Abstract
The human gut contains trillions of bacteria, the major phylae of which include Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria. Fecal microbial transplantation (FMT) has been known of for many years but only recently has been subjected to rigorous examination. We review the evidence regarding FMT for recurrent Clostridium difficile infection which has resulted in it being an approved treatment. In addition there is some evidence for its use in both irritable bowel syndrome and inflammatory bowel disease. Further research is needed in order to define the indications for FMT and the most appropriate method of administration.
Keywords: Fecal microbial transplant, Clostridium difficile, Side-effects, Indications, Metabolic disorders
Core tip: Fecal microbial transplantation is approved for the treatment of recurrent Clostridium difficile infection by either nasojejunal administration or colonoscopy. In addition there is some evidence for its use in both irritable bowel syndrome and inflammatory bowel disease. There are, however, reports of side effects including weight gain, diverticulitis and development of autoimmune disease. Treatment for non-approved conditions should be performed in the framework of clinical research trials in order to better define the indications.
INTRODUCTION
The human microbiome is defined as the collection of organisms and their genomes inhabiting locations both in and on humans. Our understanding of the gastrointestinal microbiome (GIMb) has been assisted by the Human Microbiome Project[1] and the Metagenomics of the Human Intestinal Tract investigational groups.
Trillions of bacteria are present in the human gastrointestinal tract and encompass from 2000 to 4000 different species of bacteria, both aerobic and anaerobic. The major phyla include Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria.
The human GIMb is in many ways an additional organ of the body. It has ontogeny, anatomy and physiology and its function may be disturbed in certain pathological conditions. It is possible in some instances to restore normal GIMb function by fecal microbiota transplantation (FMT).
Although the concept of fecal transplantation has become more widely practiced in recent years, it has a long history. More than 2000 years ago Ge Hong used FMT to treat food poisoning and severe diarrhea[2]. Fabricius of Acquapendente in the 16th century described the transplantation of enteric bacteria. The first report of the use of FMT in recent times in more traditional medicine was that of Eiseman et al[3] in the form of a fecal enema obtained from a healthy donor in 4 cases of pseudomembranous colitis.
It is the purpose of this paper to review the current state of fecal transplantation.
FMT FOR CLOSTRIDIUM DIFFICILE INFECTION
Clostridium difficile infection (CDI) is a gram positive anerobic bacillus that produces spores. It is present in the bowel of 4%-13% of asymptomatic people[4-6]. CDI is an increasingly recognized cause of infectious hospital-acquired diarrhea in the developed world[7]. In healthy people it has been thought that it lacks the potential to produce toxins which can result in diarrhea. However, there is an increasing recognition of C. difficile in children, healthy adults and pregnant women[8-10]. Treatment consists of antibiotic therapy with metronidazole, vancomycin or fidaxomicin[11]. About 25% of those suffering from CDI have a recurrence after the first course of treatment[12]. For those patients with a recurrent episode of infection, there is a 40% chance of experiencing another recurrence and for those who have had more than 2 episodes there is a 60% chance of a further episode[12].
In view of the need for a more effective treatment for recurrent CDI, the use of FMT has been examined. In modern clinical research, there is usually an hypothesis that is examined in laboratory animals and then tried in placebo-controlled double blind clinical trials. However, for FMT as treatment for recurrent CDI this order has been reversed. The success of FMT for recurrent CDI has been demonstrated in case series[13-15] and one randomized controlled trial[16].
Hamilton et al[13] have reported their experience with 43 consecutive patients treated with FMT for recurrent CDI at the University of Minnesota from 2009. The FMT was performed by colonoscopy. Fourteen of these patients had inflammatory bowel disease (IBD). There was a mean of 5.9 relapses and the mean success rate was 86%.
