Abstract
Spinal muscular atrophy (SMA), a leading genetic cause of pediatric death in the world, is an early-onset disease affecting the motor neurons in the anterior horn of the spinal cord. This degeneration of motor neurons leads to loss of muscle function. At the molecular level, SMA results from the loss of or mutation in the survival motor neuron 1 (SMN1) gene. The number of copies of the nearly duplicated gene SMN2 modulates the disease severity in humans as well as in transgenic mouse models for SMA. Most preclinical therapeutics trials focus on identifying ways to increase SMN2 expression and to alter its splicing. Other therapeutic strategies have investigated compounds which protect affected motor neurons and their target muscles in a SMN-independent manner. In the present study, the effect of a combination regimen of the SMN2 inducer D156844 and the protectant follistatin on the disease progression and survival was measured in the SMNΔ7 SMA mouse model. The D156844/follistatin combination treatment improved the survival of, delayed the endstage of disease in and ameliorated the growth rate of SMNΔ7 SMA mice better than follistatin treatment alone. The D156844/follistatin combination treatment, however, did not provide additional benefit over D156844 alone with respect to survival and disease endstage even though it provided some additional therapeutic benefit over D156844 alone with respect to motor phenotype.
Keywords: motor neuron disease; 2,4-diaminoquinazoline; follistatin; spinal muscular atrophy; preclinical drug trial; neonatal mouse; D156844
1. INTRODUCTION
Spinal muscular atrophy (SMA), a leading genetic cause of infant death worldwide, is an autosomal recessive degenerative disease characterized by selective loss of α motor neurons of the anterior horn of the spinal cord [1]. As a result of this loss, limb and trunk muscles atrophy. SMA results from the loss or mutation of the SMN (survival motor neuron) gene [2]. In humans, there are two SMN genes (SMN1 and SMN2) which arose from gene duplication differing by a single C-to-T transition within an exon splice enhancer of exon 7 [3;4]. The SMN1 transcripts contain exon 7 to produce full-length SMN protein while most of the transcripts produced from SMN2, lack exon 7 and yield an unstable protein known as SMNΔ7. The copy number of SMN2 modifies the severity of SMA phenotype in humans [5–7] as well as in transgenic mouse models for SMA [8–10]. SMN2, therefore, is a genetic modifier of SMA phenotype.
Numerous studies have identified many types of compounds that increase SMN2 expression [11]. C5-substituted 2,4-diaminoquinazolines (2,4-DAQs) are potent inducers of SMN2 promoter activity that were initially identified through a high-throughput drug screen [12]. D156844, a piperidine 2,4-DAQ derivative, increases SMN expression in cultured fibroblasts derived from an SMA patient and ameliorates the survival and phenotype of SMNΔ7SMA mice [13–15]. RG3039, another potent 2,4-DAQ, increases the mean lifespan in different mouse models of SMA [16;17]. 2,4-DAQs are potent inhibitors of the mRNA decapping enzyme DcpS [18].
Administration of recombinant follistatin to SMNΔ7 SMA mice increases the mean lifespan by about 30% [19]. Even though follistatin increases the mean lifespan of SMNΔ7 SMA mice, the maximum lifespan is not affected by this treatment suggesting that follistatin prevents earlier deaths in these mice. Follistatin does not affect SMN expression in the spinal cord or in the skeletal muscle of SMNΔ7 SMA mice suggesting that it exerts its ameliorative effect in a SMN-independent manner [19].
In the development of an effective therapeutic strategy for SMA, a combinatorial approach has been suggested wherein different classes of therapeutic agents are administered to elicit a multi-faceted protective effect on SMA patients. It is especially desirable to develop a cocktail of therapeutic agents that targets multiple mechanisms underlying SMA pathology, i.e. increasing SMN2 expression and protection of the motor unit—the motor neuron and the muscles it innervates. The effect of the 2,4-DAQ D156844 on the protective response of SMNΔ7 SMA mice to follistatin was examined in this study.
2. MATERIAL AND METHODS
2.1 Animals and Ethical Statement
SMNΔ7 SMA mice (SMN2+/+; SMNΔ7+/+;mSmn−/−) were generated from male and female carrier mice (SMN2+/+; SMNΔ7+/+;mSmn+/−) [20]. Since maternal diet influences the survival of SMNΔ7 SMA mice [21], the breeder mice were provided with ad libitum water and PicoLab20 Mouse diet (#5058; Purina) rodent chow. Only SMA and carrier pups were used in these experiments. All experiments were conducted in accordance with the protocols described in the National Institutes of Health Guide for the Care and Use of Animals and were approved by the Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Drugs
D156844 ([5-(1-(2-fluorobenzyl)piperidine-4-ylmethoxy]quinazoline-2,4-diamine dihydrochloride) was synthesized by deCODE chemistry as described previously [13]. D156844 was dissolved in ddH2O at a concentration of 3 mg/mL. Recombinant human follistatin (Biovision, Mountain View, CA) was reconstituted in sterile ddH2O at a concentration of 100 µg/mL.
