Abstract
Screening of pharmaceutical, chemical, and environmental compounds for their effects on reproductive health relies on in vivo studies. More robust and efficient methods to assess thes effects are needed. Here we adapted and validated an organotypic in vitro follicle growth (IVFG) assay to determine the impact of compounds on markers of ovarian function. We isolated mammalian follicles and cultured them in the presence of compounds with 1) known fertotoxicity (i.e., toxicity to the reproductive system; cyclophosphamide and cisplatin); 2) no known fertotoxicity (nalbuphine); and 3) unknown fertotoxicity (Corexit EC 9500 A; CE). In each case we assayed follicle growth, hormone production, and the ability of follicle-enclosed oocytes to resume meiosis and produce a mature egg. We found that cyclophosphamide and cisplatin caused dose-dependent disruption of follicle dynamics, whereas nalbuphine did not. The reproductive toxicity of CE, an oil dispersant used heavily during the 2010 Deepwater Horizon oil spill, has never been examined in a mammalian system. We found that CE compromised follicle morphology and functional parameters. Our findings demonstrate that this IVFG assay system can be used to distinguish fertotoxic from non-toxic compounds, providing an in vitro tool for assessing effects of chemical compounds on reproductive function and health.
Keywords: ovary, follicle, oocyte, in vitro assay, culture
Introduction
In the United States, large quantities of pharmaceuticals are produced annually, with approximately 1800 active pharmaceutical ingredients approved for prescription use as of 2009 (Federal and Drug Administration, 2013). In the chemical industry, 87,000 compounds were registered for use in United States commerce as of 2006, and 3,000 of these existed in excess of 1 million pounds each. It is estimated that 700 new industrial chemicals are introduced into commerce annually (Sutton, et al., 2010; Woodruff, et al., 2008). Despite the myriad compounds to which humans are exposed, there is no standard rapid and efficient mechanism by which the potential reproductive health effects of these compounds are screened. The guidelines for reproductive toxicity testing set forth by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use as well as the The Guidelines for Reproductive Toxicity Risk Assessment issued by the United States Environmental Protection Agency rely primarily on in vivo animal studies (European Medicines Agency, 1994; Environmental Protection Agency (EPA), 1996). There is a growing interest in developing alternatives to animal studies, which are costly, require extended periods of time to carry out, can only be used to screen a limited number of compounds, and only measure overt phenotypes such as infertility or teratogenicity (Spielmann, 1998; Zurlo, 2012).
Although in vitro follicle culture could be applied to study changes in follicle formation, follicle development, and ovarian physiology (Bhattacharya et al., 2013; Dutta et al., 2014; Igawa et al., 2009; Keating et al., 2008; Keating et al., 2008) in vitro follicle growth (IVFG) represents a more simple, rapid, and robust tool that can be applied to screening the effect of compounds on female reproductive function (Ahn et al., 2013; Cortvrindt and Smitz, 2002; Lenie et al., 2008; Lenie and Smitz, 2009; Peretz et al., 2012; Peretz and Flaws, 2013; Peretz et al., 2011; Peretz et al., 2013; Sun et al., 2008; Van Wemmel et al., 2005). The follicle is the functional unit of the ovary, comprised of germ cell (oocyte) and somatic cell (granulosa and theca cell) compartments (Albertini et al., 2001; Eppig et al., 2005). Follicle development is critical for producing a fertilization-competent gamete and for maintaining the endocrine hormone axis. Thus, the follicle represents a robust proxy of overall reproductive function and health. Several culture methods have been developed to grow ovarian follicles in vitro (Cortvrindt and Smitz, 2002; Picton et al., 2008; Xu et al., 2009; Xu et al., 2011); IVFG systems recapitulate key events of mammalian in vivo oogenesis and folliculogenesis, including follicle survival and growth, antral cavity formation, estradiol production, and the ability of the oocyte to resume meiosis and produce a mature egg (Cortvrindt and Smitz, 2002). Disruptions of these endpoints are predictive of global reproductive defects, alterations in menstrual cyclicity and hormonal function, and/or subfertility related to reduced egg quality, making IVFG a potential bioassay for reproductive toxicity testing (Figure 1).
Figure 1.
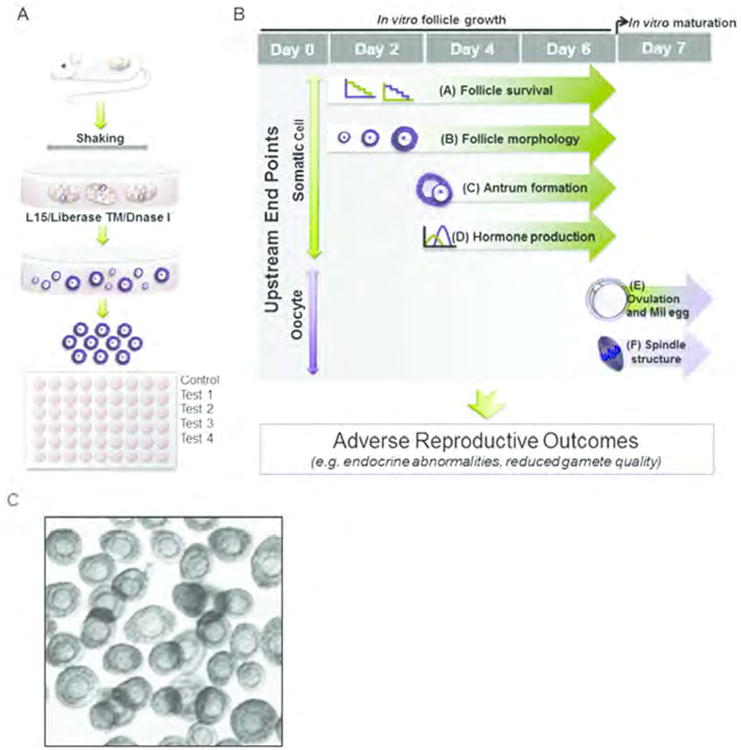
In Vitro Follicle Growth (IVFG) provides a simple and rapid method of predicting adverse reproductive outcomes in mammals in response to environmental exposures. In IVFG, (A) large numbers of ovarian follicles are collected from pre-pubertal mouse ovaries and placed individually in wells of a 96-well plate containing chemicals of interest. Follicles are cultured and monitored for up to 6 days. (B) Upstream endpoints of IVFG include: (i) follicle survival, (ii) follicle morphology, (ii) antrum formation, (iv) hormone production, (v) oocyte meiotic competence, and (F) spindle structure. These hallmarks are robust biomarkers of both endocrine and gamete quality. (C) Uniform mouse follicles obtained by enzymatic digestion (A) were cultured in the bottom of 96-well plate.