Mattila et al[14] reported a retrospective review of 70 patients from 5 medical centers in Finland from 2007-2010. The FMT was performed by colonoscopy. Thirty six (51%) of the patients had the 027 ribotype strain that is more virulent and associated with a higher rate of relapse. All of the 34 patients with the non-027 strain had a resolution within 12 wk compared to 32 of 36 patients (89%) with the 027 ribotype. The four nonresponders had serious comorbidity. Four of the patients who responded experienced a relapse after a year. Two were cured by repeat FMT and 2 by repeat antibiotic treatment.
Brandt et al[15] reported the multi-center United States experience of FMT for recurrent CDI. There were a total of 94 patients of whom there was follow-up data in 77. The primary cure rate was 91%. The secondary cure rate for the 7 patients that did not respond or relapsed was 98%. The mean follow-up period was 17 mo and was up to 68 mo.
There has recently been published a randomized controlled open-label trial of patients with at least one episode of recurrent CDI from Holland and Finland. The numbers of patients involved was small-13 in each of three groups. The groups were randomized to receive initial vancomycin for 4 d and then either bowel lavage, bowel lavage and donor feces through a nasoduodenal tube or just vancomycin alone. The study was stopped prematurely after an interim analysis revealed a resolution of CDI of 81% in the group receiving feces by nasoduodenal infusion as compared to 31% receiving vancomycin alone and 23% receiving vancomycin with bowel lavage[16] (Figure 1). These were much smaller numbers than initially planned for. Thus this study is consistent with previous case reports.
Figure 1.
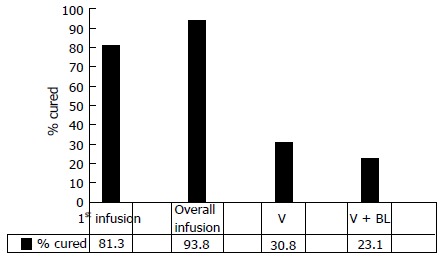
Rates of cure after treatment of recurrent C. difficile infection by nasojejunal infusion of donor feces. The data show first infusion, overall infusion after retreatment for a treatment failure, vancomycin (V) treatment, and vancomycin treatment and bowel lavage (V + BL) (16).
In view of the limited data from randomized controlled trials a systematic review of FMT involving 317 patients from 27 case series and reports has been published[17]. Two thirds of these were case series. There was resolution of the disease in 92% of cases, 89% after a single treatment. The lowest rate of resolution was by infusion via gastroscopy or nasojejunal tube. This is especially relevant regarding the above mentioned randomized trial[16], since the number of cases was very small due to the early termination of the trial mandated by the review committee. Although there is great heterogeneity between the various case reports and case series making up this systematic review, there was found to be a higher relapse rate in patients who received both bowel lavage and antibiotics before FMT (4/33, 12.1% vs 5/150, 3.3%).
In the light of this data FMT is now recognized as treatment for a third recurrence of CDI[18].
The rationale for use of FMT has been shown in a mouse model of mice treated with clindamycin and then infected with Clostridium difficile that had been isolated from patients with CDI. The mice developed chronic disease and responded to the administration of homogenized feces from healthy mice[19]. Thus the data that is available support the use of FMT for the treatment of recurrent CDI.
FMT - HOW TO DO IT
The first issue to be addressed is the donor of the feces. Initially the donors of the feces were “healthy donors” with no other details provided[3]. In some case the stools of medical residents were used. Until 2011, a partner or family member was the most frequent source of donor. It was assumed that any infectious diseases would already have been transmitted between the donor and recipient. This has not been examined by evidence-based medicine. More recently the NIH in the United States has required that donor stool be examined for C difficile toxin, enteric bacterial pathogens (including Listeria monocytogenes, Vibrio cholera and Vibrio parahemolyticus), parasites including Giardia (via antigen test), Cryptosporidium (antigen test), Isospora (acid-fast stain) and Rotavirus. In addition the donor blood needs to be screened for hepatitis A (IgM), B (HBsAg, anti-HBc-IgG and IgM, and anti-HBsAg) and C (HCV antibody) viruses, HIV type 1 and 2 and syphilis. In addition H. pylori should be tested for. The Israel Ministry of Health protocol is shown in Table 1. The tests for the donor may not be entirely covered by the medical insurance organizations since they may not be indicated for the routine medical care of the stool donor.