2.3 Drug Administration
Carrier and SMA littermate mice were divided into 3 treatment cohorts: 1) D156844 (3 mg/kg/d) and follistatin (1 mg/kg/qad (every other day)), 2) follistatin alone and 3) ddH2O. Mice were dosed daily with D156844 or vehicle via oral administration as described previously [22] while follistatin was administered intraperitoneally. Treatment began at postnatal day 4 (PND04) and continued for the lifetime of each SMA mouse. The body mass of each mouse was determined each day during treatment. The treatment cohorts were not stratified based on sex because there is no significant difference in lifespan between male and female SMNΔ7 SMA mice [23] and there are no sex-related differences in the responsiveness of SMNΔ7 SMA mice to D156844 [14].
2.4 Phenotype Assays
A cohort of SMNΔ7 SMA mice from each treatment group were assayed for changes in righting reflex success and latency, spontaneous locomotor activity and pivoting activity as described previously [14;23]. Righting reflexes were assessed on PND07 and PND11 while spontaneous locomotor activity and pivoting were monitored on PND07, PND11 and PND14. To minimize the stress on the pup, the spontaneous locomotor activity and pivoting tests were conducted simultaneously.
2.5 Statistical Analysis
Data are expressed as means ± standard errors. Kaplan-Meier curves were generated from the survival and onset of body mass loss data and tested using the Mantel-Cox log rank test. The mice in the 3 treatment groups were also compared against previously published, dietmatched D156844 data [15]. All statistical analyses were performed with SPSS v.22.0.
3. RESULTS
3.1 Effect of D156844 and Recombinant Follistatin on the Survival of SMNΔ7 SMA Mice
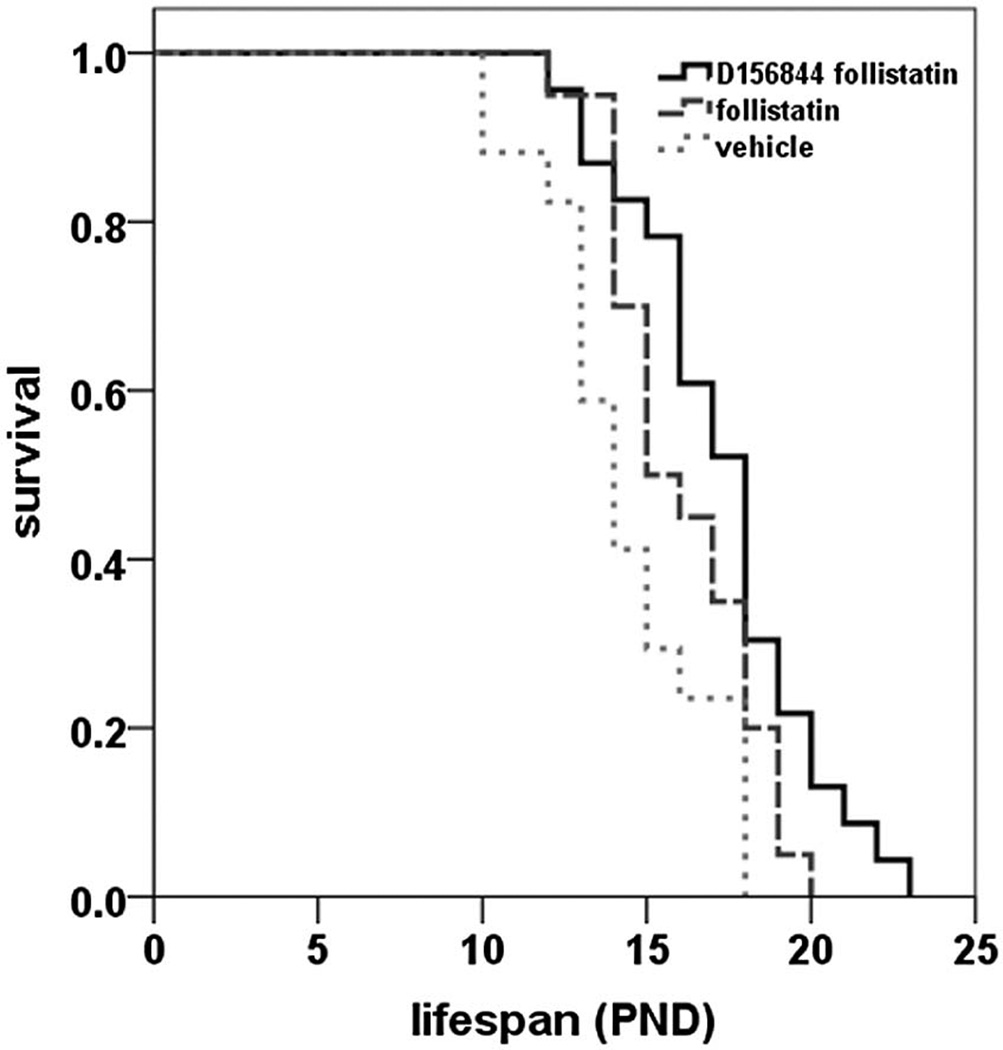
Consistent with previous findings [19], treatment of SMNΔ7 SMA mice with follistatin (n = 20) resulted in a 12% increase in mean lifespan when compared to vehicle-treated (n = 17) mice (Figure 1; 16.1 ± 0.5 days (d) vs. 14.4 ± 0.6 d; χ2 = 4.320, p = 0.038). SMNΔ7 SMA mice (n = 23) treated with both D156844 and follistatin exhibited a 20% improvement in mean survival relative to vehicle-treated mice (Figure 1; 17.3 ± 0.6 d vs. 14.4 ± 0.6 d; χ2 = 9.502, p = 0.002). When comparing the mean survival of SMNΔ7 SMA mice treated with D156844 and follistatin with those treated with follistatin alone, there was no statistically significant difference between these two treatment groups (χ2 = 2.781, p = 0.095). There was, however, a 20% increase in median lifespan in D156844/follstatin-treated SMNΔ7 SMA mice when compared to SMNΔ7 SMA mice treated with follistatin alone (Table 1). Furthermore, the maximum lifespan of SMNΔ7 SMA mice treated with D156844 and follistatin was 15% longer than in follistatin-treated SMNΔ7 SMA mice.
