Abstract
Background
An important role has emerged for calpain enzymes in regulating inflammation with one isoform, calpain-2, particularly important for macrophage activation. The goal of this study was to determine the therapeutic potential of a synthetic calpain-2 inhibitor, zLLY-CH2F, for colitis and inflammation associated colorectal cancer.
Methods
Mice were then subjected to the azoxymethane/dextran sulfate sodium (AOM/DSS) model of colitis and colitis associated cancer (CAC) incorporating intervention with daily injections of 0.75 mg/kg calpain-2 inhibitor beginning after the first signs of colitis.
Results
Calpain-2 inhibitor treatment alleviated weight loss and bloody diarrhea, and reduced inflammatory infiltration into colon tissues and inflammatory cytokine mRNA. Calpain-2 inhibitor intervention also reduced total CAC tumor volume by up to 70% in vehicle control mice and decreased cancer pathology scores of blinded histological colon tissue analyses. Mechanistic investigations showed that calpain-2 inhibition during macrophage activation reduced inhibitor of kappa beta (IκB) degradation and nuclear factor kappa beta (NFκB) nuclear localization as well as secretion of specific inflammatory cytokines. In addition, calpain-2 inhibitor treatment of CT26.WT mouse and HT-29 human colorectal cancer cells decreased proliferation, and reduced IκB degradation and NFκB translocation.
Conclusions
Overall, these findings suggest that intervention with a calpain-2 inhibitor may reduce colitis and CAC through a two-hit process of limiting macrophage activation as well as inhibiting growth of the colorectal cancer cells themselves.
Keywords: Inflammation, calpain, calpastatin, macrophage, colorectal cancer
Introduction
Chronic inflammation drives the pathogenesis of several human health disorders, including the inflammatory bowel diseases (IBD) Crohn’s disease and ulcerative colitis. A long-term complication of chronic inflammation associated with IBD is the development of colorectal cancer, which is one of the leading causes of cancer-related death in the western hemisphere.1 The cumulative risk for acquiring colorectal cancer can increase to approximately 20% in patients with IBD who live for 30 years with the disease.2,3 Moreover, clinical studies have shown that patients with colitis have a 2- to 8-fold relative risk of developing colorectal cancer compared to the general population.4 Thus, therapeutics that target both IBD and colorectal cancer cells would have a major impact on the morbidity and mortality attributed to these diseases.
The complexity of IBD and colorectal cancer require multi-targeted intervention strategies based on the underlying pathophysiology. Therapeutics for IBD include 5-aminosalicylic acids (5-ASA), topical/oral corticosteroids, thiopurines, tumour necrosis factor-α (TNF-α) blockers, and selective Janus kinase (JAK) inhibitors.5,6 A recent study covering the period of 1979–2011 found that an increasing use of thiopurines and TNF-α blockers for treating IBD over time paralleled a persistent significant decrease in surgery rate along with a significant decrease in use of 5-ASA and corticosteroids.7 However, one-third of IBD patients do not respond to anti-TNF-α and the treatment and the surgery-sparing effect of newer medications are still unclear.8,9
Our previous work identified the endogenous inhibitor of calpain enzymes, calpastatin, as a protein upregulated in human IBD tissues as well as chronically inflamed colon tissue in mice subjected to dextran sulfate sodium (DSS)-induced colitis.10 Calpastatin was found to increase as a physiological response to calpain driven inflammation and mechanistic investigations revealed a particularly important role for calpastatin in limiting macrophage activation and inflammatory pathology during colitis. Calpains are Ca2+-activated cysteine proteases that cleave specific targets to modulate cellular functions relevant to inflammation such as proliferation, migration, and apoptosis.11 The two major isoforms of this enzyme, calpain-1 (or μ-calpain) and calpain-2 (or m-calpain), require μM and mM Ca2+ concentrations for activity, respectively.12 Following activation by Ca2+, calpain cleaves a specific subset of cellular proteins, including cytoskeletal proteins and proteins involved in signal transduction.13 In macrophages, calpain-2 is the predominant isoform and calpastatin was shown to prevent the hyperactivation of macrophages and NFκB driven inflammatory mediator production during colitis10,14
The central role of calpastatin in limiting inflammation suggests that intervention with a calpain inhibitor may assist the endogenous response to inflammation and thereby serve as an effective approach to treating IBD and preventing colorectal cancer. Some limited data suggest that a synthetic pan-calpain inhibitor may reduce the severity of experimentally induced acute colitis in rats.15 However, flaws in experimental design such as pretreatment of the rats with the inhibitor prior to induction of acute colitis and lack of chronic colitis conditions limited interpretation of the data.16 Due to the role calpain-2 plays in promoting macrophage activation and inflammatory pathology, we set out to determine the effectiveness of intervention with a calpain-2 inhibitor on both colitis and colitis associated cancer (CAC) using an established mouse model involving azoxymethane (AOM)/DSS. To better model the chronology of a clinical therapeutic intervention, mice were treated with the calpain-2 inhibitor (zLLY-CH2F) after the initial symptoms of colitis appeared in the mice. Our results show that treatment with a calpain-2 inhibitor not only reduced the severity of colitis in the mice, but also reduced the development of CAC due to effects on both macrophage activation and colorectal cancer cell proliferation.
Materials and methods
AOM/DSS model
A colony generated from C57BL/6J mice (Jackson Laboratories) was maintained in the University of Hawaii vivarium. The mouse AOM/DSS induced colitis/CAC model was adapted from a previously described protocol.17 In brief, 8–10 wk old male mice were injected with AOM (A5486, Sigma) (12 mg/kg) on day 0 and were then treated with three 5 day cycles of 2% DSS (CAS#9011-18-1; MP Biomedicals, Santa Ana, CA) in drinking water alternated with regular water over 63 days. Mice in the intervention group were i.p. injected 5 days per week with 0.75 mg/kg calpain-2 inhibitor (zLLY-CH2F; Millipore EMD) beginning on day 8 and then throughout the entire protocol. This inhibitor was previously demonstrated by our group to specifically inhibit calpain-2 activity.14 Control mice were injected with AOM on day 0 and received regular drinking water throughout the protocol. For the survival study a concentration of 2.5% DSS was used throughout the protocol. All animal protocols were approved by the University of Hawaii Institutional Animal Care and Use Committee.