Table 1.
The Israel Ministry of Health protocol for donor screening for fecal microbial transplantation
| Patient eligibility for FMT |
| 1 Questionnaire to exclude potentially transmissible diseases |
| (1) Practices protected sexual intercourse (or in a monogamous relationship with a healthy partner) |
| (2) Has not had tattooing in the previous six months |
| (3) Does not have any known infectious diseases |
| (4) No drug abuse |
| (5) Has not travelled to the Far-East, India, Africa, Central or South America within the previous six months |
| (6) Has no known autoimmune disease |
| (7) There is no history of any gastrointestinal disease, including inflammatory bowel disease, celiac disease or irritable bowel disease |
| (8) Has not had any previous bowel resection |
| (9) There is no history of infectious diarrhea in the previous 12 mo |
| (10) Has not received antibiotic therapy in the previous 2 mo |
| 2 Laboratory tests |
| (1) Negative stool culture |
| (2) Negative stool microscopy, including Giardia, Cryptosporidium and Isospora beli |
| (3) Negative Clostridium difficile toxin |
| (4) Negative serological testing for HIV, HBV, HCV, HAV, VDRL, CMV |
| (5) Negative test for Helicobacter pylori (either C13 urea breath testing, stool antigen or serum antibodies) |
FMT: Fecal microbial transplantation. HCV: Hepatitis C virus; HBV: Hepatitis B virus; CMV: Cucumber mosaic virus; VDRL: Venereal disease research laboratory; HIV: Human immunodeficiency virus.
There is a growing trend for DIY stool transplants with instructions being available on the Internet- for example www.thepoweerofpoop.com. There are cases when patients refer themselves to centers performing FMT after self-administration of unscreened donor stool from a family member has failed[20]. The only systematic review of FMT suggested that stool from a related donor (spouse or intimate partner) resulted in a higher rate of cure (93.3%) as compared to an unrelated donor (84%).
The FMT program at the University of Minnesota has reported their experience with frozen/thawed or fresh fecal preparations from universal donors[13] with cure rates of 90% for frozen material and 92% for fresh material. This is higher than those reported with patient selected donors (70%), although only small numbers of patients were included in each group- 10 individual donors and 33 standard donors. A recent report of increase in weight after fecal transplantation from a related obese donor provides a note of caution[21]. For these reasons we believe that the use of individual donors should not be the first choice for obtaining feces for transplantation.
The donor needs to provide the stool sample into a clean plastic container. The amount is variable although 50 g in 250 mL of diluent is a common method. Different fluids have been used as the suspending fluid, including milk, water and saline. The resolution rates with saline and milk are 86.2% and 88.6%, with recurrence rates of 3.0% and 3.2%[17]. The use of water resulted in a resolution rate of 98.5% and recurrence of 7.8%. Three hundred milliliter is the usual dose for colonic FMT and 60 cc for upper GI tract FMT.
The patients who will receive the FMT need to have a large volume colonic lavage prior to the procedure. This is thought to cleanse the spores of C difficile that are responsible for the recurrence of the infection. There are different variations and some reports include loperamide if receiving a fecal enema[22], and proton pump inhibitor (to reduce the bactericidal effect of gastric acid) if the infusion of stools is via a nasogastric or nasojejunal route[23,24].
Some groups maintain the patient on vancomycin orally until the time of the FMT[16,25]. This practice is thought to reduce the vegetative forms of C. difficile since vancomycin has no action on the spores. However, the systematic analysis of Gough et al[17] found a higher relapse rate with the combination of bowel lavage and antibiotics. Our group policy is to discontinue antibiotics two days prior to FMT.
