Abstract
Spinal cord injury (SCI) is a traumatic event from which there is limited recovery of function, despite the best efforts of many investigators to devise realistic therapeutic treatments. Partly this is due to the multifaceted nature of SCI, where there is considerable disarray and dysfunction secondary to the initial injury. Contributing to this secondary degeneration is neurotoxicity, vascular dysfunction, glial scarring, neuroinflammation, apoptosis and demyelination. It seems logical that addressing the need for neuroprotection, regeneration and rehabilitation will require different treatment strategies that may be applied at varied stages of the post-injury response. Here we focus on a single strategy, exercise/physical training, which appears to have multiple applications and benefits for an acute or chronic SCI. Exercise has been demonstrated to be advantageous at cellular and biochemical levels, as well as being of benefit for the whole animal or human subject. Data from our lab and others will be discussed to further elucidate the many positive aspects of implementing exercise following injury and to suggest that rehabilitation is not the sole target of a training regimen following SCI.
Keywords: Spinal cord injury, exercise, neurorehabilitation, neurotrauma
Introduction
Spinal cord injury (SCI) is a traumatic event that is debilitating and results in permanent motor and sensory deficits. Between 250,000 and 500,000 people sustain a SCI each year worldwide (WHO, 2013). In the United States 12,000 people experience a SCI each year (N.S.C.I.S Center, 2013). Injuries can result from trauma, viruses or tumors, in addition to the more common motor vehicle accidents, falls, firearms, and sport injuries (Devivo, 2012). A cure for SCI has yet to be found as the cell and tissue response to injury is pervasive and progressive in nature and may require a particular sequence of therapies to correct damaged, and create new, neural connections.
Basics of spinal cord injury
An SCI can be described in two phases, the initial mechanical trauma to neurons, glial cells and their surrounding vasculature followed by a secondary expansive phase, which results in invasive degeneration of the surrounding spinal cord tissue. Events at the cellular level include apoptosis of neurons and glial cells (particularly oligodendrocytes), axon retraction, glial scarring, and recruitment of inflammatory cells, demyelination and subsequent exposure of myelin-associated inhibitory molecules, and aberrant sprouting /plasticity of spared nerve fibers/pathways (Bareyre et al., 2004; Crowe et al., 1997; Fitch and Silver, 2008; Jones et al., 2005). Biochemical changes, such as excitotoxicity, alterations to the electrophysiological properties of neurons, and the release of proinflammatory factors also are initiated once an injury has been incurred. The severity and degree of permanence of the motor and/or sensory deficits is dependent upon the location and extent of damage to the spinal cord tissue. If the initial injury is a contusion or bruising of the spinal cord, which is the most common form of SCI, then there may be some tissue sparing which can result in the retention of function. This form of injury is called an incomplete SCI. A transection through the spinal cord results in a complete interruption or separation between spinal segments causing total loss of motor and sensory function and is a model of complete SCI.
Spontaneous recovery
Even without the use of current therapeutic treatments both human subjects and animals with an incomplete SCI have demonstrated some measure of recovery. This is referred to as spontaneous recovery and is experienced to a lesser extent in patients compared to mild-moderate injury animal models. Spontaneous recovery is described as adaptive changes or plasticity and has been demonstrated in the form of change of neuronal properties (Giroux et al., 1999; Lee et al., 2007), collateral sprouting rostral and/or caudal to the injury site (Fouad et al., 2001; Murray and Goldberger, 1974; Weidner et al., 2001), alterations to cortical maps (Bruehlmeier et al., 1998; Fouad et al., 2001), or changes to spinal networks associated with the spinal pattern generator (Edgerton et al., 2008; Rossignol et al., 2008). This occurs without the use of interventions such as invasive therapy, pharmacological agents or rehabilitation training. The degree of spontaneous recovery in animal models may be difficult to determine since several groups have shown that after injury animals exhibit self-training in their home cages (Fouad et al., 2000; Kuerzi et al., 2010). Despite the occurrence of spontaneous recovery this alone will not lead to substantial functional recovery and additional interventions are needed.