Western blots, proliferation assays, invasion assays, soft agar colony assays, calpain activity assays, and cytokine analyses
Bone marrow derived macrophages (BMDM) were prepared as previously described. On day 5 of culture, 2 × 106 BMDM were seeded in 6-well plates and the following day were primed for 18 h with TNFα (20 ng/mL), followed by 1 h stimulation with E. faecalis (1 μg/mL; ATCC). Stimulation with E. faecalis was conducted in the presence of the zLLY-CH2F calpain-2 inhibitor (20 μg/mL) or DMSO as a control. Media was removed from treated BMDM, centrifuged at 300 × g for 5 min and supernatent analyzed using the Cytometric Bead Array Mouse Inflammation Kit (BD Biosciences, San Jose, CA). Cytokine data were analyzed using Flowjo software (Ashland, OR) and GraphPad Prism version 4.0 (GraphPad, La Jolla, CA). Cell pellets were lysed and analyzed for IκB and NFκB by Western blot as previously described.10 In other experiments, mouse CT-26.WT and human HT-29 colon cancer cells were plated in 96-well plates at 103 cells/well with 20 μg/mL inhibitor or DMSO, followed by analyses for IκB and NFκB by Western blot as described above for BMDM. For proliferation assays, fresh media with 20 μg/mL inhibitor or DMSO were added every 2 d. Each day 4 wells were quantified using Celltiter MTS reagent (Promega) and the resulting signal was used to generate a proliferation curve. Calpain activity was measured using a Calpain activity assay kit (BioVision, Inc., Milpitas, CA) as previously described. Enzyme-linked immunosorbant assay (ELISA) analyses of cecum hemoglobin has been previously described.10 Invasion assays were performed using 12 well 8 μm filter inserts with 40 μl matrigel. The matrigel was added to cold wells and incubated at 37°C for 30 minutes. 4 × 104 cells/well were added to the wells for both cancer cell lines with 20 μg/mL inhibitor or DMSO in 400 μl 0.2% FBS serum starvation media. 200 μl 10% FBS media was added to the lower well. The cells were incubated for 48 hrs followed by removal of cells from the upper well by q-tip. The number of invading cells was quantified using MTS reagent in the lower well with the cleaned and replaced filter-well. Soft agar colony formation assays were performed using 6-well plates. The bottom layer was 1 ml of 0.8% agar and the top layer was 0.7% agarose. The 0.7% agarose was cooled to 37°C in a water bath and 103 cells were added to the agarose before plating. 1 ml of media was refreshed every 3 days with 20 μg/mL inhibitor or DMSO for 2 weeks. The colonies were visualized with crystal violet and counted under a dissecting scope.
EMSA assay for nuclear localization of NFκB
Oligonucleotides end labeled with IR700 dye (Licor, Lincoln NE) were used to perform the EMSA. Macrophages or colon cancer cells were lysed in (10 mM Tris-HCl, 60 mM KCl, 1 mM EDTA, 1mM DTT, 0.1% NP40) spun at 4000×g for 4 min at 4°C. The nuclear pellets were lysed with (20 mM Tris-HCl, 420 mM NaCl, 1.5 mM MgCl, 0.2 mM EDTA, 25% glycerol) and centrifuged at 16,000 × g for 15 min at 4°C. The supernatant was incubated for 30 min with NFκB IR700 labeled oligonucleotide according to the Licor protocol. The samples were then run in an SDS free polyacrylamide gel in the dark in Tris glycine buffer and visualized on a Licor Odyssey® scanner.
Imaging of colon tissues and histology
On day 63, colons were dissected, cut longitudinally, and then were spread out on clear plastic. The colons were washed with PBS using transfer pipettes and images captured using an Infinity 2 microscope mounted camera (Lumenara, Madrid, Spain) in line with a Stemi 2000-C dissecting scope (Zeiss, Jena, Germany). Separate images were assembled into continuous colon images using Photoshop software (Adobe Systems Inc.). For histology, colon tissues were washed with PBS and fixed in 10% buffered formalin. Standard H&E staining of paraffin-embedded tissue samples was conducted as previously described.18 Five H&E stained sections from each mouse were blindly scored for colitis pathology as previously described using a 0–4 point scale.19 Colon cancer pathology was assessed on a four point system as follows: 1 = no tumor or dysplasia present; 2 = basally oriented nuclei, mild nuclear enlargement, nuclear crowding and hyperchromasia, decreased or loss of intracellular mucin; 3 = prominent nuclear stratification, more severe hyperchromasia and pleomorphism, marked architectural distortion; 4 = back to back glands with no intervening stroma, dysplastic epithelial cells and invasion of the colonic basement membrane. Images captured using a Zeiss Axioskop 2 Plus upright light microscope and camera. Immunohistochemistry was performed using paraffin embedded tissues. Anti-PCNA (Cells Signalling, Danvers, MA) antibody was used to stain colon tissues with HRP secondary (Jackson Research, West Grove, PA).
Statistical analyses
Comparison of two means was carried out using an unpaired Student’s t test using GraphPad Prism version 4.0 (GraphPad, La Jolla, CA). Weight curves and experiments with 3 or more groups were compared by repeated measures one-way ANOVA followed by Tukey post-test. Standard curves and regression analyses were also conducted using GraphPad Prism version 4.0. All comparisons were considered significant at P < 0.05.
Ethical Considerations
All animal studies were conducted with ethical practices as approved by the University of Hawaii Institutional Animal Care and Use Committee.
Results
Calpain-2 inhibition alleviates weight loss and bloody diarrhea
In order to determine an effective in vivo dosage of calpain-2 inhibitor we subjected mice to 2% DSS-treated drinking water combined with i.p. injections with increasing concentrations of the calpain-2 inhibitor (zLLY-CH2F at 0–1.25 mg/kg), and then calpain activity was measured in the colon tissue. The protocol was carried out for 4 days, which was consistent with our previously observation that DSS induced calpain activity peaks at 3–5 days of treatment.10 Results indicated that at 0.75 mg/kg of calpain-2 inhibitor the maximum amount of calpain activity inhibition was achieved (Figure 1A). Vehicle control had no effect on calpain activity (data not shown). It should be noted that total calpain activity was measured and only reduced by 50%, which suggests that calpain-1 may be functionally active in this tissue or that calpain-2 activity was not fully inhibited in the mouse colons.
Figure 1.