Figure 1.
Oral administration of D156844 augmented the follistatin-induced increase in survival of SMNΔ7 SMA mice. Kaplan-Meier survival plot for SMNΔ7 SMA mice receiving either vehicle (light gray dotted line; n = 17), 1 mg/kg/qad follistatin (gray dashed line; n = 20) or a combination of 3 mg/kg/d D156844 and 1 mg/kg/qad follistatin (solid line; n = 23). Treatment of SMNΔ7 SMA mice began at PND04.
Table 1.
Comparison of the effects of follistatin, D156844 and the combination of follistatin and D156844 (combo) treatments on the lifespan of SMNΔ7 SMA mice. The D156844 data were obtained from [15].
| treatment | mean lifespan ± SE (d) |
minimum lifespan (d) |
maximum lifespan (d) |
median lifespan ± SE (d) |
source |
|---|---|---|---|---|---|
| combo | 17.3 ± 0.6 | 12.0 | 23.0 | 18.0 ± 0.6 | this study |
| D156844 | 18.0 ± 0.6 | 12.0 | 22.0 | 18.0 ± 1.0 | [15] |
| follistatin | 16.1 ± 0.5 | 12.0 | 20.0 | 15.0 ± 0.8 | this study |
| vehicle | 14.4 ± 0.6 | 10.0 | 18.0 | 14.0 ± 0.7 | this study |
We also compared those mice treated with D156844 and follistatin to those treated with D156844 alone [15]; the comparison with previously published data is valid since both groups originated from the same mouse colony, were maintained on the same diet and received the same environmental conditions. Furthermore, the mean lifespan of vehicle-treated SMNΔ7 SMA mice in this study was not significantly different from the previous study [15] (14.4 ± 0.6 d vs. 15.4 ± 0.6 d).There was no difference in the mean survival of SMNΔ7 SMA mice treated with D156844 alone and those treated with D156844 and follistatin (Table 1; χ2 = 0.174; p = 0.677). There were also no differences in the median lifespan between the D156844 and the D156844/follistatin combination groups although the combination treatment did increase the maximum lifespan of treated SMNΔ7 SMA mice by 1 d (Table 1).
3.2 Effect of D156844 and Recombinant Follistatin on the Onset of Disease Endstage and Growth Rate in SMNΔ7 SMA Mice
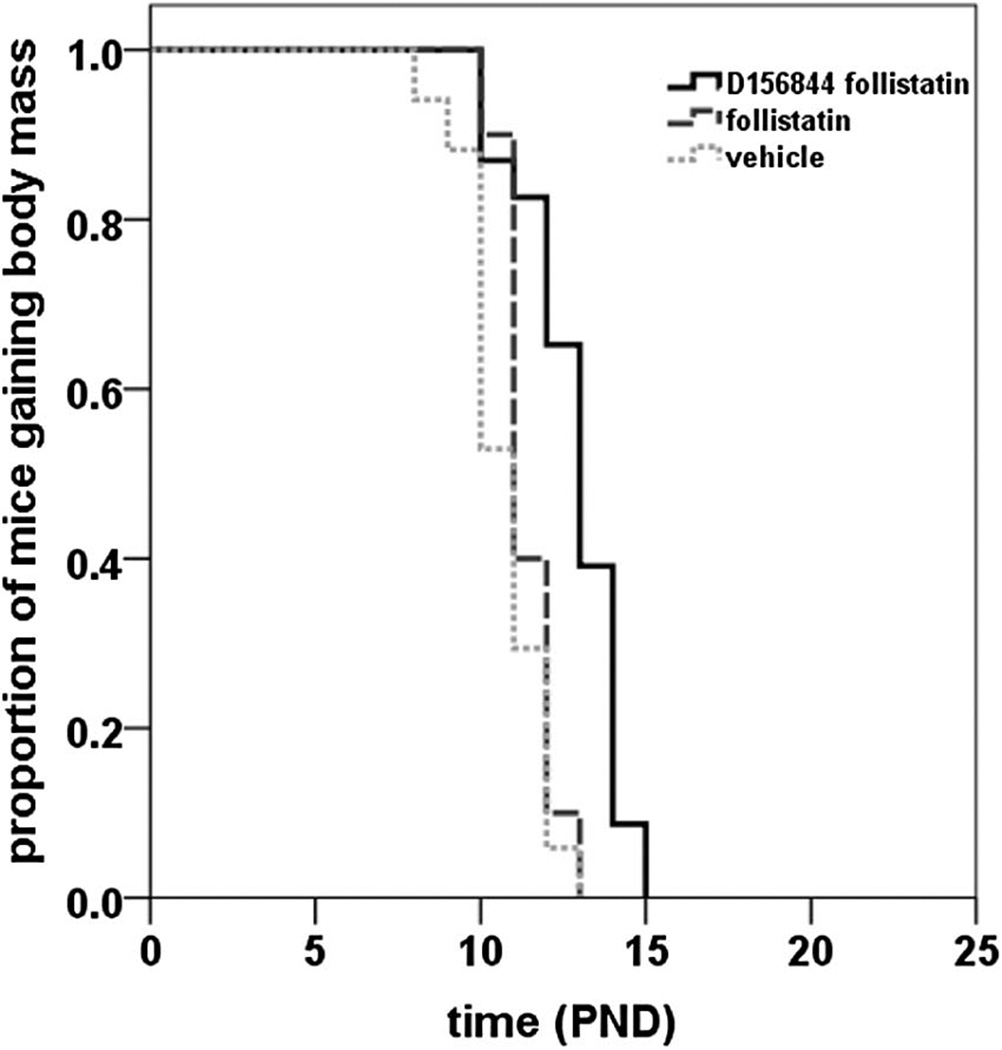