Recently, there has been a report of the use of capsules containing frozen stool. Twenty patients with recurrent CDI were treated with 15 FMT capsules daily for 2 d. The resolution rate was 70%. The 6 failures were retreated and 4 of these had resolution, resulting in an overall response rate of 90%. A total of 30% of the patients experienced mild abdominal complaints that resolved within 72 h. This is a preliminary study that needs to be repeated and expanded but is promising and if successful will probably replace the current methods of administration of the donor stools.
SAFETY AND COMPLICATIONS
Two studies have provided information regarding long-term follow up after FMT. A study from Finland of 70 patients had no data on complications[14], although the authors state “none of our patients had any serious adverse effects that could be related to fecal transplantation”. A multi-center North American study reported data from more than 3 mo follow up in 77 of the 94 eligible patients. Ninety-seven percent of the patients stated that they would choose FMT again as treatment for a recurrent CDI and 53% would prefer it as a first option. Twenty-seven percent of the patients developed abdominal pain following FMT but it was minor and resolved within 10 d. Four patients developed an autoimmune disease-peripheral neuropathy, rheumatoid arthritis, Sjogrens and thrombocytopenia[15]. We have recently treated a patient with severe CDI who required 2 consecutive FMTs and developed thrombocytopenia after each transplantation. Recently, there has been a report of diverticulitis following FMT for CDI[26].
The field of FMT is rapidly developing and as experience accumulates there are more reports of possible side effects or complications. Thus it is important to carry out these procedures in centers with approved protocols and to discourage patient or non-physician supervised self-administration.
CDI is common in patients who are immunosuppressed. Recently a retrospective study from 16 medical centers in the United States reported their experience in FMT in 80 immunosuppressed patients with severe or recurrent or refractory CDI[27]. The majority of the patients were immunosuppressed due to solid organ transplantation or treatment for IBD. The cure rate was 79% for the first time and 89% overall. There was a 15% incidence of serious adverse events within 12 wk. Five of the 36 IBD patients had post-FMT disease flare. Thus it appears that successful FMT is possible in immunosuppressed patients, although with a slightly reduced success rate and a higher rate of adverse events.
OTHER USES FOR FMT
IBD
FMT appears to be an established treatment for CDI and attention has focused also on IBD. Gastrointestinal microbiome dysbiosis has an important role in the pathogenesis of IBD[28]. In addition a recent study employing the molecular biology technique of terminal restriction fragment length polymorphism (T-RFLP) to profile the bacterial species in fecal samples has enabled the calculation of a discriminant score which was shown to be a biomarker for disease activity in UC[29]. The first FMT for ulcerative colitis (UC) was reported in 1989[30] and described the reversal of the UC that Bennet (one of the authors of the paper) had suffered from for 7 years by FMT administered as fecal enemas. Case series have suggested some role for FMT in UC[31-33]. However, a recent report[34] of a prospective study of FMT in 5 adult patients with moderate to severe UC who had failed various immunosuppressive therapies adds a note of caution. None of the patients were receiving concomitant immunosuppressive therapy. None of the patients entered clinical remission after 12 wk of follow-up and only one had some clinical improvement. Additionally all of the 5 patients had fever and an elevation of CRP after the FMT and a worsening of the diarrhea the day after the procedure. In the patient who experienced some response there was an alteration of the fecal flora after FMT. Another recent trial of FMT for UC did not demonstrate a significant effect[35] in 6 patients but there was a change in the gut microbiota. The alteration of gut microbiota was temporary and it may be necessary to undergo repeated transplantation in order to maintain the altered gut microbiota. A phase 1 trial of FMT for 9 pediatric UC patients with mild-to-moderate activity reported no serious adverse effects and found that 7 of these (79%) had responded within a week[36]. It may be that certain population sub-groups suffering from UC will derive benefit from FMT. A recent systematic review and meta-analysis of FMT therapy for IBD showed a clinical remission of 22% for UC[37].