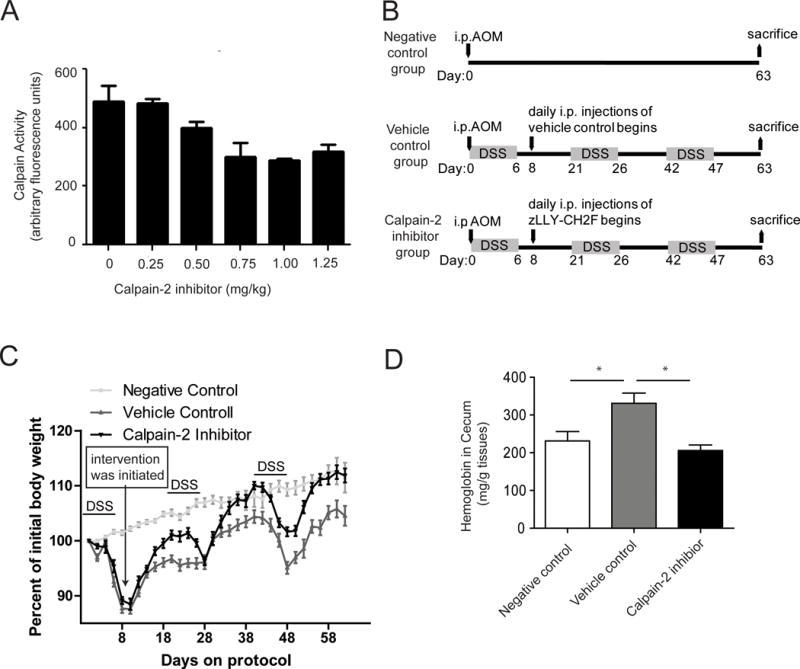
Calpain-2 inhibitor treatment reduces colitis. (A) A dose-response experiment was conducted in which mice were treated with 2% DSS for 4 days and i.p. injected daily with a calpain-2 inhibitor (zLLY-CH2F) at concentrations ranging from 0 to 1.25 mg/kg. Colons were harvested on day 4 and calpain activity measured (n = 5/group). (B) A chronic AOM/DSS protocol was modified to include intervention beginning on day 8 with daily i.p. injections of either vehicle control or 0.75 mg/kg calpain-2 inhibitor. (C) Mice were weighed every other day for the 63 d protocol (n = 20/group). Data represent mean ± SEM and weight curves were analyzed by a repeated measures one-way ANOVA (F2, 31= 82.02; P<0.0001) followed by Tukey post-test to compare groups at each day. (D) Bloody diarrhea was assessed on day 63 using an ELISA to measure hemoglobin in cecums (n = 5/group). Groups were analyzed using a one-way ANOVA followed by Tukey post-test. Data represent mean ± SEM. (E) The chronic AOM/DSS protocol was altered to 2.5% DSS for a Kaplan-Meyer survival curve (n=19/group) (p=0.234)
An established AOM/DSS model was next carried out to determine the effects of intervention with 0.75 mg/kg calpain-2 inhibitor on colitis (Figure 1B). Body weights were measured every other day and intervention with the calpain-2 inhibitor was initiated on day 8 after the initial appearance of weight loss. Results showed that the mice treated with the calpain-2 inhibitor had a significant increase in weight recovery after each administration of DSS compared to the weights of the mice injected with vehicle control (Figure 1C). Tukey post-test comparisons at each day between 16–26 showed that body weights in the calpain-2 inhibitor group were significantly higher than vehicle control group. In the later stages of the protocol, the weight recovery for mice treated with the calpain-2 inhibitor reached the levels of mice with no DSS administration. Tukey post-test comparisons at each day between 34–44 and 54–62 showed that body weights in the calpain-2 inhibitor group were significantly higher than vehicle control group but did not differ compared to the negative control group. Another sign of severe colitis for the AOM/DSS models is bloody diarrhea. Measurement of hemoglobin in the cecums by ELISA showed that mice treated with the calpain-2 inhibitor showed significantly lower levels of hemoglobin compared to vehicle control treated mice, with calpain-2 inhibitor treatment reducing hemoglobin to similar levels found in the negative control group (Figure 1D). Survival experiments were carried out following the same protocol (Supplemental Figure 1) with a 2.5% DSS concentration but the resulting Kaplain-Meier curve is was not statistically significant (p=0.234).
Colitis associated weight loss and bloody diarrhea are alleviated by inhibition of Calpain-2 activity
Histological evaluation of colon tissue in mice treated with the calpain-2 inhibitor showed a significant decrease in colitis pathology and inflammatory infiltration compared to vehicle control treated mice (Figure 2A–B). The histology scores were not completely reduced to the levels of the negative control mice, which suggests the calpain-2 inhibitor was not able to completely block tissue damage caused by DSS treatment and instead inhibited the ensuing inflammatory response that contributed to pathology. To further assess inflammation, mouse colon tissues were harvested at the end of the chronic colitis model and mRNA levels for different cytokines measured by real-time PCR. Results showed that calpain-2 inhibitor treatment lowered TNFα, interleukin (IL)-6, and monocyte chemoattractant protein (MCP)-1 mRNA levels compared to vehicle control treated mice and approached levels exhibited by the untreated control mice (Figure 2C). IFNγ also appeared to be decreased but the levels were not significantly different compared to vehicle control treated mice (p=0.211).
Figure 2.
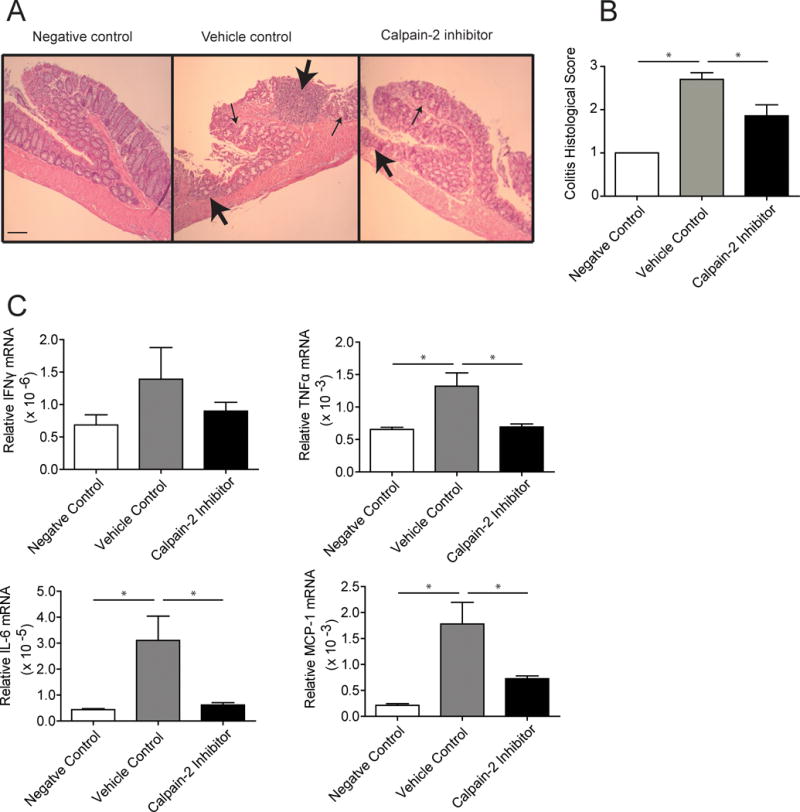
Calpain-2 inhibitor treatment reduces pathology and inflammation in colons. Mice subjected to the AOM/DSS model were sacrificed on day 63 and colons assessed by histology and cytokine mRNA analyses. (A) Paraffin embedded sections were H&E stained, with 20X representative images from each group shown (scalebar = 100 mm). Sections from vehicle control group exhibited severe colitis as indicated by a high degree of cellular infiltration into the submucosa and muscularis propria (large arrows) and highly disrupted crypt architecture (small arrows), while mice treated with the calpain-2 inhibitor showed less severe pathology. (B) Sections were blindly scored for colitis including 5 sections from each colon. (C) Real-time PCR was performed on total RNA extracted from colon tissues to assess levels of cytokine mRNAs. These experiments included 20 mice/group and were analyzed using a one-way ANOVA followed by Tukey post-test. Data represent mean + SEM with *p<0.05.
The mechanism of calpain-2 driven colon inflammation is macrophage activation through NFκB, and is independent of calpain-1
In macrophages, calpain cleaves IκB and releases NFκB that then translocates to the nucleus to activate transcription of inflammatory cytokine genes.20 and calpastatin was shown in our previous work to be important for limiting this signaling event.10 To determine if a similar mechanism may be contributing to the effect of the calpain-2 inhibition in the DSS model of colitis, macrophages were analyzed for NFκB activation and inflammatory cytokine secretion. Macrophages were primed with TNFα and activated with E. faecalis commensal bacteria in the presence of calpain-2 inhibitor and supernatents were evaluated for a panel of cytokines including interferon (IFN)γ, TNFα, IL-6, MCP-1, IL-10, and IL-12. Results showed that calpain-2, but not calpain-1, inhibitor treatment during priming and activation led to a significant decrease in the production of the inflammatory cytokines IFNγ, TNFα, IL-6, and MCP-1, while IL-10 and IL-12 were not significantly affected (Figure 3A). Importantly, those cytokines most affected by the calpain-2 are regulated by NFκB.
