Abstract
Dopaminergic projections from the ventral midbrain to the nucleus accumbens (NAc) have long been implicated in encoding associations between reward availability and environmental stimuli. As such, this circuit is instrumental in guiding behaviors towards obtaining maximal rewards based on previous experience. Cocaine acts on the dopamine system to exert its reinforcing effects and it is thought that cocaine-induced dysregulation of dopamine neurotransmission contributes to the difficulty cocaine addicts exhibit in selecting environmentally appropriate behaviors. Here we used cocaine self-administration combined with in vivo fast scan cyclic voltammetry in anesthetized rats to examine the function of ventral tegmental area (VTA) to NAc projection neurons. Over 5 days of cocaine self-administration animals increased their rate of intake (fixed-ratio 1; 1.5 mg/kg/inj; 40 inj/day). Following cocaine self-administration, there was a marked reduction in VTA-stimulated NAc dopamine release. Additionally, there was a decreased augmentation of stimulated dopamine overflow in response to a cocaine challenge. These findings demonstrate that cocaine induces a hypodopaminergic state, which may contribute to the inflexible drug taking and seeking behaviors observed in cocaine abusers. Additionally, tolerance to the ability of cocaine to elevate dopamine may lead to increased cocaine intake in order to overcome decreased effects, another hallmark of cocaine abuse.
Keywords: Hypodopamine, In Vivo, Nucleus Accumbens, Rat, Tolerance
Graphical Abstract
Introduction
The nucleus accumbens (NAc) is situated at the confluence of a wide array of afferents relaying information concerning emotional salience, predicted outcomes and contextual relevance of environmental inputs (Morgenson et al., 1980). This includes a dense innervation of dopaminergic afferents projecting from the ventral tegmental area (VTA) (Doucet et al., 1986) which respond in accordance with previously learned environmental contingences in order to guide goal-directed motor outputs (Waelti et al., 2001; Tobler et al., 2005). Proper function of this circuit is critical for the selection and performance of environmentally appropriate behaviors. Disruption of dopamine neurotransmission diminishes responding for natural (Woolverton and Virus, 1989; Thanos et al., 2008) and psychostimulant reinforcers (Woolverton and Virus, 1989; Thanos et al., 2008; Ritz et al., 1987; Roberts et al., 1980) and prevents learning of associations between rewards and the discrete and contextual cues that predict their availability (Taylor et al., 1986; Ranaldi et al., 1993). As such, drug-induced alterations in dopaminergic neurotransmission are hypothesized to, at least in part, mediate maladaptive and inelastic behaviors characteristic of drug addiction. For example, reductions in the functioning of this system following repeated drug administration may result in an inability to dynamically modulate behaviors in an environmentally appropriate manner, and thus may contribute to a cycle of repeated relapses in response to cue exposure in drug abusers despite negative physical, social and financial outcomes (Graybiel, 1995, 2008).
One phenomenon that has been observed in examinations of cocaine dependent humans is a marked decrease in the ability of cocaine to elevate NAc dopamine levels (Volkow et al., 1996, 1997, 2006). Previous work from our laboratory has effectively modeled the cocaine tolerance observed in humans by demonstrating a decreased ability of cocaine to inhibit the dopamine transporter (DAT) following cocaine self-administration in rats (Ferris et al., 2011, 2012; Calipari et al., 2013; Siciliano et al., 2015). Demonstrations of tolerance have been limited to ex vivo slice preparations, which assessed cocaine effects at the dopamine terminal in isolation. Further, dopamine signaling in response to cocaine-associated cues has been shown to decrease over the course of cocaine self-administration (Wiluhn et al., 2014); however it remains to be determined if these alterations are occurring directly in VTA dopamine neurons or are a result of deficits to afferent inputs onto dopamine neurons. Here we used fast scan cyclic voltammetry to examine the effects of cocaine self-administration on dopamine system function and cocaine potency.
