw M, Cohen P. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS letters. 1999;461(1–2):120–124. doi: 10.1016/s0014-5793(99)01434-9. [DOI] [PubMed] [Google Scholar]
- 36.Karrasch T, Spaeth T, Allard B, Jobin C. PI3K-dependent GSK3ss(Ser9)-phosphorylation is implicated in the intestinal epithelial cell wound-healing response. PloS one. 2011;6(10):e26340. doi: 10.1371/journal.pone.0026340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Brown J, Martin M. Glycogen synthase kinase 3: a point of convergence for the host inflammatory response. Cytokine. 2011;53(2):130–140. doi: 10.1016/j.cyto.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 39.Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current biology: CB. 1996;6(12):1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 40.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacology & therapeutics. 2010;128(2):281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasquali L, Busceti CL, Fulceri F, Paparelli A, Fornai F. Intracellular pathways underlying the effects of lithium. Behavioural pharmacology. 2010;21(5–6):473–492. doi: 10.1097/FBP.0b013e32833da5da. [DOI] [PubMed] [Google Scholar]
- 43.Afrazi A, Sodhi CP, Good M, Jia H, Siggers R, Yazji I, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. Journal of immunology. 2012;188(9):4543–4557. doi: 10.4049/jimmunol.1103114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Good M, Sodhi CP, Ozolek JA, Buck RH, Goehring KC, Thomas DL, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. American journal of physiology Gastrointestinal and liver physiology. 2014;306(11):G1021–1032. doi: 10.1152/ajpgi.00452.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coudriet GM, He J, Trucco M, Mars WM, Piganelli JD. Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PloS one. 2010;5(11):e15384. doi: 10.1371/journal.pone.0015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochemical research. 2007;32(4–5):577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nature immunology. 2005;6(8):777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto D, Gregorieff A, Begthel H, Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes & development. 2003;17(14):1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wahab Mohamed WA, Aseeri AM. Cord blood epidermal growth factor as a possible predictor of necrotizing enterocolitis in very low birth weight infants. Journal of neonatal-perinatal medicine. 2013;6(3):257–262. doi: 10.3233/NPM-1370813. [DOI] [PubMed] [Google Scholar]
- 50.Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, Newburg DS. Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection. The Journal of biological chemistry. 2003;278(16):14112–14120. doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- 51.Newburg DS. Neonatal protection by an innate immune system of human milk consisting of oligosaccharides and glycans. Journal of animal science. 2009;87(13 Suppl):26–34. doi: 10.2527/jas.2008-1347. [DOI] [PubMed] [Google Scholar]
- 52.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. Journal of pediatric surgery. 2007;42(1):214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137(1):221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quaroni A, Isselbacher KJ, Ruoslahti E. Fibronectin synthesis by epithelial crypt cells of rat small intestine. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(11):5548–5552. doi: 10.1073/pnas.75.11.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]