Figure 3.
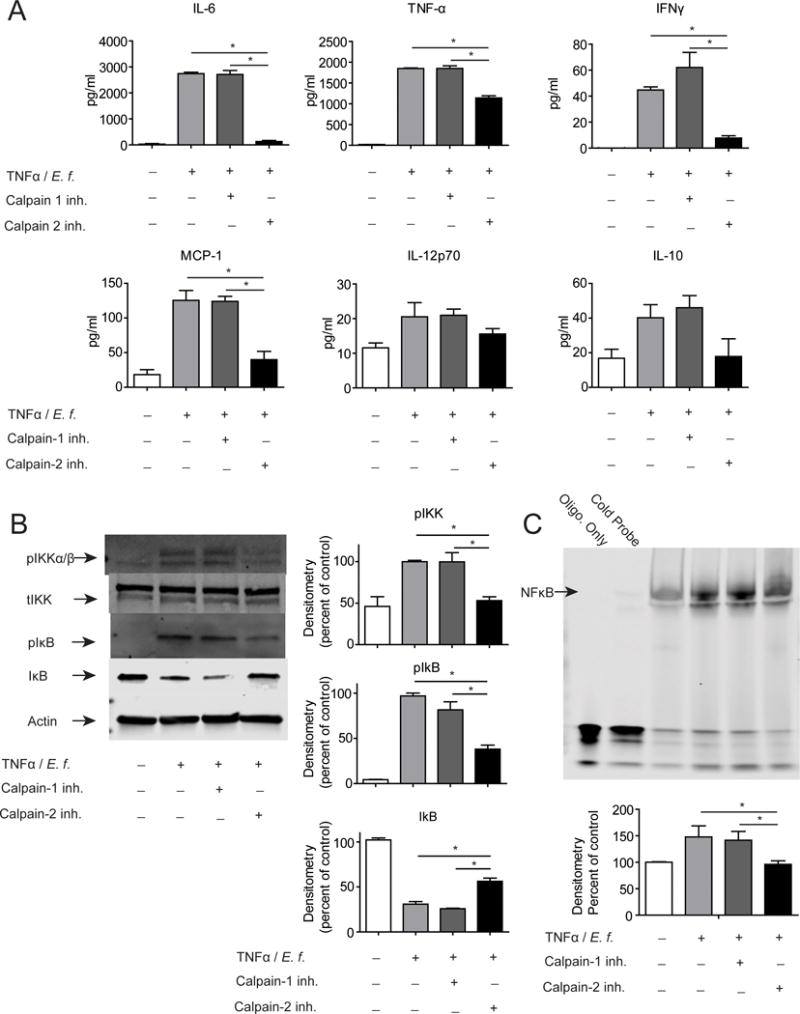
Macrophages treated with a calpain-2 inhibitor but not a calpain-1 inhibitor showed reduced secretion of inflammatory cytokines with a concurrent decrease in IκB degradation and NFκB DNA binding. BMDM were primed 18 h with TNFα (20 ng/mL) and activated with heat-killed E. faecalis (1 μg/mL) for 1 h. Calpain-2 inhibitor (20 μg/mL) or DMSO as a control were added during both priming and stimulation. (A) Cytokines in media were measured using a cytometric bead array. (B) Levels of phosphorylated and total IKKα/β and IκB in whole cell lysates were analyzed by Western blot, with β-actin serving as loading control for lysates. (C) Nuclear cell fractions were examined for NFκB DNA binding by EMSA assay using Licor IR700 oligonucleotides. Representative immunoblots are shown above with densitometric analyses shown below. Data are mean ± SEM from three independent experiments, analyzed by one-way ANOVA followed by Tukey post-test, *p < 0.05.
To analyze NFκB regulation, TNFα primed macrophages were activated with E. faecalis in the presence of the calpain-1 inhibitor, calpain-2 inhibitor, or DMSO as a control. Levels of total IκBα, phospho IκBα, total IKKα/β, and phospho IKKα/β were analyzed by Western blot with β-actin used as a loading control. Results show again that calpain-2, but not calpain-1, inhibition decreased phosphorylation of IKKα/β and IκBα leading to less degradation of IκBα (Figure 3B). Nuclear NFκB levels were analyzed by EMSA and western blots of nuclear fractions and showed decreased levels with the treatment of the calpain-2 inhibitor (Figure 3C) (Supplemental Figure 2). Calpain 1 KO mouse derived BMDM also show no inhibition of cytokines production apon activation (Supplemental Figure 3A). Specificity of the calpain-1 inhibitor was demonstrated by the addition of recombinant Selenoprotein K to calpain-1 in the presence of DMSO control, calpain-1, or calpain-2 inhibitor (Supplemental Figure 3B). Specificity of the calpain-2 inhibitor was demonstrated by the addition of recombinant IκB to calpain-2 in the presence of DMSO control, calpain-1, or calpain-2 inhibitor (Supplemental Figure 3B). Knockdown of calpains 1 and 2 with siRNA resulted in decreases in cytokine production by activated macrophages in calpain-2 knowckdowns only (Supplemental Figure 3C).
The cleavage of IκB by calpain-2 and the upstream inhibition of IKK phosphorylation may not be the only mechanisms controlling the inhibitor effects on macrophage inflammation. The AP-1 inflammatory activation pathway was interrogated by western blot to determine if calpain-2 inhibition could limit macrophage activation through inhibition of phosphorylation of ERK, JNK, or p38 and we found that none of these MAP kinases were effected by calpain-2 inhibition in macrophages (Supplemental Figure 4A). Other known targets of calpain-2 cleavage (FAK, Vimentin, Talin) were examined by western blot and their cleavage was also found to be inhibited (Supplemental Figure 4A).
Limiting colitis associated inflammation with a calpain-2 inhibitor limits downstream colon cancer
The secondary outcome of the AOM/DSS model is CAC, which was evaluated to determine effects of intervention with the calpain-2 inhibitor on colorectal cancer formation/progression. On day 63 of the AOM/DSS model the mice were sacrificed and the colons removed and imaged to determine tumor volumes. Mice treated with the calpain-2 inhibitor had significantly lower total tumor volume (reduced by 70%) compared to vehicle control treated mice (Figure 4A). A blinded histological assessment of the colons was performed using a 4-point system as described in the Methods section. The calpain-2 inhibitor treated mice showed significantly lower CAC pathology compared to vehicle control treated mice, although those mice treated with the inhibitor were not completely free of cancer (Figure 4B). For example, the mice treated with the calpain-2 inhibitor had less generalized tissue disruption, pleiomorphism, and hyperchromasia. In order to determine the effects of the calpain-2 inhibitor on proliferation in the crypts of the colon, PCNA Immunohistochemistry was performed (Figure 4C). The PCNA staining was increased in the vehicle control treated group while the calpain-2 inhibitor treated colons had the same number of positive cells per crypt as the negative control. Overall, intervention with the calpain-2 inhibitor reduced the development of CAC as assessed by macroscopic analyses and histology.
Figure 4.