We found that following cocaine self-administration, electrically-stimulated dopamine release from the VTA to NAc core projection was severely blunted. Additionally, we found that the ability of cocaine to increase electrically-stimulated dopamine release was attenuated in cocaine self-administration animals. Together, these data demonstrate that cocaine induces profound hypofunction of the mesolimbic dopamine circuit which is not ameliorated by cocaine and may underlie anhedonia during withdrawal and contribute to blunted self-reported effects of cocaine observed in cocaine addicts.
Methods
Animals
Adult, male Sprague-Dawley rats (325–375g; n = 5 control, n = 4 self-administration) were housed in pairs on a 12:12 h light:dark cycle with food and water available ad libitum. All protocols and animal care procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
Self-Administration
Rats were anesthetized and implanted with chronic indwelling jugular catheters as previously described (Calipari et al., 2014). Animals were singly housed, and each 6 hour session took place in the home cage during the active/dark cycle (0900– 1500 hours). Without any prior operant training, animals were given access on a fixed-ratio one schedule to a cocaine-paired lever, which, upon responding, initiated an intravenous injection of cocaine (1.5 mg/kg, infused over ~4s, depending on animal weight). After each response/infusion, the lever was retracted and a stimulus light was illuminated for a 20-s timeout period. Sessions lasted 6 hours or until 40 injections were taken. Under these conditions, all animals acquired a stable pattern of intake within 1 to 5 days. Acquisition (Day 1) was counted when the animal reached 35 or more responses with a stable and consistent inter-injection interval. Following acquisition, the animals were given access to 40 injections per day for a period of five consecutive days before voltammetry experiments. Control animals were naive rats housed under the same reversed light–dark light cycle for at least 1 week prior to voltammetry experiments.
In Vivo Voltammetry
Rats were anesthetized with urethane (1.5 g/kg, i.p.) and placed in a stereotaxic apparatus. A stimulating electrode was lowered into the VTA (from bregma: −5.2 A/P, +1.1 M/L, −7.0 D/V), and a carbon fiber electrode was initially lowered into the caudate putamen (from bregma: +1.3 A/P, +1.3 M/L, −4.5 D/V), until a 1 second stimulation train elicited dopamine (60 pulse; 60 Hz; monophasic; 2 ms pulse width; 7.5 μA). These stimulation parameters were chosen because preliminary studies revealed detection of dopamine using lower number of pulses was prohibitively difficult in cocaine self-administering rats (if not impossible), which speaks to the hypofunctioning dopamine system in these animals. Extracellular dopamine was recorded by applying a triangular waveform (−0.4 to +1.2 to −0.4V vs Ag/AgCl, 400 V/s) scanning every 100 ms. Once stimulator and carbon fiber electrode locations achieved adequate levels of release in the caudate putamen, the carbon fiber electrode was lowered 2 mm further into the NAc core. The NAc core was selected because of its instrumental role in guiding the selection and execution of motivated behaviors based on previously learned environmental contingencies (Humphries and Prescott, 2010). Once the peak-height of the extracellular dopamine response was stable (less than 10% variation in across three consecutive stimulations spaced 5 minutes apart), animals were given an injection of cocaine (10 mg/kg, i.p.).
Data Analysis
Demon Voltammetry and Analysis software was used (Yorgason et al., 2011) for all analysis of fast scan cyclic voltammetry data. Recording electrodes were calibrated by recording responses (in electrical current; nA) to a known concentration of dopamine (3 μM) using a flow-injection system. This was used to convert electrical current to dopamine concentration.
Statistics
Graph Pad Prism (version 6, La Jolla, CA, USA) was used to statistically analyze data sets and create graphs. Baseline dopamine release data subject to a Student’s t-test. Cocaine challenge data were subject to a repeated measures two-way analysis of variance (ANOVA) with treatment group as the between subjects factor and time as the within subjects factor. When main effects were obtained, differences between groups were tested using a Bonferroni post hoc test. p values of < 0.05 were considered to be statistically significant.