The onset of body mass loss is an indicator of the final stages of disease in the SMNΔ7 SMA mice [20;23]. Follistatin treatment did not significantly delay the mean onset of body mass loss in SMNΔ7 SMA mice (Figure 2; 11.4 ± 0.2 d vs. 10.8 ± 0.3 d; χ2 = 2.370, p = 0.124). Treatment of SMNΔ7 SMA mice with D156844 and follistatin delayed the mean onset of body mass loss by 20% relative to vehicle-treated mice (Figure 2; 12.8 ± 0.3 d vs. 10.8 ± 0.3 d; χ2 = 18.861, p < 0.001). The difference in the onset of body mass loss between D156844/follistatin-treated SMNΔ7 SMA mice and those mice treated with follistatin alone is significant (χ2 = 14.781, p < 0.001) but is not significant when compared to those mice treated with D156844 alone (Table 2; χ2 = 1.234, p = 0.267).
Figure 2.
Oral administration of D156844 augmented the follistatin-induced increase in the onset of loss in body mass of SMNΔ7 SMA mice. Kaplan-Meier onset of body mass loss plot for SMNΔ7 SMA mice receiving either vehicle (light gray dotted line; n = 17), 1 mg/kg/qad follistatin (gray dashed line; n = 20) or a combination of 3 mg/kg/d D156844 and 1 mg/kg/qad follistatin (solid line; n = 23). Treatment of SMNΔ7 SMA mice began at PND04.
Table 2.
Comparison of the effects of follistatin, D156844 and the combination of follistatin and D156844 (combo) treatments on the onset of body mass loss in SMNΔ7 SMA mice. The D156844 data were obtained from [15]
| treatment | mean onset ± SE (d) |
minimum onset (d) |
maximum onset (d) |
median onset ± SE (d) |
source |
|---|---|---|---|---|---|
| combo | 12.8 ± 0.3 | 10.0 | 15.0 | 13.0 ± 0.4 | this study |
| D156844 | 12.4 ± 0.3 | 10.0 | 16.0 | 12.0 ± 0.4 | [15] |
| follistatin | 11.4 ± 0.2 | 10.0 | 13.0 | 11.0 ± 0.2 | this study |
| vehicle | 10.7 ± 0.3 | 8.0 | 13.0 | 11.0 ± 0.4 | this study |
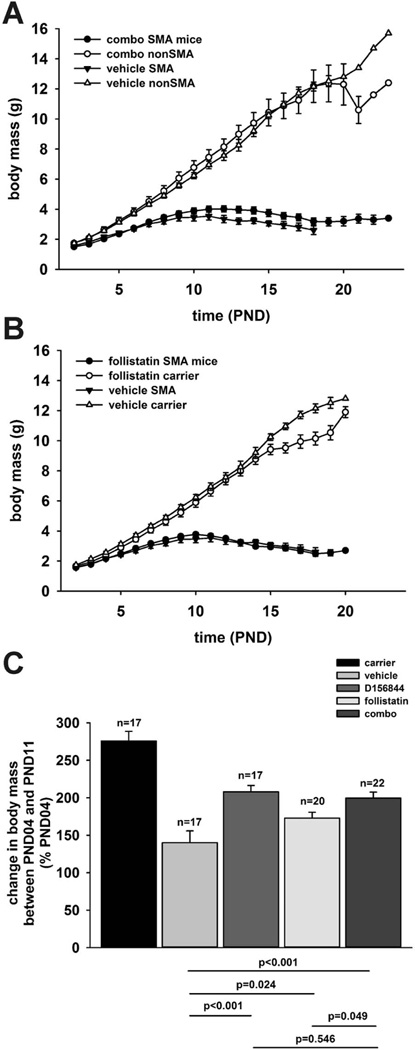
The body mass curves of SMNΔ7 SMA mice treated with D156844 and follistatin (Figure 3A; combo, closed circles) or with vehicle (closed triangles) were similar in shape. Between PND11 and PND18, SMNΔ7 SMA mice treated with D156844 and follistatin showed higher body masses than age-matched, vehicle-treated SMNΔ7 SMA mice. There were no marked differences in the body mass curves of follistatin-treated (Figure 3B, closed circles) and of vehicle-treated (closed triangles) SMNΔ7 SMA mice. SMNΔ7 SMA mice treated with D156844 showed higher body masses than vehicle-treated SMNΔ7 SMA mice between PND11 and PND16 [15].