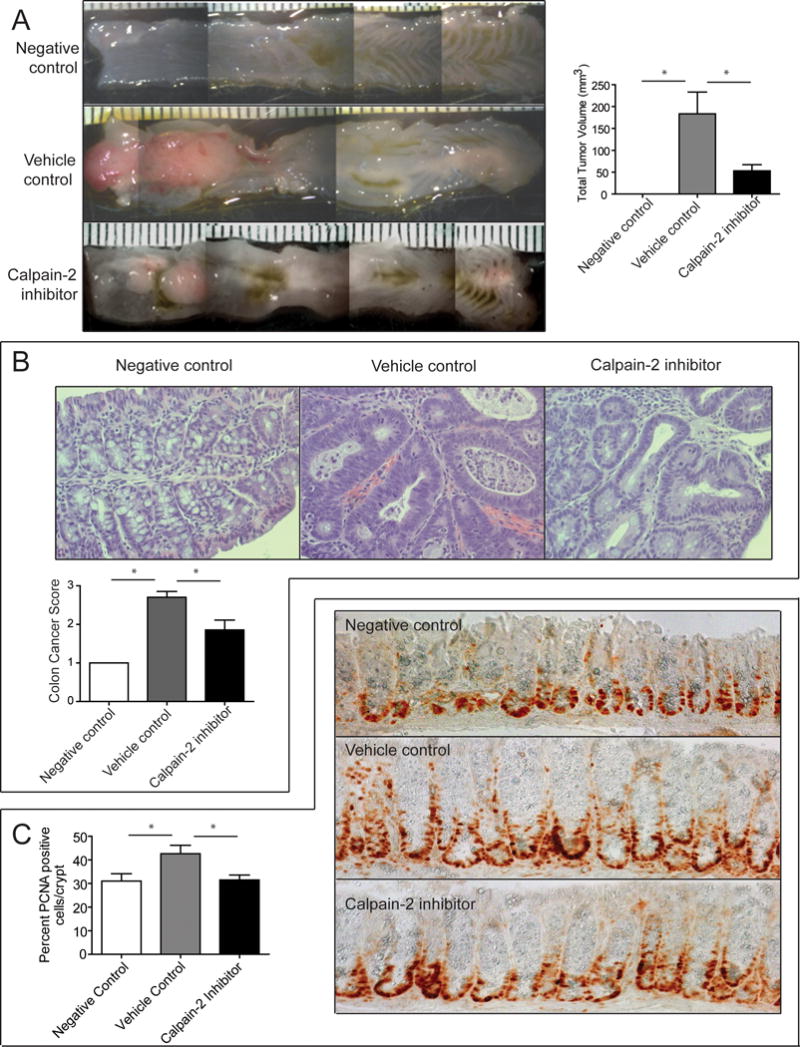
Calpain-2 inhibitor treatment results in smaller colorectal tumor volumes and reduced cancer pathology. (A) Mice subjected to the AOM/DSS model were assessed for tumor volume. Representative images show large colon tumors in the distal regions near the rectum (left side) and calpain-2 inhibitor treatment reduced the size of these tumors. The measurements of colon tumors from mice (n=20) showed a significant reduction in tumor volume. Data represent mean ± SEM analyzed by one-way ANOVA and *p<0.0001 by Tukey post-test. (B) Histological assessment of colon cancer pathology was performed at 40× as described in the Methods section. Slides were blindly scored by a Pathologist and calpain-2 inhibitor treatment reduced pathology scores compared to vehicle controls. Representative images are shown above. (C) PCNA immunohistochemical staining of crypt cells was assessed in a blinded manner. The calpain-2 inhibitor reduced the number of PCNA positive cells per crypt. Representative 40× images are shown to the right. Data represent mean + SEM (n=20) and means were compared by one-way ANOVA followed by Tukey post-test, *p<0.05.
Calpain-2 inhibition of colon cancer is due to both limitation of inflammation and inhibition of the growth of the colon cancer cells
The effect of the calpain-2 inhibitor on CAC was striking and could be attributed to reduced inflammation and/or direct inhibition of colorectal tumor progression. The fact that calpain-2 expression is upregulated in CAC and other cancers suggests that calpain-2 may function to promote the progression of the tumors.21,22 It has also been demonstrated that calpain activity can lead to more aggressive and chemotherapy resistant cancers.20,23 In order to determine if the calpain-2 inhibitor had any direct effects on CAC, we cultured mouse CT26.WT and human HT-29 colorectal cancer cell lines in the presence of the calpain-2 inhibitor. Calpain-2 activity in both cell lines was reduced which shows that calpain-2 is active in these cell lines (Figure 5A). We then examined the effects of calpain-2 inhibition on invasion, soft agar colony formation, and proliferation (Figure 5B-D). We found that each of these measures of cancer cell function had decreased by 20–35%. As shown above, treatment of activated macrophages with the calpain-2 inhibitor reduced IκB degradation and NFκB nuclear localization. Thus, Western blot analyses were performed on colorectal cancer cell lines treated with calpain-2 inhibitor to determine levels of IκBα, phospho IκBα, total IKKα/β, and phospho IKKα/β. Phospho IκBα and IKKα/β levels were undetectable by western blot but calpain-2 inhibition reduced IκB degradation by two-fold and DNA bound NFκB, as examined by EMSA, by ~25% in the colorectal cancer cell lines (Figure 5E). These results together with the results from the macrophage experiments suggest that the 70% reduction in colon tumors from the calpain-2 inhibitor is likely due to the combined effects of decreased inflammation and directly reduced tumor cell proliferation.
Figure 5.