Results
Rate of cocaine intake escalates over days
Animals completed 5 consecutive days of cocaine self-administration (1.5 mg/kg/inj), with a maximum of 40 injections per day. Injections per day were held constant to avoid potential differences in neurochemical effects due to differential cocaine intake. Consistent with previous results with this procedure, animals completed the 40 injection maximum in less time each day (Figure 1A). A one-way ANOVA revealed a main effect of session on rate of cocaine intake (Figure 1B; F4, 15 = 3.55, p = 0.0314). Bonferroni post hoc analysis revealed that infusions per hour were higher on day 5 compared to day 1 (p < 0.05), indicating that animals increased their rate of intake over days.
Figure 1. Rate of cocaine intake escalates over 5 days of self-administration.
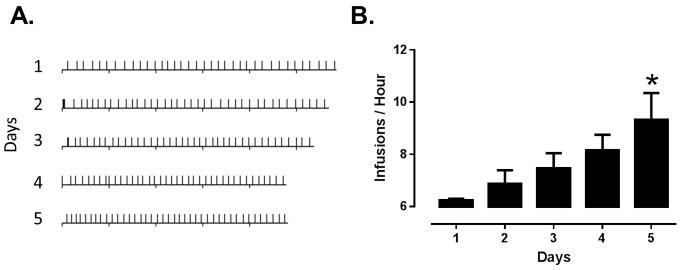
(A) Event records of 5 days of cocaine self-administration from a representative animal. Downward tics denote hours while upward tics denote infusions. Over the course of 5 days, 40 injection sessions are completed in a shorter amount of time. (B) Group data indicating that animals increased rate of cocaine intake over days. *, p < 0.05 vs. day 1
Cocaine self-administration results in attenuated stimulated dopamine
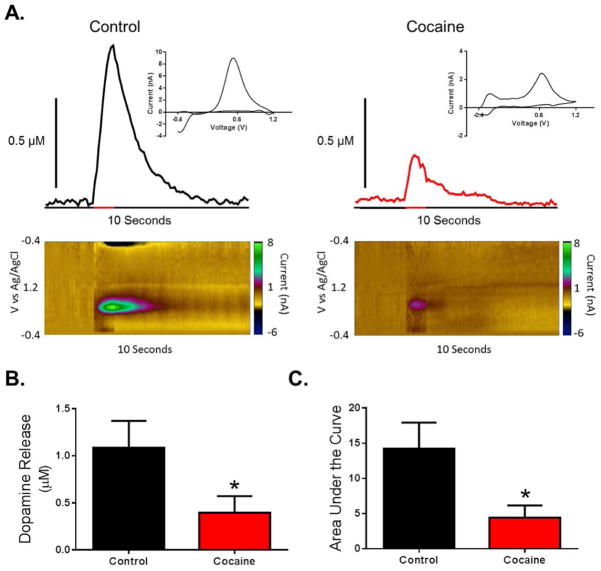
Approximately 18 hours following cessation of the final cocaine self-administration session (i.e., the following morning), animals were anesthetized and a recording electrode and stimulating electrode were lowered into the NAc core and VTA. We found that cocaine self-administration resulted in a robust hypodopaminergic state (Figure 2A). Indeed, dopamine release magnitude was greatly attenuated in cocaine self-administration animals as compared to controls (Figure 2B; t7 = 1.92, p = 0.048). Finally, we found that area under the stimulated dopamine transient curve was reduced in cocaine self-administration animals, further indicating hypofunction of the VTA to NAc projection neurons (Figure 2C; t7 = 2.18, p = 0.033).
Figure 2. Cocaine self-administration results in reduced dopamine release in the NAc following VTA stimulation.