Figure 3.
The effect of D156844 on changes in body mass of SMNΔ7 SMA mice being treated with recombinant follistatin. (A) Body mass curves of SMNΔ7 SMA mice (closed) and non-SMA mice (open) receiving 3 mg/kg/d D156844 and 1 mg/kg/qad follistatin (combo; circles) or vehicle (triangles). Treatment began at PND04. (B) Body mass curves of SMNΔ7 SMA mice (closed) and non-SMA mice (open) receiving 1 mg/kg/qad follistatin (circles) or vehicle (triangles). Treatment began at PND04. (C) Changes in body mass between PND04 and PND11 of SMNΔ7 SMA mice treated with either 3 mg/kg/d D156844 and 1 mg/kg/qad follistatin (combo), 3 mg/kg/d D156844, 1 mg/kg/qad follistatin or vehicle. The change in body mass between PND04 and PND11 of age-matched nonSMA littermates was also shown. The statistical significances for pairs of experimental groups were provided below the bar graph.
The growth rate—as measured by the change in body mass between PND04 and PND11—was diminished in SMNΔ7 SMA mice as expected (Figure 3C) [20;23]. SMNΔ7 SMA mice treated with follistatin showed a 23% increase in growth rate relative to vehicle-treated SMNΔ7 SMA mice (Figure 3C; p = 0.024). Co-administration of D156844 and follistatin to SMNΔ7 SMA mice resulted in a 42% increase in growth rate relative to vehicle-treated SMNΔ7 SMA mice (p < 0.001); the increase in growth rate of D156844 and follistatin-treated SMNΔ7 SMA mice was significantly greater (p = 0.049) than that for SMNΔ7 SMA mice treated with follistatin alone. Treatment of SMNΔ7 SMA mice with D156844 alone [15] resulted in a 48% greater growth rate when compared against vehicle-treated SMNΔ7 SMA mice. There was no difference in the PND04-to-PND11 growth rates between SMNΔ7 SMA mice treated with the D156844-follistatin combination and D156844 alone (p = 0.546).
3.3 Effect of D156844 and Recombinant Follistatin on the Motor Phenotype of SMNΔ7 SMA Mice
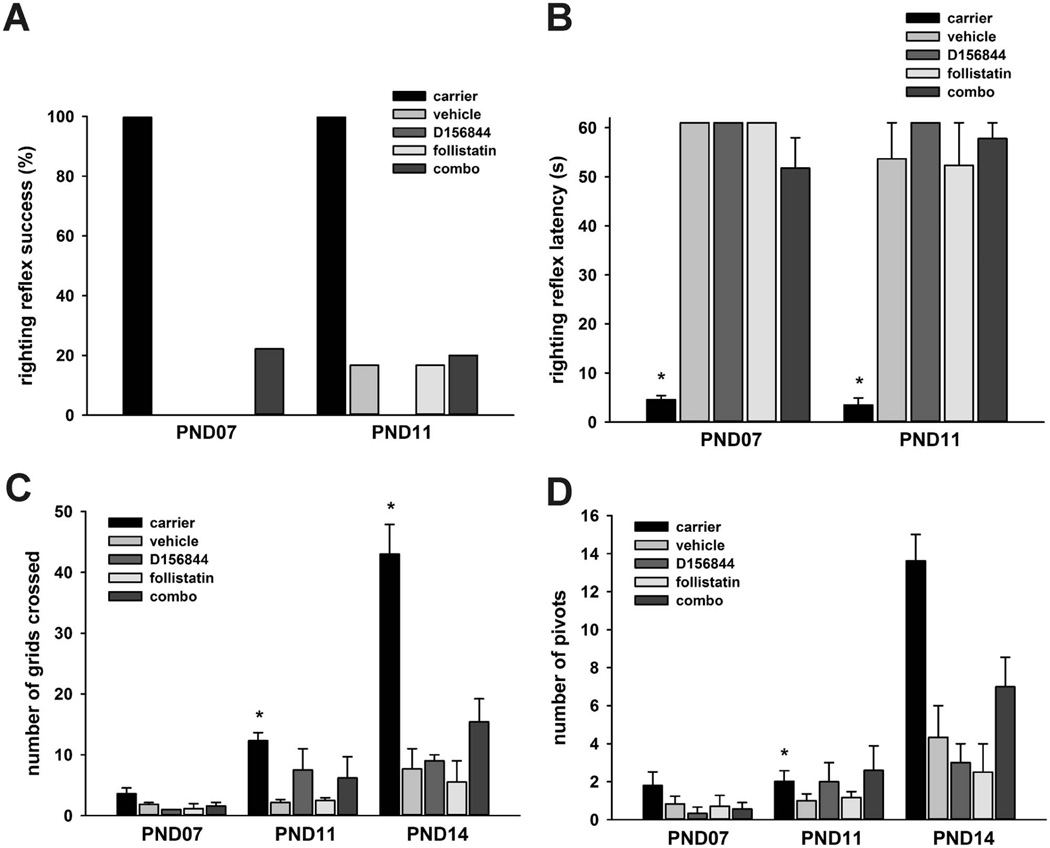
There is a progressive impairment of motor behavior in neonatal SMNΔ7 SMA mice phenotype analysis; this impairment is characterized by a loss of surface righting reflexes and reduced spontaneous locomotor activity [23]. Both D156844 [14] and follistatin [19] ameliorate the impaired motor phenotype of SMNΔ7 SMA mice. Within our treatment cohort, some of the SMNΔ7 SMA mice treated with the combination of D156844 and follistatin exhibited a successful surface righting response at PND07 while none of the SMNΔ7 SMA mice treated with D156844, follistatin or vehicle displayed this response (Figure 4A). The latency for surface righting was longer in SMNΔ7 SMA mice than in carrier littermates; there were, however, no significant differences in the surface righting latencies among the treatment groups of SMNΔ7 SMA mice at either time point examined (Figure 4B).
Figure 4.