In this study, we demonstrated that IVFG can be used to distinguish fertotoxic compounds from those that are known to have no impact on reproductive function. As a test of the system, we examined a compound with unknown reproductive effects, Corexit EC 9500 A (CE), an oil dispersant with widespread use during the 2010 Deepwater Horizon oil spill. While it is not known whether or to what extent humans were exposed to Corexit during the extensive clean up phase of the crisis, we do know that no tests have been conducted to assess the reproductive impact of this compound in mammals. Thus, Corexit serves as an interesting test case for our IVFG system. There is great urgency in developing a variety of tactics from many laboratories to provide pre-clinical information about the potential fertility threats of a variety of compounds and drugs. This assay is one addition to a growing number of tools toward this end. The eventual transfer of suitable assays to pharmaceutical companies, contract research organizations and even the FDA is a goal for the field. The data presented below are part of this field-wide intent to document reproductive safety and hazards in our environment and drug pipeline.
Materials and Methods
Drugs and chemicals
Cisplatin (CDDP) and cyclophosphamide (CTX) were obtained from Sigma-Aldrich (St. Louis, MO) and used at final concentrations of 0.2, 1, and 5 μM. Nalbuphine was obtained from the NIH Clinical Collection (South San Francisco, CA) and used at final concentrations of 0.2, 1, and 5 μM. Nalbuphine was selected as a negative control based on its known in vivo injection strength at doses of 20 mg/ml (approximately 70 μM) (FDA, 2014). Corexit EC 9500A (CE) was obtained via a Material Transfer Agreement from Nalco (Sugar Land, Texas). CE was used at final concentrations of 5, 10, 25, 50, 75, 100, 125, 150, 200, 250, 300, 350 and 400 ppm, which correspond to 4.75, 9.5, 23.75, 47.5, 71.25, 95, 118.75, 142.5, 180, 237.5, 285, 332.5, and 380 mg/L. The doses of CE were chosen based on data from evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500 (Zheng et al., 2014). The LC50 values for Corexit were 16 ppm in BL16/BL6 cells, 33 ppm in 1321N1 cells, 70 ppm in H19-7 cells, 93 ppm in HEK293 cells, and 95 ppm in HK-2 cells (Zheng et al., 2014).
Animals and follicle isolation
All experiments were performed using CD-1 female mice (Harlan Laboratories, Indianapolis, IN) that were housed in a controlled barrier facility at Northwestern University's Center of Comparative Medicine under constant temperature, humidity, and light (12h light/12h dark). Food and water were provided ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee and were performed in accordance with National Institutes of Health Guidelines. For follicle isolation, ovaries were dissected from 14- to 15-day-old mice and cut into 4 pieces. The tissue was incubated for 1 hour in L15 media (Invitrogen, Carlsbad, CA, USA) containing 2% Liberase TM (Roche, Indianapolis, IN) and 1% DNase I (Worthington, Lakewood, NJ). The enzymatic digestion was quenched by rinsing follicles in αMEM Glutamax (Invitrogen, Carlsbad, CA) containing 1% Fetal Bovine Serum (FBS, Invitrogen) and 0.5% Pen-Strep (Mediatech, Herndon, VA). Multilayer secondary follicles (140-175 μm in diameter) (Figure 1C) were manually collected and cultured individually in wells of a 96-well plate (Pedersen & Peters, 1968). An average of 30 follicles were collected from a single ovary. Follicles from different animals were pooled and grown in 100 μl of growth media comprised of αMEM Glutamax supplemented with 10 mIU/ml recombinant follicle-stimulating hormone (rFSH; A.F. Parlow, National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases), 1% Pen-Strep, 5 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium (Sigma-Aldrich).
Follicle culture, growth, and survival
Follicles were cultured at 37°C in a humidified atmosphere of 5% CO2 in air for up to 6 days. All chemical exposures were initiated on day 2 of culture. Half of the culture media was replaced with fresh growth media every other day and spent media was stored at -20°C for use in steroid hormone assays. Follicles were imaged at each media change using a Leica DM IRB inverted microscope equipped with 4× and 20× objectives (Leica Microsystems, Heidelberg, Germany) to assess survival, morphology, and growth. Live follicles were defined as those that contained an oocyte surrounded completely by somatic cells. Dead follicles where characterized by dark granulosa cells, granulosa cells that failed to proliferate, or an oocyte that had dissociated completely from the follicle structure (Lenie et al., 2008). The percent of surviving follicles was calculated as the number of live follicles out of the total number of follicles plated. Antral cavity formation was assessed morphologically and characterized by the differentiation of outer layers of mural granulosa cells and inner layers of cumulus granulosa cells surrounding the oocyte as well as the presence of a fluid-filled structure.
Hormone assays
To measure hormones secreted by cultured follicles, spent culture media from viable follicles was pooled on day 4 and day 6. Three independent measurements for each steroid at each time point were performed. 17-β-estradiol (E2) was measured using a commercially available E2 ELISA Kit (Calbiotech, Inc. Spring Valley, CA) according to the manufacturer's protocol. All assays were run in duplicate. The assay sensitivity is 3.94 pg/ml, and medium collected from wells without follicles was used as a negative control. Intra- and interassay variations were less than 5%.
In vitro oocyte maturation
In vitro maturation (IVM) was performed after 6 days of culture. Briefly, half of the medium was replaced with IVM medium (αMEM Glutamax containing 5 ng/ml epidermal growth factor, 1.5 IU/ml human chorionic gonadotropin [hCG] and 5% FBS) and follicles were incubated at 37°C in a humidified atmosphere of 5% CO2 in air for 14-16 h. Oocytes were then removed from the cumulus cells by treatment with 0.3% hyaluronidase. The meiotic stage of each oocyte was scored morphologically using the Leica DM IL microscope. Oocytes that remained arrested in prophase of meiosis I, with a visible intact germinal vesicle (GV), were classified as GV-intact oocytes. Oocytes that resumed meiosis and reached metaphase of meiosis II (MII) with polar body extrusion were classified as MII-arrested eggs. Those oocytes that resumed meiosis but did not reach MII, defined by the lack of a both a GV and a polar body, were referred to as oocytes that had undergone GV breakdown (GVBD). Degenerated oocytes were also documented. The meiotic progression distribution is reported as the number of oocytes in each stage over the total number of follicles that were matured in vitro.
Egg cytoskeleton analysis
To examine the microtubule and actin cytoskeleton, cells were processed as described previously (Hornick et al., 2012). Briefly, oocytes were fixed in 3.8% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) containing 0.1% Triton X-100 (Sigma-Aldrich) in 1X phosphate-buffered saline (PBS) for 1 hr at room temperature. After fixation, oocytes were washed with blocking buffer containing 0.3% BSA, 0.02% NaN3, and 0.01% Tween-20 in 1X PBS and then incubated in permeabilization solution containing 0.3% BSA, 0.1% Triton X-100, and 0.02% NaN3 for 15 minutes. After rinsing with blocking buffer, oocytes were incubated in a 1:100 dilution of Alexa Fluor 488-conjugated α-tubulin rabbit mAb for 1 h at room temperature to detect the microtubule cytoskeleton (Cell Signaling, Boston, MA). F-actin was detected by simultaneous incubation in a 1:50 dilution of rhodamine phalloidin (Invitrogen). All antibodies were diluted in blocking buffer. Oocytes were washed in blocking buffer after antibody incubations and mounted in Vectashield containing DAPI to detect DNA (Vector Laboratories, Burlingame, CA, USA). Images were acquired with a Leica SP5 inverted laser-scanning confocal microscope equipped with a 40× objective and Confocal LAS AF software (Leica Microsystems, Heidelberg, Germany). Additional image processing was done using Image J software (National Institutes of Health, Bethesda, MD). Spindle morphology was scored and normal spindles were defined as those that were bipolar and had chromosomes tightly aligned on the metaphase plate.