(A) Representative traces and pseudo-color plots from control (left) and cocaine self-administration (right) animals demonstrating decreased stimulated (60 pulse, 60 Hz, denoted by red bar on x-axis) dopamine release following cocaine self-administration. Inset: cyclic voltammogram from peak of representative trace. (B) Amplitude of dopamine release is decreased in cocaine self-administration animals. (C) Area under the curve is decreased in cocaine self-administration animals. *, p < 0.05 vs. control
Cocaine self-administration results in tolerance to the dopamine elevating effects of cocaine
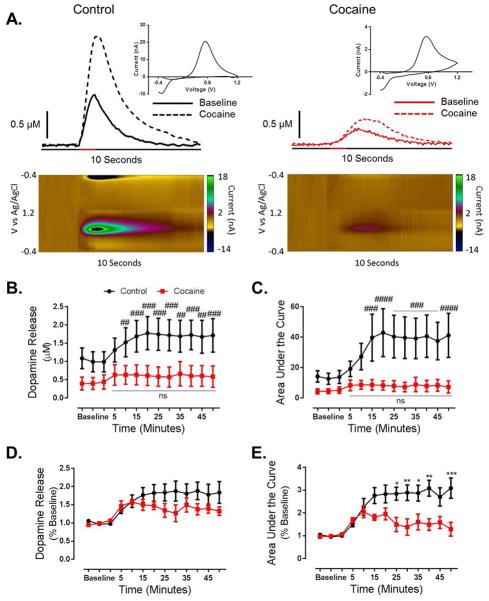
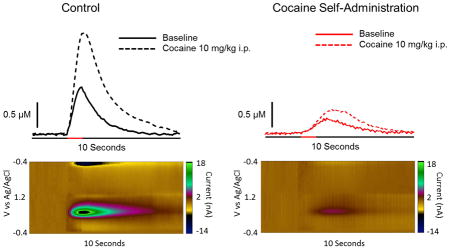
We then determined the effects of cocaine self-administration on the dopaminergic responsiveness to cocaine by administering a cocaine challenge (10 mg/kg, i.p.). Cocaine was injected immediately following the final baseline collection. We found that cocaine-induced increases in stimulated dopamine were blunted in cocaine self-administering animals (Figure 3A). Following a cocaine challenge, a two-way repeated measures ANOVA revealed a main effect of time (Figure 3B; F12, 84 = 5.83, p < 0.0001) on dopamine release, as well as a group x time interaction (F12, 84 = 2.132, p = 0.0229). Bonferroni post hoc analysis revealed that in control animals, dopamine release was elevated compared to baseline at 15 (p < 0.01), 20 (p < 0.001), 25 (p < 0.001), 30 (p < 0.001), 35 (p < 0.01), 40 (p < 0.001), 45 (p < 0.01) and 50 (p < 0.001) minutes post cocaine injection. In contrast, cocaine did not elevate stimulated dopamine release at any time point in cocaine self-administration animals, as compared to their own baseline.
Figure 3. Cocaine self-administration results in tolerance to the ability of cocaine to increase dopamine.
(A) Representative traces and pseudo-color plots from control (left) and cocaine self-administration (right) animals demonstrating decreased stimulated (60 pulse, 60 Hz, denoted by red bar on x-axis) dopamine transmission following a cocaine challenge (10 mg/kg, i.p.). Cocaine was injected immediately following the final baseline collection, and representative traces were taken 15 minutes (3 collections) following injection. Inset: cyclic voltammogram from peak of post injection representative trace. (B) Group data representing severely blunted dopamine release at baseline and following a cocaine challenge in cocaine self-administration animals as compared to controls. (C) Area under the curve is attenuated at baseline and following a cocaine challenge in cocaine animals. (D) When represented as a percent of baseline, the effect of a cocaine challenge on dopamine release is attenuated by a history of cocaine self-administration. Although an ANOVA revealed a main effect of time, and a time x group interaction, Bonferroni post hoc analysis did not reveal a significant difference at any of the time points. (E) When represented as a percent of baseline the effect of a cocaine challenge on area under the curve is blunted in cocaine animals. #, p < 0.05 vs. baseline; ##, p < 0.01 vs. baseline; ###, p < 0.001 vs. baseline; ####, p < 0.0001 vs. baseline, *, p < 0.05 vs. cocaine; **, p < 0.01 vs. cocaine; ***, p < 0.001 vs. cocaine; ****, p < 0.0001 vs. cocaine
Similarly, in regard to area under the curve, which accounts for changes in both stimulated dopamine release and uptake, a two-way repeated measures ANOVA revealed a significant effect of time (Figure 3C; F12,84 = 5.004, p < 0.0001) and a time x group interaction (F12, 84 = 3.378, p = 0.0005). Bonferroni post hoc analysis revealed that in control animals area under the curve was elevated compared to baseline line at 15 (p < 0.001), 20 (p < 0.0001), 25 (p < 0.001), 30 (p < 0.001), 35 (p < 0.001), 40 (p < 0.001), 45 (p < 0.001) and 50 (p < 0.0001) minutes post cocaine injection, while there was no effect in cocaine self-administration animals. These results indicate that cocaine self-administration animals are less responsive to cocaine effects on dopamine neurotransmission.