Crohn’s disease (CD) has also been treated by FMT. The data are limited to case reports and small case series[32,38]. The recent systematic review and meta-analysis of FMT as therapy for IBD found higher pooled estimate of clinical remission for CD, 60.5% as compared to UC 22%[37]. There have been 2 previous systematic reviews of FMT for IBD[39,40]. The success rate of FMT for adult IBD patients was 77.8%[39] but outcomes were measured by “success rates” only. There were also other methodological problems[37]. The other review[40] noted endoscopic and histologic remission of 63% in 24 patients but this too has been criticized for methodological flaws[37]. A note of caution is necessary following the report of bacteremia after FMT in a patient with Crohns and CDI[41].
Thus the jury is out regarding the utility of FMT for IBD and further work is required in order to define the effect of different methods of delivery, changes on the microbiome and the interaction between phases of the illness (induction or maintenance) and the impact of additional therapies.
Irritable bowel disease
There is evidence linking dysbiosis to irritable bowel disease (IBS)[42,43] and thus there is a question regarding the possibility of FMT for treatment. There have been reports of a favorable outcome after FMT in diarrhea - predominant IBS[32]. A recent report of single-center experience of 13 patients with IBS of whom 9 had diarrhea-predominant, 3 constipation-predominant and one mixed-type[44], found resolution or improvement in symptoms in 70% of the patients overall. Presumably, the small sample size prevented the reporting of the response rate in each group separately. This subject has recently been reviewed[45]. There is a need for further research to define the role of FMT in the treatment of this common condition.
FMT and metabolic disorders
It is now apparent that there is an interaction between the microbiome of the intestinal tract and the metabolism of the human host and that there is a link to obesity[46]. Although there are reports of changes in the ratio of Firmicutes/Bacteroides with human obesity[47] other groups have not found such changes[48,49]. There are reports in mice of the induction of a phenotype of the metabolic syndrome via fecal transplants[50]. This complex subject has been reviewed[46,51,52]. Furthermore in mice the intestinal microbiota plays a role in the development of non-alcoholic fatty liver disease[53]. Recently stools from twins discordant for obesity has been shown to promote or impair the development of obesity in adult male germ-free mice[54].
In humans there is a single study reporting that FMT using stool from lean donors improves insulin sensitivity in obese male individuals concomitant with an increase in butyrate-producing intestinal bacteria[55].
We are currently conducting a randomized controlled trial of FMT in obese individuals undergoing screening colonoscopy in order to determine if there is a clinical effect on obesity in humans (clinical trials.gov NCT02336789).
Other conditions
Immune thrombocytopenia (ITP) was reported to be reversed in a patient treated with FMT for UC - but only published in an abstract form[55]. Other fields include autoimmune disease, allergic disorders, and neuropsychiatric disorders. This exciting field has recently been reviewed[56].