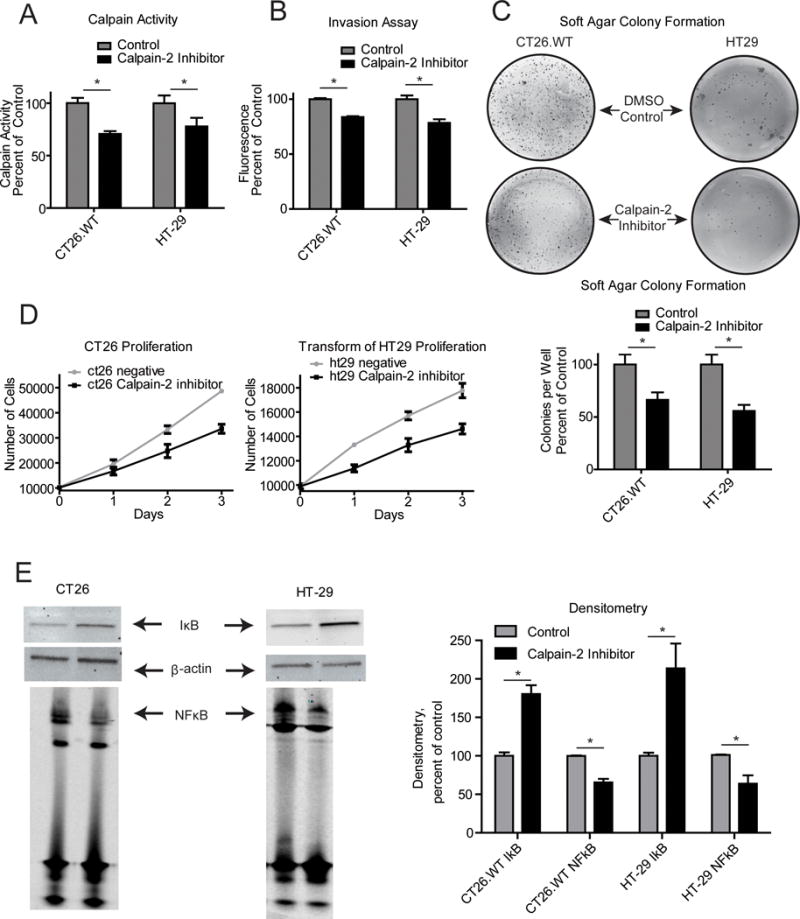
Calpain-2 inhibitor treatment of mouse and human colon cancer cells reduces proliferation with a concurrent decrease in IκB degradation and NFκB nuclear localization. (A) Cells were serum starved in RPMI with 0.2% serum in the presence of calpain-2 inhibitor or DMSO for 1 h. Calpain activity was measured using a fluorescence assay. The calpain-2 inhibitor was able to lower calpain activity in both cell lines (B) Cells were plated in matrigel coated wells in serum starved media with 10%FBS media in the larger well for a period of 24 hrs. Invasion was assessed by MTS reagent after removal of cells and matrigel from the upper well. Calpain-2 inhibition decreased invasion in both cell lines. (C) Soft agar colony formation assays were performed by plating 104 cells per well in a 6-well plate in 0.7% agarose. Media was changed with the addition of DMSO or calpain-2 inhibior every 3 days for a period of 3 wks. Calpain-2 inhibition decreased the number of colonies per well in both cell lines. (D) CT26 mouse colon cancer cells and HT-29 human colorectal cancer cells were plated at a concentration of 104 cells per well in a 96-well plate with media containing either calpain-2 inhibitor (20 μg/mL) or DMSO. Cells were enumerated each day using an MTS assay. (E) Western blots were performed on cell lysates for IκB. Equivalent loading was confirmed by detection of β-actin. Nuclear cell fractions were examined for NFκB DNA binding by EMSA assay using Licor IR700 oligonucleotides in three independent experiments. Data represent mean + SEM and means were compared by students t-test, *p<0.05.
Discussion
Emerging evidence published by our laboratory and others suggests that calpains play an important role in regulating inflammation and immune responses.24,25 The calpain protease system and its dynamic role in macrophage activation represent a promising target of potential drug intervention for inflammation driven disease. The previous studies demonstrating that calpastatin increases in chronically inflamed tissues as a physiological mechanism to limit inflammation suggested to us that assisting this process through therapeutic intervention with a calpain-2 inhibitor would suppress the pathology of colitis in the AOM/DSS model. The findings presented in this study reveal two important elements of the calpain-2 inhibitor. First, this potential therapeutic strategy may provide a two-hit effect by both reducing inflammation and directly inhibiting CAC cell proliferation. Second, initiating intervention after the appearance of symptoms was still effective in providing protection against colitis severity and CAC development. It is important to note that the survival data in Supplemental figure 1 did not reach statistical significance and this may have resulted due to one or more of the following possibilities: the model shows injury is reduced with the inhibitor, but overall numbers of mice dying from colorectal cancer was not enough to show a significant effect on survival; when we discontinued the use of the inhibitor on day 60 the cancers in the treated mice began to grow rapidly and catch up with the control group; the n of groups was not large enough to achieve statistical significance, the optimal dosage for the treatment was not achieved.
Our previous work showed that the endogenous inhibitor of calpains, calpastatin, was upregulated predominantly in macrophages isolated from inflamed colon tissues of mice subjected to the DSS model of chronic colitis.10 While our treatment with the calpain-2 inhibitor was delivered systemically, we suspect that its major anti-inflammatory effects were exerted through inhibiting NFκB signaling in intestinal macrophages. This notion was supported by our data showing calpain-2 inhibitor treatment resulted in reduced NFκB translocation and inflammatory cytokine secretion by activated BMDM. Many current studies have focused on inhibiting inflammatory mediators downstream of NFκB translocation.26,27 Inflammatory cytokines such as TNFα have been shown to increase in intestinal tissues and peripheral phagocytes of patients with IBD.28 This has led to the development of monoclonal antibodies against TNF-α for treating inflammation in IBD and other inflammatory disorders,29 although some patients do not respond to this treatment and other inflammatory mediators may contribute to this effect.8,30
Our approach using a calpain-2 inhibitor works upstream of cytokine blockers by reducing the actions of NFκB and therefore may simultaneously reduce several inflammatory mediators. By inhibiting the initial production of inflammatory mediators such as IL-6, IFNγ, and MCP-1 in addition to TNFα this may be a more effective treatment. However, calpains catalytically modulate a variety of target proteins including cytoskeletal proteins, membrane receptors, calmodulin binding proteins, G proteins, protein kinase C, other enzymes involved in signal transduction, and transcription factors.11 Thus, using a calpain inhibitor for reducing inflammatory mediators may induce side effects that depend on other calpain functions. Our use of a specific calpain-2 inhibitor reduces this potential effect by leaving calpain-1 activity intact14, but safety issues in applying our pre-clinical findings to human trials must be considered. In fact, our approach may be improved upon by modifying the inhibitor or the delivery mechanism to enhance intestine-specific delivery of the calpain-2 inhibitor. This approach has recently emerged as a very attractive means for directing the effects of therapeutics in the intestinal tissues.31,32 With this in mind, we are currently investigating methods to encapsulate the zLLY-CH2F for specific delivery and release in the intestinal tissues.
Our data showing anti-inflammatory effects of the calpain-2 inhibitor are consistent with the anti-inflammatory effects of the endogenous calpain inhibitor, calpastatin, and suggest that calpain-2 is the predominant isoform of this enzyme family driving macrophage hyperactivation and severe colitis in this model. However, we were surprised to find direct effects of zLLY-CH2F on NFκB nuclear localization in colorectal cancer cell lines and their proliferative capacity. The role of different calpain family members in cell death, apoptosis, and cancer is complex.33,34 Calpains can be phosphorylated by ERK, a classic pro-growth signaling molecule, and this phosphorylation has been shown to be related to changes in mobility and tissue invasion.35 Calpain expression has been correlated with chemotherapeutic resistance in colorectal cancer, esophageal cancer, and melanoma.21,36,37 This resistance has been specifically correlated with calpain-2 activity. In melanoma, the correlation has been extended to survival outcomes with tumors expressing high levels of calpain-2 resulting in shorter survival for patients.37,38 Importantly, calpain proteolytic activity has been shown to increase NFκB signaling in melanoma cancer cells and treatment with calpain inhibitor was able to attenuate this signaling in cisplatin resistant cells.38 Other researchers have demonstrated a correlation between calpain expression and drug resistant esophageal cancer.39 These correlations are further evidence pointing to the need for further investigation of the involvement of the calpain/calpastatin system in carcinogenesis and tumor progression. Overall, our data indicate that calpain-2 inhibition protects from chronic inflammation in IBD and can prevent pathology of the gut arising from this chronic inflammation as well as directly inhibit growth of the colon cancer cells themselves. The combination of effects on the inflammation and the colorectal cancer suggest this approach could prove to be an effective inflammation treatment.
Supplementary Material
Supplemental FIgure 1. A Kaplain-Meier survival curve was generated from an AOM and 2.5% DSS colitis model (n=20). There were no statistically significant differences in survival of the groups, Log-Rank test p=0.2831.