To further assess the effects of a history of cocaine self-administration on the effects of acute cocaine, we calculate the effects on cocaine on stimulated dopamine release and area under the curve as a percent of pre-cocaine baseline for each group. A two-way repeated measures ANOVA revealed a main effect of time on stimulated dopamine release (Figure 3D; F12, 84 = 9.768, p < 0.0001), as well as a time x group interaction (F12, 84 = 2.177, p = 0.0200); however we found no post hoc significance. In regard to cocaine-induced increases in area under the curve, we found a main effect of time (Figure 3E; F12, 84 = 11.20, p < 0.0001 and group (F1, 7 = 9.385, p = 0.0182), as well as an interaction (F12, 84 = 4.795; p < 0.0001). Bonferroni post hoc analysis revealed that area the curve was attenuated in cocaine self-administration animals as compared to controls at 25 (p < 0.05), 30 (p < 0.01), 35 (p < 0.05), 40 (p < 0.01) and 50 (p < 0.001) minutes post injection.
Discussion
Here we demonstrate in vivo that cocaine self-administration induces hypofunction of the mesolimbic dopamine pathway. We found that the amplitude of stimulated dopamine was attenuated, as was the area under the curve of the evoked dopamine curve. Additionally, we found that the dopaminergic response to an injection of cocaine was greatly reduced in cocaine self-administration animals. These data, in concert with mounting evidence in both preclinical and human investigations, suggest that hypofunction of the dopamine system is a neurochemical consequences of cocaine abuse.
Given the integral involvement of the mesolimbic dopamine pathway in guiding the selection and execution of goal directed behaviors and the maladaptive behaviors of cocaine addicts (Volkow et al., 2012; Siciliano et al., 2015), it is of critical importance to determine the way in which cocaine exposure alters its function. Here we show that the responsiveness of this projection is greatly reduced following by cocaine self-administration, which has implications for the affective state of the animal as well as the ability of the animal to modulate behavioral outputs. Indeed, decreased basal dopamine has been linked to increases in intracranial self-stimulation thresholds, indicating that animals are less sensitive to reward (Kokkinidis and McCarter, 1990), a state that is thought to model anhedonia in psychostimulant addicts during abstinence (Dackis and Gold, 1985; Markou and Koob, 1991). Anhedonia induced by cocaine use may contribute to the decreased reward experienced by detoxified cocaine addicts in response to non-drug stimuli (Siegel, 1982; Gawin et al., 1986).
In regard to the role of dopamine in selecting goal-directed behaviors, the decreased stimulated dopamine release observed here is a critical determinant in the progression of from cocaine use to addiction. Indeed, it was recently demonstrated that extended access cocaine self-administration results in decreased phasic dopamine neurotransmission in the NAc and this was highly correlated with escalation of cocaine intake (Wiluhn et al., 2014). Previous investigations have focused on cue-elicited dopamine release, without investigating if these changes are due to cocaine-induced alterations to VTA neurons per se or changes in non-dopaminergic afferent projections to the VTA or NAc. Additionally, changes in cue-elicited dopamine transient amplitude could be attributable to either reduced releasable dopamine or changes in dopamine cell firing. Determining if these changes are occurring specifically at the VTA to NAc projection is particularly important as cocaine self-administration has been shown to alter the strength of synaptic inputs to the VTA (Chen et al., 2008). Here we demonstrate that cocaine self-administration results in disruption of dopamine neurotransmission within the VTA to NAc projection, regardless of possible differences in cell firing, and that this depression of dopamine neurotransmission persists for at least 18 hours following cessation of cocaine use.