In summary, fecal microbial transplantation is now prime time treatment for refractory Cl. difficile infection. In addition, it may be of use for treating other disorders of the gastrointestinal tract including irritable bowel syndrome and IBD. Further research is needed to define the optimal method of administration of the stools as well as the indications for treatment in other conditions. In addition there needs to be vigilance for the development of side-effects related to this technique. For this reason it is important that treatments for other indications be conducted in the framework of research protocols.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 4, 2015
First decision: March 20, 2015
Article in press: April 30, 2015
P- Reviewer: Oz HS, Saniabadi AR S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang F, Luo W, Shi Y, Fan Z, Ji G Should we standardize the 1,700-year-old fecal microbiota transplantation? Am J Gastroenterol 2012; 107: 1755; author reply p.1755-p.1756. doi: 10.1038/ajg.2012.251. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman B, Silen W, Bascom GS, KauvaR AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- 4.Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut. Curr Opin Microbiol. 2011;14:82–91. doi: 10.1016/j.mib.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Vedantam G, Clark A, Chu M, McQuade R, Mallozzi M, Viswanathan VK. Clostridium difficile infection: toxins and non-toxin virulence factors, and their contributions to disease establishment and host response. Gut Microbes. 2012;3:121–134. doi: 10.4161/gmic.19399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To KB, Napolitano LM. Clostridium difficile infection: update on diagnosis, epidemiology, and treatment strategies. Surg Infect (Larchmt) 2014;15:490–502. doi: 10.1089/sur.2013.186. [DOI] [PubMed] [Google Scholar]
- 8.Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children’s hospitals in the United States, 2001-2006. Pediatrics. 2008;122:1266–1270. doi: 10.1542/peds.2008-0469. [DOI] [PubMed] [Google Scholar]
- 9.Pituch H. Clostridium difficile is no longer just a nosocomial infection or an infection of adults. Int J Antimicrob Agents. 2009;33 Suppl 1:S42–S45. doi: 10.1016/S0924-8579(09)70016-0. [DOI] [PubMed] [Google Scholar]
- 10.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chahine EB, Sucher AJ, Mantei K. Fidaxomicin: a novel macrolide antibiotic for Clostridium difficile infection. Consult Pharm. 2014;29:614–624. doi: 10.4140/TCP.n.2014.614.. [DOI] [PubMed] [Google Scholar]
- 12.Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008;359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton MJ, Weingarden AR, Sadowsky MJ, Khoruts A. Standardized frozen preparation for transplantation of fecal microbiota for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:761–767. doi: 10.1038/ajg.2011.482. [DOI] [PubMed] [Google Scholar]
- 14.Mattila E, Uusitalo-Seppälä R, Wuorela M, Lehtola L, Nurmi H, Ristikankare M, Moilanen V, Salminen K, Seppälä M, Mattila PS, et al. Fecal transplantation, through colonoscopy, is effective therapy for recurrent Clostridium difficile infection. Gastroenterology. 2012;142:490–496. doi: 10.1053/j.gastro.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, Park T, Stollman N, Rohlke F, Surawicz C. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–1087. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 16.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 17.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 18.Surawicz CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH, McFarland LV, Mellow M, Zuckerbraun BS. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498; quiz 499. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 19.Lawley TD, Clare S, Walker AW, Stares MD, Connor TR, Raisen C, Goulding D, Rad R, Schreiber F, Brandt C, et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 2012;8:e1002995. doi: 10.1371/journal.ppat.1002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandt LJ. American Journal of Gastroenterology Lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in treatment of C. difficile infection. Am J Gastroenterol. 2013;108:177–185. doi: 10.1038/ajg.2012.450. [DOI] [PubMed] [Google Scholar]
- 21.Alang N, Kelly CR. Weight Gain After Fecal Microbiota Transplantation. Open Forum Infect Dis. 2015;4:ofv004–ofv004. doi: 10.1093/ofid/ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persky SE, Brandt LJ. Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol. 2000;95:3283–3285. doi: 10.1111/j.1572-0241.2000.03302.x. [DOI] [PubMed] [Google Scholar]
- 23.Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, Kelly C, Khoruts A, Louie T, Martinelli LP, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. 2011;9:1044–1049. doi: 10.1016/j.cgh.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aas J, Gessert CE, Bakken JS. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis. 2003;36:580–585. doi: 10.1086/367657. [DOI] [PubMed] [Google Scholar]