The effects of D156844 and follistatin on the motor phenotype of SMNΔ7 SMA mice. SMNΔ7 SMA mice received either 3 mg/kg/d D156844 and 1 mg/kg/qad follistatin (combo), follistatin, D156844 or vehicle beginning at PND04. Behavior responses were also compared against age-matched healthy littermates (carrier). (A) Proportion of mice exhibiting successful righting reflex responses at PND07 and PND11. (B) Righting reflex latency in treated SMNΔ7 SMA mice. There was an arbitrary cutoff time of 61 s. (C) Spontaneous locomotor activity— measured as the number of grids crossed in 1 min—in treated SMNΔ7 SMA mice. (D) The number of pivots, or 90° turns, made in 1 min in treated SMNΔ7 SMA mice. Key: *, p ≤ 0.05 when compared against vehicle-treated SMNΔ7 SMA mice.
Spontaneous locomotor activity is impaired in SMNΔ7 SMA mice [23]. At PND11, SMNΔ7 SMA mice treated with the D156844/follistatin combination or with D156844 alone tended to cross a greater number of grids—i.e. increased spontaneous locomotor activity—than vehicle-treated (Figure 4C) but the differences were not statistically significant. The combination D156844/follistatin treatment tended to increase spontaneous locomotor activity at PND14 greater than either drug alone in the SMNΔ7 SMA mice. The pivoting activities of the treated SMNΔ7 SMA mice also showed similar tendencies with spontaneous locomotor activity (Figure 4D). Co-administration of D156844 and follistatin may improve motor impairment in SMNΔ7 SMA mice to a greater extent than either drug alone.
4. DISCUSSION
This study shows that the C5-substituted 2,4-DAQ D156844 enhances the protective effects of recombinant follistatin on SMNΔ7 SMA mice by augmenting the increased growth rate between PND04 and PND11, delaying the onset of the endstage of disease and improving their survival. In this case, the combinatorial effects of D156844 and follistatin were not additive with respect to D156844 [15]. D156844 acts as an inhibitor of the mRNA decapping enzyme DcpS [18]. It is possible that DcpS inhibition may—in addition to increasing SMN2 expression— regulate the same pathways affected by follistatin. Alternatively, DcpS inhibition by D156844 may abrogate the protective effects of follistatin. Future studies comparing the effects of D156844 and follistatin on gene expression in motor neurons and their target muscles will address this possibility.
Administration of recombinant follistatin improves the survival of SMA mice ([19] and this study); however, transgenic overexpression of follistatin does not improve survival of SMNΔ7 SMA mice [24]. One possible explanation for these disparate results is that the circulating levels of recombinant follistatin may be higher than the circulating levels of transgenic follistatin. Rose et al. [19], however, found that higher doses of recombinant follistatin do not improve the survival of SMNΔ7 SMA mice even though lower doses exhibit protective effects. Also, the follistatin transgene used in the Sumner et al. [24] study is present on the Y chromosome [25] which means that only male SMNΔ7 SMA mice would ectopically overexpress follistatin. Even though there are no differences in the SMA phenotype between the sexes [23], the location of the follistatin transgene on the Y chromosome may affect transgene expression and may partially explain the disparate results between the transgenic follistatin studies [24] and the recombinant follistatin injection studies ([19] and this study).