Supplemental FIgure 2. (A) Levels of NFκB in nuclear fractions were analyzed by Western blot, with β-actin and TATA binding protein serving as loading controls for lysates and nuclear fractions. Representative immunoblots are shown on the left with densitometric analyses shown on the right. (B) NFκB protein levels were detected by anti-NFκB membrane probe after in-gel EMSA assay to verify that the shifted bands of the EMSA could be positively identified as NFκB. Cold probe sample was positive control (TNFα nad treated with 50x unlabeled DNA probe. Data are mean ± SEM from three independent experiments, analyzed by one-way ANOVA followed by Tukey post-test, *p < 0.05.
Supplemental Figure 3. Calpain-2 siRNA knock down has similar effects in production of inflammatory cytokines to the Calpain-2 inhibitor. (A) BMDM derived from WT mice treated with calpain-2 siRNA were primed 18 h with TNFα (20 ng/mL) and activated with heat-killed E. faecalis (1 μg/mL) for 1 h. Calpain-2 inhibitor (20 μg/mL) or DMSO as a control were added during both priming and stimulation. Western blots were performed to verify protein KO or siRNA knock down with βctin as loading control. Cytokines in media were measured using a cytometric bead array. (B) Calpain-1 and 2 inhibitors were analysed for specificity with the combination of recombinant calpain-1 (1μg) with Selenoprotein K (50 μg) and calpain-2 (1 μg) with IκB (50 μg) in buffer containing 50 mM Tris-HCl pH 7.5, 10 mM CaCl2, 30 mM NaCl, 5 mM DTT. (C) Calpain-1 and 2 inhibitors were applied (20ug/ml) to lysates from mouse colons after 3 days of DSS treatment. The resulting total calpain activity was measured.
Supplemental Figure 4. Calpain-2 inhibition in BMDM and colon cancer cells show different effects on proteolytic activity. (A) Western blots were performed on BMDM treated with DMSO, calpain-1 inhibitor, or calpain-2 inhibitor. These blots were stained with antibodies for the known targets of calpain-2 specific cleavage; FAK, Vimentin, and Talin, with β-actin serving as loading control for lysates. The results show the calpain-2 inhibitor alone decreases degradation products of FAK and Vimentin. (B) CT26.WT and HT-29 colon cancer cells were serum starved in RPMI with 0.2% serum in the presence of calpain-2 inhibitor or DMSO for 1 h and western blots were performed. Representative immunoblots are shown to the left with densitometric analyses shown on the right. Data are mean ± SEM from three independent experiments, analyzed by one-way ANOVA followed by Tukey post-test or students t-test, *p < 0.05.
Acknowledgments
This work was supported by awards from the NIH and donor support to the University of Hawaii Cancer Center through the University of Hawai’i foundation.
This research was supported by the University of Hawaii Cancer Center and NIH grant R01AI089999, and core facilities supported by P20GM103516, P20RR016453, G12RR003061, G12MD007601.
Abbreviations
- 5-ASA
5-aminosalicylic acids
- AOM
azoxymethane
- BMDM
bone marrow derived macrophages
- CAC
colitis associated cancer
- DSS
dextran sulfate sodium
- ELISA
Enzyme-linked immunosorbant assay
- IFN
interferon
- IκB
inhibitor of kappa beta
- IL
interleukin
- JAK
Janus kinase
- MCP-1
monocyte chemoattractant protein −1
- NFκB
nuclear factor kappa beta
- TNF-α
tumour necrosis factor-α
Footnotes
Author Contributions: PRH was involved in study concept and design; analysis and interpretation of data; drafting of the manuscript. AHR was involved with acquisition, analysis and interpretation of data; drafting of the manuscript. ZH, CM, FWH, ASH, and PB were involved in acquisition, analysis and interpretation of data
References
- 1.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachar DB. Cancer in Crohn’s disease: dispelling the myths. Gut. 1994;35:1507–1508. doi: 10.1136/gut.35.11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–1816. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 5.Paul S, Del Tedesco E, Marotte H, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19:2568–2576. doi: 10.1097/MIB.0b013e3182a77b41. [DOI] [PubMed] [Google Scholar]
- 6.Scaldaferri F, Petito V, Lopetuso L, et al. Pre- and posttherapy assessment of intestinal soluble mediators in IBD: where we stand and future perspectives. Mediators Inflamm. 2013;2013:391473. doi: 10.1155/2013/391473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2013 doi: 10.1136/gutjnl-2013-305607. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 9.Sokol H, Seksik P, Cosnes J. Complications and surgery in the inflammatory bowel diseases biological era. Curr Opin Gastroenterol. 2014;30:378–384. doi: 10.1097/MOG.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 10.Huang Z, Rose AH, Hoffmann FW, et al. Calpastatin prevents NF-κB-mediated hyperactivation of macrophages and attenuates colitis. J Immunol. 2013;191:3778–3788. doi: 10.4049/jimmunol.1300972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saido TC, Sorimachi H, Suzuki K. Calpain: new perspectives in molecular diversity and physiological-pathological involvement. FASEB J. 1994;8:814–822. [PubMed] [Google Scholar]
- 12.Brown N, Crawford C. Structural modifications associated with the change in Ca2+ sensitivity on activation of m-calpain. FEBS Lett. 1993;322:65–68. doi: 10.1016/0014-5793(93)81112-d. [DOI] [PubMed] [Google Scholar]
- 13.duVerle D, Takigawa I, Ono Y, et al. CaMPDB: a resource for calpain and modulatory proteolysis. Genome Inform. 2010;22:202–213. [PubMed] [Google Scholar]
- 14.Huang Z, Hoffmann FW, Norton RL, et al. Selenoprotein K is a novel target of m-calpain, and cleavage is regulated by Toll-like receptor-induced calpastatin in macrophages. J Biol Chem. 2011;286:34830–34838. doi: 10.1074/jbc.M111.265520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, McDonald MC, Mazzon E, et al. Calpain inhibitor I reduces colon injury caused by dinitrobenzene sulphonic acid in the rat. Gut. 2001;48:478–488. doi: 10.1136/gut.48.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballinger A, Azooz O. Calpain inhibitor I and colonic inflammation induced by DNBS in the rat. Gut. 2002;50:440–441. doi: 10.1136/gut.50.3.440-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akitake-Kawano R, Seno H, Nakatsuji M, et al. Inhibitory role of Gas6 in intestinal tumorigenesis. Carcinogenesis. 2013;34:1567–1574. doi: 10.1093/carcin/bgt069. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann PR, Jourdan-Le Saux C, Hoffmann FW, et al. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007;179:3258–3267. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz S, Neufert C, Weigmann B, et al. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 20.Fenouille N, Grosso S, Yunchao S, et al. Calpain 2-dependent IκBα degradation mediates CPT-11 secondary resistance in colorectal cancer xenografts. J Pathol. 2012;227:118–129. doi: 10.1002/path.3034. [DOI] [PubMed] [Google Scholar]