Current treatment paradigms
Generally, use of a single intervention may result in only modest recovery, as it targets one aspect of the injury while the remaining impeding or detrimental properties of the injury are left untreated. Due to the complex nature of the injury several therapeutic strategies are combined to treat the many varied aspects of the trauma. Neuroprotection pertains to the preservation of the spared neurons and their processes immediately following the injury, since the events that occur during the secondary injury or expansion phase harm the spared, once fully functional neurons. Neuroregeneration aims to modulate the lesion site environment to promote axonal regrowth by removing inhibitory growth substances and providing a growth supportive environment. Intraspinal transplants enrich the lesion site by replacing lost cells with new neurons and/or glial cells to create and restore functional connections or provide a more permissible medium for regenerating axons. Neurorehabilitation in the form of exercise/physical training has demonstrated beneficial effects at the cellular and molecular levels that may translate into recovery of function (see review by Fouad and Tetzlaff, 2012). Possible mechanisms of action of exercise during protection and regeneration will be discussed in this review.
Exercise as a non-invasive therapy
Neurorehabilitative training such as exercise is a non-invasive treatment that passively or actively allows the patient or animal to engage in repetitive physical activity, often providing a rhythmic stimulation to affected regions of the spinal cord. Exercise has been shown to preserve muscle mass (Houle et al., 1999), restore motor and sensory function (Hutchinson et al., 2004; Sandrow-Feinberg et al., 2009), induce synaptic plasticity by way of neurotrophic factor production (Vaynman et al., 2003), increase the concentration of neurotrophic factors in spinal and muscle tissue (Gomez-Pinilla et al., 2002; Ying et al., 2005; Cote et al. 2011) and reduce inflammation around the lesion site (Sandrow-Feinberg et al., 2009). Many of the issues of post-SCI neuromuscular damage and loss of physiological activity, and the effects of exercise have been addressed by Gardiner (2001, 2011) in several comprehensive text books.
With SCI the once intact neuronal circuits undergo extensive reorganization in which inactivity leads to their pruning and a return of activity strengthens synaptic connections (Murakami et al., 1992). The tenet of “either use it or lose it” is based on the Hebbian synaptic strengthening concept that was validated by studies examining the development and plasticity of the visual cortex (Do, 1949; Hubel and Wiesel, 1963; Wiesel and Hubel, 1963). In a study of the dendritic field of motoneurons below the level of injury Gazula et al. (2004) reported a significant decrease in dendritic branches that was prevented if SCI-animals received daily exercise of the affected hind limbs. Therefore, it seems advantageous that after sustaining a SCI that some form of movement be encouraged or facilitated. But this raises a variety of questions and challenges as not all patients are in a medical condition to avail themselves of rehabilitation services. There are questions about what types of exercises are most beneficial to the patient or animal and how soon after an injury rehabilitation should be introduced. If the program is started too early, could the patient or animal experience more harm than good? What type(s) of rehabilitative strategy should be implemented? Is it necessary to include multiple types of rehabilitation in one’s training program, i.e. is a different training paradigm required for each specific task to be recovered? Magnuson and colleagues (2009) demonstrated the dramatic effect of task specific training in rats with a moderate to severe thoracic spinal cord contusion injury that were swim trained. Swimming provided an environment in which the limbs were able to move without loading forces unlike over ground locomotion, and while these animals improved their hind limb step pattern they were not able to improve the ability to bear weight.
Additionally, the post-injury time at which exercise is introduced can have beneficial or detrimental effects on the integrity of an injury. Several groups have used swim training and locomotor training to help determine these effects; Marsh et al (2011) reviewed the topic by including the use of pharmacological interventions, such as anti-Nogo-A and chondroitinase ABC alongside training, Smith et al. (2009) report that beginning swim training 3 days after SCI exacerbated an already active inflammatory response at the lesion’s epicenter and Norrie et al (2005) determined that a 3 month delay in horizontal ladder training of SCI rats failed to improve stepping performance to a level achieved with immediate training after an injury. As an important note, there also is information that delayed initiation of training post SCI does not affect performance in tasks for which the animal has not been trained (Krajacic et al., 2009). Recent studies from our lab investigated the timing at which exercise after an incomplete cervical level contusion injury affected the onset of neuropathic pain. If locomotor training by use of a forced wheel exercise was started 5 days after a C5 injury, then the incidence of neuropathic pain was reduced from 40% to ~ 5% of injured rats (Detloff et al., 2014). If exercise was delayed until the onset of pain (about 14 days post injury) there was no reversal or diminution of the established allodynia (submitted for publication).