In addition to reductions in stimulated dopamine neurotransmission, cocaine self-administration resulted in tolerance to the ability of cocaine to augment dopamine. We were unable to use kinetic modeling of the current data to isolate the contribution of release and uptake due to insufficient concentrations of dopamine release in the cocaine self-administration animals; however, as indicated by our previous ex vivo studies, the decreased effect of cocaine on dopamine neurotransmission is due to a decreased ability of cocaine to produce uptake inhibition (Ferris et al., 2011, 2012; Calipari et al., 2013). While the mechanism for decreased cocaine-induced uptake inhibition following cocaine self-administration has not been clearly defined, it is likely that extensive cocaine blockade of the DAT produces an allosteric alteration to the cocaine binding site, or to the conformational state of the DAT, which has been shown to alter cocaine effects (Kohut et al., 2014). The self-administration paradigm used in the current investigation has been shown to reduce both membrane-associated and total DAT expression (Ferris et al., 2015; Calipari et al., 2014); however, genetically increasing DAT levels and thereby dopamine uptake rate has been shown to have no effect on cocaine potency (Calipari et al. 2013; Salahpour et al., 2008). Finally, tolerance to cocaine effects at the DAT has been shown to generalize to other DAT blockers, while the potency of DAT substrates is unaffected (Ferris et al., 2011, 2012). Thus, it is unlikely that decreases in cocaine potency are due to an orthosteric alteration to DAT function or to DAT expression.
One point of interest is that differences in cocaine effects between the two groups appear to only be present ≥ 15 minutes post-injection, while early time points are not affected by cocaine self-administration history. This suggests that the decreased effect of cocaine may be due to a shift in efficacy rather than potency, whereby the maximal effect of cocaine on dopamine uptake is shifted downwards by a history of cocaine self-administration. Regardless of mechanism, given that cocaine’s actions at the DAT have been shown to mediate the discriminative stimulus effects of the drug (Cunningham and Callahan, 1991; Melia and Spealman, 1991), it is likely that tolerance of the DAT to cocaine results in reduced subjective effects of the compound. Indeed, in human studies DAT occupancy by cocaine predicts the self-reported euphoric effects of cocaine and this effect is blunted in cocaine addicts (Volkow et al., 1996, 1997, 2006). Given that animals titrate their cocaine intake based on its subjective effects, it is possible that the increase in rate of cocaine intake over days observed in these animals occurs in compensation for the decreased effects of cocaine as tolerance develops.
Together, these data give further support to the phenomenon of hypodopaminergia induced by cocaine self-administration, and demonstrate that cocaine-induced dysregulation of the mesolimbic dopamine system occurs within the mesolimbic dopamine pathway. Additionally, we have demonstrated that, as in the human cocaine addict, cocaine self-administration results in marked tolerance to the ability of cocaine to augment dopamine neurotransmission. The decreased function of the mesolimbic dopamine pathway is likely to lead to an inability of the system to respond appropriately to environmental stimuli, resulting the in the inflexible and maladaptive behaviors of psychostimulant addicts, including relapse and uncontrolled cocaine use.
Acknowledgments
This work was funded by NIH grants R01 DA021325, R01 DA030161, R01 DA014030 (SRJ), P50 DA006634 (SRJ, MJF), K99 DA031791 (MJF) and T32 AA007565, F31 DA037710 (CAS).
Abbreviations
- NAc
nucleus accumbens
- VTA
ventral tegmental area
Footnotes
Conflict of Interest: The authors have no conflicts to report.