Ovarian stromal cell isolation and survival
Ovarian stromal cell isolation was carried out as described previously with minor modifications (Tingen et al., 2011). Ovaries harvested from day 21-22 mice were placed in L15 media containing 0.5% Pen-Strep and 1% FBS. Granulosa cells were released by mechanical disruption of the ovaries using two insulin-gauge needles. The remaining tissue was dissociated in 1X Trypsin/EDTA-Hanks (Invitrogen) containing 1% Liberase TM (Roche Applied Scientific) and 1% DNase I (Sigma) with gentle rocking for 1 hr at 37°C in a humidified atmosphere of 5% CO2 in air. The enzymatic reaction was stopped by addition of 10% FBS and the solution was passed sequentially through 70-μm and 20-μm filters (Gorlitz, Germany). The flow-through was then pelleted by centrifugation for 5 min at 1500 rpm and rinsed with the following growth media: DMEM/F12 supplemented with 1M HEPES, 20 mg/ml androstenedione, 4 mg/ml hydrocortisone, 6.5 μg/ml insulin, 6.5 μg/ml transferrin, and 6.5 ng/ml selenium (Sigma-Aldrich). The cells were pelleted again and rinsed with growth media containing 10% heat-inactivated FBS (Tingen et al., 2011). Cells were then resuspended in 1-2 ml of growth media with FBS and plated in 96-well polystyrene plates at an approximate density of 50, 000 cells per well. Cells were incubated overnight at 37°C in a humidified atmosphere of 5% CO2 in air. The following day, the media was replaced with growth media containing different concentrations of CE. Growth media was changed every other day, and the MTS CellTiter 96 Aqueous Non-Radioactive One Solution Assay (Promega Corp., Madison, Wis.) was used to evaluate cell viability on Day 4 of culture according to the manufacturer's instructions.
HeLa cell culture and survival
Human cervical cancer cells (HeLa cell line; ATCC, Manassas, VA) were thawed and plated in 96-well plates at an approximate density of 50,000 cells per well. CE exposure and the cell viability assay were performed as described above for stromal cells. The only modification was that the cell viability assay was performed on Day 2 for HeLa cells, as they proliferate more rapidly than primary cell lines.
Statistical analysis
Plotting of results and statistical analysis were done using Graph Prism (La Jolla, CA, USA). Antral cavity formation, E2 production, and survival following CDDP and CTX exposure were analyzed using a two-way analysis of variance (ANOVA) followed by a Bonferroini post-hoc test for single time point comparisons. HeLa cell, stromal cell, and follicle survival were determined by sigmoidal dose-response (variable slope) curve followed by an F-test. Meiotic progression and spindle morphology were analyzed using a one-way analysis of variance (ANOVA). In all cases, a p-value of less than 0.05 was considered statistically significant.
Results
IVFG can identify chemicals with known adverse reproductive outcomes
To determine the utility of the IVFG system, we first validated the assay by determining the effects of two common chemotherapeutics, cyclophosphamide (CTX) and cisplatin (CDDP), which are known to be toxic to the ovary and to follicles specifically (Hartmann and Lipp, 2003; Kerr et al., 2012). Follicle survival decreased in a dose-dependent manner during culture in CTX and CDDP (Figure 2). Exposure to CTX resulted in a significant decrease in survival; by Day 4, survival was 95.40% for control follicles and 57.23% and 36.41% for follicles exposed to 1 μM and 5 μM CTX, respectively (Figure 2A; P<0.001). By Day 6, there was a further decrease in survival, with only 32.43% and 11.49% follicles remaining in the 1 μM and 5 μM CTX treatment groups, respectively. The difference in survival between follicles cultured in 0.2 μM CTX compared to control follicles by Day 6 was also statistically significant (Figure 2A; 71.8% vs. 95.40%, respectively, P<0.05). A similar trend in decreased follicle survival after 4 and 6 days of culture was observed following CDDP treatment compared to control follicles (Figure 2B); however, the reduction in survival with CDDP exposure was only significant at the 1 μM and 5 μM doses (Figure 2B; 76.31 and 47.83% on Day 4 and 58.38% and 12.56% on Day 6, respectively, P<0.05). Thus, both CTX and CDDP adversely impacted follicle survival in the IVFG system in a dose-dependent manner.
Figure 2.
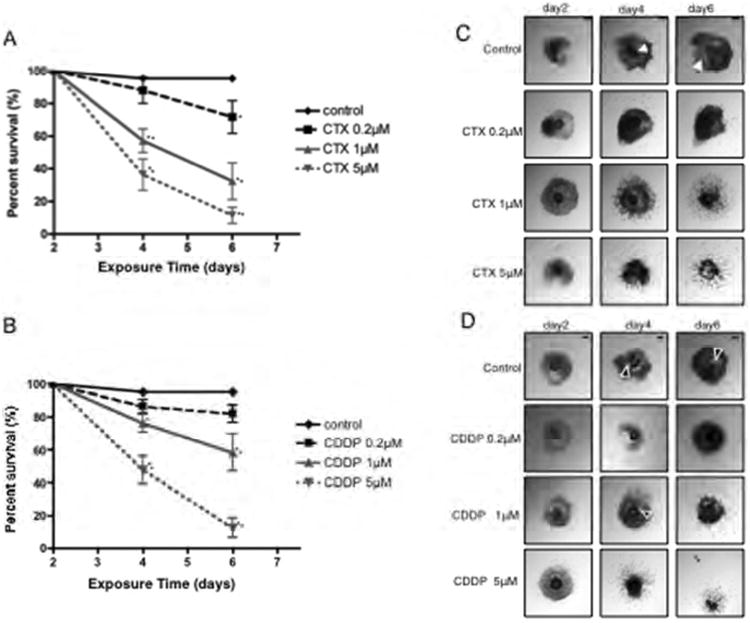
Validation of the IVFG system using two chemotherapeutics known to have adverse reproductive effects. The effects of increasing concentrations of CTX and CDDP on follicle (A, B) survival and (C, D) morphology during IVFG are shown. Follicles were treated with CTX and CDDP on day 2 of culture, and follicle survival and morphology were assessed on days 4 and 6. This experiment was repeated 4 times and a total of at least 60 follicles in each experimental group were examined. Representative images are shown. The arrowheads indicate the antral cavity, and the arrow indicates an oocyte that has been released from the follicle. Scale bar=100 μm. Statistically significant differences are denoted as follows: *P<0.05 and **P<0.001.