One example of a combination treatment strategy is epidural stimulation of the spinal cord in conjunction with pharmacological agents and step training in adult transected rats to elicit stepping on a treadmill. (Courtine et al., 2009; Ichiyama et al., 2008). Epidural stimulation provides an electrical current directly to the spinal cord to activate particular neural networks that are no longer under the control of descending spinal pathways. Placement of an electrode on the dura of the spinal cord with the proper frequency of stimulation has produced movements below the level of SCI in rodents, cats and humans (Harkema et al., 2011) The first human subject report was of an individual who had sustained a C7-T1 subluxation from a motor vehicle accident one year prior to beginning the study and was motor complete and sensory incomplete. Locomotor training continued for 2 years and was followed by placement of an epidural electrode for chronic stimulation below the T1 level. With extensive training and epidural stimulation the patient was able to weight-bear while standing for over 4 minutes and demonstrated locomotor patterns during stimulation. Interestingly, during stimulation supraspinal control of some leg movements was recovered even 7 months after implantation of the electrode. In addition, locomotor training and epidural stimulation provided this patient with long term beneficial effects with regard to regulation of body temperature, bowel and bladder function and sexual function.
Exercise as a Neuroprotective Strategy
Biochemical changes
The mammalian target of rapamycin (mTOR) pathway is responsible for crucial cellular events including differentiation, survival, proliferation, and protein synthesis (Gingras et al., 2004; Hay and Sonenberg, 2004; Jaworski and Sheng, 2006). Once an injury has occurred in the CNS the mTOR pathway becomes deregulated and suppressed. A critical negative regulator of the pathway, phosphatase and tensin homolog (PTEN), has been shown to play a role in the prevention of axonal regeneration (Park et al., 2008). By deleting the PTEN gene in a virus-assisted conditional knockout model after retinal ganglion cell injury, Park and colleagues were able to demonstrate regeneration of damaged optic nerve axons (Park et al., 2008).
MicroRNAs (miRs) are small, non-coding mRNAs that play a significant role in regulating gene expression and were studied to elucidate the change in mTOR pathway activity after a T10 spinal transection and passive cycling exercise (Liu et al., 2012) Tissue from the lesion site and caudal spinal cord demonstrated changes in miR expression as a result of the injury. Three miRs (miR21, miR216a, and miR217) that target PTEN were examined, with a significant increase only detected in levels of miR21 in animals that received cycling exercise compared to injured, non-exercised animals (Figure 1). PTEN levels were evaluated and found to be significantly decreased with exercise back to normal levels at both the 10 and 31 day time points. Another miR, miR199a-3p, was examined as it is known to target mTOR mRNA. SCI alone resulted in a significant increase in the expression of miR199a-3p at both 10 and 31 days post injury (Figure 2). With exercise the marked increase in miR199a-3p expression was prevented at both time points, and was correlated with a significant increase in mTOR mRNA and an increase in the level of phosphorylated S6 expression in spinal interneurons. Whether these exercise-dependent molecular changes associated with regulation of protein synthesis lead to an increase in the regenerative effort of injured neurons remains to be tested.
Figure 1.
Passive exercise aids in regulating miRs along with target PTEN expression leading to a decrease in PTEN protein levels (from Liu et al., Exp. Neurol. 233:447–456, 2012).
Figure 2.
Passive exercise decreases miR199-3p thereby enabling mTOR mRNA expression and protein levels (from Liu et al., Exp. Neurol. 233:447–456, 2012).
We examined the expression of plasticity specific genes such as brain derived neurotrophic factor (BDNF), glial cell-line derived neurotrophic factor (GDNF), neurotrophin- 3 (NT-3), neurotrophin-4 (NT-4) and their receptors in specific neuron populations; motoneurons in the ventral horn, intermediate grey neurons and/or large diameter sensory neurons found within the dorsal root ganglion. In addition cellular stress genes were analyzed, including heat shock proteins (HSP) 27 and 70, glial fibrillary acidic protein (GFAP), and caspases 3, 7, and 9 (Keeler et al., 2012). Gene expression was examined in laser micro dissected neuron samples after a T10 transection injury with and without the use of cycling exercise for 1 or 4 weeks. After SCI alone, tissue from the lumbar section of the cord revealed an increase in caspases 3, 7, 9, that are involved in apoptosis. The TrkB receptor, which is the tyrosine kinase receptor for BDNF/NT3, and HSP 27 and 70 that are upregulated during stress and aid the cell the protein folding, were all increased in SCI only controls. With the addition of acute and prolonged exercise after SCI, there was an increase in the mRNA of BDNF, GDNF, HSP 27 and 70 along with a decrease in caspase-7 compared to injury only. These findings of mRNA level also corresponded to changes in protein expression. Following SCI alone, there was little change in gene expression in either the motoneurons or intermediate grey matter, but after exercise a significant increase in mRNA levels of BDNF, GDNF, and NT-4 was detected (Figure 3 and 4).