- 25.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 26.Mandalia A, Kraft CS, Dhere T. Diverticulitis after fecal microbiota transplant for C. difficile infection. Am J Gastroenterol. 2014;109:1956–1957. doi: 10.1038/ajg.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly CR, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, Aroniadis O, Barto A, Borody T, Giovanelli A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 29.Fukuda K, Fujita Y. Determination of the discriminant score of intestinal microbiota as a biomarker of disease activity in patients with ulcerative colitis. BMC Gastroenterol. 2014;14:49. doi: 10.1186/1471-230X-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet. 1989;1:164. doi: 10.1016/s0140-6736(89)91183-5. [DOI] [PubMed] [Google Scholar]
- 31.Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, Siarakas S. Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol. 2004;38:475–483. doi: 10.1097/01.mcg.0000128988.13808.dc. [DOI] [PubMed] [Google Scholar]
- 32.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 33.Borody TJ, Warren EF, Leis S, Surace R, Ashman O. Treatment of ulcerative colitis using fecal bacteriotherapy. J Clin Gastroenterol. 2003;37:42–47. doi: 10.1097/00004836-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Angelberger S, Reinisch W, Makristathis A, Lichtenberger C, Dejaco C, Papay P, Novacek G, Trauner M, Loy A, Berry D. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol. 2013;108:1620–1630. doi: 10.1038/ajg.2013.257. [DOI] [PubMed] [Google Scholar]
- 35.Kump PK, Gröchenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 36.Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H, Cloney D, Kugathasan S. Safety, tolerability, and clinical response after fecal transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013;56:597–601. doi: 10.1097/MPG.0b013e318292fa0d. [DOI] [PubMed] [Google Scholar]
- 37.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon H, Harbord M. A patient with severe Crohn’s colitis responds to Faecal Microbiota Transplantation. J Crohns Colitis. 2014;8:256–257. doi: 10.1016/j.crohns.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Sha S, Liang J, Chen M, Xu B, Liang C, Wei N, Wu K. Systematic review: faecal microbiota transplantation therapy for digestive and nondigestive disorders in adults and children. Aliment Pharmacol Ther. 2014;39:1003–1032. doi: 10.1111/apt.12699. [DOI] [PubMed] [Google Scholar]
- 40.Anderson JL, Edney RJ, Whelan K. Systematic review: faecal microbiota transplantation in the management of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36:503–516. doi: 10.1111/j.1365-2036.2012.05220.x. [DOI] [PubMed] [Google Scholar]
- 41.Quera R, Espinoza R, Estay C, Rivera D. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis. 2014;8:252–253. doi: 10.1016/j.crohns.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Collins SM, Chang C, Mearin F. Postinfectious Chronic Gut Dysfunction: From Bench to Bedside. Am J Gastroenterol. 2012;1 Suppl:2–8. [Google Scholar]
- 43.Mayer EA, Savidge T, Shulman RJ. Brain-gut microbiome interactions and functional bowel disorders. Gastroenterology. 2014;146:1500–1512. doi: 10.1053/j.gastro.2014.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation the answer for irritable bowel syndrome? A single-center experience. Am J Gastroenterol. 2014;109:1831–1832. doi: 10.1038/ajg.2014.295. [DOI] [PubMed] [Google Scholar]
- 45.Pinn DM, Aroniadis OC, Brandt LJ. Is fecal microbiota transplantation (FMT) an effective treatment for patients with functional gastrointestinal disorders (FGID)? Neurogastroenterol Motil. 2015;27:19–29. doi: 10.1111/nmo.12479. [DOI] [PubMed] [Google Scholar]
- 46.Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest. 2011;121:2126–2132. doi: 10.1172/JCI58109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 48.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 49.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, Flint HJ. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes (Lond) 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 50.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 51.Shen J, Obin MS, Zhao L. The gut microbiota, obesity and insulin resistance. Mol Aspects Med. 2013;34:39–58. doi: 10.1016/j.mam.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Gangarapu V, Yıldız K, Ince AT, Baysal B. Role of gut microbiota: obesity and NAFLD. Turk J Gastroenterol. 2014;25:133–140. doi: 10.5152/tjg.2014.7886. [DOI] [PubMed] [Google Scholar]
- 53.Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 54.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.e7. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 56.Xu MQ, Cao HL, Wang WQ, Wang S, Cao XC, Yan F, Wang BM. Fecal microbiota transplantation broadening its application beyond intestinal disorders. World J Gastroenterol. 2015;21:102–111. doi: 10.3748/wjg.v21.i1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]