In addition to survival, CTX and CDP exposure affected follicle morphology. In control follicles, granulosa cells proliferated and differentiated into mural and cumulus cells concomitant with antral cavity formation (Figures 2C, D). In contrast, exposure to increasing concentrations of CTX and CDDP resulted in disorganized follicles with dark granulosa cells and little evidence of antral cavity formation (Figures 2C, D). The most severe phenotype was a complete release of the oocyte from the follicle, observed at 1 μM CTX and 5 μM CDDP (data not shown and Figure 2D). These results confirm previous findings that CTX and CCDP are toxic to follicles (Hartmann and Lipp, 2003; Kerr et al., 2012).
To confirm that the follicle morphology and survival results obtained were specific to CTX and CDDP exposure and not due to a general toxicity response, we examined how follicles behaved when exposed to the FDA-approved pharmaceutical, nalbuphine, an analgesic with no known documented reproductive toxicity (FDA, 2014). In terms of survival and morphology, secondary mouse follicles cultured in the presence of 0.2, 1, or 5 μM nalbuphine were similar to controls (Figure 3; P>0.05). Thus, the IVFG system was able to distinguish between known reproductive toxins and compounds that have no known effect on reproduction in terms of follicle health and survival.
Figure 3.
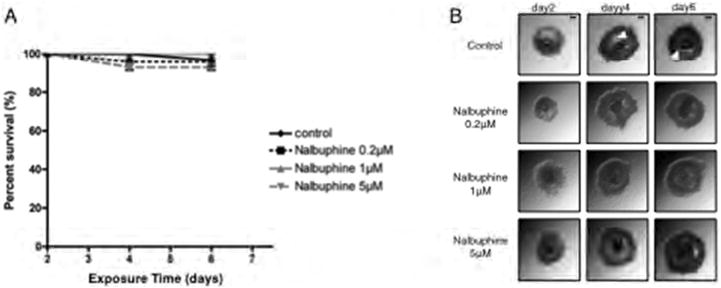
Validation of the IVFG system using two FDA-approved pharmaceuticals that are not known to have off-target reproductive effects. The effects of increasing concentrations of nalbuphine on follicle (A) survival and (B) morphology during IVFG are shown. Follicles were treated with nalbuphine on day 2 of culture, and follicle survival and morphology were assessed on days 4 and 6. Experiments with nalbuphine were repeated 5 times with a total of at least 25 follicles examined in each experimental group. Representative images are shown. The arrowheads highlight the antral cavity. Scale bar=100 μm.
Corexit exposure impacts follicle endpoints in the IVFG assay
Because the IVFG assay was used successfully to detect differences between known fertotoxic and non-fertotoxic compounds on reproductive outcomes, we applied the assay to determine how a compound with unexplored reproductive effects impact follicle growth and function. For this analysis, we chose to determine the effect of CE on IVFG parameters. Overall follicle survival decreased significantly in a dose-dependent manner after exposure to CE ranging from 0 ppm to 400 ppm (Figure 4A). By Day 4 of culture, survival was 99.08% in control follicles and 50.76%, 11.30%, and 0% in follicles cultured in 75 ppm, 100 ppm, and 125 ppm CE, respectively (Figure 4A; P<0.001). By Day 6, survival decreased to 37.10% and 0% follicles in the 75 ppm and 100 ppm CE treatment groups, respectively. The differences in survival between follicles cultured in 25 and 50 ppm compared to controls were also statistically significant (Figure 4A; 76.64%, P<0.05 and 61.83%, P<0.001, respectively). Based on these data, we determined that the concentration at which half of the follicles survived at Day 6, or the median lethal dose (LD50) with 95% confidence interval, was 57.37 ± 8.94 ppm (Figure 4B, triangles). Given this LD50, subsequent experiments were performed using CE concentrations of ≤75 ppm.
Figure 4.
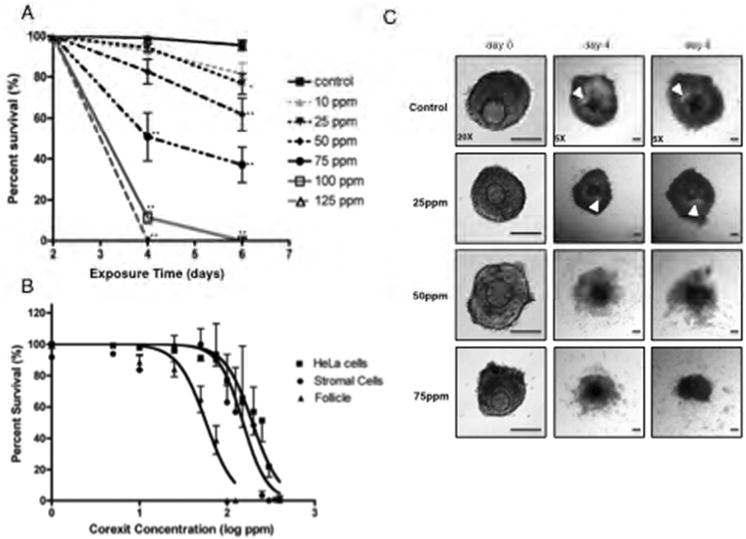
CE exposure affects ovarian follicle growth and morphology. The effect of increasing concentrations of CE on (A) follicle survival is shown. (B) The survival of follicles, primary ovarian stromal cells, and transformed HeLa cells following culture in increasing concentrations of CE was used to calculate the respective LC50 for each cell type. (C) The effect of CE exposure on follicle morphology is shown. For these experiments, follicles were treated with CE on day 2 of culture, and survival and morphology were assessed on days 4 and 6. These experiments were repeated at least 5 times with the following numbers of follicles per group: Control n=135, 5 ppm n=104, 10 ppm n=103, 25 ppm n=123, 50 ppm n=111, 75 ppm n=128, 100 ppm n=64, and 125 ppm n=51. Representative images are shown. The arrowheads indicate the antral cavity. Scale bar=100 μm. Statistically significant differences are denoted as follows: *P<0.05 and **P<0.001.
In addition to affecting follicle survival, exposure to increasing concentrations of CE altered follicle morphology and cellular differentiation (Figure 4C). Follicles grown in 10 ppm CE appeared morphologically normal and indistinguishable from controls, demonstrating appreciable granulosa cell proliferation and antral cavity formation (data not shown). Follicles exposed to 25 ppm CE also appeared healthy, but their antral cavities appeared smaller compared to those in control follicles (Figure 4C). In contrast, exposure to 50 ppm and 75 ppm CE resulted in severe follicle disorganization (Figure 4C).
To better understand how CE affected follicle growth and morphology, we quantified the percent of follicles that formed antral cavities when exposed to the dispersant (Figure 5A). Antral cavity formation was assessed between day 4 and 6 of culture, as this is when many follicles form this characteristic structure (Figures 2C, 2D, 4C). On day 4 of culture, only follicles grown in 75 ppm CE showed a significant reduction in antral cavity formation compared to controls (Figure 5A, 10.15% vs. 41.18%, respectively; P<0.05). By Day 6, however, significant reductions in antral cavity formation were also observed at lower concentrations of CE. For example, even at 25 ppm CE, the percentage of follicles that formed an antral cavity was 44.4% compared to 77.6% for control follicles (Figure 5A, P<0.001). Interestingly, in untreated control follicles, a significant increase in the percent of follicles that formed antral cavities occurred between Day 4 and Day 6 of culture (Figure 5A, from 41.18% to 70.84%; P<0.05). This increase did not occur between Day 4 and Day 6 when follicles were exposed to CE concentrations greater than 25 ppm (Figure 5A). Taken together, these data suggest that CE exposure has a dose-dependent effect on follicle morphology, with a specific consequence on cellular differentiation and antral cavity formation.