Figure 3.
Exercise modulates neurotrophic factor mRNA and neurotrophic factor receptor mRNA expression in motoneurons (from Keeler et al., Brain Research 1438: 8–21, 2012).
Figure 4.
Exercise modulates neurotrophic factor mRNA expression but not receptor mRNA in the intermediate grey matter (from Keeler et al., Brain Research 1438: 8–21, 2012).
The mRNA expression of caspases 3 and 9 within the large DRG neurons revealed an increase after SCI but with 1 week of exercise this was abated. Interestingly after a longer period of exercise (4 weeks) the levels of caspases 3 and 9 were significantly elevated. This also was evident in the caspase 3 expression of motoneurons from animals with SCI and 4 weeks of exercise. This data demonstrates that caspase 3 may be involved in synaptic plasticity (Gilman and Mattson, 2002; Gulyaeva, 2003). Taken together these results highlight the unique responses of different neuron types to injury alone and to subsequent acute or prolonged exercise.
Modulation of chloride homeostasis with SCI and exercise
Patients with SCI frequently experience spasticity, which can prevent recovery of motor function due to persistent firing of impaired pathways. The continuous firing can be attributed to the misguided release of gamma-aminobutyric acid (GABA) to inhibit post-synaptic firing. The release of the neurotransmitter GABA is dependent upon the intracellular chloride concentration, which in turn relies on two cation chloride co-transporters, Na-K-Cl co-transporter (NKCC1) and potassium-chloride co-transporter 2 (KCC2). Hyper-reflexia is a condition of heightened and unregulated response to stimulus due to the loss of descending inhibitory pathways and such spastic movements disrupt the orderly functional sequence of spared motor function. After SCI the frequency-dependent depression ratio (FDD) calculated from the H-reflex was used to measure spasticity or hyper-reflexia (Thompson et al., 1992), with the absence of FDD being indicative of a hyper-reflexive state. In a study of the effects of 2 different forms of hind limb exercise, cycling and treadmill training, after a spinal contusion injury were examined for effects on spasticity, gait and reflex components of locomotion. Bose et al. (2012) reported no spasticity in either group and an increased rate of recovery of limb placement.
A recent investigation to identify the possible mechanism behind the return of FDD focused on exercise-dependent reduction of hyper-reflexia and modulation of chloride concentrations (Côté et al., 2014). A T12 transection model was used with daily (5d/week) passive cycling exercise for 2 or 4 weeks post injury. After SCI absence of FDD was demonstrated, with an increase of NKCC1 protein and decrease of KCC2 protein in the lumbar spinal cord, caudal to the injury site. The SCI control animals revealed a gradual decrease in the ratio that plateaued by 28 days post injury. The addition of exercise significantly improved the FDD after 1 week or 4 weeks of exercise (Figure 6b). After 28 days of exercise the concentration of KCC2 expression on the motoneuron membrane was restored while there was no change in the concentration of NKCC1 expression with exercise. These findings shed light on the importance of the regulation of intracellular chloride concentrations in exercise dependent recovery. Pharmacological agents then were used to block KCC2 or NKCC1 to mimic exercise-dependent recovery of the FDD. With the addition of bumetanide, which blocks NKCC1, a return of FDD was demonstrated, resembling recovery of normal motoneuron function (Figure 5). ([Dihydroindenyl)oxy]alkanoic acid (DIOA) was used to block KCC2 during exercise and significantly suppressed the recovery gained by exercise in the 28 day group (Figure 6a) and further compromised the 7 day SCI control group.
Figure 6.
DIOA blocks KCC2, which removes exercise-dependent reflex recovery after injury (from Côté et al., J Neurosci. 34:8976–8987, 2014).
Figure 5.
Bumetanide blocks NKCC1 for H reflex recovery after SCI (from Côté et al., J Neurosci. 34:8976–8987, 2014).