Follistatin mRNA transcript levels are increased while the levels of myostatin mRNA are reduced in the hindlimb muscles of SMNΔ7 SMA mice at the endstage of disease [24]. The best known mode of action for follistatin is the inhibition of myostatin signaling by preventing myostatin from binding to its receptor [26]. Inhibiting myostatin expression, therefore, should ameliorate the SMA phenotype in these mice. Knockout of myostatin in SMNΔ7 SMA mice does not improve disease severity [27]. Administration of the myostatin inhibitor ActRIIB-Fc to SMNΔ7 SMA mice does not improve motor function or survival [24]. These studies suggest that myostatin inhibition offers no protective effect in SMA mice. Even though myostatin inhibition is the best characterized action of follistatin, it may regulate pathways aside from myostatin. In support of this premise, transgenic overexpression of follistatin more strongly increases muscle mass in myostatin nullizygous mice [28]. Future studies can identify these non-canonical pathways affected by follistatin and determine whether or not their modulation would be protective in SMA mouse models.
We used previously published results from D156844-treated SMNΔ7 SMA mice [15] to compare the effectiveness of D156844/follistatin co-administration to D156844 treatment alone. The D156844-treated SMNΔ7 SMA mice were from the same mouse colony as the mice used in this study and all of these mice were exposed to similar environmental conditions. All of the mice used in both studies were maintained on the same PicoLab20 mouse diet. Additionally, the mean survival of vehicle-treated SMNΔ7 SMA mice in this study was similar to that in the previous work [15]. Additionally, there is minimal interlitter variability in the lifespan or in the phenotype of SMNΔ7 SMA mice [23]. For these reasons, we feel that comparison of the data in this study to previously published “historical data” is valid.
DcpS inhibitors like D156844 increase the activity of the SMN2 promoter [13]. In addition to regulating its promoter, other approaches showing in vivo efficacy for increasing SMN2 expression include modulation of the splicing of its pre-mRNA so that a greater proportion of SMN2 mRNAs contain exon 7—with compounds like LDN-76070 [29] and SMN-C3 [30]—and translational read-through of SMNΔ7 mRNAs to help stabilize its protein—with compounds like geneticin [31] and TC007 [32–34]. Aside from increasing SMN2 expression, other compounds like 4-phenylbutyrate ([35;36] manuscript in preparation) and the Rho kinase inhibitor Y-27632 [37] ameliorate the phenotypes of SMA mouse models independent of SMN. Combination therapeutics will be viable strategies for treating SMA especially if these combination treatments increase SMN2 expression at different levels of gene regulation and/or protect vulnerable motor neurons.
In summary, cotreatment of SMNΔ7 SMA mice with the DcpS inhibitor D156844 and the protectant follistatin delays the onset of disease endstage and increases survival more effectively than follistatin alone. Unfortunately, this combination therapy does not provide additional therapeutic benefit when compared against D156844 treatment alone with respect to the onset of disease endstage and to survival. While this combination therapy does not significantly ameliorate disease progression or survival relative to D156844, the motor phenotype shows a trend for modest improvement in D156844/follistatin co-treated SMNΔ7 SMA mice.
HIGHLIGHTS.
D156844/follistatin treatment increases the average lifespan of and delays disease endstage in SMNΔ7 SMA mice
D156844/follistatin treatment further increases growth rate over follistatin
D156844/follistatin treatment further improved motor impairments over follistatin or D156844
ACKNOWLEDGMENTS
We would like to thank Dr. Arthur Burghes for providing laboratory space for some of these experiments. The study was supported in part by funds from Cure SMA, the Nemours Foundation and the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health (P20GM103464). Cure SMA financially supported and directed the identification and generation of the quinazoline series of compounds, including D156844.