Figure 5.
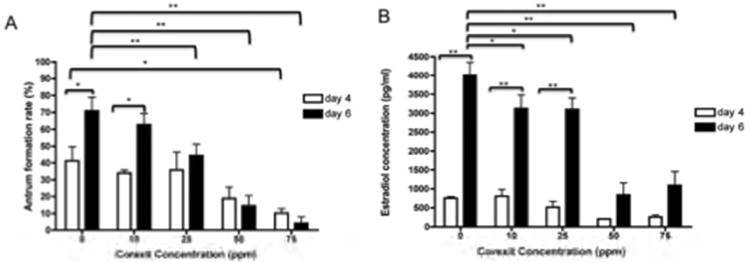
CE exposure affects follicle differentiation and function. The effects of increasing concentrations of CE on (A) antral cavity formation and (B) estradiol production are shown. These experiments were repeated at least 5 times with between 51 and 135 follicles examined per CE dose. Follicles were treated with CE on day 2 of culture, and antral cavity formation and hormone production were assessed on days 4 and 6. Statistically significant differences are denoted as follows: *P<0.05 and **P<0.001.
Corexit exposure affects follicle hormone production
E2 levels in the spent culture media of control follicles were low on Day 4 but increased dramatically by Day 6, coincident with the large increase in follicle growth and differentiation that is also observed during this time (Figures 4C, 5). CE exposure did not have a significant effect on baseline E2 levels on Day 4; but by day 6 of culture, CE exposures of 10 ppm and 25 ppm resulted in a slight but significant reduction in E2 levels compared to controls (3127 pg/ml and 3097 pg/ml compared to 4007 pg/ml, respectively, P<0.05; Figure 5B). This negative impact of CE on E2 production was even more dramatic at concentrations of 50 ppm and greater, when E2 levels were between 842.9 pg/ml and 1094 pg/ml, P<0.001; Figure 5B). In fact, the large relative average increase in E2 that typically occurs between day 4 and day 6 was abrogated significantly in follicles cultured in ≥50 ppm CE (P<0.05; Figure 5B). These results suggest that CE exposure not only affects follicular morphology and development, but also influences hormonal homeostasis.
Corexit exposure causes meiotic defects in the oocyte
In control follicles that were not exposed to CE, 78% ± 4.243% of the gametes reached MII (Figure 6B). This percentage was unchanged in follicles that were exposed to 10 ppm and 25 ppm of CE; however, at 50 ppm and 75 ppm CE, only 21.80% and 13.90% of the oocytes reached MII, respectively (P<0.001; Figure 6B). The decrease in the number of mature gametes was accompanied by a significant increase in the percent of degenerated cells (Figure 6B). CE exposure did not affect the percentage of cells that were in the GV or GVBD/MI stages (Figure 6B). These data imply that at higher concentrations, CE negatively impacts the ability of the follicle to produce a viable and mature egg.
Figure 6.
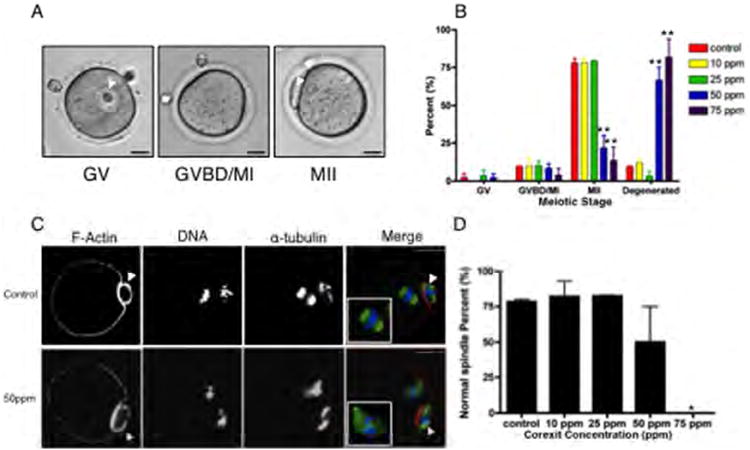
CE exposure affects oocyte meiotic competence and spindle morphology. The ability of oocytes derived from follicles exposed to CE to progress through the stages of meiotic maturation was (A) scored by light microscopy and (B) quantified. Cells were classified as germinal vesicle intact (GV), germinal vesicle breakdown/metaphase of meiosis I (GVBD/MI), or metaphase of meiosis II (MII), and representative images for each stage are shown (A). This experiment was repeated at least twice and a minimum of 25 cells were analyzed in each experimental group. (C) The actin- and microtubule-based cytoskeleton in the resulting MII eggs from control and CE-exposed follicles was examined by immunocytochemistry and confocal microscopy. An optical section encompassing the meiotic spindle is shown. In the merged images, the pseudocolor represents the following: green=α-tubulin, red=F-actin, and blue=DNA. Normal meiotic spindles were characterized by a bipolar structure with chromosomes tightly aligned on the metaphase plate (upper panel, control). Abnormal spindles were characterized by disrupted tubulin and scattered chromosomes (lower panel, 50 ppm CE). (D) The percent of normal spindles observed in the MII eggs derived from follicles exposed to increasing concentrations of CE was quantified. This experiment was repeated a minimum of 2 times, and between 18 and 29 oocytes in each experimental condition were analyzed. Representative images are shown. Arrowheads indicate the polar body. Scale bar=25μm. Statistically significant differences are denoted as follows: *P<0.05.
Although CE doses of 50 ppm and 75 ppm significantly compromised meiotic maturation, there were still some MII eggs produced. To better understand the quality of the resulting eggs, we performed a more detailed analysis of their cytoskeletal morphology (Figures 6C, D). We observed no differences in spindle structures in MII eggs from follicles exposed to 10 ppm and 25 ppm CE compared to controls. However, abnormal spindles were observed following exposure to higher concentrations of CE (Figures 6C, D). In follicles grown in 75 ppm CE, no normal spindle morphology was observed in the resulting eggs. Instead, spindles in these eggs lacked an organized bipolar structure and had significant chromosome misalignment (Figure 6C). In contrast to the meiotic spindle, which appeared to be highly sensitive to CE, cortical F-actin was not affected (Figure 6C). Therefore, although MII eggs were produced in the presence of high CE concentrations, the resulting eggs were of poor quality, further highlighting the depth at which follicle health can be assessed using IVFG and how CE affects various aspects of follicle biology.