Neuropathic pain
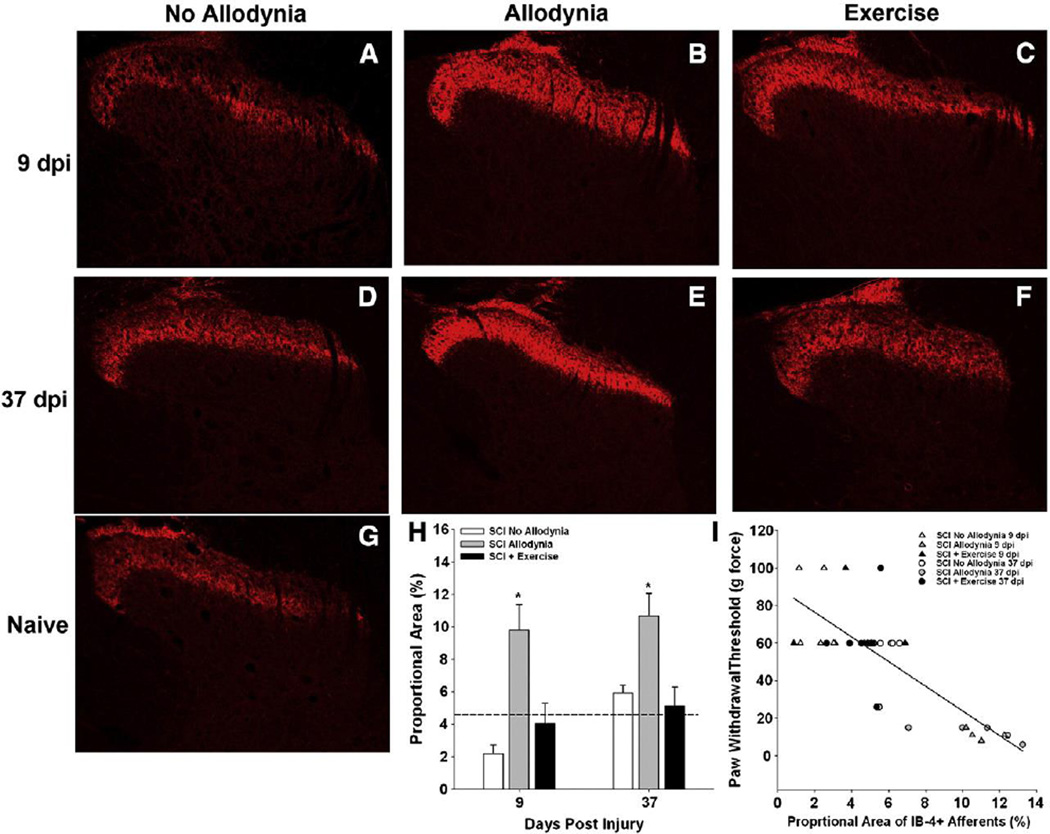
Damage of central sensory fibers during SCI frequently results in chronic debilitating neuropathic pain. After SCI intraspinal levels of neurotrophic factors decrease but with exercise there is an increase in their production both in spinal cord tissue (Côté et al., 2011; Gomez-Pinilla et al., 2001; Gomez-Pinilla et al., 2002; Sandrow-Feinberg et al., 2009). Hutchinson et al. (2004) used several types of exercise after injury to show a decrease in SCI-induced pain. By restoring the level of GDNF to baseline levels in a peripheral nerve injury, the level of neuropathic pain can be diminished (Boucher and McMahon, 2001; Hao et al., 2003; Pezet et al., 2006). In our study using a C5 unilateral contusion injury, daily exercise beginning 5 days post injury and applied 5 days per week resulted in a decrease in the frequency of allodynia (Figure 7), decrease in the labeling of nociceptive primary afferent sprouting in both the ipsilateral and contralateral dorsal horn, and reduction in GDNF and Artemin levels found in the dorsal root ganglion neurons involved in the afferent sprouting (Detloff et al., 2014) (Figures 8 and 9). Tactile allodynia at the level of the injury (C5 dermatome) was decreased significantly with 5 weeks of exercise compared to no exercise. This study demonstrates the valuable effects of an acute exercise approach for prevention of neuropathic pain at the molecular and cellular level, which translated into behavioral benefits.
Figure 7.