References
- Calipari ES, Ferris MJ, Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128(2):224–32. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC, Jones SR. Methylphenidate and cocaine self-administration produce distinct dopamine terminal alterations. Addict Biol. 2014;19(2):145–55. doi: 10.1111/j.1369-1600.2012.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun. 2013;4:2720. doi: 10.1038/ncomms3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano CA, Zimmer BA, Jones SR. Brief Intermittent Cocaine Self-Administration and Abstinence Sensitizes Cocaine Effects on the Dopamine Transporter and Increases Drug Seeking. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.238. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59(2):288–97. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Callahan PM. Monoamine reuptake inhibitors enhance the discriminative state induced by cocaine in the rat. Psychopharmacology (Berl) 1991;104(2):177–80. doi: 10.1007/BF02244175. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Gold MS. New concepts in cocaine addiction: the dopamine depletion hypothesis. Neurosci Biobehav Rev. 1985;9(3):469–77. doi: 10.1016/0149-7634(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Doucet G, Descarries L, Garcia S. Quantification of the dopamine innervation in adult rat neostriatum. Neuroscience. 1986;19:427–445. doi: 10.1016/0306-4522(86)90272-1. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology. 2012;37(7):1708–16. doi: 10.1038/npp.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. A Single Amphetamine Infusion Reverses Deficits in Dopamine Nerve-Terminal Function Caused by a History of Cocaine Self-Administration. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.45. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DC, Jones SR. Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry. 2011;69(3):201–7. doi: 10.1016/j.biopsych.2010.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawin FH, Herbert D, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Arch Gen Psychiatry. 1986;43(2):107–13. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Trends Neurosci. 1995;18(2):60–2. [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90(4):385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Kohut SJ, Hiranita T, Hong SK, Ebbs AL, Tronci V, Green J, Garcés-Ramírez L, Chun LE, Mereu M, Newman AH, Katz JL, Tanda G. Preference for distinct functional conformations of the dopamine transporter alters the relationship between subjective effects of cocaine and stimulation of mesolimbic dopamine. Biol Psychiatry. 2014;76(10):802–9. doi: 10.1016/j.biopsych.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinidis L, McCarter BD. Postcocaine depression and sensitization of brain-stimulation reward: analysis of reinforcement and performance effects. Pharmacol Biochem Behav. 1990;36(3):463–71. doi: 10.1016/0091-3057(90)90242-a. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–91. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4(1):17–26. [PubMed] [Google Scholar]
- Melia KF, Spealman RD. Pharmacological characterization of the discriminative-stimulus effects of GBR 12909. J Pharmacol Exp Ther. 1991;258(2):626–32. [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14(2–3):69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Beninger RJ. Dopamine D1 and D2 antagonists attenuate amphetamine-produced enhancement of responding for conditioned reward in rats. Psychopharmacology (Berl) 1993;113(1):110–8. doi: 10.1007/BF02244342. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A. 2008;105(11):4405–10. doi: 10.1073/pnas.0707646105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci. 2015;6(1):27–36. doi: 10.1021/cn5002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RK. Cocaine smoking. J Psychoact Drugs. 1982;14:321–337. doi: 10.1080/02791072.1982.10524303. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. 6-Hydroxydopamine lesions of the nucleus accumbens, but not of the caudate nucleus, attenuate enhanced responding with reward-related stimuli produced by intra-accumbens d-amphetamine. Psychopharmacology (Berl) 1986;90(3):390–7. doi: 10.1007/BF00179197. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Ho CW, Wang GJ, Newman AH, Heidbreder CA, Ashby CR, Jr, Gardner EL, Volkow ND. The effects of two highly selective dopamine D3 receptor antagonists (SB-277011A and NGB-2904) on food self-administration in a rodent model of obesity. Pharmacol Biochem Behav. 2008;89(4):499–507. doi: 10.1016/j.pbb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemannn R, Gatley SJ, MacGregor RR, Wolf AP. Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology. 1996;14(3):159–68. doi: 10.1016/0893-133X(95)00073-M. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–3. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D. Annu Rev Pharmacol Toxicol. 2012;52:321–36. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001;412(6842):43–8. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17(5):704–9. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Virus RM. The effects of a D1 and a D2 dopamine antagonist on behavior maintained by cocaine or food. Pharmacol Biochem Behav. 1989;32(3):691–7. doi: 10.1016/0091-3057(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–64. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]