The ovarian follicle is more sensitive to Corexit compared to cell lines
In toxicity studies, cell lines are often used for in vitro drug screening and toxicity studies (Allen et al., 2005). We determined the relative sensitivity to CE of follicles versus primary mouse ovarian stromal cells and the human HeLa cell line. For both primary ovarian stromal cells and HeLa cells, the data were fit to a variable sigmoidal dose-response curve and an F-test was performed to determine significance (P<0.0001; Figure 4B). We calculated that the LD50 with 95% confidence interval for primary ovarian stromal cells was 151.9±32.25 ppm and for HeLa cells was 194.1±22.8 ppm. This observed difference in survival between HeLa cells and primary ovarian stromal cells was statistically significant (P<0.05). Moreover, the LD50 of follicles was significantly lower than either of the somatic cell lines examined (P<0.0001; Figure 4B). These data highlight distinct differences in how cells behave in response to CE and suggest that transformed cells are more resistant to CE toxicity than primary cells. Moreover, the ovarian follicle, which contains both somatic and germ cell components, is particularly sensitive to CE.
Discussion
IVFG is a powerful model system that recapitulates key events of oogenesis and folliculogenesis. To date, IVFG assays have been used to examine the effects of certain chemicals, including chemotherapeutics, bisphenol-A, colchicine, trichlorfon, and diazepam, on follicle development (Ahn et al., 2013; Lenie et al., 2008; Lenie and Smitz, 2009; Meirow and Nugent, 2001; Peretz et al., 2012; Peretz and Flaws, 2013; Peretz et al., 2011; Peretz et al., 2013; Sun et al., 2008; Van Wemmel et al., 2005). However, several of these studies only reported negative impacts of these chemicals on follicle health without the use of negative control compounds, and so the possibility of non-specific global toxicity cannot be excluded. We were interested in whether we could apply the IVFG assay to adequately distinguish between chemicals that have known reproductive toxicity from those that do not. To do this, we exposed follicles in vitro to compounds that have well-documented in vivo reproductive side effects (CTX and CDDP) and to Nalbuphine that have no known or reported reproductive toxicities (FDA, 2014; Borovskaya et al., 2004; Kociba and Sleight, 1971; Meirow et al., 1999; Meirow and Nugent, 2001; Wallace et al., 1989; Yanulevich, 1983; Yeh et al., 2006; Yucebilgin et al., 2004). We found that this IVFG assay was, in fact, able to phenocopy the predicted outcomes, with follicles grown in the presence of CTX and CDDP exhibiting a dose-dependent and significant reduction in survival and growth. In contrast, nalbuphine did not have an effect on follicle survival and growth, even at the highest concentrations tested. Finally, CE, a compound that had no previous mammalian fertotoxic assessment, was identified as a potential reproductive toxin using the IVFG assay.
Previous studies suggested that CE exposure is toxic to marine organisms and wildlife. Both acute and chronic toxicity to CE have been reported in diatoms, coral larvae, inland silversides, and mysid shrimp (Goodbody-Gringley et al., 2013; Hemmer et al., 2011; Hook and Osborn, 2012). CE has also been shown to have embryotoxicity in mallard ducks, whereby exposure of eggs to CE resulted in significant embryo mortality and reduced hatching (Pedersen and Peters, 1968; Picton et al., 2008). Interestingly, our finding that CE is fertotoxic in the mammalian IVFG model system is consistent with what was reported in C. elegans, a soil-dwelling nematode that is highly suited for ecotoxicology studies (Cole et al., 2004; Roh et al., 2010; Sochova et al., 2007; Wang and Xing, 2008). Nematodes exposed to CE exhibited a dose-dependent impairment of reproductive function, and it was noted that the reproduction effects of CE were more pronounced than any of the growth effects observed (Zhang et al., 2013). The data from these studies suggest that the observed effects are dose-dependent and CE may have reproductive affects across species, with the more severe effects observed at the highest concentrations of exposure.
Based on the best evidence available at the time of the Deepwater Horizon accident, it was thought that the environmental effects of oil dispersants on humans would be low. Certainly authorities were balancing the unmitigated disaster caused by the oil spill against the need for containment, so the use of CE is understood. Nevertheless, there were no tools to assess the direct impact of dispersants on mammals, the routes of exposure and potential for accumulation in the body, or the effects of the dispersant alone vs. when the dispersant is mixed with oil residues. Based on our initial findings with the IVFG assay, we predict that CE could potentially result in adverse reproductive outcomes in mammalian species. Furthermore, CE appears to affect both the somatic cells and the oocyte, suggesting that adverse reproductive outcomes as a result of CE exposure could include both endocrine abnormalities and reduced gamete quality. Our data support further endocrine and fertility studies to fully understand the consequences of CE exposure on mammalian reproductive health, particularly given the widespread use of CE in oil spill mitigation.
A significant discovery made in this study was that the ovarian follicle is more sensitive to CE exposure than either primary or transformed cell lines as single units. The LD50 of primary ovarian stromal cells and HeLa cells to CE was nearly 3-times higher than that of follicles. These results have important implications in the field of toxicology testing, since cell lines—whether they are primary, transformed, or stem cell-derived—are viewed as an effective and rapid screening tool for adverse outcomes. In fact, the National Research Council's report on “Toxicity Testing in the 21st Century: A Vision and a Strategy” proposed a paradigm shift in how toxicity testing of environmental agents is performed (European Medicines Agency, 2014). The NRC explicitly called for the field to shift from in vivo animal testing towards in vitro screening assays that rely on cell lines or tissue surrogates (Andersen et al., 2010). Our results emphasize that, although a simple in vitro model is preferable for toxicity testing, it must accurately reflect the physiology of the biological system being studied. In the case of reproductive function, simple cell culture models (e.g., stromal cells) may not be the best system for toxicity screening. Instead, organotypic culture systems, which integrate the coordinated development of multiple cell types, hold tremendous promise for in vitro toxicity testing (Dash et al., 2012).
IVFG faithfully mimics characteristic aspects of ovarian biology, including oogenesis, folliculogenesis, meiosis, and ovulation, and is thereby particularly useful for reproductive toxicity testing (West-Farrell et al., 2009; Xu et al., 2009; Xu et al., 2011). IVFG is rapid, simple, and high-throughput, as ovarian follicles can be isolated in relatively large quantities and grown autonomously in a simple culture system over an extended period. Moreover, IVFG simultaneously assays both the somatic and germ cell components providing early insight into potential adverse endocrine, fertility, and even general health outcomes. In previous work, we utilized the IVFG system to evaluate the fertotoxicity of a new formulation of arsenic trioxide, and found that the effects of exposure on follicle growth and development were comparable to those seen in in vivo exposure models (Ahn et al., 2013).