Acute exercise prevents at-level tactile allodynia (from Detloff et al., Exp Neurol. 255:38–48, 2014).
Figure 8.
Exercise prevents SCI-induced redistribution of afferents in the ipsilesional dorsal horn responsive to GDNF family of ligands (from Detloff et al., Exp Neurol. 255:38–48, 2014).
Figure 9.
Exercise prevents SCI-induced redistribution of afferents in the contralesional dorsal horn responsive to GDNF family of ligands (from Detloff et al., Exp Neurol. 255:38–48, 2014).
Neuroregenerative Effects
Exercise increases number of regenerating neurons
Based upon the observed increase in the level of phosphorylated-S6 ribosomal protein (p-S6) present within spinal interneurons of transected rats that received cycling exercise (Liu et al., 2012), an experiment was designed to test whether this increase would be reflected by an increase in axonal growth by injured neurons. Using our intraspinal PNG model (Houle, 1991; Côté etal., 2011) we began cycling exercise 5 days after acute grafting into a lower thoracic transection site. After 3–4 weeks of cycling the fluorescent tracer True Blue was applied to the distal end of grafts to label by retrograde transport neurons that had regenerated their axon into the PNG. We found a 9 fold increase in the number of True Blue labeled lumbar level interneurons in exercised vs. non-exercised animals and a 3 fold increase in regenerating neurons adjacent (1–3 mm) to the lesion-graft site (submitted for publication). Future experiments will test 1) whether exercise has a positive effect on axonal regeneration by chronically injured neurons and 2) whether exercise will promote axonal growth beyond the distal end of the PNG, by overcoming the inhibitory extrinsic factors associated with scarring in the injured spinal cord.
Summary
This review addresses multiple aspects of the beneficial effects of exercise but many questions remain about exercise as a therapeutic tool; such as the time post injury when exercise is best initiated, what is the most appropriate intensity, duration and frequency and what is the best use of task-specific and non-task specific training for recovery of multiple functional modalities? Changes at molecular and cellular levels provide some insights into possible mechanisms by which exercise has a positive impact on some of the structural and functional deficits that occur with SCI. Many groups have verified that physical training is important for regaining motor and sensory function after SCI and additional studies point towards exercise as a viable treatment for several secondary consequences after SCI, including chronic inflammation (Gleeson et al., 2011), autonomic dysreflexia and cardiovascular disease (West et al., 2014) and cardiometabolic syndrome (Kressler et al., 2014). Exercise is no longer strictly a tool for rehabilitation and there are many exciting aspects of this therapy that remain to be explored.
Highlights.
Exercise is neuroprotective after SCI by preventing neuropathic pain
The number of injured neurons that regenerate their axon increases with exercise
Exercise affects the molecular, cellular and physiological make-up of the injured spinal cord
Acknowledgments
We gratefully recognize the valuable contributions of our animal care technicians and trainers who made this work possible: Kassi Miller, Daniel Qiuros Molina, Scarlett Austin and Danielle Kulich. Our work described here was supported by grants from NIH, NINDS (NS026380, NS055976).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Boucher TJ, McMahon SB. Neurotrophic factors and neuropathic pain. Curr Opin Pharmacol. 2001;1:66–72. doi: 10.1016/s1471-4892(01)00010-8. [DOI] [PubMed] [Google Scholar]
- Bose PK, Hou J, Parmer R, Reier PJ, Thompson FJ. Altered patterns of reflex excitability, balance, and locomotion following spinal cord injury and locomotor training. Front Physiol. 2012;18:1–17. doi: 10.3389/fphys.2012.00258. Article 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J, Curt A. How does the human brain deal with a spinal cord injury? Eur J Neurosci. 1998;10:3918–3922. doi: 10.1046/j.1460-9568.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- Côté MP, Azzam GA, Lemay MA, Zhukareva V, Houle JD. Activity-dependent increase in neurotrophic factors is associated with an enhanced modulation of spinal reflexes after spinal cord injury. J Neurotrauma. 2011;28:299–309. doi: 10.1089/neu.2010.