ABBREVIATIONS
- 2,4,-DAQ
2,4-diaminoquinazoline
- PND
postnatal day
- qad
quaque altera die (every other day)
- SMA
spinal muscular atrophy
- SMN
survival motor neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS CONFLICTS OF INTEREST
None
REFERENCES
- 1.Crawford TO, Pardo CA. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. doi: 10.1006/nbdi.1996.0010. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 3.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing an is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. U. S. A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monani UR, Lorson CL, Parsons DW, et al. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 5.Coovert DD, Le TT, McAndrew PE, et al. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- 6.Lefebvre S, Burlet P, Liu Q, et al. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat. Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- 7.McAndrew PE, Parsons DW, Simard LR, et al. Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am. J. Hum. Genet. 1997;60:1411–1422. doi: 10.1086/515465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monani UR, Sendtner M, Coovert DD, et al. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn-/- mice and results in a mouse with spinal muscular atrophy. Hum. Mol. Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh-Li HM, Chang JG, Jong YJ, et al. A mouse model for spinal muscular atrophy. Nat. Genet. 2000;24:66–70. doi: 10.1038/71709. [DOI] [PubMed] [Google Scholar]
- 10.Michaud M, Arnoux T, Bielli S, et al. Neuromuscular defects and breathing disorders in a new mouse model of spinal muscular atrophy. Neurobiol. Dis. 2010;38:125–135. doi: 10.1016/j.nbd.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Cherry JJ, Kobayashi DT, Lynes MM, et al. Assays for the identification and prioritization of drug candidates for spinal muscular atrophy. Assay Drug Dev. Technol. 2014;12:315–341. doi: 10.1089/adt.2014.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarecki J, Chen X, Bernardino A, et al. Diverse small-molecule modulators of SMN expression found by high-throughput compound screening: early leads towards a therapeutic for spinal muscular atrophy. Hum. Mol. Genet. 2005;14:2003–2018. doi: 10.1093/hmg/ddi205. [DOI] [PubMed] [Google Scholar]
- 13.Thurmond J, Butchbach MER, Palomo M, et al. Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. Med. Chem. J. 2008;51:449–469. doi: 10.1021/jm061475p. [DOI] [PubMed] [Google Scholar]
- 14.Butchbach MER, Singh J, Þorsteindóttir M, et al. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:454–467. doi: 10.1093/hmg/ddp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butchbach MER, Singh J, Gurney ME, Burghes AHM. The effect of diet on the protective action of D156844 observed in spinal muscular atrophy mice. Exp. Neurol. 2014;256:1–6. doi: 10.1016/j.expneurol.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Meerbeke JP, Gibbs RM, Plasterer HL, et al. The DcpS inhibitor RG3039 improves motor function in SMA mice. Hum. Mol. Genet. 2013;22:4074–4083. doi: 10.1093/hmg/ddt257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gogliotti RG, Cardona H, Singh J, et al. The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models. Hum. Mol. Genet. 2013;22:4084–4101. doi: 10.1093/hmg/ddt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh J, Salcius M, Liu SW, et al. DcpS as a therapeutic target for spinal muscular atrophy. ACS Chem. Biol. 2008;3:711–722. doi: 10.1021/cb800120t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose FF, Jr, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le TT, Pham LT, Butchbach MER, et al. SMND7, the major product of the centromeric survival motor neuron gene (SMN2), extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet. 2005;14:845–857. doi: 10.1093/hmg/ddi078. [DOI] [PubMed] [Google Scholar]
- 21.Butchbach MER, Rose FF, Jr, Rhoades S, et al. Effect of diet on the survival and phenotype of a mouse model for spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2010;391:835–840. doi: 10.1016/j.bbrc.2009.11.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butchbach MER, Edwards JD, Schusslers KR, Burghes AHM. novel method for oral delivery of compounds to the neonatal SMND7 model of spinal muscular atrophy. J. Neurosci. Methods. 2007;161:285–290. doi: 10.1016/j.jneumeth.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butchbach MER, Edwards JD. AHM Burghes, Abnormal motor phenotype in the SMND7 mouse model of spinal muscular atrophy. Neurobiol. Dis. 2007;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner CJ, Wee CD, Warsing LC, et al. Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Hum. Mol. Genet. 2009;18:3145–3152. doi: 10.1093/hmg/ddp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rindt H, Buckley DM, Vale SM, et al. Transgenic inactivation of murine myostatin does not decrease the severity of disease in a model of spinal muscular atrophy. Neuromuscul. Disord. 2012;22:277–285. doi: 10.1016/j.nmd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Lee SJ. Quadrupling muscle mass in mice by targeting TGF-b signaling pathways. PLoS ONE. 2007;8:e789. doi: 10.1371/journal.pone.0000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherry JJ, Osman EY, Evans MC, et al. Enhancement of SMN protein levels in a mouse model of spinal muscular atrophy using novel drug-like compounds. EMBO Mol. Med. 2013;5:1103–1118. doi: 10.1002/emmm.201202305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naryshkin NA, Weetall M, Dakka A, et al. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science. 2014;345:688–693. doi: 10.1126/science.1250127. [DOI] [PubMed] [Google Scholar]
- 31.Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function of SMA mice in vivo. Hum. Mol. Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Delivery of a read-through inducing compound, TC007, lessens the severity of a SMA animal model. Hum. Mol. Genet. 2009;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattis VB, Fosso MY, Chang CW, Lorson CL. Sucutaneous administration of TC007 reduces disease severity in an animal model of SMA. BMC Neurosci. 2009;10:142. doi: 10.1186/1471-2202-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattis VB, Chang CWT, Lorson CL. Analysis of a read-through promoting compound in a severe mouse model of spinal muscular atrophy. Neurosci. Lett. 2012;525:72–75. doi: 10.1016/j.neulet.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butchbach MER, Le TT, Burghes AHM. Protective effects of butyrate analogues and prodrugs on a mouse model for spinal muscular atrophy. Society for Neuroscience 2004 Abstract Viewer/Itinerary Planner. 2004:226.17. [Google Scholar]
- 36.Butchbach MER, Simard LR, Burghes AHM. SMN-independent amelioration of mouse spinal muscular atrophy by butyrate prodrugs. Society for Neuroscience 2005 Abstract Viewer/Itinerary Planner. 2005:338.7. [Google Scholar]
- 37.Bowerman M, Beauvais A, Anderson CL, Kothary R. Rho-kinase inactivation prolongs survival of an intermediate SMA mouse model. Hum. Mol. Genet. 2010;19:1468–1478. doi: 10.1093/hmg/ddq021. [DOI] [PubMed] [Google Scholar]