The conclusions we can make regarding the fertotoxicity of CE in humans are limited by the lack of available information about human exposure to CE; however, it is hoped that our findings in the mouse follicle IVFG system, using CE concentrations based on data from evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500 (Zheng et al., 2014) will justify a closer investigation of human exposure levels in the environment and the potential reproductive effects of CE exposure in affected human populations. A recently published study showed that Corexit is toxic to three cell lines by altering intracellular oxidative function leading to mitochondrial dysfunction and apoptosis. The in vitro cell line study, together with the present data, build the case for additional research (Zheng et al., 2014). Despite the overall limitation regarding human uptake, the current study demonstrates the feasibility of applying IVFG-based assays in preclinical toxicology testing. Our preliminary findings suggest that IVFG may be a valuable, rapid, first-line tool in the setting of both environmental chemical exposure assessment and pharmaceutical drug screening to evaluate female reproductive toxicity in mammals. Future studies will continue to validate the system using additional compounds with known reproductive toxicology data.
Conclusions
In conclusion, we demonstrate that IVFG is a robust, organotypic model system that can be applied to rapidly assess potential adverse reproductive outcomes following chemical exposures. IVFG assays represent a novel approach to gathering critical data on female reproductive toxicity without long-term costly in vivo studies. Data from initial IVFG assays could be used to determine whether additional in-depth research on the impact of specific exposures on human reproductive health is warranted. We showed for the first time that the commonly used oil dispersant, CE, can alter follicle quality and function in vitro, indicating a need for additional research on the female reproductive health impact of CE that will inform protection guidelines and limitations to minimize work place exposures. Studies are ongoing to expand IVFG from just follicles to a system that integrates the entire reproductive system and hormone-responsive organs to acquire a more detailed reproductive toxicity profile. These studies will provide the field with a useful tool for characterizing or mitigating effects of pharmaceutical, chemical, and environmental compounds on human reproductive function.
Acknowledgments
We are grateful to Nalco (Chicago, IL) for providing us with Corexit EC 9500A for the following studies. We also thank Dr. Jessica Hornick for her technical assistance, Marilia Cordeiro for her scientific illustrations, and Dr. Stacey Tobin and Dr. Beth Sefton for editorial assistance. This study was supported by funds through NIH/NIEHS/ORWH/NCATS UH2ES022920 and the Watkins Endowed Chair in Obstetrics and Gynecology.
This study was supported by funding from NIH/NIEHS/ORWH/NCATS UH2ES022920 and the Watkins Endowed Chair in Obstetrics and Gynecology.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
References
- Ahn RW, Barrett SL, Raja MR, Jozefik JK, Spaho L, Chen H, et al. Woodruff TK. Nano-encapsulation of arsenic trioxide enhances efficacy against murine lymphoma model while minimizing its impact on ovarian reserve in vitro and in vivo. PLoS One. 2013;8(3):e58491. doi: 10.1371/journal.pone.0058491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertini DF, Combelles CM, Benecchi E, Carabatsos MJ. Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121(5):647–653. doi: 10.1530/rep.0.1210647. [DOI] [PubMed] [Google Scholar]
- Allen DD, Caviedes R, Cardenas AM, Shimahara T, Segura-Aguilar J, Caviedes PA. Cell lines as in vitro models for drug screening and toxicity studies. Drug Dev Ind Pharm. 2005;31(8):757–768. doi: 10.1080/03639040500216246. [DOI] [PubMed] [Google Scholar]
- Andersen ME, Al-Zoughool M, Croteau M, Westphal M, Krewski D. The future of toxicity testing. J Toxicol Environ Health B Crit Rev. 2010;13(2-4):163–196. doi: 10.1080/10937404.2010.483933. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Madden JA, Sen N, Hoyer PB, Keating AF. Glutathione S-transferase class mu regulation of apoptosis signal-regulating kinase 1 protein during VCD-induced ovotoxicity in neonatal rat ovaries. Toxicol Appl Pharmacol. 2013;267(1):49–56. doi: 10.1016/j.taap.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovskaya TG, Goldberg VE, Fomina TI, Pakhomova AV, Kseneva SI, Poluektova ME, Goldberg ED. Morphological and functional state of rat ovaries in early and late periods after administration of platinum cytostatics. Bull Exp Biol Med. 2004;137(4):331–335. doi: 10.1023/b:bebm.0000035121.85533.08. [DOI] [PubMed] [Google Scholar]
- Cole RD, Anderson GL, Williams PL. The nematode Caenorhabditis elegans as a model of organophosphate-induced mammalian neurotoxicity. Toxicol Appl Pharmacol. 2004;194(3):248–256. doi: 10.1016/j.taap.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Cortvrindt RG, Smitz JE. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8(3):243–254. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- Dash A, Blackman BR, Wamhoff BR. Organotypic systems in drug metabolism and toxicity: challenges and opportunities. Expert Opin Drug Metab Toxicol. 2012;8(8):999–1014. doi: 10.1517/17425255.2012.693161. [DOI] [PubMed] [Google Scholar]
- Dutta S, Mark-Kappeler CJ, Hoyer PB, Pepling ME. The steroid hormone environment during primordial follicle formation in perinatal mouse ovaries. Biol Reprod. 2014;91(3):68. doi: 10.1095/biolreprod.114.119214. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73(2):351–357. doi: 10.1095/biolreprod.105.041798. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Detection of Toxicity to reproduction for medicinal products & Toxicity to male fertility. Mar, 1994. [Accessed July 26, 2014]. www.ema.europa.edu . Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002809.pdf.
- Federal and Drug Administration. Approved Drug Products with Therapeutic Equivalence Evaluations. 2009. [Accessed July 26, 2014]. www.fda.gov .