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Gandhi S, Zambrotta M, Houle JD. Exercise modulates chloride homeostasis after spinal cord injury. J Neurosci. 2014;34:8976–8987. doi: 10.1523/JNEUROSCI.0678-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12:1333–1342. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Detloff MR, Smith EJ, Quiros Molina D, Ganzer PD, Houle JD. Acute exercise prevents the development of neuropathic pain and the sprouting of non-peptidergic (GDNF- and artemin-responsive) c-fibers after spinal cord injury. Exp Neurol. 2014;255:38–48. doi: 10.1016/j.expneurol.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012;50:365–372. doi: 10.1038/sc.2011.178. [DOI] [PubMed] [Google Scholar]
- DO H. Organization of Behavior: A Neuropsychological Theory. New York: John Wiley and Sons Inc; 1949. Vol. [Google Scholar]
- Edgerton VR, Courtine G, Gerasimenko YP, Lavrov I, Ichiyama RM, Fong AJ, Cai LL, Otoshi CK, Tillakaratne NJ, Burdick JW, Roy RR. Training locomotor networks. Brain Res Rev. 2008;57:241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Metz GA, Merkler D, Dietz V, Schwab ME. Treadmill training in incomplete spinal cord injured rats. Behav Brain Res. 2000;115:107–113. doi: 10.1016/s0166-4328(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME, Brosamle C. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol. 2001;11:1766–1770. doi: 10.1016/s0960-9822(01)00535-8. [DOI] [PubMed] [Google Scholar]
- Fouad K, Tetzlaff W. Rehabilitative training and plasticity following spinal cord injury. Exp Neurol. 2012;235:91–99. doi: 10.1016/j.expneurol.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Neuromuscular Aspects of Physical Activity. Pub: Human Kinetics. 2001 [Google Scholar]
- Gardiner PF. Advanced Nueromuscular Exercise Physiology. Pub: Human Kinetics. 2011 [Google Scholar]
- Gazula VR, Roberts M, Luzzio C, Jawad AF, Kalb RG. Effects of limb exercise after spinal cord injury on motor neuron dendrite structure. J Comp Neurol. 2004;476:130–145. doi: 10.1002/cne.20204. [DOI] [PubMed] [Google Scholar]
- Gilman CP, Mattson MP. Do apoptotic mechanisms regulate synaptic plasticity and growth-cone motility? Neuromolecular Med. 2002;2:197–214. doi: 10.1385/NMM:2:2:197. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–197. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- Giroux N, Rossignol S, Reader TA. Autoradiographic study of alpha1- and alpha2-noradrenergic and serotonin1A receptors in the spinal cord of normal and chronically transected cats. J Comp Neurol. 1999;406:402–414. [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Gulyaeva NV. Non-apoptotic functions of caspase-3 in nervous tissue. Biochemistry (Mosc) 2003;68:1171–1180. doi: 10.1023/b:biry.0000009130.62944.35. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. HSV-mediated gene transfer of the glial cell-derived neurotrophic factor provides an antiallodynic effect on neuropathic pain. Mol Ther. 2003;8:367–375. doi: 10.1016/s1525-0016(03)00185-0. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Houle JD. Demonstration of the potential for chronically injured neurons to regenerate axons into intraspinal peripheral nerve grafts. Exp Neurol. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- Houle JD, Morris K, Skinner RD, Garcia-Rill E, Peterson CA. Effects of fetal spinal cord tissue transplants and cycling exercise on the soleus muscle in spinalized rats. Muscle Nerve. 1999;22:846–856. doi: 10.1002/(sici)1097-4598(199907)22:7<846::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ichiyama RM, Courtine G, Gerasimenko YP, Yang GJ, van den Brand R, Lavrov IA, Zhong H, Roy RR, Edgerton VR. Step training reinforces specific spinal locomotor circuitry in adult spinal rats. J Neurosci. 2008;28:7370–7375. doi: 10.1523/JNEUROSCI.1881-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Jones TB, McDaniel EE, Popovich PG. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–1236. doi: 10.2174/1381612053507468. [DOI] [PubMed] [Google Scholar]
- Keeler BE, Liu G, Siegfried RN, Zhukareva V, Murray M, Houle JD. Acute and prolonged hindlimb exercise elicits different gene expression in motoneurons than sensory neurons after spinal cord injury. Brain Res. 2012;1438:8–21. doi: 10.1016/j.brainres.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajacic A, Ghosh M, Puentes R, Pearse DD, Fouad K. Advantages of delaying the onset of rehabilitative reaching training in rats with incomplete spinal cord injury. Eur J Neurosci. 2009;29:641–651. doi: 10.1111/j.1460-9568.2008.06600.x. [DOI] [PubMed] [Google Scholar]