- 21.Leloup L, Wells A. Calpains as potential anti-cancer targets. Expert Opinion on Therapeutic Targets. 2011;15:309–323. doi: 10.1517/14728222.2011.553611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu T, Mendes DE, Berkman CE. Prolonged androgen deprivation leads to overexpression of calpain 2: implications for prostate cancer progression. Int J Oncol. 2014;44:467–472. doi: 10.3892/ijo.2013.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Storr SJ, Safuan S, Woolston CM, et al. Calpain-2 expression is associated with response to platinum based chemotherapy, progression-free and overall survival in ovarian cancer. J Cell Mol Med. 2012;16:2422–2428. doi: 10.1111/j.1582-4934.2012.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deshpande RV, Goust JM, Chakrabarti AK, et al. Calpain expression in lymphoid cells. Increased mRNA and protein levels after cell activation. J Biol Chem. 1995;270:2497–2505. doi: 10.1074/jbc.270.6.2497. [DOI] [PubMed] [Google Scholar]
- 25.Svensson L, McDowall A, Giles KM, et al. Calpain 2 controls turnover of LFA-1 adhesions on migrating T lymphocytes. PLoS ONE. 2010;5:e15090. doi: 10.1371/journal.pone.0015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perrier C, Rutgeerts P. New drug therapies on the horizon for IBD. Dig Dis. 2012;30(Suppl 1):100–105. doi: 10.1159/000341133. [DOI] [PubMed] [Google Scholar]
- 27.Allocca M, Jovani M, Fiorino G, et al. Anti-IL-6 treatment for inflammatory bowel diseases: next cytokine, next target. Curr Drug Targets. 2013;14:1508–1521. doi: 10.2174/13894501113146660224. [DOI] [PubMed] [Google Scholar]
- 28.Zipperlen K, Peddle L, Melay B, et al. Association of TNF-alpha polymorphisms in Crohn disease. Hum Immunol. 2005;66:56–59. doi: 10.1016/j.humimm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Lapadula G, Marchesoni A, Armuzzi A, et al. Adalimumab in the treatment of immune-mediated diseases. Int J Immunopathol Pharmacol. 2014;27:33–48. doi: 10.1177/03946320140270S103. [DOI] [PubMed] [Google Scholar]
- 30.Bank S, Andersen PS, Burisch J, et al. Associations between functional polymorphisms in the NFκB signaling pathway and response to anti-TNF treatment in Danish patients with inflammatory bowel disease. Pharmacogenomics J. 2014 doi: 10.1038/tpj.2014.19. [DOI] [PubMed] [Google Scholar]
- 31.Suneela D, Gaurav V, Himanshu R. Design and development of novel azo prodrugs using various permutations and combinations of 5- and 4-aminosalicylic acids for inflammatory bowel disease: a colon-targeted approach. Inflamm Allergy Drug Targets. 2013;12:328–340. doi: 10.2174/18715281113129990059. [DOI] [PubMed] [Google Scholar]
- 32.Adebisi A, Conway BR. Gastroretentive microparticles for drug delivery applications. J Microencapsul. 2011;28:689–708. doi: 10.3109/02652048.2011.590613. [DOI] [PubMed] [Google Scholar]
- 33.Łopatniuk P, Witkowski JM. Conventional calpains and programmed cell death. Acta Biochim Pol. 2011;58:287–296. [PubMed] [Google Scholar]
- 34.Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. doi: 10.1016/s1357-2725(02)00069-9. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Deng X. Protein Kinase Cι Promotes Nicotine-induced Migration and Invasion of Cancer Cells via Phosphorylation of μ- and m-Calpains. J Biol Chem. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- 36.Ho W, Pikor L, Gao Y, et al. Calpain 2 Regulates Akt-FoxO-p27Kip1 Protein Signaling Pathway in Mammary Carcinoma. J Biol Chem. 2012;287:15458–15465. doi: 10.1074/jbc.M112.349308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raimbourg Q, Perez J, Vandermeersch S, et al. The Calpain/Calpastatin System Has Opposing Roles in Growth and Metastatic Dissemination of Melanoma. In: Mattei F, editor. PLoS ONE. Vol. 8. 2013. p. e60469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Młynarczuk-Biały I, Roeckmann H, Kuckelkorn U, et al. Combined Effect of Proteasome and Calpain Inhibition on Cisplatin-Resistant Human Melanoma Cells. Cancer Res. 2006;66:7598–7605. doi: 10.1158/0008-5472.CAN-05-2614. [DOI] [PubMed] [Google Scholar]
- 39.Liu T-L, Shimada H, Ochiai T, et al. Enhancement of chemosensitivity toward peplomycin by calpastatin-stabilized NF-κB p65 in esophageal carcinoma cells: possible involvement of Fas/Fas-L synergism. Apoptosis. 2006;11:1025–1037. doi: 10.1007/s10495-006-6353-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental FIgure 1. A Kaplain-Meier survival curve was generated from an AOM and 2.5% DSS colitis model (n=20). There were no statistically significant differences in survival of the groups, Log-Rank test p=0.2831.
Supplemental FIgure 2. (A) Levels of NFκB in nuclear fractions were analyzed by Western blot, with β-actin and TATA binding protein serving as loading controls for lysates and nuclear fractions. Representative immunoblots are shown on the left with densitometric analyses shown on the right. (B) NFκB protein levels were detected by anti-NFκB membrane probe after in-gel EMSA assay to verify that the shifted bands of the EMSA could be positively identified as NFκB. Cold probe sample was positive control (TNFα nad treated with 50x unlabeled DNA probe. Data are mean ± SEM from three independent experiments, analyzed by one-way ANOVA followed by Tukey post-test, *p < 0.05.
Supplemental Figure 3. Calpain-2 siRNA knock down has similar effects in production of inflammatory cytokines to the Calpain-2 inhibitor. (A) BMDM derived from WT mice treated with calpain-2 siRNA were primed 18 h with TNFα (20 ng/mL) and activated with heat-killed E. faecalis (1 μg/mL) for 1 h. Calpain-2 inhibitor (20 μg/mL) or DMSO as a control were added during both priming and stimulation. Western blots were performed to verify protein KO or siRNA knock down with βctin as loading control. Cytokines in media were measured using a cytometric bead array. (B) Calpain-1 and 2 inhibitors were analysed for specificity with the combination of recombinant calpain-1 (1μg) with Selenoprotein K (50 μg) and calpain-2 (1 μg) with IκB (50 μg) in buffer containing 50 mM Tris-HCl pH 7.5, 10 mM CaCl2, 30 mM NaCl, 5 mM DTT. (C) Calpain-1 and 2 inhibitors were applied (20ug/ml) to lysates from mouse colons after 3 days of DSS treatment. The resulting total calpain activity was measured.
Supplemental Figure 4. Calpain-2 inhibition in BMDM and colon cancer cells show different effects on proteolytic activity. (A) Western blots were performed on BMDM treated with DMSO, calpain-1 inhibitor, or calpain-2 inhibitor. These blots were stained with antibodies for the known targets of calpain-2 specific cleavage; FAK, Vimentin, and Talin, with β-actin serving as loading control for lysates. The results show the calpain-2 inhibitor alone decreases degradation products of FAK and Vimentin. (B) CT26.WT and HT-29 colon cancer cells were serum starved in RPMI with 0.2% serum in the presence of calpain-2 inhibitor or DMSO for 1 h and western blots were performed. Representative immunoblots are shown to the left with densitometric analyses shown on the right. Data are mean ± SEM from three independent experiments, analyzed by one-way ANOVA followed by Tukey post-test or students t-test, *p < 0.05.