- Federal and Drug Administration. [Accessed July, 2014]. www.fda.gov. (Drug@FDA)
- Goodbody-Gringley G, Wetzel DL, Gillon D, Pulster E, Miller A, Ritchie KB. Toxicity of Deepwater Horizon source oil and the chemical dispersant, Corexit(R) 9500, to coral larvae. PLoS One. 2013;8(1):e45574. doi: 10.1371/journal.pone.0045574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4(6):889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- Hemmer MJ, Barron MG, Greene RM. Comparative toxicity of eight oil dispersants, Louisiana sweet crude oil (LSC), and chemically dispersed LSC to two aquatic test species. Environ Toxicol Chem. 2011;30(10):2244–2252. doi: 10.1002/etc.619. [DOI] [PubMed] [Google Scholar]
- Hook SE, Osborn HL. Comparison of toxicity and transcriptomic profiles in a diatom exposed to oil, dispersants, dispersed oil. Aquat Toxicol. 2012;124-125:139–151. doi: 10.1016/j.aquatox.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Hornick JE, Duncan FE, Shea LD, Woodruff TK. Isolated primate primordial follicles require a rigid physical environment to survive and grow in vitro. Hum Reprod. 2012;27(6):1801–1810. doi: 10.1093/humrep/der468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Evaluation of ovotoxicity induced by 7, 12-dimethylbenz[a]anthracene and its 3,4-diol metabolite utilizing a rat in vitro ovarian culture system. Toxicol Appl Pharmacol. 2009;234(3):361–369. doi: 10.1016/j.taap.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Rajapaksa KS, Sipes IG, Hoyer PB. Effect of CYP2E1 gene deletion in mice on expression of microsomal epoxide hydrolase in response to VCD exposure. Toxicol Sci. 2008;105(2):351–359. doi: 10.1093/toxsci/kfn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sipes IG, Hoyer PB. Expression of ovarian microsomal epoxide hydrolase and glutathione S-transferase during onset of VCD-induced ovotoxicity in B6C3F(1) mice. Toxicol Appl Pharmacol. 2008;230(1):109–116. doi: 10.1016/j.taap.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, Brogan L, Myers M, Hutt KJ, Mladenovska T, Ricardo S, et al. Findlay JK. The primordial follicle reserve is not renewed after chemical or gamma-irradiation mediated depletion. Reproduction. 2012;143(4):469–476. doi: 10.1530/REP-11-0430. [DOI] [PubMed] [Google Scholar]
- Kociba RJ, Sleight SD. Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother Rep. 1971;55(1):1–8. [PubMed] [Google Scholar]
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651(1-2):71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Lenie S, Smitz J. Steroidogenesis-disrupting compounds can be effectively studied for major fertility-related endpoints using in vitro cultured mouse follicles. Toxicol Lett. 2009;185(3):143–152. doi: 10.1016/j.toxlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- Meirow D, Lewis H, Nugent D, Epstein M. Subclinical depletion of primordial follicular reserve in mice treated with cyclophosphamide: clinical importance and proposed accurate investigative tool. Hum Reprod. 1999;14(7):1903–1907. doi: 10.1093/humrep/14.7.1903. [DOI] [PubMed] [Google Scholar]
- Meirow D, Nugent D. The effects of radiotherapy and chemotherapy on female reproduction. Hum Reprod Update. 2001;7(6):535–543. doi: 10.1093/humupd/7.6.535. [DOI] [PubMed] [Google Scholar]
- Pedersen T, Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968;17(3):555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012;87(3):63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol. 2013;271(2):249–256. doi: 10.1016/j.taap.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119(1):209–217. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J, Neese SL, Flaws JA. Mouse strain does not influence the overall effects of bisphenol a-induced toxicity in adult antral follicles. Biol Reprod. 2013;89(5):108. doi: 10.1095/biolreprod.113.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton HM, Harris SE, Muruvi W, Chambers EL. The in vitro growth and maturation of follicles. Reproduction. 2008;136(6):703–715. doi: 10.1530/REP-08-0290. [DOI] [PubMed] [Google Scholar]
- Roh JY, Park YK, Park K, Choi J. Ecotoxicological investigation of CeO(2) and TiO(2) nanoparticles on the soil nematode Caenorhabditis elegans using gene expression, growth, fertility, and survival as endpoints. Environ Toxicol Pharmacol. 2010;29(2):167–172. doi: 10.1016/j.etap.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Sochova I, Hofman J, Holoubek I. Effects of seven organic pollutants on soil nematode Caenorhabditis elegans. Environ Int. 2007;33(6):798–804. doi: 10.1016/j.envint.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Spielmann H. Reproduction and development. Environ Health Perspect. 1998;106 Suppl 2:571–576. doi: 10.1289/ehp.98106571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Betzendahl I, Van Wemmel K, Cortvrindt R, Smitz J, Pacchierotti F, Eichenlaub-Ritter U. Trichlorfon-induced polyploidy and nondisjunction in mouse oocytes from preantral follicle culture. Mutat Res. 2008;651(1-2):114–124. doi: 10.1016/j.mrgentox.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Sutton P, Giudice LC, Woodruff TJ. Reproductive environmental health. Curr Opin Obstet Gynecol. 2010;22(6):517–524. doi: 10.1097/GCO.0b013e3283404e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen CM, Kiesewetter SE, Jozefik J, Thomas C, Tagler D, Shea L, Woodruff TK. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141(6):809–820. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Guidelines for reproductivetoxicity risk assessment (FRL-5360-6) 1996. [Accessed July 26, 2014]. www.epa.gov . Available at http://www.epa.gov/raf/publications/guidelines-reproductive-tox-risk-assessment.htm.
- Van Wemmel K, Gobbers E, Eichenlaub-Ritter U, Smitz J, Cortvrindt R. Ovarian follicle bioassay reveals adverse effects of diazepam exposure upon follicle development and oocyte quality. Reprod Toxicol. 2005;20(2):183–193. doi: 10.1016/j.reprotox.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Shalet SM, Crowne EC, Morris-Jones PH, Gattamaneni HR, Price DA. Gonadal dysfunction due to cis-platinum. Med Pediatr Oncol. 1989;17(5):409–413. doi: 10.1002/mpo.2950170510. [DOI] [PubMed] [Google Scholar]
- Wang D, Xing X. Assessment of locomotion behavioral defects induced by acute toxicity from heavy metal exposure in nematode Caenorhabditis elegans. J Environ Sci (China) 2008;20(9):1132–1137. doi: 10.1016/s1001-0742(08)62160-9. [DOI] [PubMed] [Google Scholar]
- West-Farrell ER, Xu M, Gomberg MA, Chow YH, Woodruff TK, Shea LD. The mouse follicle microenvironment regulates antrum formation and steroid production: alterations in gene expression profiles. Biol Reprod. 2009;80(3):432–439. doi: 10.1095/biolreprod.108.071142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Carlson A, Schwartz JM, Giudice LC. Proceedings of the Summit on Environmental Challenges to Reproductive Health and Fertility: executive summary. Fertil Steril. 2008;89(2 Suppl):e1–e20. doi: 10.1016/j.fertnstert.2008.01.065. [DOI] [PubMed] [Google Scholar]
- Xu M, Banc A, Woodruff TK, Shea LD. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103(2):378–386. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Fazleabas AT, Shikanov A, Jackson E, Barrett SL, Hirshfeld-Cytron J, et al. Woodruff TK. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84(4):689–697. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanulevich J. Outpatient anesthesia with nalbuphine hydrochloride. AANA J. 1983;51(4):395–397. [PubMed] [Google Scholar]
- Yeh J, Kim B, Liang YJ, Peresie J. Mullerian inhibiting substance as a novel biomarker of cisplatin-induced ovarian damage. Biochem Biophys Res Commun. 2006;348(2):337–344. doi: 10.1016/j.bbrc.2006.06.195. [DOI] [PubMed] [Google Scholar]
- Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, Erhan Y. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004;44(1):6–9. doi: 10.1111/j.1479-828X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen D, Ennis AC, Polli JR, Xiao P, Zhang B, et al. Pan X. Chemical dispersant potentiates crude oil impacts on growth, reproduction, and gene expression in Caenorhabditis elegans. Arch Toxicol. 2013;87(2):371–382. doi: 10.1007/s00204-012-0936-x. [DOI] [PubMed] [Google Scholar]
- Zheng M, Ahuja M, Bhattacharya D, Clement TP, Hayworth JS, Dhanasekaran M. Evaluation of differential cytotoxic effects of the oil spill dispersant Corexit 9500. Life Sci. 2014;95(2):108–117. doi: 10.1016/j.lfs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Zurlo J. No animals harmed: toward a paradigm shift in toxicity testing. Hastings Cent Rep, Suppl. 2012:S23–26. doi: 10.1002/hast.104. [DOI] [PubMed] [Google Scholar]