- Kressler J, Cowan RE, Bigford GE, Nash MS. Reducing cardiometabolic disease in spinal cord injury. Phys Med Rehabil Clin N Am. 2014;25:573–604. doi: 10.1016/j.pmr.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, Smith RR, Magnuson DS. Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010;224:178–187. doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Johnson CS, Wrathall JR. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol. 2007;203:502–511. doi: 10.1016/j.expneurol.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Detloff MR, Miller KN, Santi L, Houle JD. Exercise modulates microRNAs that affect the PTEN/mTOR pathway in rats after spinal cord injury. Exp Neurol. 2012;233:447–456. doi: 10.1016/j.expneurol.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson DS, Smith RR, Brown EH, Enzmann G, Angeli C, Quesada PM, Burke D. Swimming as a model of task-specific locomotor retraining after spinal cord injury in the rat. Neurorehabil Neural Repair. 2009;23:535–545. doi: 10.1177/1545968308331147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BC, Astill SL, Utley A, Ichiyama RM. Movement rehabilitation after spinal cord injuries: emerging concepts and future directions. Brain Res Bull. 2011;84:327–336. doi: 10.1016/j.brainresbull.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Murakami F, Song WJ, Katsumaru H. Plasticity of neuronal connections in developing brains of mammals. Neurosci Res. 1992;15:235–253. doi: 10.1016/0168-0102(92)90045-e. [DOI] [PubMed] [Google Scholar]
- Murray M, Goldberger ME. Restitution of function and collateral sprouting in the cat spinal cord: the partially hemisected animal. J Comp Neurol. 1974;158:19–36. doi: 10.1002/cne.901580103. [DOI] [PubMed] [Google Scholar]
- Norrie BA, Nevett-Duchcherer JM, Gorassini MA. Reduced functional recovery by delaying motor training after spinal cord injury. J Neurophysiol. 2005;94:255–264. doi: 10.1152/jn.00970.2004. [DOI] [PubMed] [Google Scholar]
- N.S.C.I.S. Center. Spinal cord injury facts and figures at a glance. 2013 doi: 10.1179/1079026813Z.000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, Krzyzanowska A, Wong LF, Grist J, Mazarakis ND, Georgievska B, McMahon SB. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther. 2006;13:1101–1109. doi: 10.1016/j.ymthe.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Barriere G, Frigon A, Barthelemy D, Bouyer L, Provencher J, Leblond H, Bernard G. Plasticity of locomotor sensorimotor interactions after peripheral and/or spinal lesions. Brain Res Rev. 2008;57:228–240. doi: 10.1016/j.brainresrev.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Sandrow-Feinberg HR, Izzi J, Shumsky JS, Zhukareva V, Houle JD. Forced exercise as a rehabilitation strategy after unilateral cervical spinal cord contusion injury. J Neurotrauma. 2009;26:721–731. doi: 10.1089/neu.2008.0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrow HR, Shumsky JS, Amin A, Houle JD. Aspiration of a cervical spinal contusion injury in preparation for delayed peripheral nerve grafting does not impair forelimb behavior or axon regeneration. Exp Neurol. 2008;210:489–500. doi: 10.1016/j.expneurol.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Brown EH, Shum-Siu A, Whelan A, Burke DA, Benton RL, Magnuson DS. Swim training initiated acutely after spinal cord injury is ineffective and induces extravasation in and around the epicenter. J Neurotrauma. 2009;26:1017–1027. doi: 10.1089/neu.2008-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson FJ, Reier PJ, Lucas CC, Parmer R. Altered patterns of reflex excitability subsequent to contusion injury of the rat spinal cord. J Neurophysiol. 1992;68:1473–1486. doi: 10.1152/jn.1992.68.5.1473. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Weidner N, Ner A, Salimi N, Tuszynski MH. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–3518. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CR, Crawford MA, Poormasjedi-Meibod MS, Currie KD, Fallavollita A, Yuen V, McNeill JH, Krassioukov AV. Passive hind-limb cycling improves cardiac function and reduces cardiovascular disease risk in experimental spinal cord injury. J Physiol. 2014;592:1771–1783. doi: 10.1113/jphysiol.2013.268367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- W.H.O. Spinal Cord Injury Fact Sheet N. 2013;384 [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]